Dissection of Maize Drought Tolerance at the Flowering Stage Using Genome-Wide Association Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Phenotyping for Drought-Stress-Related Traits
2.3. Association Analysis for ASI, EBM, and PH
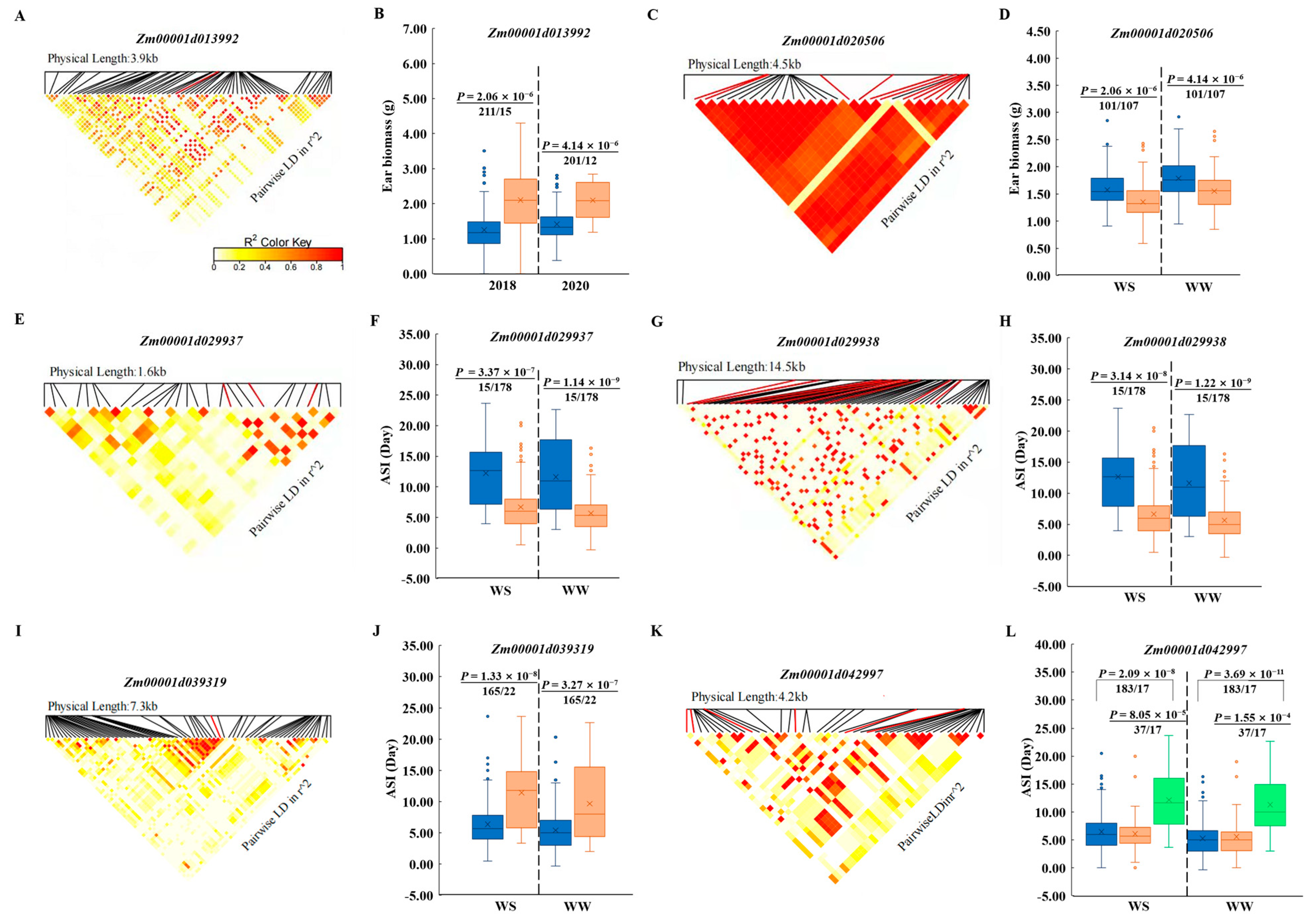
2.4. Drought Responsive, Linkage Disequilibrium, and Haplotype Analysis of Candidate Genes
2.5. Statistical Analysis
3. Results
3.1. Performance of Drought-Tolerant Phenotypes in the Association Panel
3.2. Correlations among Drought-Related Traits
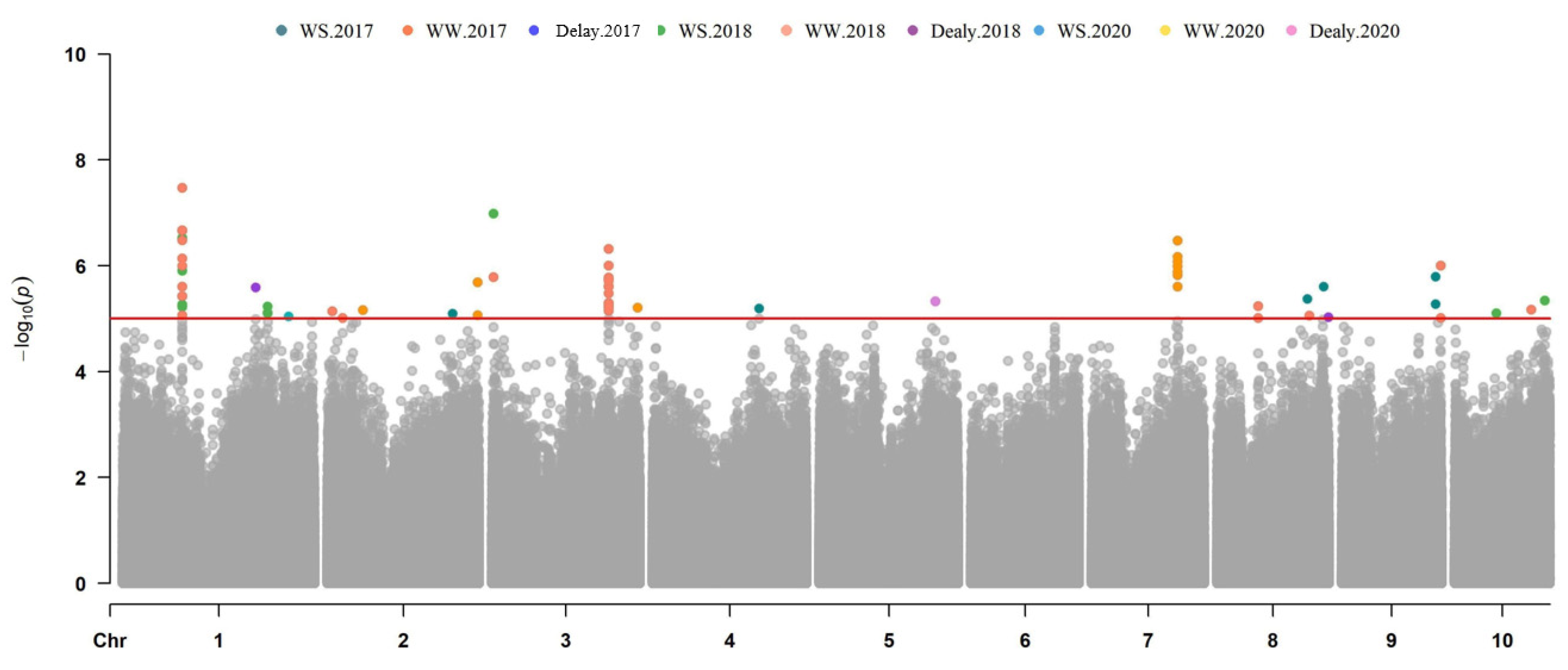
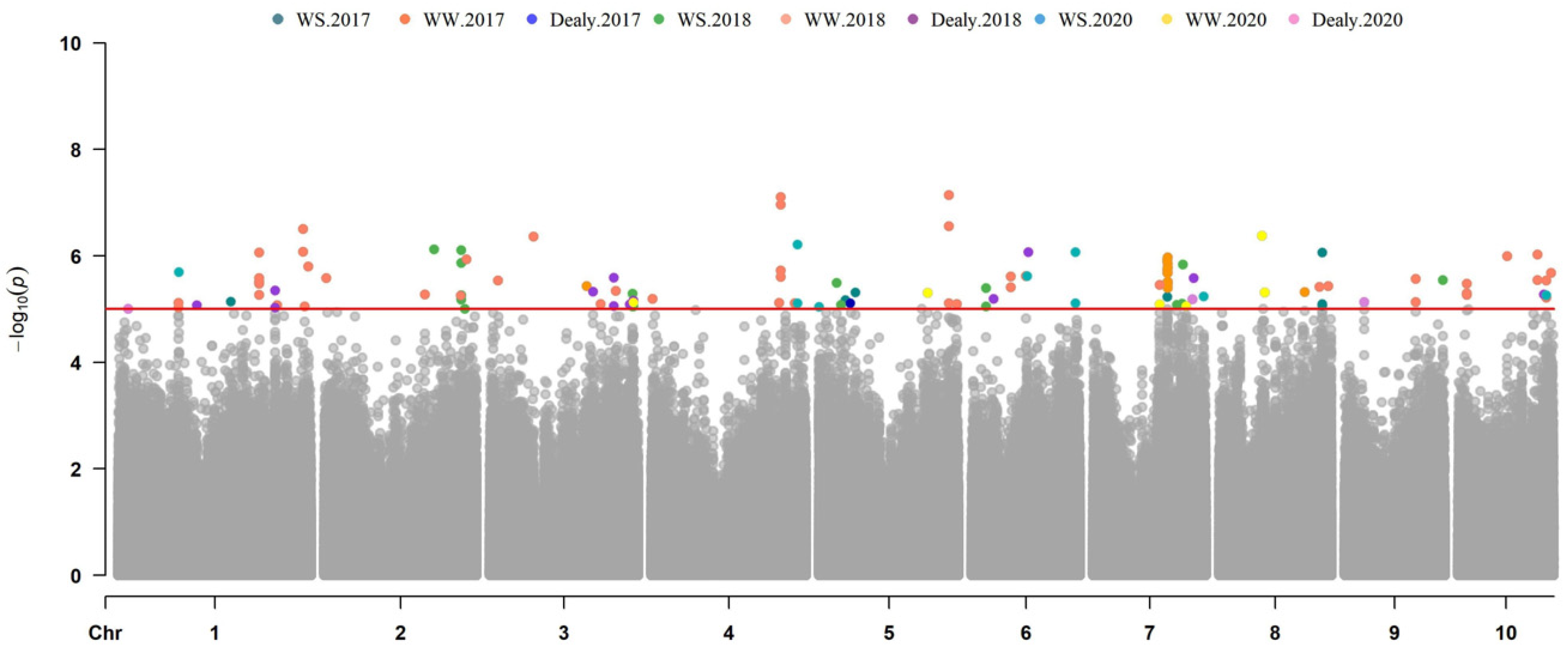
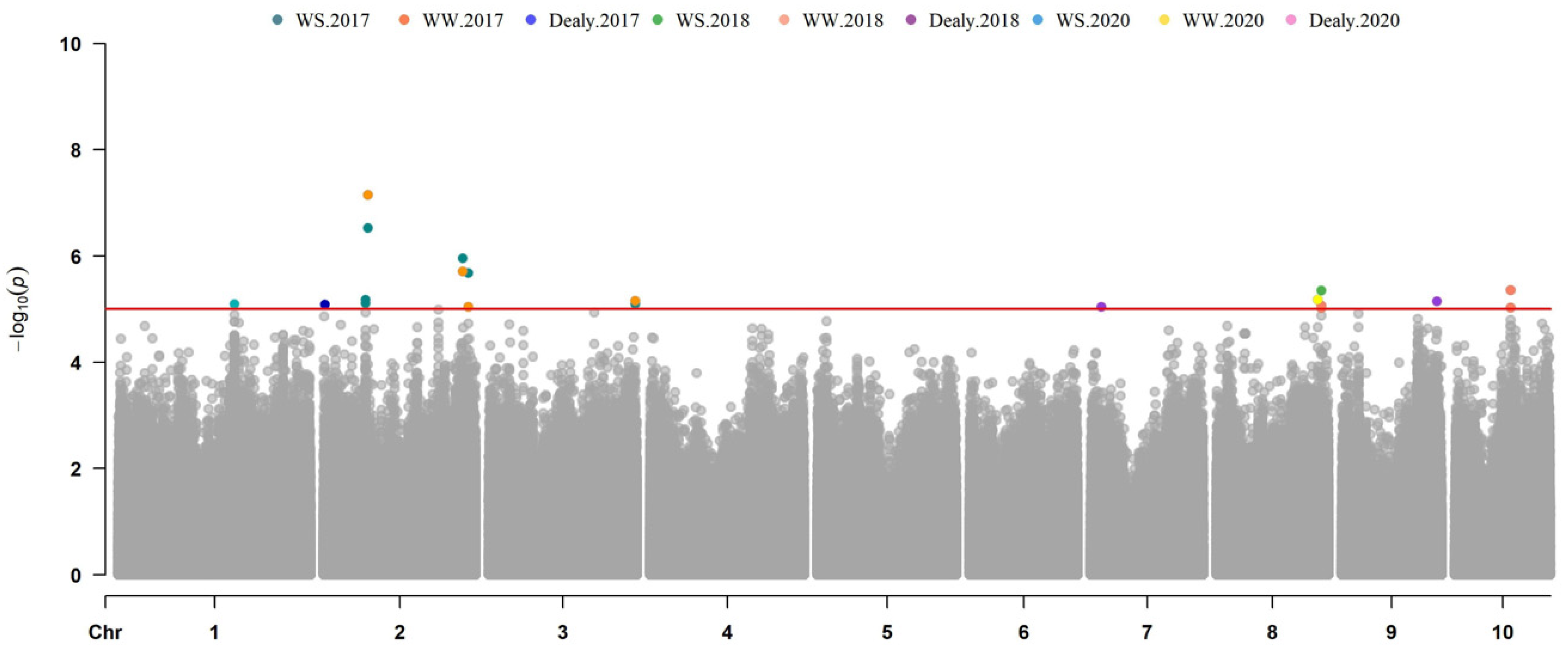
3.3. GWAS for Maize Drought Tolerance Genes
3.4. Common Genes Identified for Ear Development across Multiple Years or Conditions
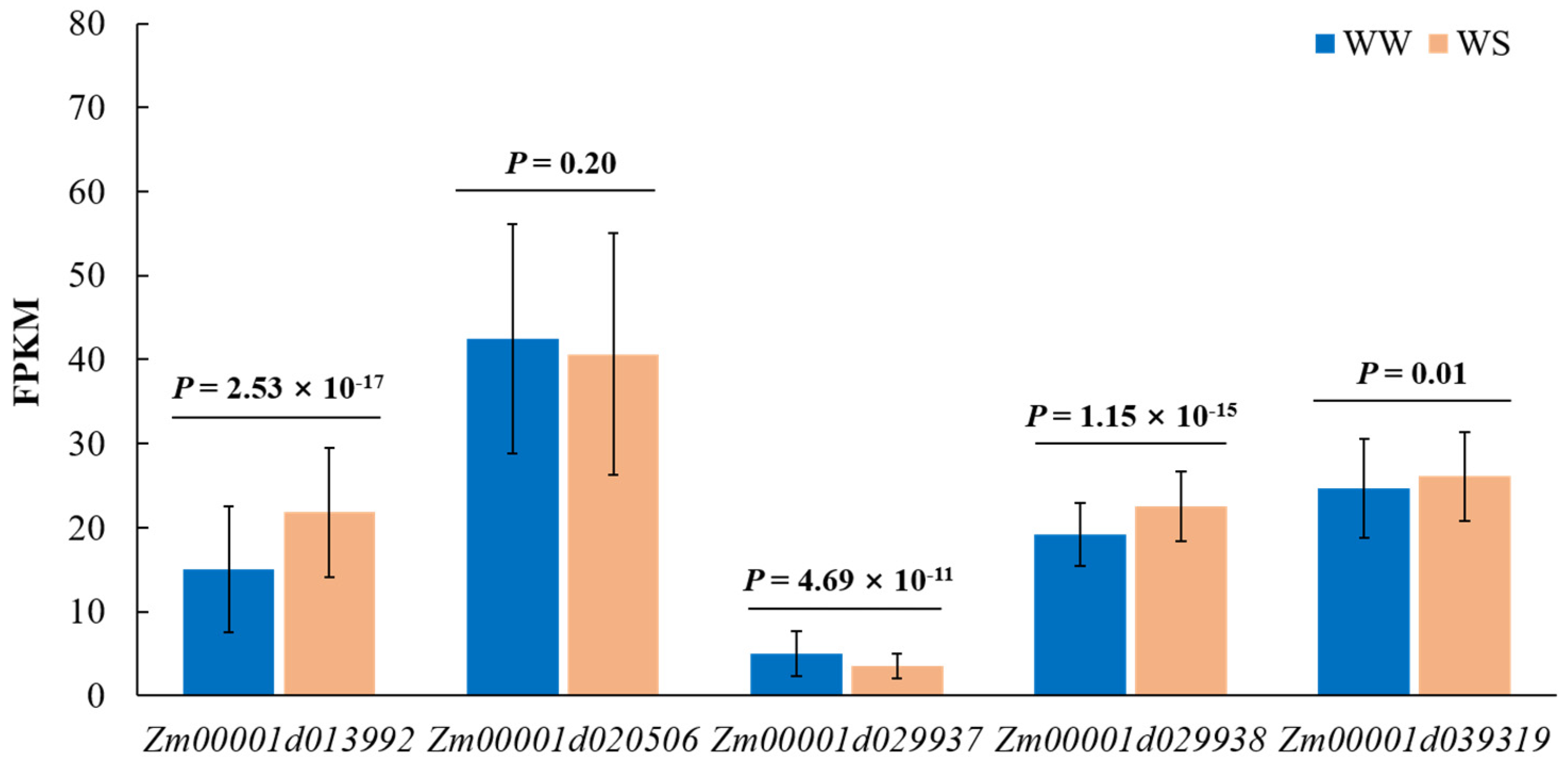
3.5. Candidate Genes Drought Responsive Pattern
3.6. Allele Effects of Common Candidate Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef] [Green Version]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Global synthesis of drought effects on maize and wheat production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Bänziger, M.; Edmeades, G.O.; Beck, D.; Bellon, M. Breeding for Drought and Nitrogen Stress Tolerance in Maize: From Theory to Practice; CIMMYT: Mexico, DF, USA, 2000; pp. 1–68. [Google Scholar]
- Wu, X.; Feng, H.; Wu, D.; Yan, S.; Zhang, P.; Wang, W.; Zhang, J.; Ye, J.; Dai, G.; Fan, Y.; et al. Using high-throughput multiple optical phenotyping to decipher the genetic architecture of maize drought tolerance. Genome Biol. 2021, 22, 1–26. [Google Scholar] [CrossRef]
- NeSmith, D.S.; Ritchie, J.T. Effects of soil water-deficits during tassel emergence on development and yield component of maize (Zea mays). Field Crops Res. 1992, 28, 251–256. [Google Scholar] [CrossRef]
- Saini, H.S.; Westgate, M.E. Reproductive development in grain crops during drought. Adv. Agron. 1999, 68, 59–96. [Google Scholar]
- Bolaños, J.; Edmeades, G.O. The importance of the anthesis-silking interval in breeding for drought tolerance in tropical maize. Field Crops Res. 1996, 48, 65–80. [Google Scholar] [CrossRef]
- Bruce, W.B.; Edmeades, G.O.; Barker, T.C. Molecular and physiological approaches to maize improvement for drought tolerance. J. Exp. Bot. 2002, 53, 13–25. [Google Scholar] [CrossRef]
