Is COL1A1 Gene rs1107946 Polymorphism Associated with Sport Climbing Status and Flexibility?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study I
2.1.1. Subjects
2.1.2. Genotyping
Japanese Climbers and Controls and Polish Climbers
Polish Controls
Russian Climbers and Controls
2.2. Study II
2.2.1. Subjects
2.2.2. Genotyping
2.3. Statistical Analysis
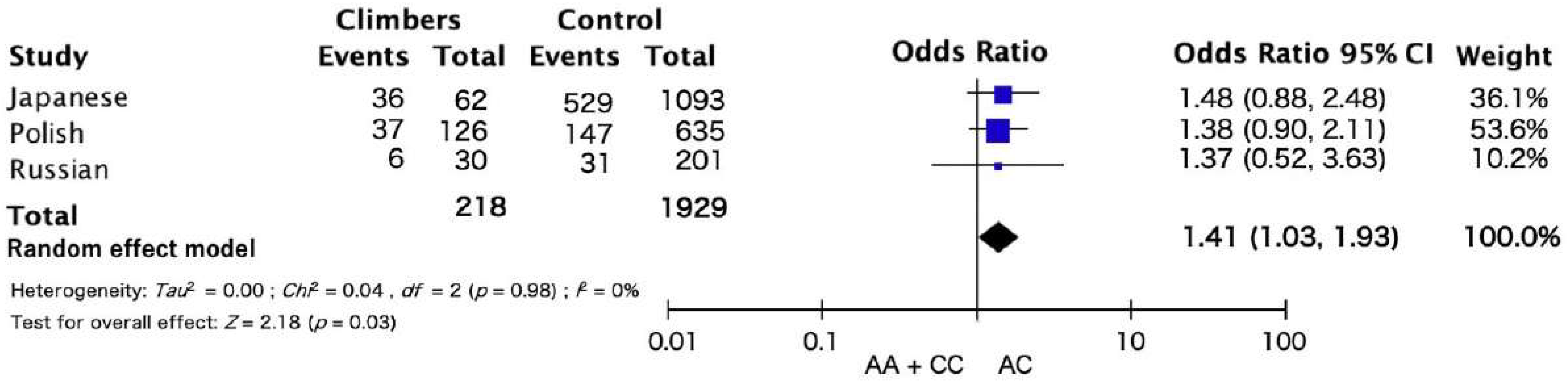
3. Results
3.1. Study I
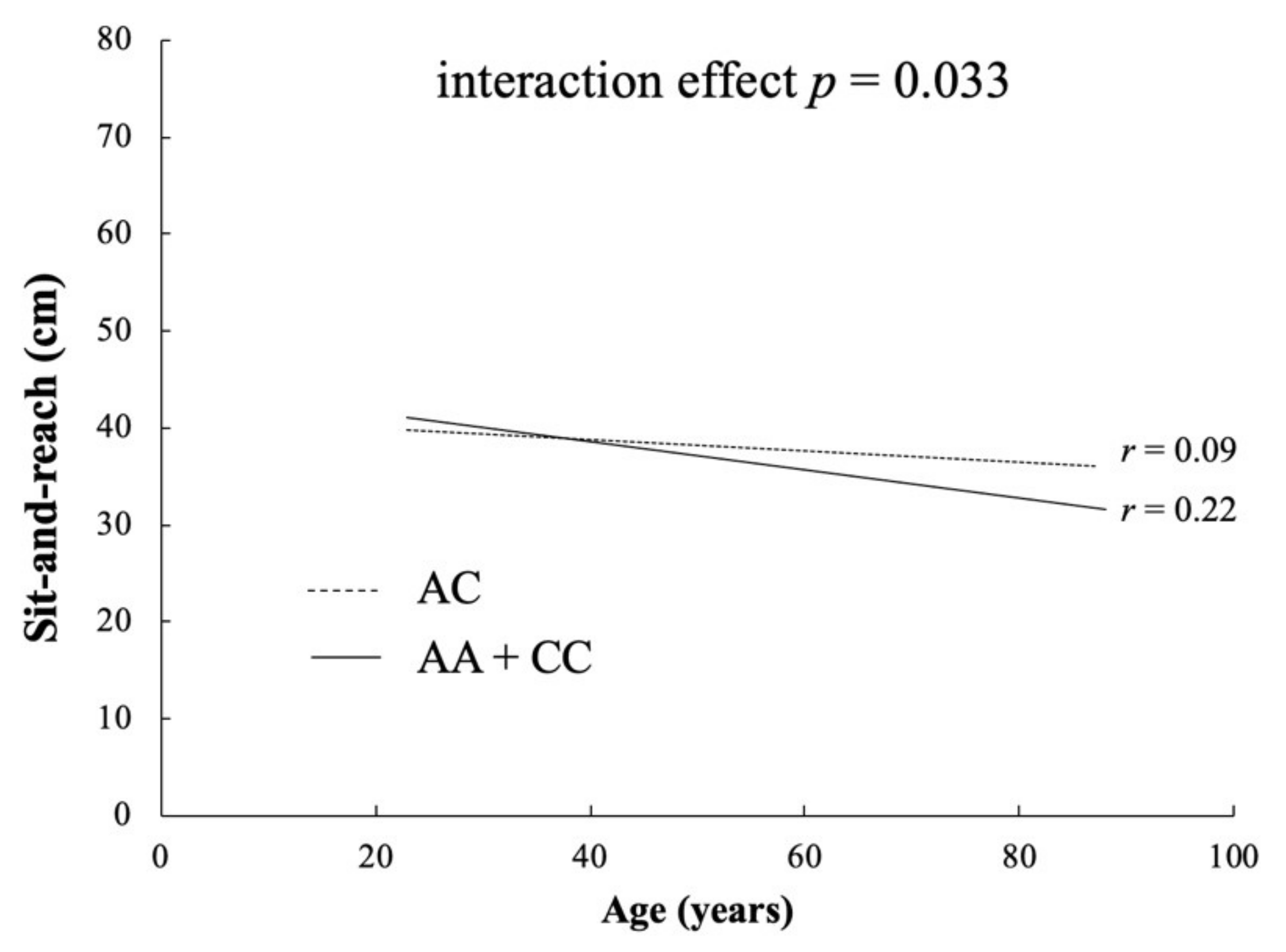
3.2. Study II
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacKenzie, R.; Monaghan, L.; Masson, R.A.; Werner, A.K.; Caprez, T.S.; Johnston, L.; Kemi, O.J. Physical and Physiological Determinants of Rock Climbing. Int. J. Sports Physiol. Perform. 2020, 15, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Philippe, M.; Wegst, D.; Muller, T.; Raschner, C.; Burtscher, M. Climbing-specific finger flexor performance and forearm muscle oxygenation in elite male and female sport climbers. Eur. J. Appl. Physiol. 2012, 112, 2839–2847. [Google Scholar] [CrossRef] [PubMed]
- Ozimek, M.; Krawczyk, M.; Zadarko, E.; Barabasz, Z.; Ambrozy, T.; Stanula, A.; Mucha, D.K.; Jurczak, A.; Mucha, D. Somatic Profile of the Elite Boulderers in Poland. J. Strength Cond. Res. 2017, 31, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Giles, D.; Barnes, K.; Taylor, N.; Chidley, C.; Chidley, J.; Mitchell, J.; Torr, O.; Gibson-Smith, E.; Espana-Romero, V. Anthropometry and performance characteristics of recreational advanced to elite female rock climbers. J. Sports Sci. 2021, 39, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Draper, N.; Brent, S.; Hodgson, C.; Blackwell, G. Flexibility assessment and the role of flexibility as a determinant of performance in rock climbing. Int. J. Perform. Anal. Sport 2009, 9, 67–89. [Google Scholar] [CrossRef]
