Chimeric RNAs Discovered by RNA Sequencing and Their Roles in Cancer and Rare Genetic Diseases
Abstract
1. Introduction
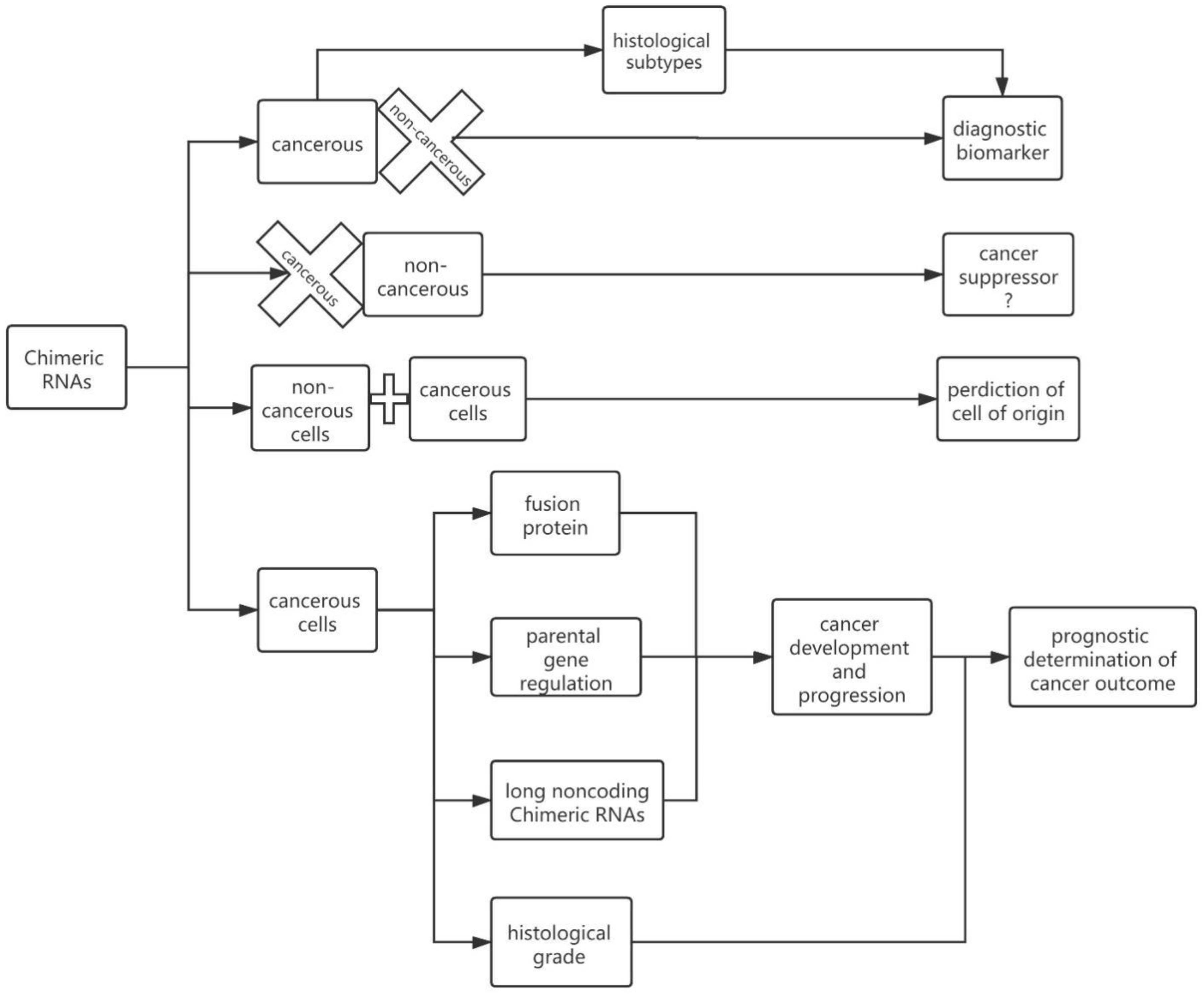
2. Chimeric RNAs
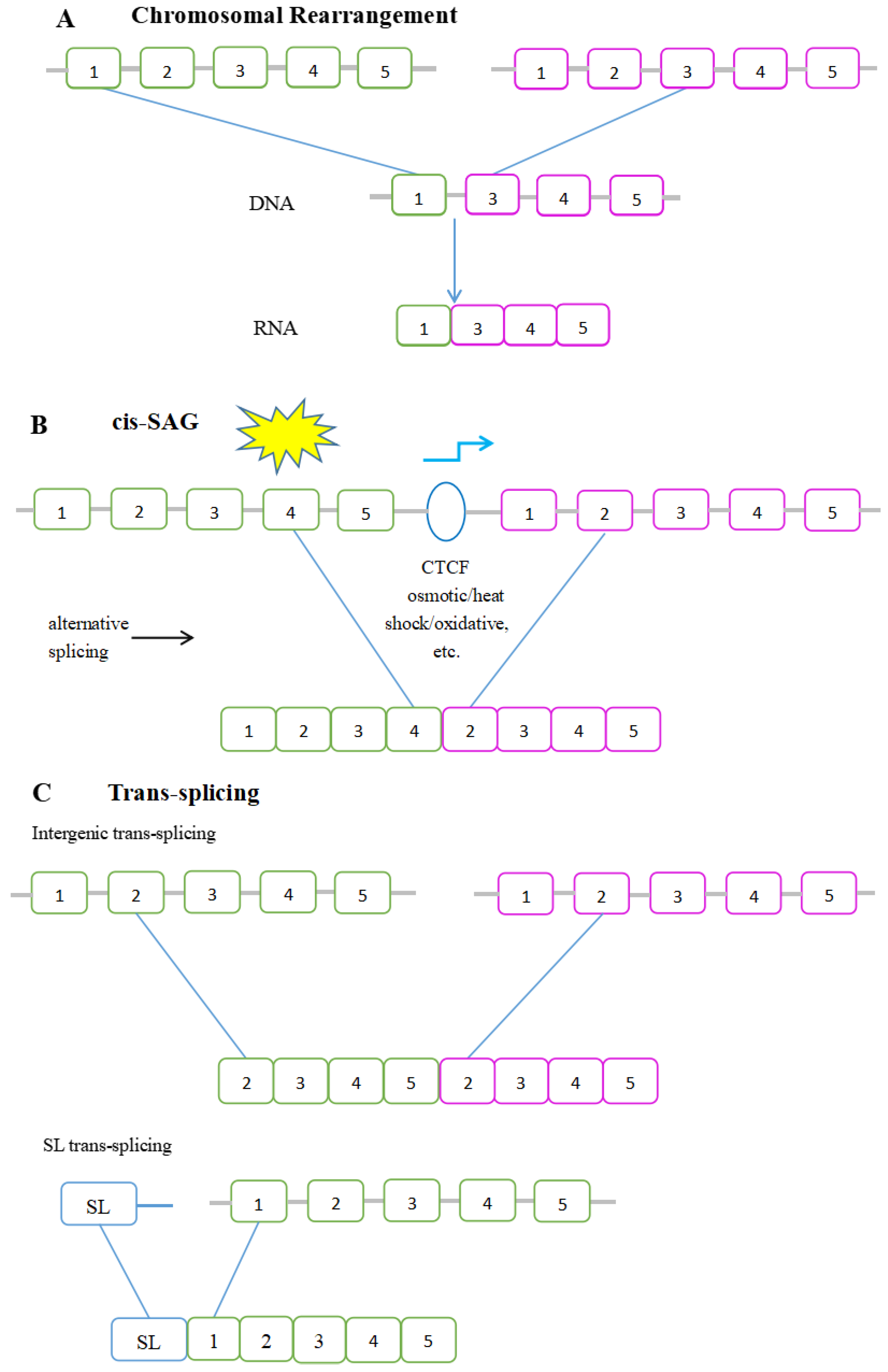
3. Formation of Chimeric RNAs
3.1. Cis-Splicing of Adjacent Genes
Poly(A) Signal and Transcription Termination in Transcriptional Read Through
3.2. Trans-Splicing
3.3. Gene Fusion
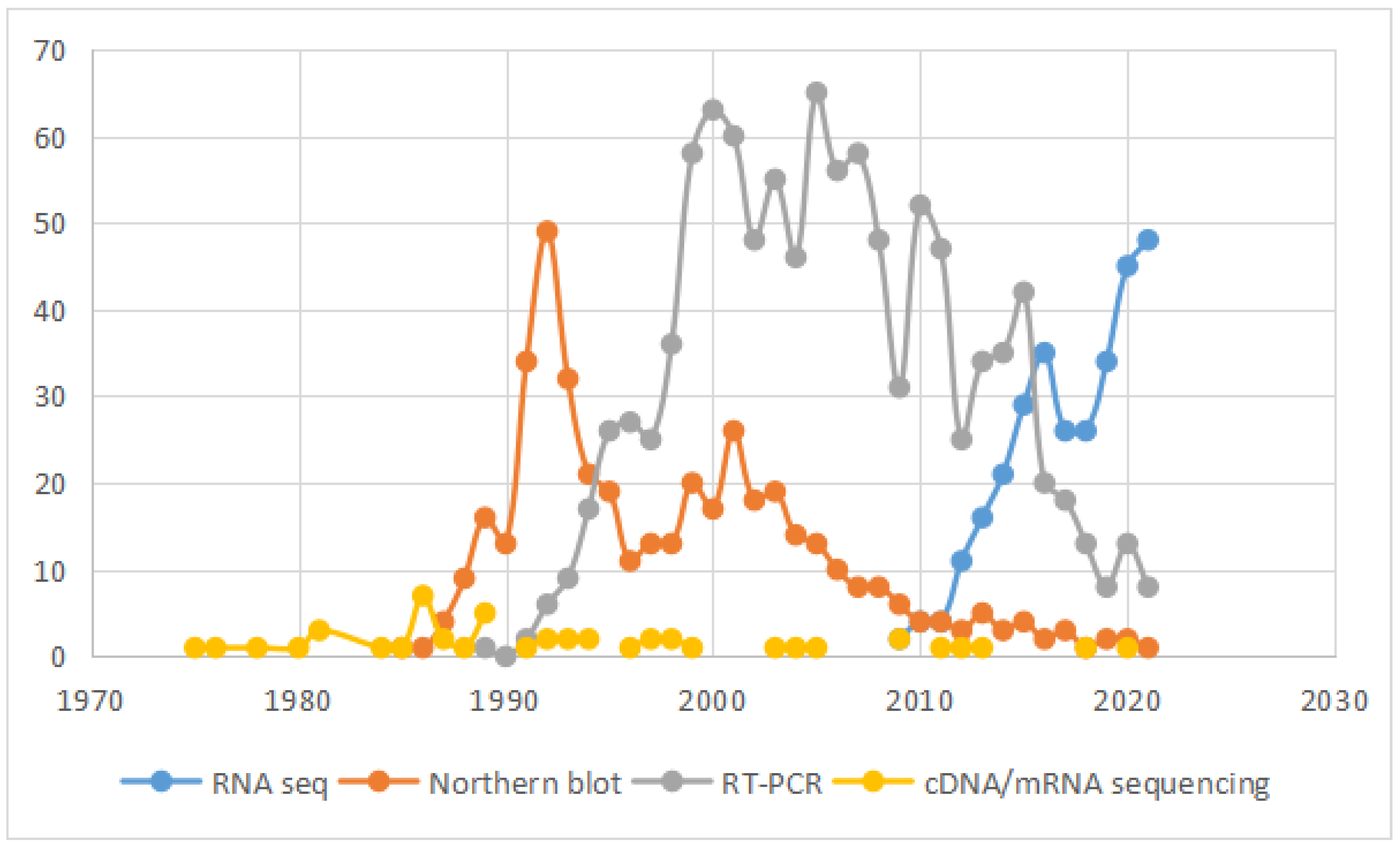
4. RNA Sequencing
4.1. Applications of RNA-Seq in Chimeric RNAs
4.2. Computational Methods for Identifying Chimeric RNAs
5. Chimeric RNAs in Cancer
5.1. Esophageal Cancer
5.2. NSCLC
5.3. Gastric Cancer
5.4. Colorectal Cancer
5.5. Tumors of Reproductive System
5.6. Prostate Cancer
5.7. Renal Cell Carcinoma
5.8. Bladder Cancer
5.9. Head and Neck Squamous Cell Carcinoma
5.10. Sarcoma
5.11. Others
6. Rare Genetic Diseases and Psychological Disorders
6.1. Autism
6.2. Schizophrenia
6.3. Intellectual Disability
7. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Xiang, Y.; Ye, Y.; Zhang, Z.; Han, L. Maximizing the Utility of Cancer Transcriptomic Data. Trends Cancer 2018, 4, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.; Marks, L.; May, G.H.W.; Wilson, J.B. The genetic basis of disease. Essays Biochem. 2018, 62, 643–723. [Google Scholar] [CrossRef] [PubMed]
- Strynatka, K.A.; Gurrola-Gal, M.C.; Berman, J.N.; McMaster, C.R. How Surrogate and Chemical Genetics in Model Organisms Can Suggest Therapies for Human Genetic Diseases. Genetics 2018, 208, 833–851. [Google Scholar] [CrossRef]
- Eeles, R.A. Screening for hereditary cancer and genetic testing, epitomized by breast cancer. Eur. J. Cancer 1999, 35, 1954–1962. [Google Scholar] [CrossRef]
- Shi, X.; Singh, S.; Lin, E.; Li, H. Chimeric RNAs in cancer. Adv. Clin. Chem. 2021, 100, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Finta, C.; Zaphiropoulos, P.G. Intergenic mRNA molecules resulting from trans-splicing. J. Biol. Chem. 2002, 277, 5882–5890. [Google Scholar] [CrossRef]
- Singh, S.; Qin, F.; Kumar, S.; Elfman, J.; Lin, E.; Pham, L.; Yang, A.; Li, H. The landscape of chimeric RNAs in non-diseased tissues and cells. Nucleic Acids Res. 2020, 48, 1764–1778. [Google Scholar] [CrossRef]
- Mukherjee, S.; Frenkel-Morgenstern, M. Evolutionary impact of chimeric RNAs on generating phenotypic plasticity in human cells. Trends Genet. 2022, 38, 4–7. [Google Scholar] [CrossRef]
- Akiva, P.; Toporik, A.; Edelheit, S.; Peretz, Y.; Diber, A.; Shemesh, R.; Novik, A.; Sorek, R. Transcription mediated gene fusion in the human genome. Genome Res. 2006, 16, 30–36. [Google Scholar] [CrossRef]
- Kim, D.S.; Huh, J.W.; Kim, H.S. HYBRIDdb: A database of hybrid genes in the human genome. BMC Genom. 2007, 8, 128. [Google Scholar] [CrossRef]
- Parra, G.; Reymond, A.; Dabbouseh, N.; Dermitzakis, E.T.; Castelo, R.; Thomson, T.M.; Antonarakis, S.E.; Guigó, R. Tandem chimerism as a means to increase protein complexity in the human genome. Genome Res. 2006, 16, 37–44. [Google Scholar] [CrossRef]
- Audano, P.A.; Sulovari, A.; Graves-Lindsay, T.A.; Cantsilieris, S.; Sorensen, M.; Welch, A.E.; Dougherty, M.L.; Nelson, B.J.; Shah, A.; Dutcher, S.K.; et al. Characterizing the major structural variant alleles of the human genome. Cell 2019, 176, 663–675.e19. [Google Scholar] [CrossRef]
- Chwalenia, K.; Facemire, L.; Li, H. Chimeric RNAs in cancer and normal physiology. Wiley Interdiscip. Rev. RNA 2017, 8, e1427. [Google Scholar] [CrossRef]
- Nowacki, M.; Vijayan, V.; Zhou, Y.; Schotanus, K.; Doak, T.G.; Landweber, L.F. RNA-mediated epigenetic programming of a genome-rearrangement pathway. Nature 2008, 451, 153–158. [Google Scholar] [CrossRef]
- Fang, W.; Landweber, L.F. RNA-mediated genome rearrangement: Hypotheses and evidence. Bioessays 2013, 35, 84–87. [Google Scholar] [CrossRef]
- Yan, Z.; Huang, N.; Wu, W.; Chen, W.; Jiang, Y.; Chen, J.; Huang, X.; Wen, X.; Xu, J.; Jin, Q.; et al. Genome-wide colocalization of RNA-DNA interactions and fusion RNA pairs. Proc. Natl. Acad. Sci. USA 2019, 116, 3328–3337. [Google Scholar] [CrossRef]
- Friedrich, S.; Sonnhammer, E.L.L. Fusion transcript detection using spatial transcriptomics. BMC Med. Genom. 2020, 13, 110. [Google Scholar] [CrossRef]
- Chwalenia, K.; Qin, F.; Singh, S.; Li, H. A cell-based splicing reporter system to identify regulators of cis-splicing between adjacent genes. Nucleic Acids Res. 2019, 47, e24. [Google Scholar] [CrossRef]