- Barutçular, C.; Dizlek, H.; El-Sabagh, A.; Sahin, T.; EL-Sabagh, M.; Islam, M.S. Nutritional quality of maize in response to drought stress during grain-filling stages in mediterranean climate condition. J. Exp. Biol. Agric. Sci. 2016, 4, 644–652. [Google Scholar] [CrossRef]
- Lopes, M.S.; Araus, J.L.; Van Heerden, P.D.R.; Foyer, C.H. Enhancing drought tolerance in C4 crops. J. Exp. Bot. 2011, 62, 3135–3153. [Google Scholar] [CrossRef] [PubMed]
- Sari-Gorla, M.; Krajewski, P.; Di Fonzo, N.; Villa, M.; Frova, C. Genetic analysis of drought tolerance in maize by molecular markers. II. plant height and flowering. Theor. Appl. Genet. 1999, 99, 289–295. [Google Scholar] [CrossRef]
- Mackay, T.F.C. Quantitative trait loci in drosophila. Nat. Rev. Genet. 2001, 2, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Buckler, E.S. Genetic association mapping and genome organization of maize. Curr. Opin. Biotechnol. 2006, 17, 155–160. [Google Scholar] [CrossRef]
- Rosenberg, N.A.; Huang, L.; Jewett, E.M.; Szpiech, Z.A.; Jankovic, I.; Boehnke, M. Genome-wide association studies in diverse populations. Nat. Rev. Genet. 2010, 11, 356–366. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Warburton, M.; Crouch, J. Association mapping for enhancing maize (Zea mays L.) genetic improvement. Crop Sci. 2011, 51, 433–449. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Liu, S.; Ferjani, A.; Li, J.; Yan, J.; Yang, X.; Qin, F. Genetic variation in ZmVPP1 contributes to drought tolerance in maize seedlings. Nat. Genet. 2016, 48, 1233–1241. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Wang, H.; Xin, H.; Yang, X.; Yan, J.; Li, J.; Tran, L.S.; Shinozaki, K.; Yamaguchi-Shinozaki, K.; et al. Genome-wide analysis of ZmDREB genes and their association with natural variation in drought tolerance at seedling stage of Zea mays L. PLoS Genet. 2013, 9, e1003790. [Google Scholar] [CrossRef] [Green Version]
- Mao, H.; Wang, H.; Liu, S.; Li, Z.; Yang, X.; Yan, J.; Li, J.; Tran, L.S.; Qin, F. A transposable element in a NAC gene is associated with drought tolerance in maize seedlings. Nat. Commun. 2015, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Y.; Sun, X.; Gao, S.; Qin, F.; Dai, M. Deletion of an endoplasmic reticulum stress response element in a ZmPP2C-A gene facilitates drought tolerance of maize seedlings. Mol. Plant 2017, 10, 456–469. