Behavior Problems and Social Competence in Fragile X Syndrome: A Systematic Review
Abstract
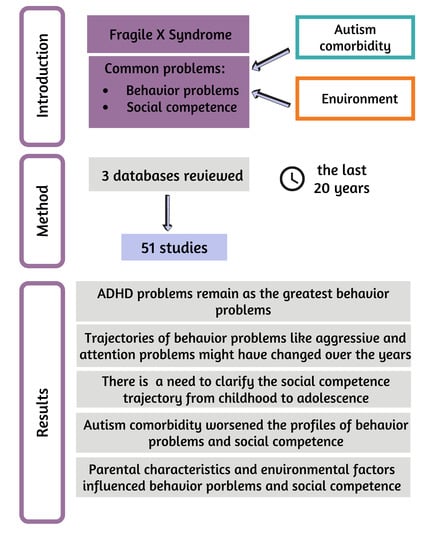
1. Introduction
1.1. Comorbidities
1.2. Behavior Problems
1.3. Social Skills and Social Competence
1.4. Environmental Factors
1.5. Current Study
- What behavior problems have been researched in individuals with fragile X syndrome in the last 20 years?
- What social competence problems have been researched in individuals with fragile X syndrome in the last 20 years?
- What differences have been found in behavior problems and social competence when comparing individuals with fragile X syndrome with typically developing individuals (TD) and individuals with other IDs?
- What differences have been found in behavior problems and social competence when comparing individuals with fragile X syndrome with comorbid individuals with fragile X syndrome and autism?
- How might environmental factors affect behavior and social problems in individuals with fragile X syndrome?
2. Materials and Methods
Search Strategy
3. Results
3.1. Researched Profile in Behavior Problems
Developmental Trajectories in Behavior Problems
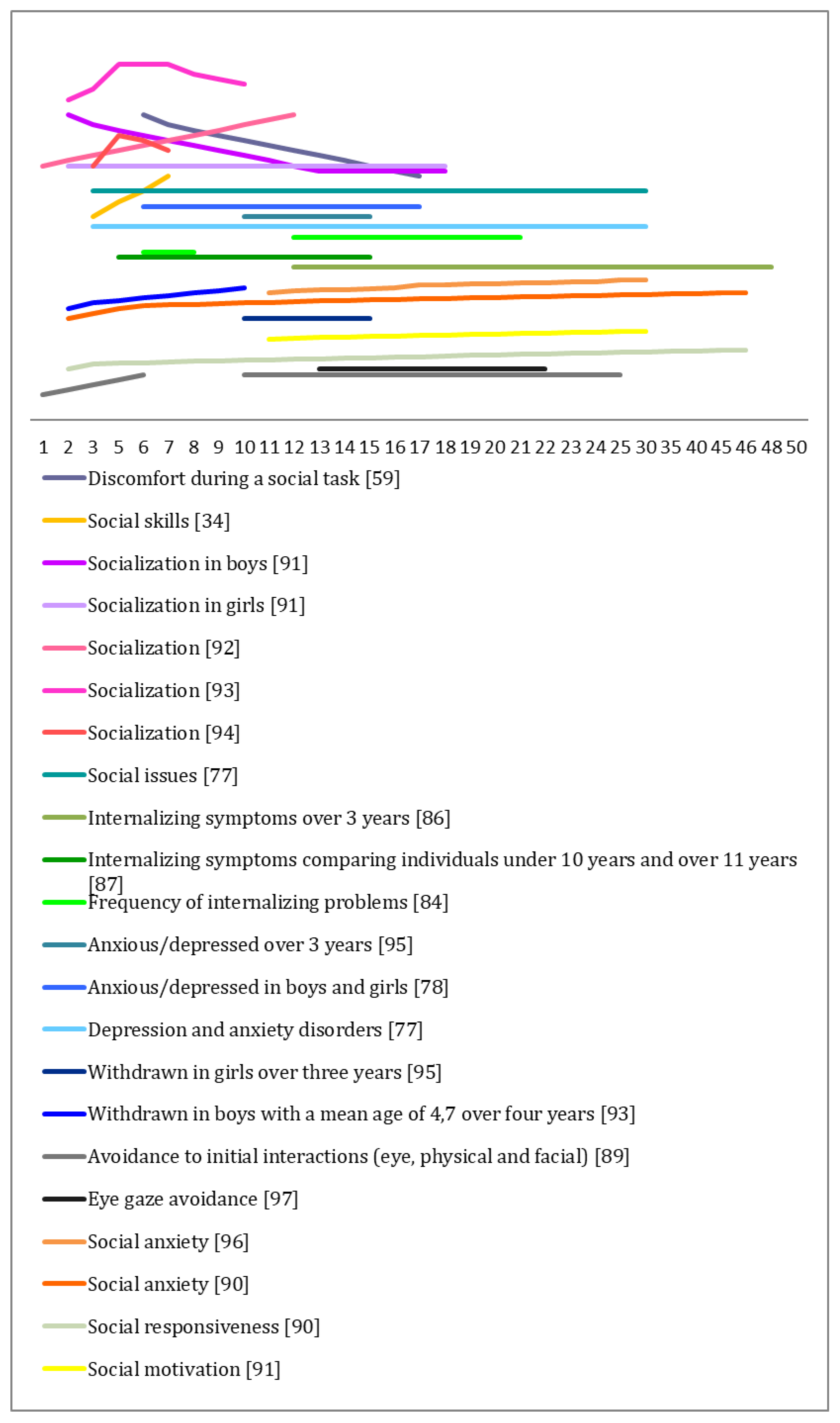
3.2. Researched Profile in Social Competence
Developmental Trajectories in Social Competence
3.3. Differences in Behavior Problems and Social Competence, Comparing Individuals with Fragile X Syndrome with Individuals with TD and Individuals with Other IDs
3.3.1. Comparison of Behavior Problems between Individuals with FXS and Individuals with TD
3.3.2. Comparisons of Behavior Problems between Individuals with FXS and Individuals with Other IDs
3.3.3. Comparisons of Social Competence between Individuals with FXS and Individuals with TD
3.3.4. Comparisons of Social Competence between Individuals with FXS and Individuals with Other IDs
3.4. Differences in Behavior Problems and Social Competence between Individuals with FXS Only and Those with Comorbid ASD
3.4.1. Differences in Behavior Problems
3.4.2. Differences in Social Competence
3.5. Environmental Factors Affecting Behavior and Social Problems in FXS
3.5.1. Environmental Factors Affecting Behavior Problems
3.5.2. Environmental Factors Affecting Social Competence
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Research Paper Assessed for: | Total Score: |
|---|---|
| 1. Control group | 0 = No control group 1 = Comparisons between non-genetically distinct groups or utilized standardized assessment tools 2 = Genetically distinct control group |
| 2. Controls by autism comorbidity | 0 = No 1 = Yes, statistically 2 = Yes, removing those with comorbid ASD |
| 3. Sample size | 0 = Fewer than 15 participants 1 = 15 or more participants 2 = 30 or more participants |
| 4. Recruitment | 0 = Participants selected by clinician(s) 1 = Participants recruited either through charity or medical clinic 2 = Multiple methods, multiple clinics, or multiple charities are used for recruitment |
| 5. Syndrome diagnosis | 0 = Based on reports from the parents 1 = Diagnosis based on physical features or sibling diagnosis 2 = Diagnosis based on appropriate genetic test (PCR or blood test) |
| 6. Methodology | 0 = No validated measures are used 1 = Use validated and/or standardized assessment tools 2 = Validated and/or standardized measures are used alongside new measures, observations, or other methodologies |
| 7. Considerations for development | 0 = Participants are compared as a whole 1 = The study considers age as a variable for at least one aspect of behavior or social competence 2 = Age is considered as a variable in relation to behavior and social competence |
| 8. Appropriate statistics/comparisons | 0 = Data not analyzed 1 = Descriptive statistics are used 2 = Appropriate comparative/correlative statistics are reported |
| Behavior Problem/ Comorbid Condition | Study | Gender | Age | Prevalence % | Borderline/ Clinical Concern % |
|---|---|---|---|---|---|
| Attention | Hessl et al. (2001) [66] | Boys | 6–17 years | 62 | |
| Girls | 47.5 | ||||
| Hatton et al., 2002 [22] | Boys | 4–12 years | 56 | ||
| DaWalt et al., 2021 [78] | Boys and girls | 6 years | 66 | ||
| 12 years | 46.67 | ||||
| 18 years | 44.44 | ||||
| 18 years | 20 | ||||
| Talisa et al., 2014 [63] | Boys | 3–11 years >11 years | 74.3 85.5 | ||
| ADHD | Von-Gontard et al., 2002 [87] | Boys | 5.7–16.10 years | 73.5 | |
| Attention and hyperactivity | Côte et al., 2020 [77] | Boys and girls | 3–30 years | 15 | |
| Hyperactivity/ impulsivity | Talisa et al., 2014 [63] | Boys | 3–11 years >11 years | 58.9 68.9 | |
| Self-injurious behavior | Talisa et al., 2014 [63] | Boys | 3–11 years >11 years | 35.7 44.7 | |
| Hall et al., 2016 [80] | Boys | 11–18 years | 70.6 | ||
| Depression | Talisa et al., 2014 [63] | Boys | 3–11 years >11 years | 1.2 16 | |
| Thought | Talisa et al., 2014 [63] | Boys | 6–17 years | 54.4 | |
| Hessl et al.,2001 [66] | Girls | 6–17 years | 25 | ||
| Boys | 57 | ||||
| Hatton et al., 2002 [22] | Boys | 4–12 years | 57 | ||
| Social problems | Hatton et al., 2002 [22] | Boys | 4–12 years | 26 | |
| Hessl et al.,2001 [66] | Girls | 6–17 years | 40 | ||
| Boys | 41.8 | ||||
