Identification of Endosymbiotic Virus in Small Extracellular Vesicles Derived from Trichomonas vaginalis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trichomonas vaginalis Culture
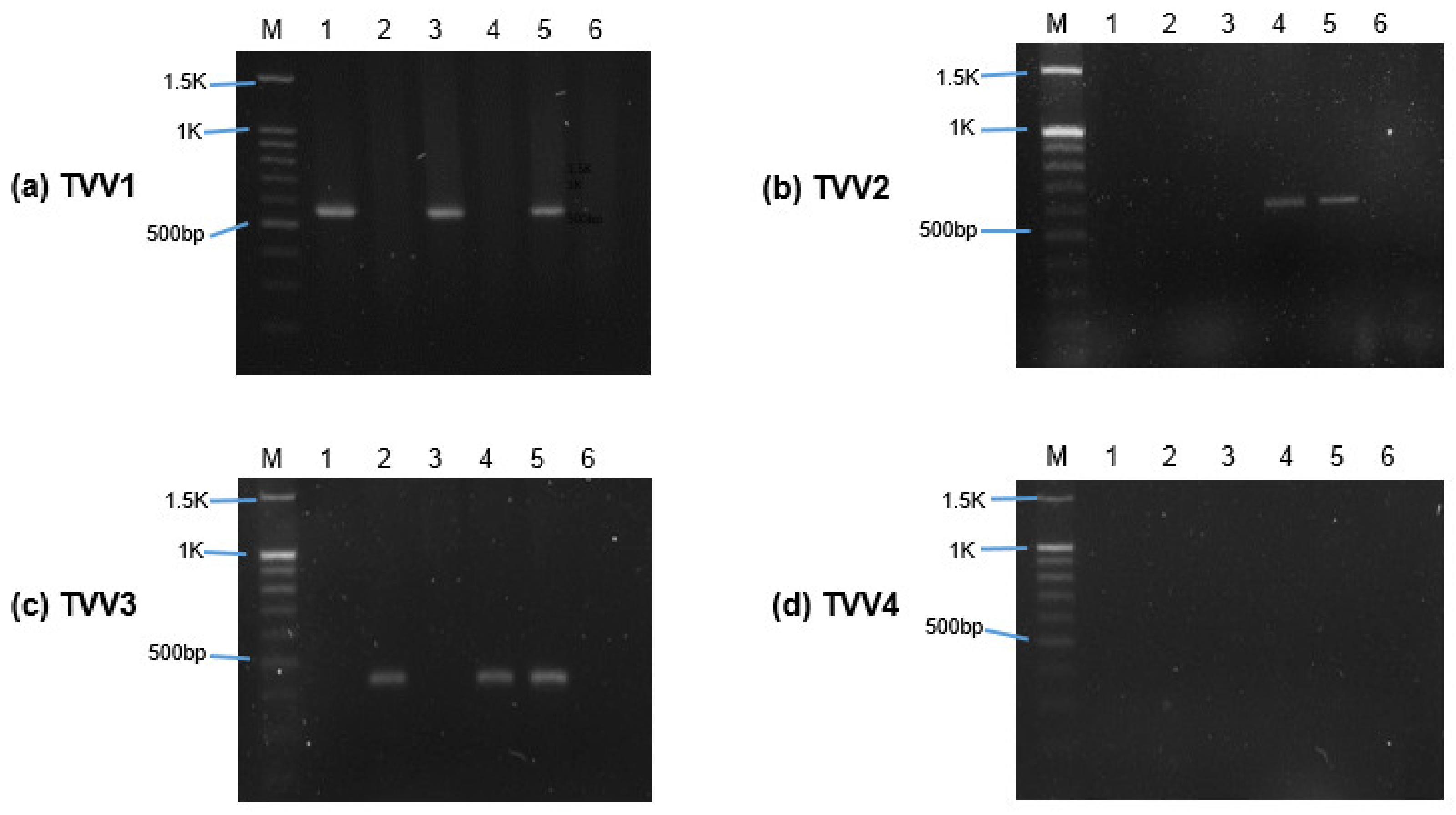
2.2. Detection of TVV through Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.3. The Isolation of Small Extracellular Vesicles
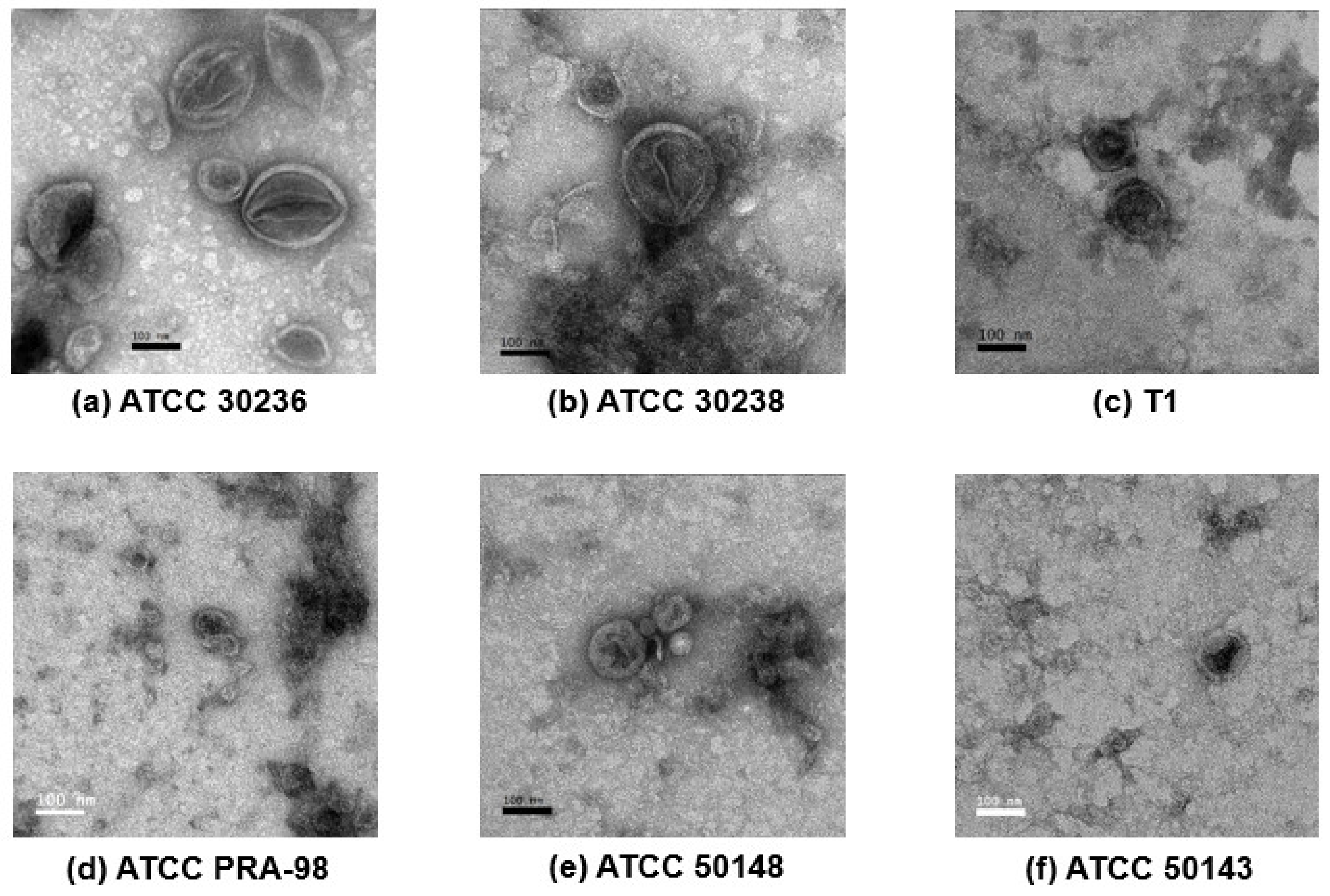
2.4. Transmission Electron Microscopy (TEM)
2.5. Proteomics Data Analysis
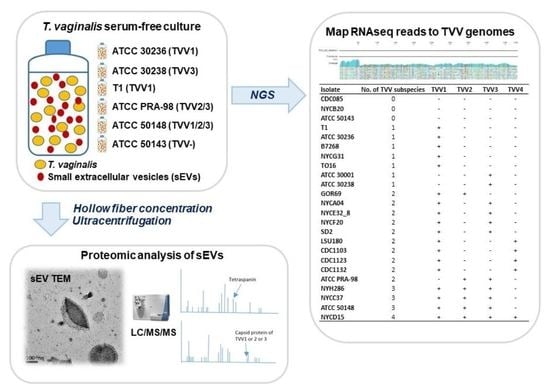
2.6. Identification of TVV Fragments by RNAseq and New-Generation Sequencing (NGS)
3. Results
3.1. Trichomonasvirus Detection by RT-PCR
3.2. Morphology of T. vaginalis Small Extracellular Vesicles
3.3. Proteomics Analysis of Trichomonasvirus and Tetraspanin by LC-MS/MS
3.4. Identification of TVV Fragments by RNAseq and NGS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sexually Transmitted Infections (STIs). Available online: https://www.who.int/en/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) (accessed on 22 November 2021).
- Wendel, K.A.; Rompalo, A.M.; Erbelding, E.J.; Chang, T.H.; Alderete, J.F. Double-stranded RNA viral infection of Trichomonas vaginalis infecting patients attending a sexually transmitted diseases clinic. J. Infect. Dis. 2002, 186, 558–561. [Google Scholar] [CrossRef] [Green Version]
- Patel, E.U.; Gaydos, C.A.; Packman, Z.R.; Quinn, T.C.; Tobian, A.A.R. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin. Infect. Dis. 2018, 67, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swygard, H.; Seña, A.C.; Hobbs, M.M.; Cohen, M.S. Trichomoniasis: Clinical manifestations, diagnosis and management. Sex. Transm. Infect. 2004, 80, 91–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreisel, K.M.; Spicknall, I.H.; Gargano, J.W.; Lewis, F.M.T.; Lewis, R.M.; Markowitz, L.E.; Roberts, H.; Johnson, A.S.; Song, R.; St Cyr, S.B.; et al. Sexually transmitted infections among US women and men: Prevalence and incidence estimates, 2018. Sex Transm. Dis. 2021, 48, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Twu, O.; de Miguel, N.; Lustig, G.; Stevens, G.C.; Vashisht, A.A.; Wohlschlegel, J.A.; Johnson, P.J. Trichomonas vaginalis exosomes deliver cargo to host cells and mediate host:parasite interactions. PLoS Pathog. 2013, 9, e1003482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrera-Bravo, C.; Koh, E.Y.; Tan, K.S.W. The roles of parasite-derived extracellular vesicles in disease and host-parasite communication. Parasitol. Int. 2021, 83, 102373. [Google Scholar] [CrossRef]