- Barresi, V.; Cosentini, I.; Scuderi, C.; Napoli, S.; Bella, V.D.; Spampinato, G.; Condorelli, D.F. Fusion Transcripts of Adjacent Genes: New Insights into the World of Human Complex Transcripts in Cancer. Int. J. Mol. Sci. 2019, 20, 5252. [Google Scholar] [CrossRef]
- Qin, F.; Song, Y.; Zhang, Y.; Facemire, L.; Frierson, H.; Li, H. Role of CTCF in Regulating SLC45A3-ELK4 Chimeric RNA. PLoS ONE 2016, 11, e0150382. [Google Scholar] [CrossRef]
- Qin, F.; Song, Z.; Babiceanu, M.; Song, Y.; Facemire, L.; Singh, R.; Adli, M.; Li, H. Discovery of CTCF-sensitive Cis-spliced fusion RNAs between adjacent genes in human prostate cells. PLoS Genet. 2015, 11, e1005001. [Google Scholar] [CrossRef]
- Chwalenia, K.; Qin, F.; Singh, S.; Tangtrongstittikul, P.; Li, H. Connections between transcription downstream of genes and cis-SAGe chimeric RNA. Genes 2017, 8, 338. [Google Scholar] [CrossRef]
- Vilborg, A.; Sabath, N.; Wiesel, Y.; Nathans, J.; Levy-Adam, F.; Yario, T.A.; Steitz, J.A.; Shalgi, R. Comparative analysis reveals genomic features of stress-induced transcriptional readthrough. Proc. Natl. Acad. Sci. USA 2017, 114, E8362–E8371. [Google Scholar] [CrossRef]
- Lai, J.; An, J.; Seim, I.; Walpole, C.; Hoffman, A.; Moya, L.; Srinivasan, S.; Perry-Keene, J.L.; Wang, C. Fusion transcript loci share many genomic features with nonfusion loci. BMC Genom. 2015, 16, 1021. [Google Scholar] [CrossRef]
- Vilborg, A.; Passarelli, M.C.; Yario, T.A.; Tycowski, K.T.; Steitz, J.A. Widespread Inducible Transcription Downstream of Human Genes. Mol. Cell 2015, 59, 449–461. [Google Scholar] [CrossRef]
- Hennig, T.; Michalski, M.; Rutkowski, A.J.; Djakovic, L.; Whisnant, A.W.; Friedl, M.S.; Jha, B.A.; Baptista, M.A.P.; L’Hernault, A.; Erhard, F.; et al. HSV-1-induced disruption of transcription termination resembles a cellular stress response but selectively increases chromatin accessibility downstream of genes. PLoS Pathog. 2018, 14, e1006954. [Google Scholar] [CrossRef]
- Duc, C.; Sherstnev, A.; Cole, C.; Barton, G.J.; Simpson, G.G. Transcription termination and chimeric RNA formation controlled by Arabidopsis thaliana FPA. PLoS Genet. 2013, 9, e1003867. [Google Scholar] [CrossRef]
- Taniue, K.; Akimitsu, N. Fusion Genes and RNAs in Cancer Development. Noncoding RNA 2021, 7, 10. [Google Scholar] [CrossRef]
- Hong, E.M.; Ingemarsdotter, C.K.; Lever, A.M.L. Therapeutic applications of trans-splicing. Br. Med. Bull. 2020, 136, 4–20. [Google Scholar] [CrossRef]
- Zaphiropoulos, P.G. Trans-splicing in higher eukaryotes: Implications for cancer development? Front. Genet. 2011, 2, 92. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Ma, X.; Sklar, J. Gene fusions and RNA trans-splicing in normal and neoplastic human cells. Cell Cycle 2009, 8, 218–222. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Mor, G.; Sklar, J. A Neoplastic Gene Fusion Mimics Trans-Splicing of RNAs in Normal Human Cells. Science 2008, 321, 1357–1361. [Google Scholar] [CrossRef]
- Xie, Z.; Tang, Y.; Su, X.; Cao, J.; Zhang, Y.; Li, H. PAX3-FOXO1 escapes miR-495 regulation during muscle differentiation. RNA Biol. 2019, 16, 144–153. [Google Scholar] [CrossRef]
- Yuan, H.; Qin, F.; Movassagh, M.; Park, H.; Golden, W.; Xie, Z.; Zhang, P.; Sklar, J.; Li, H. A chimeric RNA characteristic of rhabdomyosarcoma in normal myogenesis process. Cancer Discov. 2013, 3, 1394–1403. [Google Scholar] [CrossRef]
- Kim, P.; Zhou, X. FusionGDB: Fusion gene annotation DataBase. Nucleic Acids Res. 2019, 47, D994–D1004. [Google Scholar] [CrossRef]
- Rowley, J.D. Chromosomal patterns in myelocytic leukemia. N. Engl. J. Med. 1973, 289, 220–221. [Google Scholar] [CrossRef]
- Lucas, G.S.; Ardern, J.C. BCR-ABL rearrangements in acute lymphoblastic leukaemia. Lancet 1991, 337, 1548. [Google Scholar] [CrossRef]
- Yang, K.; Fu, L. Mechanisms of resistance to BCR-ABL TKIs and the therapeutic strategies: A review. Crit. Rev. Oncol. Hematol. 2015, 93, 277–292. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Barr, F.G. Therapeutic Approaches Targeting PAX3-FOXO1 and Its Regulatory and Transcriptional Pathways in Rhabdomyosarcoma. Molecules 2018, 23, 2798. [Google Scholar] [CrossRef]