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Xu, Z.; Xu, L.; Galli, M.; Gallavotti, A.; Dooner, H.K.; Chuck, G. Necrotic upper tips1 mimics heat and drought stress and encodes a protoxylem-specific transcription factor in maize. Proc. Natl. Acad. Sci. USA 2020, 117, 20908–20919. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, B.; Yang, Z.; Liu, Y.; Yang, S.; Shi, Y.; Jiang, C.; Qin, F. Manipulating ZmEXPA4 expression ameliorates the drought-induced prolonged anthesis and silking interval in maize. Plant Cell 2021, 33, 2058–2071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, H.; Ma, X.; Zhou, G.; Ruan, H.; Cui, H.; Pang, J.; Siffat, U.K.; Zong, N.; Wang, R.; et al. Genome-wide association study and metabolic pathway prediction of barrenness in maize as a response to high planting density. J. Integr. Agric. 2021, in press. [Google Scholar]
- Fu, J.; Cheng, Y.; Linghu, J.; Yang, X.; Kang, L.; Zhang, Z.; Zhang, J.; He, C.; Du, X.; Peng, Z. RNA sequencing reveals the complex regulatory network in the maize kernel. Nat. Commun. 2013, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pang, J.; Fu, J.; Zong, N.; Wang, J.; Song, D.; Zhang, X.; He, C.; Fang, T.; Zhang, H.; Fan, Y.; et al. Kernel size-related genes revealed by an integrated eQTL analysis during early maize kernel development. Plant J. 2019, 98, 19–32. [Google Scholar] [CrossRef]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Holland, J.B.; Nyquist, W.E.; Cervantes-Martínez, C.T. Estimating and Interpreting Heritability for Plant Breeding: An Update; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Messmer, R.; Fracheboud, Y.; Bänziger, M.; Vargas, M.; Stamp, P.; Ribaut, J.M. Drought stress and tropical maize: QTL-by-environment interactions and stability of QTLs across environments for yield components and secondary traits. Theor. Appl. Genet. 2009, 119, 913–930. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Warburton, M.L.; Sawkins, M.; Zhang, X.; Setter, T.; Xu, Y.; Grudloyma, P.; Gethi, J.; Ribaut, J.M.; Li, W. Genome-wide association analysis for nine agronomic traits in maize under well-watered and water-stressed conditions. Theor. Appl. Genet. 2013, 126, 2587–2596. [Google Scholar] [CrossRef]