- Charrin, S.; Jouannet, S.; Boucheix, C.; Rubinstein, E. Tetraspanins at a glance. J. Cell Sci. 2014, 127, 3641–3648. [Google Scholar] [CrossRef] [Green Version]
- Hemler, M.E. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu. Rev. Cell Dev. Biol. 2003, 19, 397–422. [Google Scholar] [CrossRef]
- Pols, M.S.; Klumperman, J. Trafficking and function of the tetraspanin CD63. Exp. Cell Res. 2009, 315, 1584–1592. [Google Scholar] [CrossRef]
- Olmos-Ortiz, L.M.; Barajas-Mendiola, M.A.; Barrios-Rodiles, M.; Castellano, L.E.; Arias-Negrete, S.; Avila, E.E.; Cuéllar-Mata, P. Trichomonas vaginalis exosome-like vesicles modify the cytokine profile and reduce inflammation in parasite-infected mice. Parasite Immunol. 2017, 39, e12426. [Google Scholar] [CrossRef] [PubMed]
- Flegr, J.; Cerkasov, J.; Kulda, J.; Tachezy, J.; Stokrová, J. The dsRNA of Trichomonas vaginalis is associated with virus-like particles and does not correlate with metronidazole resistance. Folia Microbiol. 1987, 32, 345–348. [Google Scholar] [CrossRef]
- Wang, A.L.; Wang, C.C. The double-stranded RNA in Trichomonas vaginalis may originate from virus-like particles. Proc. Natl. Acad. Sci. USA 1986, 83, 7956–7960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, J.-H.; Ip, C.-F. The cDNA sequence of Trichomonas vaginalis virus-T1 double-stranded RNA. Virology 1995, 206, 773–777. [Google Scholar] [CrossRef] [Green Version]
- Bokharaei-Salim, F.; Esteghamati, A.; Khanaliha, K.; Esghaei, M.; Donyavi, T.; Salemi, B. The first detection of co-infection of double-stranded RNA virus 1, 2 and 3 in Iranian isolates of Trichomonas vaginalis. Iran. J. Parasitol. 2020, 15, 357–363. [Google Scholar] [CrossRef]