- Grant, S.; Hynes, V.; Whittaker, A.; Aitchison, T. Anthropometric, strength, endurance and flexibility characteristics of elite and recreational climbers. J. Sports Sci. 1996, 14, 301–309. [Google Scholar] [CrossRef]
- De Moor, M.H.; Spector, T.D.; Cherkas, L.F.; Falchi, M.; Hottenga, J.J.; Boomsma, D.I.; De Geus, E.J. Genome-wide linkage scan for athlete status in 700 British female DZ twin pairs. Twin Res. Hum. Genet. 2007, 10, 812–820. [Google Scholar] [CrossRef]
- Saito, M.; Ginszt, M.; Semenova, E.A.; Massidda, M.; Huminska-Lisowska, K.; Michałowska-Sawczyn, M.; Homma, H.; Cięszczyk, P.; Okamoto, T.; Larin, A.K.; et al. Genetic profile of sports climbing athletes from three different ethnicities. Biol. Sport 2022, 39, 913–919. [Google Scholar] [CrossRef]
- Kikuchi, N.; Zempo, H.; Fuku, N.; Murakami, H.; Sakamaki-Sunaga, M.; Okamoto, T.; Nakazato, K.; Miyachi, M. Association between ACTN3 R577X Polymorphism and Trunk Flexibility in 2 Different Cohorts. Int. J. Sports Med. 2017, 38, 402–406. [Google Scholar] [CrossRef]
- Okuda, E.; Horii, D.; Kano, T. Genetic and Environmental Effects on Physical Fitness and Motor Performance. Int. J. Sport Health Sci. 2005, 3, 1–9. [Google Scholar] [CrossRef]
- Maes, H.H.; Beunen, G.P.; Vlietinck, R.F.; Neale, M.C.; Thomis, M.; Vanden Eynde, B.; Lysens, R.; Simons, J.; Derom, C.; Derom, R. Inheritance of physical fitness in 10-yr-old twins and their parents. Med. Sci. Sports Exerc. 1996, 28, 1479–1491. [Google Scholar] [CrossRef]
- Satipati Chatterjee, N.D. Physical and Motor Fitness in Twins. Jpn. J. Physiol. 1995, 45, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto-Mikami, E.; Kumagai, H.; Tanisawa, K.; Taga, Y.; Hirata, K.; Kikuchi, N.; Kamiya, N.; Kawakami, R.; Midorikawa, T.; Kawamura, T.; et al. Female Athletes Genetically Susceptible to Fatigue Fracture Are Resistant to Muscle Injury: Potential Role of COL1A1 Variant. Med. Sci. Sports Exerc. 