- Ommer, J.; Selfe, J.L.; Wachtel, M.; O’Brien, E.M.; Laubscher, D.; Roemmele, M.; Kasper, S.; Delattre, O.; Surdez, D.; Petts, G.; et al. Aurora A Kinase Inhibition Destabilizes PAX3-FOXO1 and MYCN and Synergizes with Navitoclax to Induce Rhabdomyosarcoma Cell Death. Cancer Res. 2020, 80, 832–842. [Google Scholar] [CrossRef]
- Tao, H.; Shi, L.; Zhou, A.; Li, H.; Gai, F.; Huang, Z.; Che, N.; Liu, Z. Distribution of EML4-ALK fusion variants and clinical outcomes in patients with resected non-small cell lung cancer. Lung Cancer 2020, 149, 154–161. [Google Scholar] [CrossRef]
- Su, Y.; Long, X.; Song, Y.; Chen, P.; Li, S.; Yang, H.; Wu, P.; Wang, Y.; Bing, Z.; Cao, Z.; et al. Distribution of ALK fusion variants and correlation with clinical outcomes in Chinese patients with non-small cell lung cancer treated with Crizotinib. Target 2019, 14, 159–168. [Google Scholar] [CrossRef]
- Wu, X.; Wang, W.; Zou, B.; Li, Y.; Yang, X.; Liu, N.; Ma, Q.; Zhang, X.; Wang, Y.; Li, D. Novel NLRC4-ALK and EML4-ALK double fusion mutations in a lung adenocarcinoma patient: A case report. Thorac. Cancer 2020, 11, 1695–1698. [Google Scholar] [CrossRef]
- Stefano, A.L.D.; Picca, A.; Saragoussi, E.; Bielle, F.; Ducray, F.; Villa, C.; Eoli, M.; Paterra, R.; Bellu, L.; Mathon, B.; et al. Clinical, molecular, and radiomic profile of gliomas with FGFR3-TACC3 fusions. Neuro Oncol. 2020, 22, 1614–1624. [Google Scholar] [CrossRef]
- Lee, J.R.; Kwon, C.H.; Choi, Y.; Park, H.J.; Kim, H.S.; Jo, H.J.; Oh, N.; Park, D.Y. Transcriptome analysis of paired primary colorectal carcinoma and liver metastases reveals fusion transcripts and similar gene expression profiles in primary carcinoma and liver metastases. BMC Cancer 2016, 16, 539. [Google Scholar] [CrossRef]
- Micci, F.; Gorunova, L.; Gatius, S.; Matias-Guiu, X.; Davidson, B.; Heim, S. Panagopoulos I.MEAF6/PHF1 is a recurrent gene fusion in endometrial stromal sarcoma. Cancer Lett. 2014, 347, 75–78. [Google Scholar] [CrossRef]
- Dewaele, B.; Przybyl, J.; Quattrone, A.; Ferreiro, J.F.; Vanspauwen, V.; Geerdens, E.; Gianfelici, V.; Kalender, Z.; Wozniak, A.; Moerman, P.; et al. Identification of a novel, recurrent MBTD1-CXorf67 fusion in low-grade endometrial stromal sarcoma. Int. J. Cancer 2014, 134, 1112–1122. [Google Scholar] [CrossRef]
- Honeyman, J.N.; Simon, E.P.; Robine, N.; Chiaroni-Clarke, R.; Darcy, D.G.; Lim, I.I.; Gleason, C.E.; Murphy, J.M.; Rosenberg, B.R.; Teegan, L.; et al. Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science 2014, 343, 1010–1014. [Google Scholar] [CrossRef]
- Lorenz, S.; Barøy, T.; Sun, J.; Nome, T.; Vodák, D.; Bryne, J.C.; Håkelien, A.-M.; Fernandez-Cuesta, L.; Möhlendick, B.; Rieder, H.; et al. Unscrambling the genomic chaos of osteosarcoma reveals extensive transcript fusion, recurrent rearrangements and frequent novel TP53 aberrations. Oncotarget 2016, 7, 5273–5288. [Google Scholar] [CrossRef]
- Kumar, S.; Vo, A.D.; Qin, F.; Li, H. Comparative assessment of methods for the fusion transcripts detection from RNA-Seq data. Sci. Rep. 2016, 6, 21597. [Google Scholar] [CrossRef]
- Kumar, S.; Razzaq, S.K.; Vo, A.D.; Gautam, M.; Li, H. Identifying fusion transcripts using next generation sequencing. Wiley Interdiscip. Rev. RNA 2016, 7, 811–823. [Google Scholar] [CrossRef]
- Krappinger, J.C.; Bonstingl, L.; Pansy, K.; Sallinger, K.; Wreglesworth, N.I.; Grinninger, L.; Deutsch, A.; El-Heliebi, A.; Kroneis, T.; Mcfarlane, R.J.; et al. Non-coding Natural Antisense Transcripts: Analysis and Application. J. Biotechnol. 2021, 340, 75–101. [Google Scholar] [CrossRef]
- Marco-Puche, G.; Lois, S.; Benítez, J.; Trivino, J.C. RNA-Seq Perspectives to Improve Clinical Diagnosis. Front. Genet. 2019, 10, 1152. [Google Scholar] [CrossRef]
- Docking, T.R.; Parker, J.D.K.; Jädersten, M.; Duns, G.; Chang, L.; Jiang, J.; Pilsworth, J.A.; Swanson, L.A.; Chan, S.K.; Chiu, R.; et al. A clinical transcriptome approach to patient stratification and therapy selection in acute myeloid leukemia. Nat. Commun. 2021, 12, 2474. [Google Scholar] [CrossRef]
- Oliver, G.R.; Tang, X.; Schultz-Rogers, L.E.; Vidal-Folch, N.; Jenkinson, W.G.; Schwab, T.L.; Gaonkar, K.; Cousin, M.A.; Nair, A.; Basu, S.; et al. A tailored approach to fusion transcript identification increases diagnosis of rare inherited disease. PLoS ONE 2019, 14, e0223337. [Google Scholar] [CrossRef]
- Conesa, A.; Madrigal, P.; Tarazona, S.; Gomez-Cabrero, D.; Cervera, A.; McPherson, A.; Szcześniak, M.W.; Gaffney, D.J.; Elo, L.L.; Zhang, X.; et al. A survey of best practices for RNA-seq data analysis. Genome Biol. 2016, 17, 13. [Google Scholar] [CrossRef]