- Goodman, R.P.; Freret, T.S.; Kula, T.; Geller, A.M.; Talkington, M.W.; Tang-Fernandez, V.; Suciu, O.; Demidenko, A.A.; Ghabrial, S.A.; Beach, D.H.; et al. Clinical isolates of Trichomonas vaginalis concurrently infected by strains of up to four Trichomonasvirus species (family Totiviridae). J. Virol. 2011, 85, 4258–4270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jehee, I.; van der Veer, C.; Himschoot, M.; Hermans, M.; Bruisten, S. Direct detection of Trichomonas vaginalis virus in Trichomonas vaginalis positive clinical samples from the Netherlands. J. Virol. Methods 2017, 250, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.P.; Ghabrial, S.A.; Fichorova, R.N.; Nibert, M.L. Trichomonasvirus: A new genus of protozoan viruses in the family Totiviridae. Arch. Virol. 2011, 156, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Provenzano, D.; Khoshnan, A.; Alderete, J.F. Involvement of dsRNA virus in the protein composition and growth kinetics of host Trichomonas vaginalis. Arch. Virol. 1997, 142, 939–952. [Google Scholar] [CrossRef]
- Arroyo, R.; Alderete, J.F. Two Trichomonas vaginalis surface proteinases bind to host epithelial cells and are related to levels of cytoadherence and cytotoxicity. Arch. Med. Res. 1995, 26, 279–285. [Google Scholar] [PubMed]
- Lustig, G.; Ryan, C.M.; Secor, W.E.; Johnson, P.J. Trichomonas vaginalis contact-dependent cytolysis of epithelial cells. Infect. Immun. 2013, 81, 1411–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoshnan, A.; Alderete, J.F. Trichomonas vaginalis with a double-stranded RNA virus has upregulated levels of phenotypically variable immunogen mRNA. J. Virol. 1994, 68, 4035–4038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.; Wang, C.C.; Alderete, J.F. Trichomonas vaginalis phenotypic variation occurs only among trichomonads infected with the double-stranded RNA virus. J. Exp. Med. 1987, 166, 142–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govender, Y.; Chan, T.; Yamamoto, H.S.; Budnik, B.; Fichorova, R.N. The role of small extracellular vesicles in viral-protozoan symbiosis: Lessons from Trichomonasvirus in an isogenic host parasite model. Front. Cell. Infect. Microbiol. 2020, 10, 591172. [Google Scholar] [CrossRef]
- Diamond, L.S.; Clark, C.G.; Cunnick, C.C. YI-S, a casein-free medium for axenic cultivation of Entamoeba histolytica, related Entamoeba, Giardia intestinalis and Trichomonas vaginalis. J. Eukaryot. Microbiol. 1995, 42, 277–278. [Google Scholar] [CrossRef]