2021, 53, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, H.; Miyamoto-Mikami, E.; Hirata, K.; Kikuchi, N.; Kamiya, N.; Hoshikawa, S.; Zempo, H.; Naito, H.; Miyamoto, N.; Fuku, N. ESR1 rs2234693 Polymorphism Is Associated with Muscle Injury and Muscle Stiffness. Med. Sci. Sports Exerc. 2019, 51, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, N.; Miyamoto-Mikami, E.; Hirata, K.; Kimura, N.; Fuku, N. Association analysis of the ACTN3 R577X polymorphism with passive muscle stiffness and muscle strain injury. Scand. J. Med. Sci. Sports 2018, 28, 1209–1214. [Google Scholar] [CrossRef]
- Abrahams, S.; Posthumus, M.; Collins, M. A polymorphism in a functional region of the COL5A1 gene: Association with ultraendurance-running performance and joint range of motion. Int. J. Sports Physiol. Perform. 2014, 9, 583–590. [Google Scholar] [CrossRef]
- Gajdosik, R.L. Passive extensibility of skeletal muscle: Review of the literature with clinical implications. Clin. Biomech. 2001, 16, 87–101. [Google Scholar] [CrossRef]
- Light, N.; Champion, A.E. Characterization of muscle epimysium, perimysium and endomysium collagens. Biochem. J. 1984, 219, 1017–1026. [Google Scholar] [CrossRef]
- Alter, M.J. Science of Flexibility; Human Kinetics: Champaign, IL, USA, 1996. [Google Scholar]
- McKay, M.J.; Baldwin, J.N.; Ferreira, P.; Simic, M.; Vanicek, N.; Burns, J. Normative reference values for strength and flexibility of 1,000 children and adults. Neurology 2017, 88, 36–43. [Google Scholar] [CrossRef]