- Parkhomchuk, D.; Borodina, T.; Amstislavskiy, V.; Banaru, M.; Hallen, L.; Krobitsch, S.; Lehrach, H.; Soldatov, A. Transcriptome analysis by strand-specific sequencing of complementary DNA. Nucleic Acids Res. 2009, 37, e123. [Google Scholar] [CrossRef]
- Singh, S.; Li, H. Comparative study of bioinformatic tools for the identification of chimeric RNAs from RNA Sequencing. RNA Biol. 2021, 18, 254–267. [Google Scholar] [CrossRef]
- Ma, C.; Shao, M.; Kingsford, C. SQUID: Transcriptomic structural variation detection from RNA-seq. Genome Biol. 2018, 19, 52. [Google Scholar] [CrossRef]
- Lorenzi, C.; Barriere, S.; Villemin, J.P.; Bretones, L.D.; Mancheron, A.; Ritchie, W. iMOKA: K-mer based software to analyze large collections of sequencing data. Genome Biol. 2020, 21, 261. [Google Scholar] [CrossRef]
- Carrara, M.; Beccuti, M.; Cavallo, F.; Donatelli, S.; Lazzarato, F.; Cordero, F.; Calogero, R.A. State of art fusion-finder algorithms are suitable to detect transcription-induced chimeras in normal tissues? BMC Bioinform. 2013, 14 (Suppl. S7), S2. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Q.; Li, F.; Zhao, W.; Xu, H.; Zhang, W.; Deng, H.; Yang, X. Identification of the cross-strand chimeric RNAs generated by fusions of bi-directional transcripts. Nat. Commun. 2021, 12, 4645. [Google Scholar] [CrossRef]
- Spiller, D.G.; Giles, R.V.; Grzybowski, J.; Tidd, D.M.; Clark, R.E. Improving the intracellular delivery and molecular efficacy of antisense oligonucleotides in chronic myeloid leukemia cells: A comparison of streptolysin-O permeabilization, electroporation, and lipophilic conjugation. Blood 1998, 91, 4738–4746. [Google Scholar] [CrossRef]
- Carey, M.F.; Peterson, C.L.; Smale, S.T. The RNase protection assay. Cold Spring Harb. Protoc. 2013, 3, pdb.prot071910. [Google Scholar] [CrossRef]
- Eastel, J.M.; Lam, K.W.; Lee, N.L.; Lok, W.Y.; Tsang, A.H.F.; Pei, X.M.; Chan, A.K.C.; Cho, W.C.S.; Wong, S.C.C. Application of NanoString technologies in companion diagnostic development. Expert Rev. Mol. Diagn. 2019, 19, 591–598. [Google Scholar] [CrossRef]
- Feng, L.; Lintula, S.; Ho, T.H.; Anastasina, M.; Paju, A.; Haglund, C.; Stenman, U.-H.; Hotakainen, K.; Orpana, A.; Kainov, D.; et al. Technique for strand-specific gene-expression analysis and monitoring of primer-independent cDNA synthesis in reverse transcription. Biotechniques 2012, 52, 263–270. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, Y.; Yang, M.; Liao, D.J. New methods as alternative or corrective measures for the pitfalls and artifacts of reverse transcription and polymerase chain reactions (RT-PCR) in cloning chimeric or antisense-accompanied RNA. RNA Biol. 2013, 10, 958–967. [Google Scholar] [CrossRef]
- Lei, Q.; Li, C.; Zuo, Z.; Huang, C.; Cheng, H.; Zhou, R. Evolutionary Insights into RNA trans-Splicing in Vertebrates. Genome Biol. Evol. 2016, 8, 562–577. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, W.; Kannan, K.; Luo, L.; Li, J.; Chao, P.; Wang, Y.; Chen, Y.-P.; Gu, J.; Yen, L. Aberrant chimeric RNA GOLM1-MAK10 encoding a secreted fusion protein as a molecular signature for human esophageal squamous cell carcinoma. Oncotarget 2013, 4, 2135–2143. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, X.; Yao, Z.; Zhu, J.; Lin, Y.; Lin, W.; Li, K.; Xu, X.; Guo, Y.; Chen, Y.; et al. Chimeric RNA ASTN2-PAPPA as aggravates tumor progression and metastasis in human esophageal cancer. Cancer Lett. 2021, 501, 1–11. [Google Scholar] [CrossRef]
- Kawakami, M.; Ishikawa, R.; Amano, Y.; Sunohara, M.; Watanabe, K.; Ohishi, N.; Yatomi, Y.; Nakajima, J.; Fukayama, M.; Nagase, T.; et al. Detection of novel paraja ring finger 2-fer tyrosine kinase mRNA chimeras is associated with poor postoperative prognosis in non-small cell lung cancer. Cancer Sci. 2013, 104, 1447–1454. [Google Scholar] [CrossRef]