| Withdrawn | Hessl et al., 2001 [66] | Boys | 6–17 years | 21.5 | |
| Girls | 17.5 | ||||
| Hatton et al., 2002 [22] | Boys | 4–12 years | 17 | ||
| Aggressive | Hessl et al.,2001 [66] | Boys | 6–17 years | 12.7 | |
| Girls | 12.5 | ||||
| Hatton et al., 2002 [22] | Boys and girls | 6 years | 33 | ||
| Talisa et al., 2014 [63] | Boys | 3–11 years >11 years | 29.2 41.7 | ||
| DaWalt et al., 2021 [78] | Boys and girls | 6 years | 16.67 | ||
| 12 years | 26.67 | ||||
| 18 years | 6.25 | ||||
| Hall et al., 2016 [80] | Boys | 11–18 years | 82.4% | ||
| Anxious/depressed | DaWalt et al., 2021 [78] | Boys and girls | 6 years | 8.33 | |
| 12 years | 33.33 | ||||
| 18 years | 18.7 | ||||
| 18 years | 20 | ||||
| Delinquent behaviors | Hessl et al.,2001 [66] | Boys | 6–17 years | 2.5 | |
| Girls | 5 | ||||
| Total behavior problems | Hessl et al.,2001 [66] | Boys | 6–17 years | 44 | |
| Girls | 47.5 | ||||
| Hatton et al., 2002 [22] | Boys and girls | 6 years | 44 | ||
| Von-Gontard et al., 2002 [87] | Boys | 5.7–16.10 years | 89.8 | ||
| Externalizing | Hessl et al.,2001 [66] | Boys | 6–17 years | 26.6 | |
| Girls | 25 | ||||
| Hatton et al., 2002 [22] | Boys and girls | 6 years | 19 | ||
| Von-Gontard et al., 2002 [87] | Boys | 5.7–16.10 years | 67.3 | ||
| Internalizing | Hessl et al.,2001 [66] | Boys | 6–17 years | 34.2 | |
| Girls | 40 | ||||
| Hatton et al., 2002 [22] | Boys and girls | 6 years | 17 | ||
| Von-Gontard et al., 2002 [87] | Boys | 5.7–16.10 years | 63.3 | ||
| Stereotypy | Hall et al., 2016 [80] | Boys | 11–18 years | 90.6 | |
| Property destruction | Hall et al., 2016 [80] | Boys | 11–18 years | 62.4 |

References
- Bear, M.F.; Huber, K.M.; Warren, S.T. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004, 27, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, R.J.; Rivera, S.M.; Hagerman, P.J. The fragile X family of disorders: A model for autism and targeted treatments. Curr. Pediatr. Rev. 2008, 4, 40–52. [Google Scholar] [CrossRef]
- Hagerman, R.; Hagerman, P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 2013, 12, 786–798. [Google Scholar] [CrossRef]
- Bassell, G.J.; Warren, S.T. Fragile X Syndrome: Loss of Local mRNA Regulation Alters Synaptic Development and Function. Neuron 2008, 60, 201–214. [Google Scholar] [CrossRef]
- Nolin, S.L.; Brown, W.T.; Glicksman, A.; Houck, J.G.E.; Gargano, A.D.; Sullivan, A.; Biancalana, V.; Bröndum-Nielsen, K.; Hjalgrim, H.; Holinski-Feder, E.; et al. Expansion of the Fragile X CGG Repeat in Females with Premutation or Intermediate Alleles. Am. J. Hum. Genet. 2003, 72, 454–464. [Google Scholar] [CrossRef]
- Hessl, D.; Tassone, F.; Loesch, D.Z.; Berry-Kravis, E.; Leehey, M.A.; Gane, L.W.; Barbato, I.; Rice, C.; Gould, E.; Hall, D.A.; et al. Abnormal elevation ofFMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2005, 139B, 115–121. [Google Scholar] [CrossRef]
- Hunter, J.E.; Epstein, M.P.; Tinker, S.W.; Abramowitz, A.; Sherman, S.L. The FMR1 Premutation and Attention-Deficit Hyperactivity Disorder (ADHD): Evidence for a Complex Inheritance. Behav. Genet. 2011, 42, 415–422. [Google Scholar] [CrossRef]
- Wang, J.Y.; Hessl, D.H.; Hagerman, R.J.; Tassone, F.; Rivera, S.M. Age-Dependent Structural Connectivity Effects in Fragile X Premutation. Arch. Neurol. 2012, 69, 482–489. [Google Scholar] [CrossRef]
- Hessl, D.; Rivera, S.; Koldewyn, K.; Cordeiro, L.; Adams, J.; Tassone, F.; Hagerman, P.J.; Hagerman, R.J. Amygdala dysfunction in men with the fragile X premutation. Brain 2006, 130, 404–416. [Google Scholar] [CrossRef]
- Shelton, A.L.; Cornish, K.; Fielding, J. Long term verbal memory recall deficits in fragile X premutation females. Neurobiol. Learn. Mem. 2017, 144, 131–135. [Google Scholar] [CrossRef]
- Hagerman, R.J.; Jackson, C.; Amiri, K.; O’Connor, R.; Sobesky, W.; Silverman, A.C. Girls with Fragile X Syndrome: Physical and Neurocognitive Status and Outcome. Pediatrics 1992, 89, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Bartholomay, K.L.; Lee, C.H.; Bruno, J.L.; Lightbody, A.A.; Reiss, A.L. Closing the Gender Gap in Fragile X Syndrome: Review of Females with Fragile X Syndrome and Preliminary Research Findings. Brain Sci. 2019, 9, 11. [Google Scholar] [CrossRef]
- Wang, Z.; Taylor, A.K.; A Bridge, J. FMR1 fully expanded mutation with minimal methylation in a high functioning fragile X male. J. Med. Genet. 1996, 33, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Artigas-Pallarés, J.; Brun, C.; Gabau, E. Aspectos médicos y neuropsicológicos del síndrome X frágil. Rev. Neurol. 2001, 2, 42–54. [Google Scholar]
- Tassone, F.; Hagerman, R.J.; Iklé, D.N.; Dyer, P.N.; Lampe, M.; Willemsen, R.; Oostra, B.A.; Taylor, A.K. FMRP expression as a potential prognostic indicator in fragile X syndrome. Am. J. Med. Genet. 1999, 84, 250–261. [Google Scholar] [CrossRef]
- Loesch, D.Z.; Huggins, R.M.; Hagerman, R.J. Phenotypic variation and FMRP levels in fragile X. Ment. Retard. Dev. Disabil. Res. Rev. 2004, 10, 31–41. [Google Scholar] [CrossRef]
- National Fragile X Foundation. Available online: https://fragilex.org/understanding-fragile-x/fragile-x-101/prevalence/ (accessed on 12 September 2021).