- Kim, E.; Dear, A.; Ferguson, S.L.; Seo, D.; Bemben, M.G. Effects of 4 weeks of traditional resistance training vs. superslow strength training on early phase adaptations in strength, flexibility, and aerobic capacity in college-aged women. J. Strength Cond. Res. 2011, 25, 3006–3013. [Google Scholar] [CrossRef]
- Monteiro, W.D.; Simao, R.; Polito, M.D.; Santana, C.A.; Chaves, R.B.; Bezerra, E.; Fleck, S.J. Influence of strength training on adult women’s flexibility. J. Strength Cond. Res. 2008, 22, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.; Rhea, M.R.; Simao, R.; Dias, I.; Salles, B.F.; Novaes, J.; Leite, T.; Blair, J.C.; Bunker, D.J. Influence of moderately intense strength training on flexibility in sedentary young women. J. Strength Cond. Res. 2010, 24, 3144–3149. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, D.M.; Cini, A.; Sbruzzi, G.; Lima, C.S. Influence of static stretching on hamstring flexibility in healthy young adults: Systematic review and meta-analysis. Physiother. Theory Pract. 2016, 32, 438–445. [Google Scholar] [CrossRef]
- Welle, S.; Brooks, A.; Thornton, C.A. Senescence-related changes in gene expression in muscle: Similarities and differences between mice and men. Physiol. Genom. 2001, 5, 67–73. [Google Scholar] [CrossRef]
- Podolsky, M.J.; Yang, C.D.; Valenzuela, C.L.; Datta, R.; Huang, S.K.; Nishimura, S.L.; Dallas, S.L.; Wolters, P.J.; Le Saux, C.J.; Atabai, K. Age-dependent regulation of cell-mediated collagen turnover. JCI Insight 2020, 5, e137519. [Google Scholar] [CrossRef]
- Grant, S.; Hasler, T.; Davies, C.; Aitchison, T.C.; Wilson, J.; Whittaker, A. A comparison of the anthropometric, strength, endurance and flexibility characteristics of female elite and recreational climbers and non-climbers. J. Sports Sci. 2001, 19, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Training. Available online: http://tech.cochrane.org/revman (accessed on 3 December 2021).