- Hejnák, V.; Tatar, Ö.; Atasoy, G.D.; Martinková, J.; Çelen, A.E.; Hnilička, F.; Skalický, M. Growth and Photosynthesis of upland and pima cotton: Response to drought and heat stress. Plant Soil Environ. 2015, 62, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Zafar, S.A.; Patil, S.B.; Uzair, M.; Fang, J.; Zhao, J.; Guo, T.; Yuan, S.; Uzair, M.; Luo, Q.; Shi, J.; et al. DEGENERATED PANICLE AND PARTIAL STERILITY 1 (DPS 1) encodes a cystathionine β–synthase domain containing protein required for anther cuticle and panicle development in rice. New Phytol. 2020, 225, 356–375. [Google Scholar] [CrossRef] [Green Version]
- Edmeades, G.O.; Bolanos, J.; Elings, A.; Ribaut, J.M.; Bänziger, M.; Westgate, M.E. The role and regulation of the anthesis-silking interval in maize. Physiol. Modeling Kernel Set Maize 2000, 29, 43–73. [Google Scholar]
- Setter, T.L. Analysis of constituents for phenotyping drought tolerance in crop improvement. Front. Physiol. 2012, 3, 180. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Sun, B.; Li, Y.; Liu, C.; Wu, X.; Zhang, D.; Shi, Y.; Song, Y.; Buckler, E.S.; Zhang, Z.; et al. Numerous genetic loci identified for drought tolerance in the maize nested association mapping populations. BMC Genom. 2016, 17, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farfan, I.D.; De La Fuente, G.N.; Murray, S.C.; Isakeit, T.; Huang, P.C.; Warburton, M.; Williams, P.; Windham, G.L.; Kolomiets, M. Genome wide association study for drought, aflatoxin resistance, and important agronomic traits of maize hybrids in the sub-tropics. PLoS ONE 2015, 10, e0117737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Z.; Li, X.; Xie, C.; Weng, J.; Li, M.; Zhang, D. Identification of functional genetic variations underlying drought tolerance in maize using SNP markers. J. Integr. Plant Biol. 2011, 53, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, S.; Shah, T.; Xie, C.; Hao, Z.; Li, X.; Farkhari, M.; Ribaut, J.M.; Cao, M.; Rong, T. Joint linkage-linkage disequilibrium mapping is a powerful approach to detecting quantitative trait loci underlying drought tolerance in maize. Proc. Natl. Acad. Sci. USA 2010, 107, 19585–19590. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Wang, G.; Du, X.; Zhang, H.; Xu, Z.; Wang, J.; Chen, G.; Wang, B.; Li, X.; Chen, X.; et al. QTL analysis across multiple environments reveals promising chromosome regions associated with yield-related traits in maize under drought conditions. Crop J. 2021, 9, 759–766. [Google Scholar] [CrossRef]