- McKechanie, A.; Barnicoat, A.; Trender-Gerhard, I.; Allison, M.; Stanfield, A. Fragile X-associated conditions: Implications for the whole family. Br. J. Gen. Pract. 2019, 69, 460–461. [Google Scholar] [CrossRef]
- Baumgardner, T.L.; Reiss, A.L.; Freund, L.S.; Abrams, M.T. Specification of the Neurobehavioral Phenotype in Males with Fragile X Syndrome. Pediatrics 1995, 95, 744–752. [Google Scholar] [CrossRef]
- Faraone, S.V.; Biederman, J.; Mick, E. The age-dependent decline of attention deficit hyperactivity disorder: A meta-analysis of follow-up studies. Psychol. Med. 2005, 36, 159–165. [Google Scholar] [CrossRef]
- Fryns, J.P.; Jacobs, J.; Kleczkowska, A.; Berghe, H.V.D. The psychological profile of the fragile X syndrome. Clin. Genet. 2008, 25, 131–134. [Google Scholar] [CrossRef]
- Hatton, D.D.; Hooper, S.R.; Bailey, D.B.; Skinner, M.L.; Sullivan, K.M.; Wheeler, A. Problem behavior in boys with fragile X syndrome. Am. J. Med. Genet. 2002, 108, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, R.J.; Hagerman, P.J. The fragile X premutation: Into the phenotypic fold. Curr. Opin. Genet. Dev. 2002, 12, 278–283. [Google Scholar] [CrossRef]
- Torrioli, M.; Vernacotola, S.; Setini, C.; Bevilacqua, F.; Martinelli, D.; Snape, M.; Hutchison, J.A.; Di Raimo, F.R.; Tabolacci, E.; Neri, G. Treatment with valproic acid ameliorates ADHD symptoms in fragile X syndrome boys. Am. J. Med. Genet. Part A 2010, 152A, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.W.; Hessl, D.; Goodlin-Jones, B.; Ferranti, J.; Bacalman, S.; Barbato, I.; Tassone, F.; Hagerman, P.J.; Herman, K.; Hagerman, R.J. Autism Profiles of Males with Fragile X Syndrome. Am. J. Ment. Retard. 2008, 113, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.J.; Wehner, E.A.; Hagerman, R. The Behavioral Phenotype in Fragile X: Symptoms of Autism in Very Young Children with Fragile X Syndrome, Idiopathic Autism, and Other Developmental Disorders. J. Dev. Behav. Pediatr. 2001, 22, 409–417. [Google Scholar] [CrossRef]
- DeMark, J.L.; Feldman, M.A.; Holden, J.J.A. Behavioral Relationship between Autism and Fragile X Syndrome. Am. J. Ment. Retard. 2003, 108, 314–326. [Google Scholar] [CrossRef]
- Clifford, S.; Dissanayake, C.; Bui, Q.M.; Huggins, R.; Taylor, A.K.; Loesch, D.Z. Autism Spectrum Phenotype in Males and Females with Fragile X Full Mutation and Premutation. J. Autism Dev. Disord. 2006, 37, 738–747. [Google Scholar] [CrossRef]
- Reddy, K.S. Cytogenetic abnormalities and fragile-x syndrome in Autism Spectrum Disorder. BMC Med. Genet. 2005, 6, 3. [Google Scholar] [CrossRef]
- Harris, S.; Goodlin-Jones, B.; Nowicki, S.; Bacalman, S.; Tassone, F.; Hagerman, R. Autism Profiles of Young Males with Fragile X Syndrome. J. Dev. Behav. Pediatr. 2005, 26, 464. [Google Scholar] [CrossRef]
- Hagerman, R. Fragile X syndrome. In Neuro-Developmental Disorders: Diagnosis and Treatment; Hagerman, R., Ed.; Oxford University Press: New York, NY, USA, 1999; pp. 61–132. [Google Scholar]
- Merenstein, S.A.; Sobesky, W.E.; Taylor, A.K.; Riddle, J.E.; Tran, H.X.; Hagerman, R.J. Molecular-clinical corre-lations in males with an expanded FMR1 mutation. Am. J. Med. Genet. 1996, 64, 388–394. [Google Scholar] [CrossRef]
- Hagerman, R.J. The physical and behavioral phenotype. In Fragile X Syndrome: Diagnosis, Treatment, and Research, 3rd ed.; Hagerman, R.J., Hagerman, P.J., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2002; pp. 206–248. [Google Scholar]
- Reisinger, D.L.; Roberts, J.E. Differential Relationships of Anxiety and Autism Symptoms on Social Skills in Young Boys with Fragile X Syndrome. Am. J. Intellect. Dev. Disabil. 2017, 122, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E.; Barker, E.T.; Seltzer, M.M.; Abbeduto, L.; Greenberg, J.S. Behavioral Phenotype of Fragile X Syndrome in Adolescence and Adulthood. Am. J. Intellect. Dev. Disabil. 2012, 117, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Abbeduto, L.; McDuffie, A.; Thurman, A.J. The fragile X syndrome–autism comorbidity: What do we really know? Front. Genet. 2014, 5, 355. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.B., Jr.; Mesibov, G.B.; Hatton, D.D.; Clark, R.D.; Roberts, J.E.; Mayhew, L. Autistic behavior in young boys with frag-ile X syndrome. J. Autism Dev. Disord. 1998, 28, 499–508. [Google Scholar] [CrossRef]
- Cordeiro, L.; Ballinger, E.; Hagerman, R.; Hessl, D. Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: Prevalence and characterization. J. Neurodev. Disord. 2010, 3, 57–67. [Google Scholar] [CrossRef]
- Bailey, D.B., Jr.; Raspa, M.; Olmsted, M.; Holiday, D.B. Co-occurring conditions associated with FMR1 gene variations: Findings from a national parent survey. Am. J. Med. Genet. Part A 2008, 146A, 2060–2069. [Google Scholar] [CrossRef]
- Martin, J.P.; Bell, J. A Pedigree of Mental Defect Showing Sex-Linkage. J. Neurol. Neurosurg. Psychiatry 1943, 6, 154–157. [Google Scholar] [CrossRef]
- Dykens, E.M. Measuring behavioral phenotypes: Provocations from the “new genetics”. Am. J. Ment. Retard. 1995, 99, 522–532. [Google Scholar]
- Oakes, A.; Thurman, A.J.; McDuffie, A.; Bullard, L.; Hagerman, R.J.; Abbeduto, L. Characterising repetitive behaviours in young boys with fragile X syndrome. J. Intellect. Disabil. Res. 2015, 60, 54–67. [Google Scholar] [CrossRef]
- Cohen, I.L.; Fisch, G.S.; Sudhalter, V.; Wolf-Schein, E.G.; Hanson, D.; Hagerman, R.; Jenkins, E.C.; Brown, W.T. Social gaze, social avoidance, and repetitive behavior in fragile X males: A controlled study. Am. J. Ment. Retard. 1988, 92, 436–446. [Google Scholar]
- Boyle, L.; Kaufman, W.E. The behavioral phenotype of FMR1 mutations. Am. J. Med. Genet. C Semin. Med. Genet. 2010, 154C, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, R.J. Physical and behavioral phenotype. In Fragile X Syndrome: Diagnosis, Treatment, and Research; Hagerman, R.J., Cronister, A., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 1996; pp. 3–88. [Google Scholar]
- Kau, A.S.; Tierney, E.; Bukelis, I.; Stump, M.H.; Kates, W.R.; Trescher, W.H.; Kaufmann, W.E. Social behavior profile in young males with fragile X syndrome: Characteristics and specificity. Am. J. Med. Genet. 2004, 126A, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.J.; Bodfish, J.W.; Hazlett, H.C.; Lightbody, A.A.; Reiss, A.L.; Piven, J. Evidence of a Distinct Behavioral Phenotype in Young Boys with Fragile X Syndrome and Autism. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, W.E.; Cortell, R.; Kau, A.S.; Bukelis, I.; Tierney, E.; Gray, R.M.; Cox, C.; Capone, G.T.; Stanard, P. Autism spectrum disorder in fragile X syndrome: Communication, social interaction, and specific behaviors. Am. J. Med. Genet. 2004, 129A, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Cross, E.M.; Hare, D.J. Behavioural phenotypes of the mucopolysaccharide disorders: A systematic literature review of cognitive, motor, social, linguistic and behavioural presentation in the MPS disorders. J. Inherit. Metab. Dis. 2013, 36, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Raspa, M.; Wheeler, A.C.; Riley, C. Public Health Literature Review of Fragile X Syndrome. Pediatrics 2017, 139, S153–S171. [Google Scholar] [CrossRef]
- Usher, L.V.; Dawalt, L.S.; Hong, J.; Greenberg, J.S.; Mailick, M.R. Trajectories of Change in the Behavioral and Health Phenotype of Adolescents and Adults with Fragile X Syndrome and Intellectual Disability: Longitudinal Trends over a Decade. J. Autism Dev. Disord. 2020, 50, 2779–2792. [Google Scholar] [CrossRef]
- Iarocci, G.; Yager, J.; Rombough, A.; McLaughlin, J. The Development of Social Competence among Persons with Down Syndrome: From Survival to Social Inclusion. Int. Rev. Res. Ment. Retard. 2007, 35, 87–119. [Google Scholar] [CrossRef]
- Vaughn, S.; Hogan, A. The Social Competence of Students with Learning Disabilities over Time. J. Learn. Disabil. 1994, 27, 292–303. [Google Scholar] [CrossRef]
- Eisenberg, N.; Haris, J.D. Social Competence—A Developmental Perspective. Sch. Psychol. Rev. 1984, 13, 267–277. [Google Scholar] [CrossRef]
- Gresham, F.M. Social skills and learning disabilities: Causal, concomitant, or correlational? Sch. Sychol. Rev. 1992, 21, 348–360. [Google Scholar] [CrossRef]
- American Association on Intellectual and Developmental Disabilities. Available online: https://www.aaidd.org/intellectual-disability/definition (accessed on 30 October 2021).