- Haus, J.M.; Carrithers, J.A.; Trappe, S.W.; Trappe, T.A. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J. Appl. Physiol. 2007, 103, 2068–2076. [Google Scholar] [CrossRef]
- Wood, L.K.; Kayupov, E.; Gumucio, J.P.; Mendias, C.L.; Claflin, D.R.; Brooks, S.V. Intrinsic stiffness of extracellular matrix increases with age in skeletal muscles of mice. J. Appl. Physiol. 2014, 117, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, N.; Yoshida, S.; Min, S.K.; Lee, K.; Sakamaki-Sunaga, M.; Okamoto, T.; Nakazato, K. The ACTN3 R577X genotype is associated with muscle function in a Japanese population. Appl. Physiol. Nutr. Metab. 2015, 40, 316–322. [Google Scholar] [CrossRef]
- Zempo, H.; Tanabe, K.; Murakami, H.; Iemitsu, M.; Maeda, S.; Kuno, S. Age differences in the relation between ACTN3 R577X polymorphism and thigh-muscle cross-sectional area in women. Genet. Test. Mol. Biomarkers. 2011, 15, 639–643. [Google Scholar] [CrossRef]
- Alfuraih, A.M.; Tan, A.L.; O’Connor, P.; Emery, P.; Wakefield, R.J. The effect of ageing on shear wave elastography muscle stiffness in adults. Aging Clin. Exp. Res. 2019, 31, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Gentry, B.A.; Ferreira, J.A.; McCambridge, A.J.; Brown, M.; Phillips, C.L. Skeletal muscle weakness in osteogenesis imperfecta mice. Matrix Biol. 2010, 29, 638–644. [Google Scholar] [CrossRef]
- Leznicka, K.; Zyzniewska-Banaszak, E.; Gebska, M.; Machoy-Mokrzynska, A.; Krajewska-Pedzik, A.; Maciejewska-Skrendo, A.; Leonska-Duniec, A. Interactions between Gene Variants within the COL1A1 and COL5A1 Genes and Musculoskeletal Injuries in Physically Active Caucasian. Genes 2021, 12, 1056. [Google Scholar] [CrossRef] [PubMed]
- Samsami, D.A.; Doroudchi, M.; Kalantari, T.; Pezeshki, A.M.; Ghaderi, A. Heterozygosity in CTLA-4 gene and severe preeclampsia. Int. J. Gynaecol. Obstet. 2005, 88, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, P.; Singh, S.; Juneja, P.K.; Kaur, T. A haplotype derived from the common variants at the −1997G/T and Sp1 binding site of the COL1A1 gene influences risk of postmenopausal osteoporosis in India. Rheumatol. Int. 2013, 33, 501–506. [Google Scholar] [CrossRef]
- Kostik, M.M.; Smirnov, A.M.; Demin, G.S.; Mnuskina, M.M.; Scheplyagina, L.A.; Larionova, V.I. Genetic polymorphisms of collagen type I alpha1 chain (COL1A1) gene increase the frequency of low bone mineral density in the subgroup of children with juvenile idiopathic arthritis. EPMA J. 2013, 4, 15. [Google Scholar] [CrossRef][Green Version]
- Stewart, T.L.; Jin, H.; McGuigan, F.E.; Albagha, O.M.; Garcia-Giralt, N.; Bassiti, A.; Grinberg, D.; Balcells, S.; Reid, D.M.; Ralston, S.H. Haplotypes defined by promoter and intron 1 polymorphisms of the COLIA1 gene regulate bone mineral density in women. J. Clin. Endocrinol. Metab. 2006, 91, 3575–3583. [Google Scholar] [CrossRef]
| Genotype | p-Value | Allele | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| AA | AC | CC | A | C | ||||
| Japanese | ||||||||
| Climbers | 4 (6) | 36 (58) | 22 (36) | 0.164 | 44 (35) | 80 (65) | 0.558 | |
| Controls | 152 (14) | 529 (48) | 412 (38) | 833 (38) | 1353 (62) | |||