- Maspero, D.; Dassano, A.; Pintarelli, G.; Noci, S.; Cecco, L.D.; Incarbone, M.; Tosi, D.; Santambrogio, L.; A Dragani, T.; Colombo, F. Read-through transcripts in lung: Germline genetic regulation and correlation with the expression of other genes. Carcinogenesis 2020, 41, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.M.; Yoon, K.; Lee, S.; Kim, E.; Kong, S.H.; Choe, J.; Kang, J.M.; Han, T.-S.; Kim, P.; Choi, Y.; et al. PPP1R1B-STARD3 chimeric fusion transcript in human gastric cancer promotes tumorigenesis through activation of PI3K/AKT signaling. Oncogene 2014, 33, 5341–5347. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Cho, G.A.; Han, S.W.; Shin, J.Y.; Jeong, E.G.; Song, S.H.; Lee, W.-C.; Lee, K.-H.; Bang, D.; Seo, J.-S.; et al. Novel fusion transcripts in human gastric cancer revealed by transcriptome analysis. Oncogene 2014, 33, 5434–5441. [Google Scholar] [CrossRef]
- Nacu, S.; Yuan, W.; Kan, Z.; Bhatt, D.; Rivers, C.S.; Stinson, J.; Peters, B.A.; Modrusan, Z.; Jung, K.; Seshagiri, S.; et al. Deep RNA sequencing analysis of readthrough gene fusions in human prostate adenocarcinoma and reference samples. BMC Med. Genom. 2011, 4, 11. [Google Scholar] [CrossRef]
- Babiceanu, M.; Qin, F.; Xie, Z.; Jia, Y.; Lopez, K.; Janus, N.; Facemire, L.; Kumar, S.; Pang, Y.; Qi, Y.; et al. Recurrent chimeric fusion RNAs in non-cancer tissues and cells. Nucleic Acids Res. 2016, 44, 2859–2872. [Google Scholar] [CrossRef]
- Tang, Y.; Guan, F.; Li, H. Case Study: The Recurrent Fusion RNA DUS4L-BCAP29 in Noncancer Human Tissues and Cells. Methods Mol. Biol. 2020, 2079, 243–258. [Google Scholar] [CrossRef]
- Wu, H.; Singh, S.; Xie, Z.; Li, X.; Li, H. Landscape characterization of chimeric RNAs in colorectal cancer. Cancer Lett. 2020, 489, 56–65. [Google Scholar] [CrossRef]
- Li, N.; Zheng, J.; Li, H.; Deng, J.; Hu, M.; Wu, H.; Li, W.; Li, F.; Lan, X.; Lu, J.; et al. Identifification of chimeric TSNAX-DISC1 resulting from intergenic splicing in endometrial carcinoma through high-throughput RNA sequencing. Carcinogenesis 2014, 35, 2687–2697. [Google Scholar] [CrossRef]
- Wu, P.; Yang, S.; Singh, S.; Qin, F.; Kumar, S.; Wang, L.; Ma, D.; Li, H. The Landscape and Implications of Chimeric RNAs in Cervical Cancer. EBioMedicine 2018, 37, 158–167. [Google Scholar] [CrossRef]
- Kannan, K.; Wang, L.; Wang, J.; Ittmann, M.M.; Li, W.; Yen, L. Recurrent chimeric RNAs enriched in human prostate cancer identified by deep sequencing. Proc. Natl. Acad. Sci. USA 2011, 108, 9172–9177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gong, M.; Yuan, H.; Park, H.G.; Frierson, H.F.; Li, H. Chimeric transcript generated by cis-splicing of adjacent genes regulates prostate cancer cell proliferation. Cancer Discov. 2012, 2, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Song, Z.; Chang, M.; Song, Y.; Frierson, H.; Li, H. Recurrent cis-SAGe chimeric RNA, D2HGDH-GAL3ST2, in prostate cancer. Cancer Lett. 2016, 380, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, M.; Ichikawa, H.; Arai, E.; Chiku, S.; Sakamoto, H.; Fujimoto, H.; Hiramoto, M.; Nammo, T.; Yasuda, K.; Yoshida, T.; et al. Comprehensive exploration of novel chimeric transcripts in clear cell renal cell carcinomas using whole transcriptome analysis. Chromosomes Cancer 2014, 53, 1018–1032. [Google Scholar] [CrossRef]
- Grosso, A.R.; Leite, A.P.; Carvalho, S.; Matos, M.R.; Martins, F.B.; Vítor, A.C.; Desterro, J.; Carmo-Fonseca, M.; De Almeida, S.F. Pervasive transcription read-through promotes aberrant expression of oncogenes and RNA chimeras in renal carcinoma. Elife 2015, 4, e09214. [Google Scholar] [CrossRef]
- Pflueger, D.; Sboner, A.; Storz, M.; Roth, J.; Compérat, E.; Bruder, E.; Rubin, M.; Schraml, P.; Moch, H. Identification of molecular tumor markers in renal cell carcinomas with TFE3 protein expression by RNA sequencing. Neoplasia 2013, 15, 1231–1240. [Google Scholar] [CrossRef]
- Pflueger, D.; Mittmann, C.; Dehler, S.; Rubin, M.A.; Moch, H.; Schraml, P. Functional characterization of BC039389-GATM and KLK4-KRSP1 chimeric read-through transcripts which are up-regulated in renal cell cancer. BMC Genom. 2015, 16, 247. [Google Scholar] [CrossRef]
- Zhu, D.; Singh, S.; Chen, X.; Zheng, Z.; Huang, J.; Lin, T.; Li, H. The landscape of chimeric RNAs in bladder urothelial carcinoma. Int. J. Biochem. Cell Biol. 2019, 110, 50–58. [Google Scholar] [CrossRef]
- Kekeeva, T.; Tanas, A.; Kanygina, A.; Alexeev, D.; Shikeeva, A.; Zavalishina, L.; Andreeva, Y.; Frank, G.; Zaletaev, D. Novel fusion transcripts in bladder cancer identified by RNA-seq. Cancer Lett. 2016, 374, 224–228. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Y.; Li, J.; Chang, I.; Wang, C.Y. A novel read-through transcript JMJD7-PLA2G4B regulates head and neck squamous cell carcinoma cell proliferation and survival. Oncotarget 2017, 8, 1972–1982. [Google Scholar] [CrossRef]