- Mazzocco, M.M. Advances in research on the fragile X syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 2000, 6, 96–106. [Google Scholar] [CrossRef]
- Williams, T.A.; Langdon, R.; Porter, M.A. Hyper-reactivity in fragile X syndrome females: Generalised or specific to social-ly-salient stimuli? A skin conductance study. Int. J. Psychophysiol. 2013, 88, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Hessl, D.; Glaser, B.; Dyer-Friedman, J.; Reiss, A.L. Social behavior and cortisol reactivity in children with fragile X syndrome. J. Child Psychol. Psychiatry 2005, 47, 602–610. [Google Scholar] [CrossRef]
- Farzin, F.; Rivera, S.M.; Hessl, D. Brief Report: Visual Processing of Faces in Individuals with FragileX Syndrome: An Eye Tracking Study. J. Autism Dev. Disord. 2009, 39, 946–952. [Google Scholar] [CrossRef]
- Bailey, D.B., Jr.; Hatton, D.D.; Skinner, M.; Mesibov, G. Autistic behavior, FMR1 protein, and developmental trajectories in young males with fragile X syndrome. J. Autism Dev. Disord. 2001, 31, 165–174. [Google Scholar] [CrossRef]
- Carter, A.S.; Davis, N.O.; Klin, A.; Volkmar, F.R. Social Development in Autism. In Handbook of Autism and Pervasive Develop-mental Disorders, Diagnosis, Development, Neurobiology, and Behavior; Volkmar, F.R., Paul, R., Klin, A., Cohen, D., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005; pp. 312–334. [Google Scholar]
- Talisa, V.B.; Boyle, L.; Crafa, D.; Kaufmann, W.E. Autism and anxiety in males with fragile X syndrome: An exploratory analysis of neurobehavioral profiles from a parent survey. Am. J. Med. Genet. Part A 2014, 164, 1198–1203. [Google Scholar] [CrossRef]
- Thompson, J.R.; Bradley, V.J.; Buntinx, W.H.E.; Schalock, R.L.; Shogren, K.A.; Snell, M.E.; Wehmeyer, M.L.; Borthwick-Duffy, S.; Coulter, D.L.; Craig, E.; et al. Conceptualizing Supports and the Support Needs of People with Intellectual Disability. Intellect. Dev. Disabil. 2009, 47, 135–146. [Google Scholar] [CrossRef]
- Luckasson, R.; Borthwick-Duffy, S.; Buntix, W.H.E.; Coulter, D.L.; Craig, E.M.; Reeve, A.; Schalock, R.L.; Snell, M.E.; Spit-alnik, D.M.; Spreat, S.; et al. Mental Retardation: Definition, Classification, and Systems of Supports, 10th ed.; American Association on Mental Retardation: Washington, DC, USA, 2002. [Google Scholar]
- Hessl, D.; Dyer-Friedman, J.; Glaser, B.; Wisbeck, J.; Barajas, R.G.; Taylor, A.; Reiss, A.L. The Influence of Environmental and Genetic Factors on Behavior Problems and Autistic Symptoms in Boys and Girls with Fragile X Syndrome. Pediatrics 2001, 108, e88. [Google Scholar] [CrossRef]
- Sterling, A.M.; Barnum, L.; Skinner, D.; Warren, S.F.; Fleming, K. Parenting Young Children with and without Fragile X Syndrome. Am. J. Intellect. Dev. Disabil. 2012, 117, 194–206. [Google Scholar] [CrossRef]
- Fielding-Gebhardt, H.; Warren, S.F.; Brady, N.C. Child Challenging Behavior Influences Maternal Mental Health and Relationship Quality over Time in Fragile X Syndrome. J. Autism Dev. Disord. 2019, 50, 779–797. [Google Scholar] [CrossRef] [PubMed]
- Hartley, S.L.; Seltzer, M.M.; Hong, J.; Greenberg, J.S.; Smith, L.; Almeida, D.; Coe, C.; Abbeduto, L. Cortisol response to be-havior problems in FMR1 premutation mothers of adolescents and adults with fragile X syndrome: A diathesis-stress model. Int. J. Behav. Dev. 2012, 36, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, M.M.; Barker, E.T.; Greenberg, J.S.; Hong, J.; Coe, C.; Almeida, D. Differential sensitivity to life stress in FMR1 premutation carrier mothers of children with Fragile X Syndrome. Health Psychol. 2012, 31, 612–622. [Google Scholar] [CrossRef]
- Summers, J.A.; Poston, D.J.; Turnbull, A.P.; Marquis, J.; Hoffman, L.; Mannan, H.; Wang, M. Conceptualizing and measuring family quality of life. J. Intellect. Disabil. Res. 2005, 49, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Pardini, D. Novel insights into longstanding theories of bidirectional parent-child influences: Introduction to the special sec-tion. J. Abnorm. Child Psychol. 2008, 36, 627–631. [Google Scholar] [CrossRef]
- Thurman, A.J.; McDuffie, A.; Hagerman, R.; Abbeduto, L. Psychiatric symptoms in boys with fragile X syndrome: A comparison with nonsyndromic autism spectrum disorder. Res. Dev. Disabil. 2014, 35, 1072–1086. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Tranfaglia, M.R. The Psychiatric Presentation of Fragile X: Evolution of the Diagnosis and Treatment of the Psychiatric Comorbidities of Fragile X Syndrome. Dev. Neurosci. 2011, 33, 337–348. [Google Scholar] [CrossRef]
- Ciaccio, C.; Fontana, L.; Milani, D.; Tabano, S.; Miozzo, M.; Esposito, S. Fragile X syndrome: A review of clinical and molecular diagnoses. Ital. J. Pediatr. 2017, 43, 39. [Google Scholar] [CrossRef]
- Côté, V.; Knoth, I.S.; Lalancette, È.; Lavergne, J.-A.; Côté, L.; Major, P.; Lippé, S. Behavioural Characteristics Related to Adaptive Functioning in Young Persons with Tuberous Sclerosis Complex, Down Syndrome and Fragile x Syndrome. J. Dev. Phys. Disabil. 2020, 33, 279–296. [Google Scholar] [CrossRef]
- DaWalt, L.S.; Fielding-Gebhardt, H.; Fleming, K.K.; Warren, S.F.; Brady, N. Change in Behavior Problems from Childhood through Adolescence for Children with Fragile X Syndrome. J. Autism Dev. Disord. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Steinhausen, H.-C.; von Gontard, A.; Spohr, H.-L.; Hauffa, B.P.; Eiholzer, U.; Backes, M.; Willms, J.; Malin, Z. Behavioral phenotypes in four mental retardation syndromes: Fetal alcohol syndrome, Prader-Willi syndrome, fragile X syndrome, and tuberosis sclerosis. Am. J. Med. Genet. 2002, 111, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.S.; Barnett, R.P.; Hustyi, K.M. Problem behaviour in adolescent boys with fragile X syndrome: Relative prevalence, frequency and severity. J. Intellect. Disabil. Res. 2016, 60, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Crawford, H.; Karakatsani, E.; Singla, G.; Oliver, C. The Persistence of Self-injurious and Aggressive Behavior in Males with Fragile X Syndrome over 8 Years: A Longitudinal Study of Prevalence and Predictive Risk Markers. J. Autism Dev. Disord. 2019, 49, 2913–2922. [Google Scholar] [CrossRef] [PubMed]
- Langthorne, P.; McGill, P. An Indirect Examination of the Function of Problem Behavior Associated with Fragile X Syndrome and Smith-Magenis Syndrome. J. Autism Dev. Disord. 2011, 42, 201–209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rice, L.J.; Gray, K.M.; Howlin, P.; Taffe, J.; Tonge, B.J.; Einfeld, S.L. The developmental trajectory of disruptive behavior in Down syndrome, fragile X syndrome, Prader–Willi syndrome and Williams syndrome. Am. J. Med. Genet. Part C Semin. Med. Genet. 2015, 169, 182–187. [Google Scholar] [CrossRef]
- Greenberg, J.S.; Seltzer, M.M.; Baker, J.K.; Smith, L.E.; Warren, S.F.; Brady, N.; Hong, J. Family Environment and Behavior Problems in Children, Adolescents, and Adults with Fragile X Syndrome. Am. J. Intellect. Dev. Disabil. 2012, 117, 331–346. [Google Scholar] [CrossRef]
- Achenbach, T.M. Manual for the Child Behavior Checklist/4-18 and 1991 Profile; Department of Psychiatry, University of Vermont: Burlington, VT, USA, 1991. [Google Scholar]
- Smith, L.E.; Hong, J.; Greenberg, J.S.; Mailick, M.R. Change in the Behavioral Phenotype of Adolescents and Adults with FXS: Role of the Family Environment. J. Autism Dev. Disord. 2016, 46, 1824–1833. [Google Scholar] [CrossRef]
- Von Gontard, A.; Backes, M.; Laufersweiler-Plass, C.; Wendland, C.; Lehmkuhl, G.; Zerres, K.; Rudnik-Schoneborn, S. Psychopathology and familial stress—Comparison of boys with Fragile X syndrome and Spinal Muscular Atrophy. J. Child Psychol. Psychiatry 2002, 43, 949–957. [Google Scholar] [CrossRef]
- Crawford, H.; Moss, J.; Stinton, C.; Singla, G.; Oliver, C. Overactivity, impulsivity and repetitive behaviour in males with fragile X syndrome: Contrasting developmental trajectories in those with and without elevated autism symptoms. J. Intellect. Disabil. Res. 2018, 62, 672–683. [Google Scholar] [CrossRef]