- Almeida, G.D.; Nair, S.; Borém, A.; Cairns, J.; Trachsel, S.; Ribaut, J.M.; Bänziger, M.; Prasanna, B.M.; Crossa, J.; Babu, R. Molecular mapping across three populations reveals a QTL hotspot region on chromosome 3 for secondary traits associated with drought tolerance in tropical maize. Mol. Breed. 2014, 34, 701–715. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.N.; Li, X.H.; George, M.L.; Li, M.S.; Zhang, S.H.; Zheng, Y.L. Quantitative trait locus analysis of drought tolerance and yield in maize in China. Plant Mol. Biol. Rep. 2005, 23, 155–165. [Google Scholar] [CrossRef]
- Cai, H.; Chu, Q.; Gu, R.; Yuan, L.; Liu, J.; Zhang, X.; Chen, F.; Mi, G.; Zhang, F. Identification of QTLs for plant height, ear height and grain yield in maize (Zea mays L.) in response to nitrogen and phosphorus supply. Plant Breed. 2012, 131, 502–510. [Google Scholar] [CrossRef]
- Messmer, R.; Fracheboud, Y.; Bänziger, M.; Stamp, P.; Ribaut, J.M. Drought stress and tropical maize: QTLs for leaf greenness, plant senescence, and root capacitance. Field Crops Res. 2011, 124, 93–103. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Z.; Li, R.; Weng, J.; Zhang, Q.; Li, X.; Wang, B.; Zhang, W.; Song, W.; Li, X. Mapping QTL for flowering time-related traits under three plant densities in maize. Crop J. 2021, 9, 372–379. [Google Scholar] [CrossRef]
- Leng, P.; Ouzunova, M.; Landbeck, M.; Wenzel, G.; Eder, J.; Darnhofer, B.; Lübberstedt, T. Quantitive trait loci mapping of forage agronomic traits in six mapping populations derived from European elite maize germplasm. Plant Breed. 2018, 137, 370–378. [Google Scholar] [CrossRef]
- de Lorenzo, L.; Merchan, F.; Blanchet, S.; Megias, M.; Frugier, F.; Crespi, M.; Sousa, C. Differential expression of the TFIIIA regulatory pathway in response to salt stress between Medicago truncatula genotypes. Plant Physiol. 2007, 145, 1521–1532. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Jia, J.; Yan, X.; Shi, H.; Han, Y. Arabidopsis KHZ1 and KHZ2, two novel non-tandem CCCH zinc-finger and K-homolog domain proteins, have redundant roles in the regulation of flowering and senescence. Plant Mol. Biol. 2017, 95, 549–565. [Google Scholar] [CrossRef]