- Wang, J.; Xie, G.F.; He, Y.; Deng, L.; Long, Y.K.; Yang, X.H.; Ma, J.-J.; Gong, R.; Cen, W.-J.; Ye, Z.-L.; et al. Interfering expression of chimeric transcript SEPT7P2-PSPH promotes cell proliferation in patients with nasopharyngeal carcinoma. J. Oncol. 2019, 2019, 1654724. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Chen, R.H.; Wang, F.; Zeng, J.Y.; Yu, S.T.; Xu, L.H.; Cai, Q.; Liang, F.-Y.; Xia, T.-L.; Lin, Z.-R.; et al. Novel chimeric transcript RRM2-c2orf48 promotes metastasis in nasopharyngeal carcinoma. Cell Death Dis. 2017, 8, e3047. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Babiceanu, M.; Kumar, S.; Jia, Y.; Qin, F.; Barr, F.G.; Li, H. Fusion transcriptome profiling provides insights into alveolar rhabdomyosarcoma. Proc. Natl. Acad. Sci. USA 2016, 113, 13126–13131. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Barnhill, R.L.; Lee, S.; Li, Y.; Shao, Y.; Easton, J.; Dalton, J.; Zhang, J.; Pappo, A.; Bahrami, A. The landscape of fusion transcripts in spitzoid melanoma and biologically indeterminate spitzoid tumors by RNA sequencing. Mod. Pathol. 2016, 29, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Gonorazky, H.D.; Naumenko, S.; Ramani, A.K.; Nelakuditi, V.; Mashouri, P.; Wang, P.; Kao, D.; Ohri, K.; Viththiyapaskaran, S.; Tarnopolsky, M.A.; et al. Expanding the Boundaries of RNA Sequencing as a Diagnostic Tool for Rare Mendelian Disease. Am. J. Hum. Genet. 2019, 104, 466–483. [Google Scholar] [CrossRef]
- Yamada, M.; Suzuki, H.; Watanabe, A.; Uehara, T.; Takenouchi, T.; Mizuno, S.; Kosaki, K. Role of chimeric transcript formation in the pathogenesis of birth defects. Congenit. Anom. 2021, 61, 76–81. [Google Scholar] [CrossRef]
- Cousin, M.A.; Smith, M.J.; Sigafoos, A.N.; Jin, J.J.; Murphree, M.I.; Boczek, N.J.; Blackburn, P.R.; Oliver, G.R.; Aleff, R.A.; Clark, K.; et al. Utility of DNA, RNA, Protein, and Functional Approaches to Solve Cryptic Immunodeficiencies. J. Clin. Immunol. 2018, 38, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Oliver, G.R.; Blackburn, P.R.; Ellingson, M.S.; Conboy, E.; Pinto, E.V.F.; Webley, M.; Thorland, E.; Ferber, M.; Van Hul, E.; Van Der Werf, I.M.; et al. RNA-Seq detects a SAMD12-EXT1 fusion transcript and leads to the discovery of an EXT1 deletion in a child with multiple osteochondromas. Mol. Genet. Genom. Med. 2019, 7, e00560. [Google Scholar] [CrossRef]
- Oliver, G.R.; Jenkinson, G.; Klee, E.W. Computational Detection of Known Pathogenic Gene Fusions in a Normal Tissue Database and Implications for Genetic Disease Research. Front. Genet. 2020, 11, 173. [Google Scholar] [CrossRef]
- Loi, E.; Moi, L.; Blois, S.; Bacchelli, E.; Vega Benedetti, A.F.; Cameli, C.; Fadda, R.; Maestrini, E.; Carta, M.; Doneddu, G.; et al. ELMOD3-SH2D6 gene fusion as a possible co-star actor in autism spectrum disorder scenario. J. Cell. Mol. Med. 2020, 24, 2064–2069. [Google Scholar] [CrossRef]
- Ceroni, F.; Sagar, A.; Simpson, N.H.; Gawthrope, A.J.; Newbury, D.F.; Pinto, D.; Francis, S.; Tessman, D.C.; Cook, E.H.; Monaco, A.; et al. A deletion involving CD38 and BST1 results in a fusion transcript in a patient with autism and asthma. Autism Res. 2014, 7, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Pagnamenta, A.T.; Bacchelli, E.; de Jonge, M.V.; Mirza, G.; Scerri, T.S.; Minopoli, F.; Chiocchetti, A.; Ludwig, K.U.; Hoffmann, P.; Paracchini, S.; et al. Characterization of a Family with Rare Deletions in CNTNAP5 and DOCK4 Suggests Novel Risk Loci for Autism and Dyslexia. Biol. Psychiatry 2010, 68, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.; Sykes, N.H.; Conceição, I.C.; Cazier, J.B.; Anney, R.J.; Oliveira, G.; Gallagher, L.; Vicente, A.; Monaco, A.; Pagnamenta, A.T. CNVs leading to fusion transcripts in individuals with autism spectrum disorder. Eur. J. Hum. Genet. 2012, 20, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Masini, E.; Loi, E.; Vega-Benedetti, A.F.; Carta, M.; Doneddu, G.; Fadda, R.; Zavattari, P. An Overview of the Main Genetic, Epigenetic and Environmental Factors Involved in Autism Spectrum Disorder Focusing on Synaptic Activity. Int. J. Mol. Sci. 2020, 21, 8290. [Google Scholar] [CrossRef] [PubMed]