- Roberts, J.E.; Crawford, H.; Will, E.A.; Hogan, A.L.; McQuillin, S.; Tonnsen, B.L.; O’Connor, S.; Roberts, D.A.; Brewe, A.M. Infant Social Avoidance Predicts Autism but Not Anxiety in Fragile X Syndrome. Front. Psychiatry 2019, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.; Oliver, C.; Stefanidou, C.; Apperly, I.; Moss, J. An Observational Study of Social Interaction Skills and Behaviors in Cornelia de Lange, Fragile X and Rubinstein-Taybi Syndromes. J. Autism Dev. Disord. 2020, 50, 4001–4010. [Google Scholar] [CrossRef] [PubMed]
- Klaiman, C.; Quintin, E.-M.; Jo, B.; Lightbody, A.A.; Hazlett, H.C.; Piven, J.; Hall, S.S.; Reiss, A.L. Longitudinal Profiles of Adaptive Behavior in Fragile X Syndrome. Pediatrics 2014, 134, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Hatton, D.D.; Wheeler, A.C.; Skinner, M.L.; Bailey, D.B.; Sullivan, K.M.; Roberts, J.E.; Mirrett, P.; Clark, R.D. Adaptive Behavior in Children with Fragile X Syndrome. Am. J. Ment. Retard. 2003, 108, 373–390. [Google Scholar] [CrossRef]
- Warren, S.F.; Brady, N.; Fleming, K.K.; Hahn, L. The Longitudinal Effects of Parenting on Adaptive Behavior in Children with Fragile X Syndrome. J. Autism Dev. Disord. 2017, 47, 768–784. [Google Scholar] [CrossRef]
- Hernandez, R.N.; Feinberg, R.L.; Vaurio, R.; Passanante, N.M.; Thompson, R.E.; Kaufmann, W.E. Autism spectrum disorder in fragile X syndrome: A longitudinal evaluation. Am. J. Med. Genet. Part A 2009, 149A, 1125–1137. [Google Scholar] [CrossRef]
- Soriano, L.D.H.; Thurman, A.J.; Abbeduto, L. Specificity: A Phenotypic Comparison of Communication-Relevant Domains between Youth with Down Syndrome and Fragile X Syndrome. Front. Genet. 2018, 9, 424. [Google Scholar] [CrossRef]
- Crawford, H.; Moss, J.; Groves, L.; Dowlen, R.; Nelson, L.; Reid, D.; Oliver, C. A Behavioural Assessment of Social Anxiety and Social Motivation in Fragile X, Cornelia de Lange and Rubinstein-Taybi Syndromes. J. Autism Dev. Disord. 2019, 50, 127–144. [Google Scholar] [CrossRef]
- Murphy, M.M.; Abbeduto, L.; Schroeder, S.; Serlin, R. Contribution of Social and Information-Processing Factors to Eye-Gaze Avoidance in Fragile X Syndrome. Am. J. Ment. Retard. 2007, 112, 349–360. [Google Scholar] [CrossRef]
- Hatton, D.D.; Wheeler, A.; Sideris, J.; Sullivan, K.; Reichardt, A.; Roberts, J.; Clark, R.; Bailey, D.B. Developmental Trajectories of Young Girls with Fragile X Syndrome. Am. J. Intellect. Dev. Disabil. 2009, 114, 161–171. [Google Scholar] [CrossRef]
- Caravella, K.E.; Roberts, J.E. Adaptive skill trajectories in infants with fragile X syndrome contrasted to typical controls and infants at high risk for autism. Res. Autism Spectr. Disord. 2017, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.S.; Burns, D.D.; Reiss, A.L. Modeling Family Dynamics in Children with Fragile X Syndrome. J. Abnorm. Child Psychol. 2006, 35, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Cornish, K.; Munir, F.; Wilding, J. Perfil neuropsicológico y conductual de los déficits de atención en síndrome de X frágil. Rev. Neurol. 2001, 33 (Suppl. S1), S24–S29. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.; Powis, L.; Moss, J.; Stinton, C.; Nelson, L.; Oliver, C. Prospective study of autism phenomenology and the behavioural phenotype of Phelan-McDermid syndrome: Comparison to fragile X syndrome, Down syndrome and idiopathic autism spectrum disorder. J. Neurodev. Disord. 2017, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Britton, T.C.; Wilkinson, E.H.; Hall, S.S. Examining the Specificity of Forms and Functions of Aggressive Behavior in Boys with Fragile X Syndrome. Am. J. Intellect. Dev. Disabil. 2020, 125, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Kau, A.S.; Reider, E.E.; Payne, L.; Meyer, W.A.; Freund, L. Early behavior signs of psychiatric phenotypes in fragile X syn-drome. Am. J. Ment. Retard. 2000, 105, 286–299. [Google Scholar] [CrossRef]
- Bailey, J.D.B.; Hatton, D.D.; Mesibov, G.; Ament, N.; Skinner, M. Early Development, Temperament, and Functional Impairment in Autism and Fragile X Syndrome. J. Autism Dev. Disord. 2000, 30, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, W.; Zhan, J.; Hu, L.; Wu, L.; Zhao, Z. Adaptive behaviour of Chinese boys with fragile X syndrome. J. Intellect. Disabil. Res. 2015, 60, 1–8. [Google Scholar] [CrossRef]
- Roberts, J.E.; Clarke, M.A.; Alcorn, K.; Carter, J.C.; Long, A.C.; Kaufman, W.E. Autistic behavior in boys with fragile X syn-drome: Social approach and HPA- axis dysfunction. J. Neurodev. Disord. 2009, 1, 283. [Google Scholar] [CrossRef]
- Russo-Ponsaran, N.M.; Berry-Kravis, E.; McKown, C.A.; Lipton, M. A Pilot Study of Social Information Processing Skills in Girls with Fragile X Syndrome. J. Ment. Heal. Res. Intellect. Disabil. 2014, 7, 143–168. [Google Scholar] [CrossRef]
- Tonnsen, B.; Scherr, J.; Reisinger, D.; Roberts, J. Behavioral Markers of Emergent Stranger Anxiety in Infants and Toddlers with Fragile X Syndrome. J. Autism Dev. Disord. 2017, 47, 3646–3658. [Google Scholar] [CrossRef] [PubMed]
- Dawalt, L.S.; Usher, L.V.; Greenberg, J.S.; Mailick, M.R. Friendships and social participation as markers of quality of life of adolescents and adults with fragile X syndrome and autism. Autism 2019, 23, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, W.E.; Kidd, S.A.; Andrews, H.F.; Budimirovic, D.B.; Esler, A.; Haas-Givler, B.; Stackhouse, T.; Riley, C.; Peacock, G.; Sherman, S.L.; et al. Autism Spectrum Disorder in Fragile X Syndrome: Cooccurring Conditions and Current Treatment. Pediatrics 2017, 139, S194–S206. [Google Scholar] [CrossRef] [PubMed]
- Budimirovic, D.B.; Bukelis, I.; Cox, C.; Gray, R.M.; Tierney, E.; Kaufmann, W.E. Autism spectrum disorder in Fragile X syndrome: Differential contribution of adaptive socialization and social withdrawal. Am. J. Med. Genet. Part A 2006, 140A, 1814–1826. [Google Scholar] [CrossRef]
- Martin, G.E.; Barstein, J.; Hornickel, J.; Matherly, S.; Durante, G.; Losh, M. Signaling of noncomprehension in communication breakdowns in fragile X syndrome, Down syndrome, and autism spectrum disorder. J. Commun. Disord. 2017, 65, 22–34. [Google Scholar] [CrossRef]
- Barkley, R.A. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol. Bull. 1997, 121, 65–94. [Google Scholar] [CrossRef]
- Mazzocco, M.M.M.; Baumgardner, T.; Freund, L.S.; Reiss, A.L. Social functioning among girls with fragile X or Turner syndrome and their sisters. J. Autism Dev. Disord. 1998, 28, 509–517. [Google Scholar] [CrossRef]
- Backes, M.; Genç, J.B.; Schreck, W.; Doerfler, G.; Lemkuhl, A.; Von Gontard, A. Cognitive and behavioral profile of fragile x boys: Correlations to molecular data. Am. J. Med. Genetics. 2000, 95, 150–156. [Google Scholar] [CrossRef]
- Wheeler, A.C.; Raspa, M.; Bishop, E.; Bailey, D.B. Aggression in fragile X syndrome. J. Intellect. Disabil. Res. 2015, 60, 113–125. [Google Scholar] [CrossRef]
- Hardiman, R.L.; McGill, P. How common are challenging behaviours amongst individuals with Fragile X Syndrome? A systematic review. Res. Dev. Disabil. 2018, 76, 99–109. [Google Scholar] [CrossRef]
- Symons, F.J.; Clark, R.D.; Roberts, J.P.; Bailey, D.B., Jr. Classroom Behavior of Elementary School-Age Boys with Fragile X Syndrome. J. Spec. Educ. 2001, 34, 194–202. [Google Scholar] [CrossRef]
- Symons, F.J.; Clark, R.D.; Hatton, D.D.; Skinner, M.; Bailey, D.B., Jr. Self-injurious behavior in young boys with fragile X syndrome. Am. J. Med. Genet. 2003, 118A, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Symons, F.J.; Byiers, B.J.; Raspa, M.; Bishop, E.; Bailey, D.B. Self-Injurious Behavior and Fragile X Syndrome: Findings from the National Fragile X Survey. Am. J. Intellect. Dev. Disabil. 2010, 115, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.S.; Hustyi, K.M.; Barnett, R.P. Examining the influence of social-environmental variables on self-injurious behaviour in adolescent boys with fragile X syndrome. J. Intellect. Disabil. Res. 2018, 62, 1072–1085. [Google Scholar] [CrossRef] [PubMed]