- Gupta, S.; Rawat, S.; Arora, V.; Kottarath, S.K.; Dinda, A.K.; Vaishnav, P.K.; Nayak, B.; Mohanty, S. An improvised one-step sucrose cushion ultracentrifugation method for exosome isolation from culture supernatants of mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 180–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.-C.; Huang, M.-N.; Chang, J.-F.; Liu, C.-C.; Chen, C.-K.; Hsieh, C.-H. Snake venom proteome and immuno-profiling of the hundred-pace viper, Deinagkistrodon acutus, in Taiwan. Acta Trop. 2019, 189, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Hsu, C.-W.; Chen, C.-D.; Yu, C.-J.; Chang, K.-P.; Tai, D.-I.; Liu, H.-P.; Su, W.-H.; Chang, Y.-S.; Yu, J.-S. Candidate serological biomarkers for cancer identified from the secretomes of 23 cancer cell lines and the human protein atlas. Mol. Cell. Proteom. 2010, 9, 1100–1117. [Google Scholar] [CrossRef] [Green Version]
- Nievas, Y.R.; Coceres, V.M.; Midlej, V.; de Souza, W.; Benchimol, M.; Pereira-Neves, A.; Vashisht, A.A.; Wohlschlegel, J.A.; Johnson, P.J.; De Miguel, N. Membrane-shed vesicles from the parasite Trichomonas vaginalis: Characterization and their association with cell interaction. Cell. Mol. Life Sci. 2018, 75, 2211–2226. [Google Scholar] [CrossRef]
- de Miguel, N.; Riestra, A.; Johnson, P.J. Reversible association of tetraspanin with Trichomonas vaginalis flagella upon adherence to host cells. Cell. Microbiol. 2012, 14, 1797–1807. [Google Scholar] [CrossRef] [Green Version]
- Coceres, V.M.; Alonso, A.M.; Nievas, Y.R.; Midlej, V.; Frontera, L.; Benchimol, M.; Johnson, P.J.; de Miguel, N. The C-terminal tail of tetraspanin proteins regulates their intracellular distribution in the parasite Trichomonas vaginalis. Cell. Microbiol. 2015, 17, 1217–1229. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Wang, C.C. Discovery of a specific double-stranded RNA virus in Giardia lamblia. Mol. Biochem. Parasitol. 1986, 21, 269–276. [Google Scholar] [CrossRef]
- Widmer, G.; Dooley, S. Phylogenetic analysis of Leishmania RNA virus and Leishmania suggests ancient virus-parasite association. Nucleic Acids Res. 1995, 23, 2300–2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nalçacı, M.; Karakuş, M.; Yılmaz, B.; Demir, S.; Özbilgin, A.; Özbel, Y.; Töz, S. Detection of Leishmania RNA virus 2 in Leishmania species from Turkey. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 410–417. [Google Scholar] [CrossRef]