- Rippey, C.; Walsh, T.; Gulsuner, S.; Brodsky, M.; Nord, A.S.; Gasperini, M.; Pierce, S.; Spurrell, C.; Coe, B.P.; Krumm, N.; et al. Formation of chimeric genes by copy-number variation as a mutational mechanism in schizophrenia. Am. J. Hum. Genet. 2013, 93, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Geyer, M.A.; Kelsoe, J.R. Does disrupted-in-schizophrenia (DISC1) generate fusion transcripts? Mol. Psychiatry 2008, 13, 361–363. [Google Scholar] [CrossRef][Green Version]
- Zhou, X.; Chen, Q.; Schaukowitch, K.; Kelsoe, J.R.; Geyer, M.A. Insoluble DISC1-Boymaw fusion proteins generated by DISC1 translocation. Mol. Psychiatry 2010, 15, 669–672. [Google Scholar] [CrossRef]
- Ji, B.; Higa, K.K.; Kim, M.; Zhou, L.; Young, J.W.; Geyer, M.A.; Zhou, X. Inhibition of protein translation by the DISC1-Boymaw fusion gene from a Scottish family with major psychiatric disorders. Hum. Mol. Genet. 2014, 23, 5683–5705. [Google Scholar] [CrossRef]
- Yue, Y.; Grossmann, B.; Holder, S.E.; Haaf, T. De novo t(7;10)(q33;q23) translocation and closely juxtaposed microdeletion in a patient with macrocephaly and developmental delay. Hum. Genet. 2005, 117, 1–8. [Google Scholar] [CrossRef]
- Backx, L.; Seuntjens, E.; Devriendt, K.; Vermeesch, J.; Van Esch, H. A balanced translocation t(6;14)(q25.3;q13.2) leading to reciprocal fusion transcripts in a patient with intellectual disability and agenesis of corpus callosum. Cytogenet. Genome Res. 2011, 132, 135–143. [Google Scholar] [CrossRef]
- Córdova-Fletes, C.; Domínguez, M.G.; Delint-Ramirez, I.; Martínez-Rodríguez, H.G.; Rivas-Estilla, A.M.; Barros-Núñez, P.; Ortiz-Lopez, R.; Neira, V.A. A de novo t(10;19)(q22.3;q13.33) leads to ZMIZ1/PRR12 reciprocal fusion transcripts in a girl with intellectual disability and neuropsychiatric alterations. Neurogenetics 2015, 16, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Moysés-Oliveira, M.; Guilherme, R.S.; Meloni, V.A.; Di Battista, A.; de Mello, C.B.; Bragagnolo, S.; Moretti-Ferreira, D.; Kosyakova, N.; Liehr, T.; Carvalheira, G.M.; et al. X-linked intellectual disability related genes disrupted by balanced X-autosome translocations. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2015, 168, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Hackmann, K.; Matko, S.; Gerlach, E.M.; von der Hagen, M.; Klink, B.; Schrock, E.; Rump, A.; Di Donato, N. Partial deletion of GLRB and GRIA2 in a patient with intellectual disability. Eur. J. Hum. Genet. 2013, 21, 112–114. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mayo, S.; Monfort, S.; Roselló, M.; Orellana, C.; Oltra, S.; Caro-Llopis, A.; Martínez, F. Chimeric Genes in Deletions and Duplications Associated with Intellectual Disability. Int. J. Genom. 2017, 2017, 4798474. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, M.; Wang, Q.; Zhang, J.; Zhou, T.; Sun, X. Single-cell transcriptome-based multilayer network biomarker for predicting prognosis and therapeutic response of gliomas. Brief. Bioinform. 2020, 21, 1080–1097. [Google Scholar] [CrossRef]
- Haile, S.; Corbett, R.D.; LeBlanc, V.G.; Wei, L.; Pleasance, S.; Bilobram, S.; Nip, K.M.; Brown, K.; Trinh, E.; Smith, J.; et al. A Scalable Strand-Specific Protocol Enabling Full-Length Total RNA Sequencing From Single Cells. Front. Genet. 2021, 12, 665888. [Google Scholar] [CrossRef]
- Rao, A.; Barkley, D.; França, G.S.; Yanai, I. Exploring tissue architecture using spatial transcriptomics. Nature 2021, 596, 211–220. [Google Scholar] [CrossRef]
| Type of Cell/Tissue | Chimeric RNAs | Formation | Function | ||
|---|---|---|---|---|---|
| Cancer | Esophageal Cancer | GOLM1-MAK10 | cis-SAGe | correlates with histologic differentiation; lymph node metastasis; encodes a secreted fusion protein | |
| ASTN2-PAPPA | splicing of exons and intron antisense of two neighboring genes | aggravates tumor progression and metastasis | |||
| NSCLC | Pe1-Fe3 | alteration at the transcriptome level (trans-splicing) | correlates with poor postoperative survival periods | ||
| EML4-ALK | Chromosomal rearrangement/trans-splicing | promotes NSCLC tumorigenesis | |||
| Gastric Cancer | PPP1R1B-STARD3 | cis-SAGe | promotes tumorigenesis through activation of PI3K/AKT signaling | ||
| DUS4L-BCAP29 | cis-SAGe | promotes cell growth and motility | |||
| Colorectal Cancer | RRM2-C2orf48 | cis-SAGe | promotes cell proliferation and correlates with poor clinical outcomes | ||