- Huang, X.; Chao, D.; Gao, J.; Zhu, M.; Shi, M.; Lin, H. A previously unknown zinc finger protein DST regulates drought and salt tolerance in rice via stomatal aperture control. Gene. Dev. 2009, 23, 1805–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, C.; Chen, N.; Li, X. F-box Protein arabidillo-1 promotes lateral root development by depressing the functioning of GA3 in Arabidopsis. J. Plant Biol. 2010, 53, 374–380. [Google Scholar] [CrossRef]
- Sharma, M.; Singh, A.; Shankar, A.; Pandey, A.; Baranwal, V.; Kapoor, S.; Tyagi, A.K.; Pandey, G.K. Comprehensive expression analysis of rice armadillo gene family during abiotic stress and development. DNA Res. 2014, 21, 267–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nibau, C.; Gibbs, D.J.; Bunting, K.A.; Moody, L.A.; Smiles, E.J.; Tubby, J.A.; Bradshaw, S.J.; Coates, J.C. ARABIDILLO proteins have a novel and conserved domain structure important for the regulation of their stability. Plant Mol. Biol. 2011, 75, 77–92. [Google Scholar] [CrossRef]

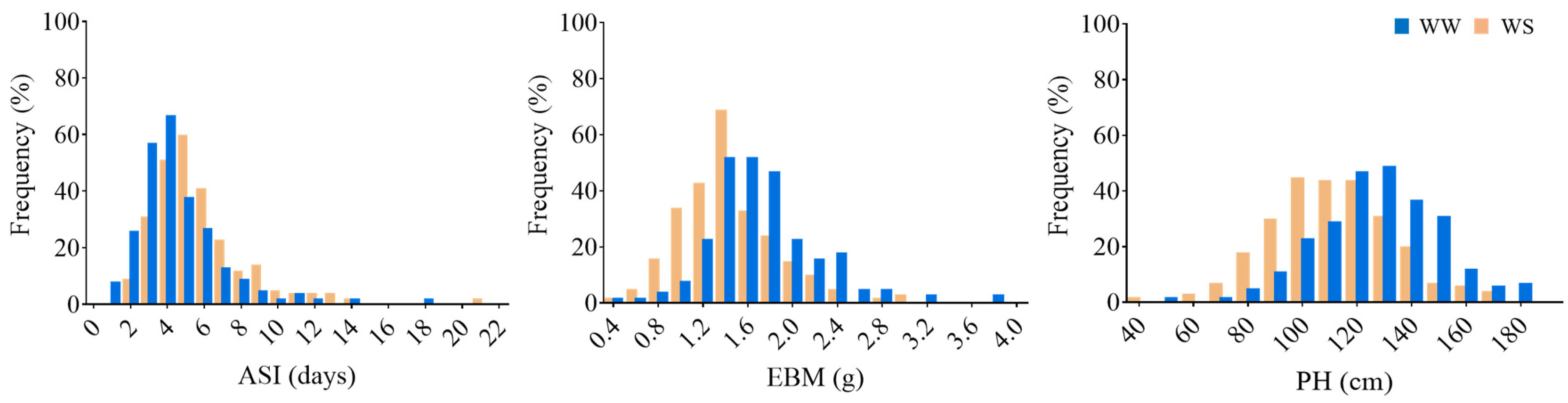
| Traits | Range | Mean ± SD | CV (%) | (H2) % | p Value |
|---|---|---|---|---|---|
| ASI-WS | 1.67–21.42 | 5.58 ± 2.39 | 42.97 | 89.31 | 2.6 × 10−3 |
| ASI-WW | 0.56–18.15 | 4.43 ± 2.17 | 48.89 | 87.45 | |
| EBM-WS | 0.48–3.08 | 1.42 ± 0.41 | 29.15 | 86.78 | 3.2 × 10−5 |
| EBM-WW | 0.48–3.81 | 1.71 ± 0.47 | 27.20 | 86.64 | |
| PH-WS | 43.21–169.72 | 111.36 ± 21.54 | 19.34 | 92.52 | 2.7 × 10−4 |
| PH-WW | 51.23–183.74 | 127.22 ± 21.53 | 16.92 | 94.60 |
| Trait | ASI | EBM | PH |
|---|---|---|---|
| ASI | −0.09 | 0.13 * | |
| EBM | −0.04 | 0.22 ** | |
| PH | 0.15 * | 0.18 ** |
| Traits | Marker | Chr. | Position | p Value | R2 | Gene ID | Annotation |
|---|---|---|---|---|---|---|---|
| ASI-WS-18 | S1_93513564 | 1 | 93513564 | 5.38 × 10−6 | 0.08207 | Zm00001d029938 | Protein ARABIDILLO 1 |
| S1_93277641 | 1 | 93277641 | 6.06 × 10−6 | 0.08113 | Zm00001d029937 | Glycoprotein | |
| PZE-103003226 | 3 | 2449913 | 1.03 × 10−7 | 0.14322 | Zm00001d039319 | Tic22-like family protein | |
| chr3.S_183263192 | 3 | 183319292 | 1.01 × 10−5 | 0.07963 | Zm00001d042997 | HIT-type zinc-finger family protein | |
| ASI-WW-18 | S1_93277641 | 1 | 93277641 | 2.20 × 10−7 | 0.1079 | Zm00001d029937 | Glycoprotein |
| S1_93277775 | 1 | 93277775 | 3.28 × 10−7 | 0.10511 | |||
| S1_93278150 | 1 | 93278150 | 7.29 × 10−7 | 0.09852 | |||
| S1_93513564 | 1 | 93513564 | 1.01 × 10−6 | 0.09549 | Zm00001d029938 | Protein ARABIDILLO 1 | |