- Charon, J.; Grigg, M.J.; Eden, J.S.; Piera, K.A.; Rana, H.; William, T.; Rose, K.; Davenport, M.P.; Anstey, N.M.; Holmes, E.C. Novel RNA viruses associated with Plasmodium vivax in human malaria and Leucocytozoon parasites in avian disease. PLoS Pathog. 2019, 15, e1008216. [Google Scholar] [CrossRef] [Green Version]
- Khramtsov, N.V.; Woods, K.M.; Nesterenko, M.V.; Dykstra, C.C.; Upton, S.J. Virus-like, double-stranded RNAs in the parasitic protozoan Cryptosporidium parvum. Mol. Microbiol. 1997, 26, 289–300. [Google Scholar] [CrossRef]
- Nibert, M.L.; Woods, K.M.; Upton, S.J.; Ghabrial, S.A. Cryspovirus: A new genus of protozoan viruses in the family Partitiviridae. Arch. Virol. 2009, 154, 1959–1965. [Google Scholar] [CrossRef] [Green Version]
- Atayde, V.D.; da Silva Lira Filho, A.; Chaparro, V.; Zimmermann, A.; Martel, C.; Jaramillo, M.; Olivier, M. Exploitation of the Leishmania exosomal pathway by Leishmania RNA virus 1. Nat. Microbiol. 2019, 4, 714–723. [Google Scholar] [CrossRef]
- Parent, K.N.; Takagi, Y.; Cardone, G.; Olson, N.H.; Ericsson, M.; Yang, M.; Lee, Y.; Asara, J.M.; Fichorova, R.N.; Baker, T.S.; et al. Structure of a protozoan virus from the human genitourinary parasite Trichomonas vaginalis. MBio 2013, 4, e00056-13. [Google Scholar] [CrossRef] [Green Version]
- Rai, A.K.; Johnson, P.J. Trichomonas vaginalis extracellular vesicles are internalized by host cells using proteoglycans and caveolin-dependent endocytosis. Proc. Natl. Acad. Sci. USA 2019, 116, 21354–21360. [Google Scholar] [CrossRef]
- Marti, M.; Johnson, P.J. Emerging roles for extracellular vesicles in parasitic infections. Curr. Opin. Microbiol. 2016, 32, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, L.; Li, J.; Wang, L.; Wu, Z.; Sun, X. Extracellular vesicle-mediated communication within host-parasite interactions. Front. Immunol. 2019, 9, 3066. [Google Scholar] [CrossRef] [PubMed]