- Meguid, N.; Effat, S.; Abou, E.I.; Hassan, H.; Amin, W. Behavioral Patterns of Down Syndrome and Fragile-X Syndrome in Egyptian Samples. Int. J. Child Neuropsychiatry 2009, 6, 1–9. [Google Scholar]
- Lachiewicz, A.M. Abnormal behaviors of young girls with fragile X syndrome. Am. J. Med. Genet. 1992, 43, 72–77. [Google Scholar] [CrossRef]
- Lesniak-Karpiak, K.; Mazzocco, M.M.M.; Ross, J.L. Behavioral assessment of social anxiety in females with Turner or fragile X syndrome. J. Autism Dev. Disord. 2003, 33, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.E.; Weisenfield, L.A.H.; Hatton, D.D.; Heath, M.; Kaufman, W.E. Social approach and autistic behavior in children with fragile X syndrome. J. Autism Dev. Disord. 2007, 37, 1748–1760. [Google Scholar] [CrossRef] [PubMed]
- Cote, S.M.; Vaillancourt, T.; LeBlanc, J.C.; Nagin, D.S.; Tremblay, R.E. The development of physical aggression from toddler-hood to pre-adolescence: A nation wide longitudinal study of Canadian children. J. Abnorm. Child Psychol. 2006, 34, 71–85. [Google Scholar] [CrossRef]
- Salcedo-Arellano, M.J.; Lozano, R.; Tassone, F.; Hagerman, R.J.; Saldarriaga, W. Alcohol use dependence in fragile X syndrome. Intractable Rare Dis. Res. 2016, 5, 207–213. [Google Scholar] [CrossRef]
- Cornish, K.; Cole, V.; Longhi, E.; Karmiloff-Smith, A.; Scerif, G. Do behavioural inattention and hyperactivity exacerbate cognitive difficulties associated with autistic symptoms? Longitudinal profiles in fragile X syndrome. Int. J. Dev. Disabil. 2013, 59, 80–94. [Google Scholar] [CrossRef]
- Wheeler, A.; Raspa, M.; Bann, C.; Bishop, E.; Hessl, D.; Sacco, P.; Bailey, D.B., Jr. Anxiety, attention problems, hyperactivity, and the Aberrant Behavior Checklist in fragile X syndrome. Am. J. Med. Genet. Part A 2013, 164, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Haessler, F.; Gaese, F.; Huss, M.; Kretschmar, C.; Brinkman, M.; Peters, H.; Elstner, S.; Colla, M.; Pittrow, D. Characterization, treatment patterns, and patient-related outcomes of patients with Fragile X syndrome in Germany: Final results of the observational EXPLAIN-FXS study. BMC Psychiatry 2016, 16, 318. [Google Scholar] [CrossRef] [PubMed]
- Doherty, B.R.; Longhi, E.; Cole, V.; Karmiloff-Smith, A.; Cornish, K.; Scerif, G. Disentangling autism spectrum and attention-deficit/hyperactivity symptoms over development in fragile X syndrome. Res. Dev. Disabil. 2020, 104, 103692. [Google Scholar] [CrossRef]
- Cornish, K.; Cole, V.; Longhi, E.; Karmiloff-Smith, A.; Scerif, G. Does Attention Constrain Developmental Trajectories in Fragile X Syndrome? A 3-Year Prospective Longitudinal Study. Am. J. Intellect. Dev. Disabil. 2012, 117, 103–120. [Google Scholar] [CrossRef]
- Frolli, A.; Piscopo, S.; Conson, M. Developmental changes in cognitive and behavioural functioning of adolescents with fragile-X syndrome. J. Intellect. Disabil. Res. 2014, 59, 613–621. [Google Scholar] [CrossRef]
- Hustyi, K.M.; Hall, S.S.; Jo, B.; Lightbody, A.A.; Reiss, A.L. Longitudinal trajectories of aberrant behavior in fragile X syn-drome. Res. Dev. Disabil. 2014, 35, 2691–2701. [Google Scholar] [CrossRef]
- Hahn, L.; Brady, N.C.; Warren, S.F.; Fleming, K.K. Do Children with Fragile X Syndrome Show Declines or Plateaus in Adaptive Behavior? Am. J. Intellect. Dev. Disabil. 2015, 120, 412–432. [Google Scholar] [CrossRef]
- Bellanti, C.J.; Bierman, K.L. Disentangling the Impact of Low Cognitive Ability and Inattention on Social Behavior and Peer Relationships. J. Clin. Child Psychol. 2000, 29, 66–75. [Google Scholar] [CrossRef]
- Arron, K.; Oliver, C.; Moss, J.; Berg, K.; Burbidge, C. The prevalence and phenomenology of self-injurious and aggressive behaviour in genetic syndromes. J. Intellect. Disabil. Res. 2010, 55, 109–120. [Google Scholar] [CrossRef]
- Turk, J. Fragile X Syndrome and Attentional Deficits. J. Appl. Res. Intellect. Disabil. 1998, 11, 175–191. [Google Scholar] [CrossRef]
- Turk, J.; Graham, P. Fragile X Syndrome, Autism and Autistic Features. Autism 1997, 1, 175–197. [Google Scholar] [CrossRef]
- Cregenzán-Royo, O.; Brun-Gasca, C.; Fornieles-Deu, A. Expressed emotion and impulsiveness in mothers of children with Fragile X Syndrome and Down Syndrome: The relation to behavioral problems in their offspring. Res. Dev. Disabil. 2018, 83, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, D.; Granero, R.; Gallastegui, F.; Pérez-Jurado, L.; Brun-Gasca, C. Behavioral features of Williams Beuren syndrome compared to Fragile X syndrome and subjects with intellectual disability without defined etiology. Res. Dev. Disabil. 2011, 32, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Artigas-Pallarés, J.; Brun-Gasca, C. ¿Se puede atribuir el fenotipo conductual del síndrome X frágil al retraso mental y al tras-torno por déficit de atención/hiperactividad? Rev. Neur. 2004, 38, 7–11. [Google Scholar]
- Woodcock, K.A.; Oliver, C.; Humphreys, G.W. Task-switching deficits and repetitive behaviour in genetic neurodevelopmental disorders: Data from children with Prader–Willi syndrome chromosome 15 q11–q13 deletion and boys with Fragile X syndrome. Cogn. Neuropsychol. 2009, 26, 172–194. [Google Scholar] [CrossRef]
- Hagerman, R.J. Biomedical advances in developmental psychology: The case of fragile X syndrome. Dev. Psychol. 1996, 32, 416–424. [Google Scholar] [CrossRef]
- Klusek, J.; Moser, C.; Schmidt, J.; Abbeduto, L.; Roberts, J.E. A novel eye-tracking paradigm for indexing social avoidance-related behavior in fragile X syndrome. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2019, 183, 5–16. [Google Scholar] [CrossRef]
- American Psychiatric Association. Manual Diagnóstico y Estadístico de Los Trastornos Mentales DSM-5, 5th ed.; Editorial Médica Panamericana: Madrid, Spain, 2014. [Google Scholar]
- Hughes, K.R.; Hogan, A.L.; Roberts, J.E.; Klusek, J. Gesture Frequency and Function in Infants with Fragile X Syndrome and Infant Siblings of Children with Autism Spectrum Disorder. J. Speech Lang. Hear. Res. 2019, 62, 2386–2399. [Google Scholar] [CrossRef]
- Goyal, S.; Srivastava, K.; Bansal, V. Study of prevalence of depression in adolescent students of a public school. Ind. Psychiatry J. 2009, 18, 43–46. [Google Scholar] [CrossRef]
- Moss, J.; Nelson, L.; Powis, L.; Richards, C.; Waite, J.; Oliver, C. A comparative study of sociability in Angelman, Cornelia de Lange, fragile X, Down and Rubinstein-Taybi syndromes and autism spectrum disorders. Am. J. Intellect. Dev. Disabil. 2016, 121, 465–486. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.S.; Frank, M.; Pusiol, G.T.; Farzin, F.; Lightbody, A.A.; Reiss, A.L. Quantifying naturalistic social gaze in fragile X syndrome using a novel eye tracking paradigm. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2015, 168, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Burack, J.A.; Volkmar, F.R. Development of low- and high-functioning autistic children. J. Child Psychol. Psychiatry 1992, 33, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.; Ritvo, E.R.; Yokota, A.; Childs, J.; Pollard, J. WISC-R and Vineland Adaptive Behavior Scale Scores in Autistic Children. J. Am. Acad. Child Adolesc. Psychiatry 1988, 27, 428–429. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.-Y.; Chen, C.-L.; Chen, Y.-J.; Chen, C.-H.; Lee, L.-F.; Chiang, T.-M. Features of developmental functions and autistic profiles in children with fragile X syndrome. Chang Gung Med. J. 2005, 28, 551–558. [Google Scholar]
- Budimirovic, D.; Kaufmann, W.E. What Can We Learn about Autism from Studying Fragile X Syndrome? Dev. Neurosci. 2011, 33, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.P.; Eckert, E.M.; Pedapati, E.V.; Shaffer, R.C.; Dominick, K.C.; Wink, L.K.; Sweeney, J.A.; Erickson, C.A. Differentiating social preference and social anxiety phenotypes in fragile X syndrome using an eye gaze analysis: A pilot study. J. Neurodev. Disord. 2019, 11, 1. [Google Scholar] [CrossRef]
- Achenbach, T.M.; Edelbrock, C.S. The Child Behavior Profile: II. Boys aged 12–16 and girls aged 6–11 and 12–16. J. Consult. Clin. Psychol. 1979, 47, 223–233. [Google Scholar] [CrossRef]