| S1_93507046 | 1 | 93507046 | 2.48 × 10−6 | 0.08831 | |||
| S1_93505855 | 1 | 93505855 | 3.76 × 10−6 | 0.08489 | |||
| S1_93509892 | 1 | 93509892 | 3.76 × 10−6 | 0.08489 | |||
| S1_93510646 | 1 | 93510646 | 3.76 × 10−6 | 0.08489 | |||
| S1_93511155 | 1 | 93511155 | 3.76 × 10−6 | 0.08489 | |||
| S1_93510058 | 1 | 93510058 | 8.64 × 10−6 | 0.07831 | |||
| S1_93511521 | 1 | 93511521 | 8.64 × 10−6 | 0.07831 | |||
| S1_93513096 | 1 | 93513096 | 8.64 × 10−6 | 0.07831 | |||
| PZE-103003226 | 3 | 2449913 | 1.64 × 10−6 | 0.10835 | Zm00001d039319 | Tic22-like family protein | |
| chr3.S_183263192 | 3 | 183319292 | 1.66 × 10−6 | 0.09449 | Zm00001d042997 | HIT-type zinc-finger family protein | |
| S3_183315457 | 3 | 183315457 | 1.91 × 10−5 | 0.09027 | |||
| S3_183315658 | 3 | 183315658 | 1.91 × 10−6 | 0.09027 | |||
| S3_183316916 | 3 | 183316916 | 1.91 × 10−6 | 0.09027 | |||
| S3_183318642 | 3 | 183318642 | 1.91 × 10−6 | 0.09027 | |||
| S3_183315400 | 3 | 183315400 | 5.78 × 10−6 | 0.08148 | |||
| S3_183311733 | 3 | 183311733 | 7.14 × 10−6 | 0.07982 | |||
| S3_183311777 | 3 | 183311777 | 7.14 × 10−6 | 0.07982 | |||
| EBM-WS-17 | chr7.S_116288756 | 7 | 116316709 | 5.92 × 10−6 | 0.1034 | Zm00001d020506 | 26S proteasome non-ATPase regulatory subunit 9 |
| chr7.S_116288791 | 7 | 116316744 | 5.92 × 10−6 | 0.1034 | |||
| chr7.S_116288792 | 7 | 116316745 | 5.92 × 10−6 | 0.1034 | |||
| chr7.S_116285652 | 7 | 116313605 | 1.01 × 10−5 | 0.09798 | |||
| chr7.S_116285655 | 7 | 116313608 | 1.01 × 10−5 | 0.09798 | |||
| EBM-WW-17 | S7_116315576 | 7 | 116315576 | 1.17 × 10−6 | 0.11793 | Zm00001d020506 | 26S proteasome non-ATPase regulatory subunit 9 |
| S7_116316425 | 7 | 116316425 | 1.17 × 10−6 | 0.11793 | |||
| S7_116316559 | 7 | 116316559 | 1.17 × 10−6 | 0.11793 | |||
| chr7.S_116288756 | 7 | 116316709 | 1.29 × 10−6 | 0.12102 | |||
| chr7.S_116288791 | 7 | 116316744 | 1.29 × 10−6 | 0.12102 | |||
| chr7.S_116288792 | 7 | 116316745 | 1.29 × 10−6 | 0.12102 | |||
| chr7.S_116285652 | 7 | 116313605 | 3.42 × 10−6 | 0.11084 | |||
| chr7.S_116285655 | 7 | 116313608 | 3.42 × 10−6 | 0.11084 | |||
| S7_116314423 | 7 | 116314423 | 1.81 × 10−6 | 0.11403 | |||
| S7_116316667 | 7 | 116316667 | 2.11 × 10−6 | 0.11193 | |||
| EBM-WS-18 | S5_27121944 | 5 | 27121944 | 3.25 × 10−6 | 0.08944 | Zm00001d013992 | Pyridoxal phosphate-dependent transferase family protein |