| Tumors of Reproductive System | EC | TSNAX-DISC1 | cis-SAGe | induces G1-S cell cycle progression and enhance cell growth | |
| cervical cancer tissues | LHX6-NDUFA8 | cis-SAGe | Diagnostic biomarker | ||
| Prostate Cancer | TMEM79-SMG5 | splicing | Diagnostic biomarker | ||
| SLC45A3-ELK4 | cis-SAGe | correlates with disease progression and metastases | |||
| D2HGDH-GAL3ST2 | cis-SAGe | Promotes cell proliferation and migration | |||
| Renal Cell Carcinoma | CTSC-RAB38 | cis-SAGe | |||
| TMED6-COG8 | cis-SAGe | Diagnostic biomarker | |||
| BC039389-GATM | cis-SAGe | Diagnostic biomarker | |||
| KLK4-KRSP1 | cis-SAGe | Diagnostic biomarker; associates with worse clinical outcome, larger tumors, high grade tumors, the histological subtype | |||
| Bladder Cancer | BCL2L2-PABPN1 | cis-SAGe | Diagnostic biomarker | ||
| CHFR-GOLGA3 | cis-SAGe | Diagnostic biomarker | |||
| SYT8-TNNI2 | cis-SAGe | Diagnostic biomarker | |||
| HNSCC | JMJD7-PLA2G4B | cis-SAGe | promotes cells proliferation by inhibiting cell cycle arrest in G1 phase; controls AKT phosphorylation to promote SCC cell survival | ||
| nasopharyngeal carcinoma | SEPT7P2-PSPH | trans-splicing | promote cell proliferation and metastasis/invasion by up-regulating the expression of the downstream gene PSPH | ||
| osteosarcoma | EIF5A-HMGN2 | trans-splicing | |||
| EEF1A1-VIM | trans-splicing | ||||
| spitzoid tumors | CDC5L-BTBD9 | no structural rearrangement | |||
| Non-cancer | diverse non-cancerous cell lines (mammary gland, lung epithelial, and foreskin fibroblast, etc.) | DUS4L-BCAP2 | cis-SAGe | promotes cell growth and motility | |
| endometrial stromal cells | JAZF1-SUZ12 | trans-splicing | increases cell proliferation | ||
| normal skeletal muscle differentiation (myogenesis) | PAX3-FOXO1 | trans-splicing | interferes with the muscle differentiation process; contributes to tumorigenesis | ||
| normal bone and primary osteoblasts | EIF5A-HMGN2 EEF1A1-VIM | trans-splicing | |||
| non-involved lung tissue of lung adenocarcinoma | CHIA-PIFO | cis-SAGe | plays a functional role in asthma and possibly other lung inflammatory conditions | ||
| CTSC-RAB38 | cis-SAGe | Maintains lung surfactant homeostasis and lamellar body morphology | |||
| ELAVL1-TIMM44 | cis-SAGe | involves in lung cancer cell apoptosis | |||
| NFATC3-PLA2G15 | cis-SAGe | epithelial–mesenchymal transition (EMT) | |||
| IFNAR2-IL10RB | cis-SAGe | ||||
| KIAA1841-C2ORF74 | cis-SAGe | Relates to ciliated epithelial cells | |||
| SHANK3-ACR | cis-SAGe | Involves in cell growth, angiogenesis and epithelial–mesenchymal transition | |||
| SIRPB1-SIRPD | cis-SAGe | ||||
| Diagnosis | Chimeric RNA | |
|---|---|---|
| Birth defects | Mowat–Wilson syndrome | ZEB2-GTDC1 |
| Birk–Barel syndrome | KCNK9-TRAPPC9 | |
| Immunodeficiencies (NBS SCID) with T cell lymphopenia (TCL) | ATM-SLC35F2 | |
| SAMD12-EXT1 | ||
| Rubinstein–Taybi syndrome | PDPK1-PRSS21 | |
| epilepsy phenotype | NARS2-TENM4 | |
| Dyggve–Melchior–Clausen disease | C18orf32-DYM | |
| Nemaline myopathy | ARL5A-NEB | |
| ZTTK syndrome | SON-FCRL3 | |
| Unresolved | PDPK1-PRSS21 | |
| Unresolved | SAMD12-EXT1 | |
| Autism | BST1-CD38 | |
| DOCK4-IMMP2L | ||
| EEP1-POLR1A | ||
| KIAA0319-TDP2 | ||
| MAPKAPK5-ACAD10 | ||
| ELMOD3-SH2D6 | ||
| Schizophrenia | MAP3K3-DDX42 | |
| DNAJA2-NETO2 | ||
| PLEKHD1-SLC39A9 | ||
| MATK-ZFR2 | ||
| DISC1-Boymaw and Boymaw-DISC1 | ||
| Intellectual disability | PTEN-SEC8L1 | |
| MRPP3-ARID1B | ||
| PRR12-ZMIZ1 | ||
| ZNF611-IL1RAPL1 | ||
| GLRB--GRIA2 | ||
| LIMS1-RANBP2 | ||
| ARID1B-ZDHHC14 | ||
| ZNF451-KIAA1586 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Li, H. Chimeric RNAs Discovered by RNA Sequencing and Their Roles in Cancer and Rare Genetic Diseases. Genes 2022, 13, 741. https://doi.org/10.3390/genes13050741
Sun Y, Li H. Chimeric RNAs Discovered by RNA Sequencing and Their Roles in Cancer and Rare Genetic Diseases. Genes. 2022; 13(5):741. https://doi.org/10.3390/genes13050741
Chicago/Turabian StyleSun, Yunan, and Hui Li. 2022. "Chimeric RNAs Discovered by RNA Sequencing and Their Roles in Cancer and Rare Genetic Diseases" Genes 13, no. 5: 741. https://doi.org/10.3390/genes13050741
APA StyleSun, Y., & Li, H. (2022). Chimeric RNAs Discovered by RNA Sequencing and Their Roles in Cancer and Rare Genetic Diseases. Genes, 13(5), 741. https://doi.org/10.3390/genes13050741