- Muller, K.; Brady, N.C.; Warren, S.F.; Fleming, K.K. Mothers’ perspectives on challenging behaviours in their children with fragile X syndrome. J. Intellect. Dev. Disabil. 2018, 44, 481–491. [Google Scholar] [CrossRef]
- Reisinger, D.L.; Shaffer, R.C.; Tartaglia, N.; Berry-Kravis, E.; Erickson, C.A. Delineating Repetitive Behavior Profiles across the Lifespan in Fragile X Syndrome. Brain Sci. 2020, 10, 239. [Google Scholar] [CrossRef]
- Newman, I.; Leader, G.; Chen, J.L.; Mannion, A. An analysis of challenging behavior, comorbid psychopathology, and Attention-Deficit/Hyperactivity Disorder in Fragile X Syndrome. Res. Dev. Disabil. 2015, 38, 7–17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McDuffie, A.; Thurman, A.J.; Hagerman, R.J.; Abbeduto, L. Symptoms of Autism in Males with Fragile X Syndrome: A Comparison to Nonsyndromic ASD Using Current ADI-R Scores. J. Autism Dev. Disord. 2014, 45, 1925–1937. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brock, M.; Hatton, D. Distinguishing features of autism in boys with fragile X syndrome. J. Intellect. Disabil. Res. 2010, 54, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.; Hatton, D.; Reichardt, A.; Bailey, D. Correlates of maternal behaviours in mothers of children with fragile X syndrome. J. Intellect. Disabil. Res. 2007, 51, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, E.; Polli, R.; Lunghi, M.; Murgia, A. Impact of the COVID-19 Italian Lockdown on the Physiological and Psychological Well-Being of Children with Fragile X Syndrome and Their Families. Int. J. Environ. Res. Public Health 2021, 18, 5752. [Google Scholar] [CrossRef] [PubMed]
- McCary, L.M.; Roberts, J.E. Early identification of autism in fragile X syndrome: A review. J. Intellect. Disabil. Res. 2012, 57, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Schopler, E.; Reichler, R.; Renner, B. The Childhood Autism Rating Scale (CARS); Western Psychological Services: Los Angeles, CA, USA, 1988. [Google Scholar]
- McDevitt, S.C.; Carey, W.B. Behavioral Style Questionnaire; Behavioral-Developmental Initiatives: Scottsdale, AZ, USA, 1978. [Google Scholar]
- Newborg, J.; Stock, J.; Wnek, L.; Guidubaldi, J.; Svinicki, J. The Battelle Developmental Inventory; DLM/Teaching Resources: Allen, TX, USA, 1984. [Google Scholar]
- Achenbach, T.M. Manual for the Child Behavior Checklist/2–3 and 1992 Profile; University of Vermont: Burlington, VT, USA, 1992. [Google Scholar]
- Achenbach, T.M.; Edelbrock, C.S. Manual for the Child Behavior Checklist and Revised Child Behavior Profile; Department of Psychiatry, University of Vermont: Burlington, VT, USA, 1983. [Google Scholar]
- Aman, M.G.; Singh, N.N. Aberrant Behavior Checklist—Community Manual; Slosson Educational Publications: East Aurora, NY, USA, 1986. [Google Scholar]
- Aman, M.G.; Singh, N.B. Aberrant Behavior Checklist—Community, Supplementary Manual; Slosson Educational Publications: East Aurora, NY, USA, 1994. [Google Scholar]
- Windle, M.; Lerner, R.M. Reassessing the dimensions of temperamental individuality across the life span: The Revised Di-mensions of Temperament Survey (DOTS-R). J. Adolesc. Res. 1986, 1, 213–230. [Google Scholar] [CrossRef]
- Sparrow, S.S.; Balla, D.A.; Cicchetti, D.V. Vineland Adaptive Behavior Scales; American Guidance Service: Circle Pines, MN, USA, 1984. [Google Scholar]
- Caldwell, B.M.; Bradley, R.H. Home Observation for Measurement of the Environment—Revised Edition; University of Arkansas at Little Rock: Little Rock, AR, USA, 1984. [Google Scholar]
- Krug, D.A.; Arick, J.R.; Almond, P.J. Autism Screening Instrument for Educational Planning; Western Psychological Services: Los Angeles, CA, USA, 1993. [Google Scholar]
- Ullmann, R.K.; Sleator, E.K.; Sprague, R.L. A new rating scale for diagnosis and monitoring of ADD children. Psychopharmacol. Bull. 1984, 20, 160–164. [Google Scholar] [PubMed]
- Achenbach, T.M. Manual for the Teacher’s Report form and 1991 Profile; University of Vermont: Burlington, VT, USA, 1991. [Google Scholar]
- Lord, C.; Rutter, M.; Le Couteur, A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef]
- Lord, C.; Rutter, M.; DiLavore, P.C.; Risi, S. Autism Diagnostic Observation Schedule—WPS Edition; Western Psychological Services: Los Angeles, CA, USA, 1999. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Einfeld, S.L.; Tonge, B.J. Manual for the Developmental Behaviour Checklist: Primary Care Version (DBC-P); University of New South Wales and Monash University: Melbourne, Australia, 1994. [Google Scholar]
- Einfeld, S.L.; Tonge, B. The Developmental Behavior Checklist: The development and validation of an instrument to assess behavioral and emotional disturbance in children and adolescents with mental retardation. J. Autism Dev. Disord. 1995, 25, 81–104. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Margraf, J.; Unnewehr, S. Kinder-DIPS: Diagnostisches Interview bei Psychischen Storungen von Kindern und Jugendlichen; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Sommer, G.; Fydrich, T. Soziale Unterstutzung. Diagnostik, Konzepte, F-SOZU (DGVT-Materialie Nr. 22); Deutsche Gesellschaft fur Verhaltenstherapie: Tubingen, Germany, 1989. [Google Scholar]
- Holroyd, J. Manual for the Questionnaire on Resources and Stress; Neuropsychiatric Institute: Los Angeles, CA, USA, 1974. [Google Scholar]
- Friedrich, W.N.; Greenberg, M.T.; Crnic, K. A short-form of the Questionnaire on Resources and Stress. Am. J. Ment. Defic. 1983, 88, 41–48. [Google Scholar] [PubMed]
- McCubbin, H.J.; Larson, A.S.; Olson, D.H. F-COPES. In Family Inventories; Family Social Science; Olson, D.H., Ed.; University of Minnesota: St Paul, MN, USA, 1982. [Google Scholar]
- Aman, M.G.; Singh, N.N.; Stewart, A.W.; Field, C.J. The aberrant behavior checklist: A behavior rating scale for the assessment of treatment effects. Am. J. Ment. Defic. 1985, 89, 485–491. [Google Scholar] [PubMed]
- Derogatis, L.R. Symptom Checklist-90-R: Administration, Scoring, and Procedures Manual, 3rd ed.; National Computer Systems, Inc.: Minneapolis, MN, USA, 1994. [Google Scholar]
- Gadow, K.D.; Sprafkin, J. Child Symptom Inventory 4: Screening and Norms Manual; Checkmate Plus: Stony Brook, NY, USA, 2002. [Google Scholar]
- Moos, R.H.; Moos, B.S. Family Environment Scale Manual; Consulting Psychologists Press Inc.: Palo Alto, CA, USA, 1994. [Google Scholar]
- Matson, J.L.; Vollmer, T.R. User’s Guide: Questions about Behavioral Function (QABF); Scientific Publishers Inc.: Baton Rouge, LA, USA, 1995. [Google Scholar]
- Sparrow, S. Vineland Adaptive Behavior Scale-Screener. Unpublished Manuscript. 2000. [Google Scholar]
- Magaña, A.B.; Goldstein, M.J.; Karno, M.; Miklowitz, D.J.; Jenkins, J.; Falloon, I.R. A brief method for assessing expressed emotion in relatives of psychiatric patients. Psychiatry Res. 1986, 17, 203–212. [Google Scholar] [CrossRef]
- Achenbach, T.M.; Rescorla, L.A. Manual for the ASEBA School-Age Forms and Profiles; Research Center for Children, Youth, and Families, University of Vermont: Burlington, VT, USA, 2001. [Google Scholar]
- Achenbach, T.M.; Rescorla, L.A. Manual for ASEBA Adult Forms & Profiles; Research Center for Children, Youth, and Families, University of Vermont: Burlington, VT, USA, 2003. [Google Scholar]
- Rutter, M.; Bailey, A.; Lord, C. Social Communication Questionnaire (SCQ); Western Psychological Services: Los Angeles, CA, USA, 2001. [Google Scholar]
- Bruininks, R.H.; Woodcock, R.W.; Weatherman, R.F.; Hill, B.K. Scales of Independent Behavior—Revised; Riverside: Itasca, IL, USA, 1996. [Google Scholar]
- Lord, C.; Rutter, M.; DiLavore, P.C.; Risi, S. Autism Diagnostic Observation Schedule; Western Psychological Services: Los Angeles, CA, USA, 2002. [Google Scholar]
- Bodfish, J.W.; Symons, F.J.; Parker, D.E.; Lewis, M.H. Varieties of repetitive behavior in autism: Comparisons to mental retar-dation. J. Autism Dev. Disord. 2000, 30, 237–243. [Google Scholar] [CrossRef]