- Masha, S.C.; Cools, P.; Crucitti, T.; Sanders, E.J.; Vaneechoutte, M. Molecular typing of Trichomonas vaginalis isolates by actin gene sequence analysis and carriage of T. vaginalis viruses. Parasit. Vectors 2017, 10, 537. [Google Scholar] [CrossRef] [Green Version]
- Da Luz Becker, D.; dos Santos, O.; Frasson, A.P.; de Vargas Rigo, G.; Macedo, A.J.; Tasca, T. High rates of double-stranded RNA viruses and Mycoplasma hominis in Trichomonas vaginalis clinical isolates in South Brazil. Infect. Genet. Evol. 2015, 34, 181–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera, W.L.; Justo, C.A.C.; Relucio-San Diego, M.A.C.V.; Loyola, L.M. Detection and molecular characterization of double-stranded RNA viruses in Philippine Trichomonas vaginalis isolates. J. Microbiol. Immunol. Infect. 2017, 50, 669–676. [Google Scholar] [CrossRef] [Green Version]
- Margarita, V.; Marongiu, A.; Diaz, N.; Dessì, D.; Fiori, P.L.; Rappelli, P. Prevalence of double-stranded RNA virus in Trichomonas vaginalis isolated in Italy and association with the symbiont Mycoplasma hominis. Parasitol Res. 2019, 118, 3565–3570. [Google Scholar] [CrossRef]
- Fichorova, R.N.; Lee, Y.; Yamamoto, H.S.; Takagi, Y.; Hayes, G.R.; Goodman, R.P.; Chepa-Lotrea, X.; Buck, O.R.; Murray, R.; Kula, T.; et al. Endobiont viruses sensed by the human host—Beyond conventional antiparasitic therapy. PLoS ONE 2012, 7, e48418. [Google Scholar] [CrossRef]
- Fichorova, R.; Fraga, J.; Rappelli, P.; Fiori, P.L. Trichomonas vaginalis infection in symbiosis with Trichomonasvirus and Mycoplasma. Res. Microbiol. 2017, 168, 882–891. [Google Scholar] [CrossRef]
- Narayanasamy, R.K.; Rada, P.; Zdrha, A.; van Ranst, M.; Neyts, J.; Tachezy, J. Cytidine nucleoside analog is an effective antiviral drug against Trichomonasvirus. J. Microbiol. Immunol. Infect. 2021. In press. [Google Scholar] [CrossRef]
| Isolate | Trichomonasvirus Subspecies | |||
|---|---|---|---|---|
| TVV1 | TVV2 | TVV3 | TVV4 | |
| ATCC 30236 | + | − | − | − |
| ATCC 30238 | − | − | + | − |
| T1 | + | − | − | − |
| ATCC PRA-98 | − | − | + | − |
| ATCC 50148 | + | + | + | − |
| ATCC 50143 | − | − | − | − |
| Isolate | Type of Tetraspanin | ||
|---|---|---|---|
| TvTSP1 | TvTSP6 | TvTSP8 | |
| ATCC 30236 | + | + | − |
| ATCC 30238 | + | − | + |
| T1 | + | − | + |
| ATCC PRA-98 | + | − | − |