| EBM-WS-20 | S5_27121944 | 5 | 27121944 | 9.15 × 10−6 | 0.09491 | Zm00001d013992 | Pyridoxal phosphate-dependent transferase family protein |
| PH-WS-17 | chr2.S_68691618 | 2 | 69321921 | 2.98 × 10−7 | 0.14098 | Zm00001d003939 | 11-ß-hydroxysteroid dehydrogenase |
| chr2.S_68691621 | 2 | 69321924 | 2.98 × 10−7 | 0.14098 | |||
| S2_218026770 | 2 | 218026770 | 1.11 × 10−6 | 0.11601 | Zm00001d007189 | Uncharacterised | |
| S2_226449870 | 2 | 226449870 | 2.08 × 10−6 | 0.10972 | GRMZM2G070937 | Leu-rich repeat protein kinase family protein | |
| PH-WW-17 | chr2.S_68691618 | 2 | 69321921 | 7.15 × 10−8 | 0.15528 | Zm00001d003939 | 11-ß-hydroxysteroid dehydrogenase |
| chr2.S_68691621 | 2 | 69321924 | 7.15 × 10−8 | 0.15528 | |||
| S2_218026770 | 2 | 218026770 | 1.95 × 10−6 | 0.11069 | Zm00001d007189 | Uncharacterised | |
| S2_226449870 | 2 | 226449870 | 9.07 × 10−6 | 0.09541 | GRMZM2G070937 | Leu-rich repeat protein kinase family protein | |
| PH-WS-18 | S8_163927011 | 8 | 163927011 | 4.47 × 10−6 | 0.07836 | Zm00001d012167 | Silk fibroin (SF16) protein |
| PH-WW-18 | S8_163927011 | 8 | 163927011 | 8.67 × 10−6 | 0.07275 | Zm00001d012167 | Silk fibroin (SF16) protein |
| S8_163927012 | 8 | 163927012 | 9.66 × 10−6 | 0.07196 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.U.; Zheng, Y.; Chachar, Z.; Zhang, X.; Zhou, G.; Zong, N.; Leng, P.; Zhao, J. Dissection of Maize Drought Tolerance at the Flowering Stage Using Genome-Wide Association Studies. Genes 2022, 13, 564. https://doi.org/10.3390/genes13040564
Khan SU, Zheng Y, Chachar Z, Zhang X, Zhou G, Zong N, Leng P, Zhao J. Dissection of Maize Drought Tolerance at the Flowering Stage Using Genome-Wide Association Studies. Genes. 2022; 13(4):564. https://doi.org/10.3390/genes13040564
Chicago/Turabian StyleKhan, Siffat Ullah, Yanxiao Zheng, Zaid Chachar, Xuhuan Zhang, Guyi Zhou, Na Zong, Pengfei Leng, and Jun Zhao. 2022. "Dissection of Maize Drought Tolerance at the Flowering Stage Using Genome-Wide Association Studies" Genes 13, no. 4: 564. https://doi.org/10.3390/genes13040564
APA StyleKhan, S. U., Zheng, Y., Chachar, Z., Zhang, X., Zhou, G., Zong, N., Leng, P., & Zhao, J. (2022). Dissection of Maize Drought Tolerance at the Flowering Stage Using Genome-Wide Association Studies. Genes, 13(4), 564. https://doi.org/10.3390/genes13040564