| Polish | ||||||||
| Climbers | 2 (2) | 37 (29) | 87 (69) | 0.328 | 41 (16) | 211 (84) | 0.240 | |
| Controls | 12 (2) | 147 (23) | 476 (75) | 171 (13) | 1099 (87) | |||
| Russian | ||||||||
| Climbers | 1 (3) | 6 (20) | 23 (77) | 0.715 | 8 (13) | 52 (87) | 0.385 | |
| Controls | 4 (2) | 31 (15) | 166 (83) | 39 (10) | 363 (90) | |||
| Japanese | Polish | Russian | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | p-Value | OR | 95%CI | p-Value | OR | 95%CI | p-Value | ||
| Dominant | ||||||||||
| CC | 1.00 | 0.73 | 1.00 | 0.17 | 1.00 | 0.45 | ||||
| AA + AC | 0.91 | 0.53–1.55 | 0.75 | 0.49–1.13 | 0.69 | 0.28–1.74 | ||||
| Recessive | ||||||||||
| CC + AC | 1.00 | 0.068 | 1.00 | 0.81 | 1.00 | 0.66 | ||||
| AA | 2.34 | 0.84–6.54 | 1.19 | 0.26–5.40 | 0.59 | 0.06–5.45 | ||||
| Over dominant | ||||||||||
| AA + CC | 1.00 | 0.14 | 1.00 | 0.14 | 1.00 | 0.53 | ||||
| AC | 0.68 | 0.40–1.14 | 0.72 | 0.47–1.11 | 0.73 | 0.28–1.93 | ||||
| n | Age | Height | Weight | Sit-and-Reach | ||
|---|---|---|---|---|---|---|
| Genotype | AA | 152 | 55.3 ± 15.3 | 161.2 ± 8.2 | 60.1 ± 10.1 | 35.6 ± 10.2 |
| CA | 529 | 55.1 ± 15.2 | 161.9 ± 9.4 | 59.7 ± 12.1 | 37.9 ± 10.1 | |
| CC | 412 | 53.0 ± 15.1 | 162.7 ± 8.7 | 60.8 ± 12.1 | 37.0 ± 10.1 | |
| Dominant | AA + AC | 681 | 55.1 ± 15.2 | 161.7 ± 9.1 | 59.8 ± 11.7 | 37.4 ± 10.2 |
| Recessive | CC + AC | 941 | 54.1 ± 15.1 | 162.3 ± 9.1 | 60.2 ± 12.1 | 37.5 ± 10.1 |
| Over dominant | AA + CC | 564 | 53.6 ± 15.2 | 162.3 ± 8.6 | 60.6 ± 11.6 | 36.6 ± 10.1 |
| p-value | Genotype | 0.071 | 0.152 | 0.33 | 0.034 * | |
| Dominant | 0.022 | 0.076 | 0.149 | 0.435 * | ||
| Recessive | 0.375 | 0.194 | 0.919 | 0.035 * | ||
| Over dominant | 0.108 | 0.412 | 0.184 | 0.026 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, M.; Ginszt, M.; Semenova, E.A.; Massidda, M.; Huminska-Lisowska, K.; Michałowska-Sawczyn, M.; Homma, H.; Cięszczyk, P.; Okamoto, T.; Larin, A.K.; et al. Is COL1A1 Gene rs1107946 Polymorphism Associated with Sport Climbing Status and Flexibility? Genes 2022, 13, 403. https://doi.org/10.3390/genes13030403
Saito M, Ginszt M, Semenova EA, Massidda M, Huminska-Lisowska K, Michałowska-Sawczyn M, Homma H, Cięszczyk P, Okamoto T, Larin AK, et al. Is COL1A1 Gene rs1107946 Polymorphism Associated with Sport Climbing Status and Flexibility? Genes. 2022; 13(3):403. https://doi.org/10.3390/genes13030403
Chicago/Turabian StyleSaito, Mika, Michał Ginszt, Ekaterina A. Semenova, Myosotis Massidda, Kinga Huminska-Lisowska, Monika Michałowska-Sawczyn, Hiroki Homma, Paweł Cięszczyk, Takanobu Okamoto, Andrey K. Larin, and et al. 2022. "Is COL1A1 Gene rs1107946 Polymorphism Associated with Sport Climbing Status and Flexibility?" Genes 13, no. 3: 403. https://doi.org/10.3390/genes13030403
APA StyleSaito, M., Ginszt, M., Semenova, E. A., Massidda, M., Huminska-Lisowska, K., Michałowska-Sawczyn, M., Homma, H., Cięszczyk, P., Okamoto, T., Larin, A. K., Generozov, E. V., Majcher, P., Nakazato, K., Ahmetov, I. I., & Kikuchi, N. (2022). Is COL1A1 Gene rs1107946 Polymorphism Associated with Sport Climbing Status and Flexibility? Genes, 13(3), 403. https://doi.org/10.3390/genes13030403