| ATCC 50148 | + | − | + |
| ATCC 50143 | + | + | + |
| Isolate | No. of TVV Subspecies | TVV1 | TVV2 | TVV3 | TVV4 |
|---|---|---|---|---|---|
| CDC085 | 0 | − | − | − | − |
| NYCB20 | 0 | − | − | − | − |
| ATCC 50143 | 0 | − | − | − | − |
| T1 | 1 | + | − | − | − |
| ATCC 30236 | 1 | + | − | − | − |
| B7268 | 1 | + | − | − | − |
| NYCG31 | 1 | + | − | − | − |
| TO16 | 1 | + | − | − | − |
| ATCC 30001 | 1 | − | − | + | − |
| ATCC 30238 | 1 | − | − | + | − |
| GOR69 | 2 | + | + | − | − |
| NYCA04 | 2 | + | − | + | − |
| NYCE32_8 | 2 | + | − | + | − |
| NYCF20 | 2 | + | − | + | − |
| SD2 | 2 | + | − | + | − |
| LSU180 | 2 | + | − | − | + |
| CDC1103 | 2 | + | − | − | + |
| CDC1123 | 2 | + | − | − | + |
| CDC1132 | 2 | + | − | − | + |
| ATCC PRA-98 | 2 | − | + | + | − |
| NYH286 | 3 | + | + | + | − |
| NYCC37 | 3 | + | + | + | − |
| ATCC 50148 | 3 | + | + | + | − |
| NYCD15 | 4 | + | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, S.-C.; Cheng, W.-H.; Ku, F.-M.; Tsai, C.-Y.; Huang, P.-J.; Lee, C.-C.; Yeh, Y.-M.; Rada, P.; Hrdý, I.; Narayanasamy, R.K.; et al. Identification of Endosymbiotic Virus in Small Extracellular Vesicles Derived from Trichomonas vaginalis. Genes 2022, 13, 531. https://doi.org/10.3390/genes13030531
Ong S-C, Cheng W-H, Ku F-M, Tsai C-Y, Huang P-J, Lee C-C, Yeh Y-M, Rada P, Hrdý I, Narayanasamy RK, et al. Identification of Endosymbiotic Virus in Small Extracellular Vesicles Derived from Trichomonas vaginalis. Genes. 2022; 13(3):531. https://doi.org/10.3390/genes13030531
Chicago/Turabian StyleOng, Seow-Chin, Wei-Hung Cheng, Fu-Man Ku, Chih-Yu Tsai, Po-Jung Huang, Chi-Ching Lee, Yuan-Ming Yeh, Petr Rada, Ivan Hrdý, Ravi Kumar Narayanasamy, and et al. 2022. "Identification of Endosymbiotic Virus in Small Extracellular Vesicles Derived from Trichomonas vaginalis" Genes 13, no. 3: 531. https://doi.org/10.3390/genes13030531
APA StyleOng, S.-C., Cheng, W.-H., Ku, F.-M., Tsai, C.-Y., Huang, P.-J., Lee, C.-C., Yeh, Y.-M., Rada, P., Hrdý, I., Narayanasamy, R. K., Smutná, T., Lin, R., Luo, H.-W., Chiu, C.-H., Tachezy, J., & Tang, P. (2022). Identification of Endosymbiotic Virus in Small Extracellular Vesicles Derived from Trichomonas vaginalis. Genes, 13(3), 531. https://doi.org/10.3390/genes13030531