- Rutter, M.; Bailey, A.; Lord, C.; Berument, S.K. Social Communication Questionnaire; Western Psychological Services: Los An-geles, CA, USA, 2003. [Google Scholar]
- Lord, C.; Rutter, M.; Goode, S.; Heemsbergen, J.; Jordan, H.; Mawhood, L.; Schopler, E. Austism diagnostic observation schedule: A standardized observation of communicative and social behavior. J. Autism Dev. Disord. 1989, 19, 185–212. [Google Scholar] [CrossRef]
- Esbensen, A.J.; Rojahn, J.; Aman, M.G.; Ruedrich, S. Reliability and Validity of an Assessment Instrument for Anxiety, Depression, and Mood Among Individuals with Mental Retardation. J. Autism Dev. Disord. 2003, 33, 617–629. [Google Scholar] [CrossRef]
- Einfeld, S.; Tonge, B. Manual for the Developmental Behaviour Checklist; University of New South Wales: Sydney, Australia; Monash University: Melbourne, Australia, 1994. [Google Scholar]
- Iwata, B.A.; DeLeon, I.G.; Roscoe, E.M. Reliability and Validity of the Functional Analysis Screening Tool. J. Appl. Behav. Anal. 2013, 46, 271–284. [Google Scholar] [CrossRef]
- Radloff, L. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Zuo, Q.H.; Lei, Z.W. Standardization of Infants-junior middle school students’ social-life abilities scales. Zhongguo Lin Chuang Xin Li Xue Za Zhi 1995, 1, 12–15, 63. [Google Scholar]
- Bryson, S.E.; Zwaigenbaum, L.; McDermott, C.; Rombough, V.; Brian, J. The Autism Observation Scale for Infants: Scale Development and Reliability Data. J. Autism Dev. Disord. 2007, 38, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Rutter, M.; DiLavore, P.C.; Risi, S.; Gotham, K.; Bishop, S. Autism Diagnostic Observation Schedule (ADOS-2) Manual, 2nd ed.; Western Psychological Services: Torrance, CA, USA, 2012. [Google Scholar]
- Sparrow, S.S.; Cicchetti, D.V.; Balla, D.A. Vineland-II Adaptive Behavior Scales: Survey Forms Manual; AGS Publishing: Circle Pines, MN, USA, 2005. [Google Scholar]
- Gresham, F.M.; Elliott, S.N. Social Skills Rating System: Preschool, Elementary Level; American Guidance Service: Circle Pines, MN, USA, 1990. [Google Scholar]
- Frey, J.R.; Elliott, S.N.; Gresham, F.M. Preschoolers’ Social Skills: Advances in Assessment for Intervention Using Social Be-havior Ratings. Sch. Ment. Health 2011, 3, 179–190. [Google Scholar] [CrossRef]
- Burbidge, C.; Oliver, C.; Moss, J.; Arron, K.; Berg, K.; Furniss, F.; Hill, L.; Trusler, K.; Woodcock, K. The association between repetitive behaviours, impul-sivity and hyperactivity in people with intellectual disability. J. Intellect. Disabil. Res. 2010, 54, 1078–1092. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.; Oliver, C.; Arron, K.; Burbidge, C.; Berg, K.; Oliver, C. The Prevalence and Phenomenology of Repetitive Behavior in Genetic Syndromes. J. Autism Dev. Disord. 2008, 39, 572–588. [Google Scholar] [CrossRef]
- Berument, S.K.; Rutter, M.; Lord, C.; Pickles, A.; Bailey, A. Autism screening questionnaire: Diagnostic validity. Br. J. Psychiatry 1999, 175, 444–451. [Google Scholar] [CrossRef]
- Lord, C.; Rutter, M.; DeLavore, P.C.; Risi, S. Autism Diagnostic Observation Schedule; Western Psychological Services: Los Angeles, CA, USA, 2001. [Google Scholar]
- Achenbach, T.M.; Rescorla, L.A. Manual for the ASEBA Preschool Forms and Profiles; Research Center for Children, Youth, and Families, University of Vermont: Burlington, VT, USA, 2000. [Google Scholar]
- Goldsmith, H.H.; Rothbart, M. The Laboratory Temperament Assessment Battery; University of Wisconsin: Madison, WI, USA, 1996. [Google Scholar]
- Burbidge, C.; Oliver, C. Activity Questionnaire: Manual for Administration and Scorer Interpretation; University of Birmingham: Birmingham, UK, 2008. [Google Scholar]
- Hyman, P.; Oliver, C.; Hall, S. Self-injurious behaviour, self- restraint, and compulsive behaviours in Cornelia de Lange syn- drome. Am. J. Ment. Retard. 2002, 107, 146–154. [Google Scholar] [CrossRef]
- Gilliam, J.E. Attention-Deficit/Hyperactivity Disorder Test; Pro-Ed: Austin, TX, USA, 1995. [Google Scholar]
- Aman, M.G.; Burrow, W.H.; Wolford, P.L. The Aberrant Behavior Checklist-Community: Factor validity and effect of subject variables for adults in group homes. Am. J. Ment. Retard. 1995, 100, 283–292. [Google Scholar]
- . Bumpass, L.; Sweet, J. A national survey of families and households. Russell Stage Foundation: New York, NY, USA, 1987. [Google Scholar]
- Zarit, S.; Reever, K.; Bach-Peterson, J. Relatives of the impaired elderly: Correlates of feelings of burden. Gerontology 1980, 20, 649–655. [Google Scholar] [CrossRef]
- Moss, J.; Howlin, P.; Hastings, R.P.; Beaumont, S.; Griffith, G.M.; Petty, J.; Tunnicliffe, P.; Yates, R.; Villa, D.; Oliver, C. Social Behavior and Characteristics of Autism Spectrum Disorder in Angelman, Cornelia de Lange, and Cri du Chat Syndromes. Am. J. Intellect. Dev. Disabil. 2013, 118, 262–283. [Google Scholar] [CrossRef]
- Moss, J.; Oliver, C.; Nelson, L.; Richards, C.; Hall, S. Delineating the Profile of Autism Spectrum Disorder Characteristics in Cornelia de Lange and Fragile X Syndromes. Am. J. Intellect. Dev. Disabil. 2013, 118, 55–73. [Google Scholar] [CrossRef]
- Oliver, C.; Royston, R.; Crawford, H.; Moss, J.; Waite, J.; Arron, K. Informant Assessments of Behavior and Affect for People with Intellectual Disability. 2019. Available online: https://secure.viewer.zmags.com/publication/9964d37c#/9964d37c/14 (accessed on 27 January 2022).
- Bodfish, J.; Symons, F.; Lewis, M. The Repetitive Behavior Scale; Western Carolina Center Research Reports; Western Carolina Center: Morganton, NC, USA, 1999. [Google Scholar]
- Lam, K.S.L.; Aman, M.G. The Repetitive Behavior Scale-Revised: Independent Validation in Individuals with Autism Spectrum Disorders. J. Autism Dev. Disord. 2006, 37, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Constantino, J.; Gruber, C. Social Responsive Scale (SRS) Manual; Western Psychological Services: Los Angeles, CA, USA, 2005. [Google Scholar]
- Bölte, S.; Poustka, F.; Constantino, J.N. Assessing autistic traits: Cross-cultural validation of the social responsiveness scale (SRS). Autism Res. 2008, 1, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, T.M. The child behavior checklist and related instruments. In the Use of Psychological Testing for Treatment Planning and Outcomes Assessment, 2nd ed.; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 1999; pp. 429–466. [Google Scholar]
- Derogatis, L.R.; Spencer, P. Brief Symptom Inventory: BSI; Pearson: Upper Saddle River, NJ, USA, 1993. [Google Scholar]
- Harrison, P.; Oakland, T. Adaptive Behavior Assessment—Second Edition Manual (ABAS-II); Harcourt Assessment: San Antonio, TX, USA, 2003. [Google Scholar]
- Chromik, L.C.; Quintin, E.M.; Lepage, J.F.; Hustyi, K.M.; Lightbody, A.A.; Reiss, A.L. The influence of hyperactivity, impulsivity, and attention problems on social functioning in adolescents and young adults with fragile X syndrome. J. Atten. Disord. 2019, 23, 181–188. [Google Scholar] [CrossRef]
- Sullivan, K.; Hatton, D.; Hammer, J.; Sideris, J.; Hooper, S.; Ornstein, P.; Bailey, D., Jr. ADHD symptoms in children with FXS. Am. J. Med. Genet. Part A 2006, 140A, 2275–2288. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cregenzán-Royo, O.; Brun-Gasca, C.; Fornieles-Deu, A. Behavior Problems and Social Competence in Fragile X Syndrome: A Systematic Review. Genes 2022, 13, 280. https://doi.org/10.3390/genes13020280
Cregenzán-Royo O, Brun-Gasca C, Fornieles-Deu A. Behavior Problems and Social Competence in Fragile X Syndrome: A Systematic Review. Genes. 2022; 13(2):280. https://doi.org/10.3390/genes13020280
Chicago/Turabian StyleCregenzán-Royo, Olga, Carme Brun-Gasca, and Albert Fornieles-Deu. 2022. "Behavior Problems and Social Competence in Fragile X Syndrome: A Systematic Review" Genes 13, no. 2: 280. https://doi.org/10.3390/genes13020280
APA StyleCregenzán-Royo, O., Brun-Gasca, C., & Fornieles-Deu, A. (2022). Behavior Problems and Social Competence in Fragile X Syndrome: A Systematic Review. Genes, 13(2), 280. https://doi.org/10.3390/genes13020280