MiR-612, miR-637, and miR-874 can Regulate VEGFA Expression in Hepatocellular Carcinoma Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. MiRNA Prediction
2.3. Transfection of miRNAs in HepG2 and HuH-7 Cell Lines
2.4. RNA and miRNA Extraction
2.5. Quantitative Real-Time PCR for Expression of VEGFA Gene and miRNAs
2.6. Extraction and Quantification of Protein
2.7. Statistical Analysis
3. Results
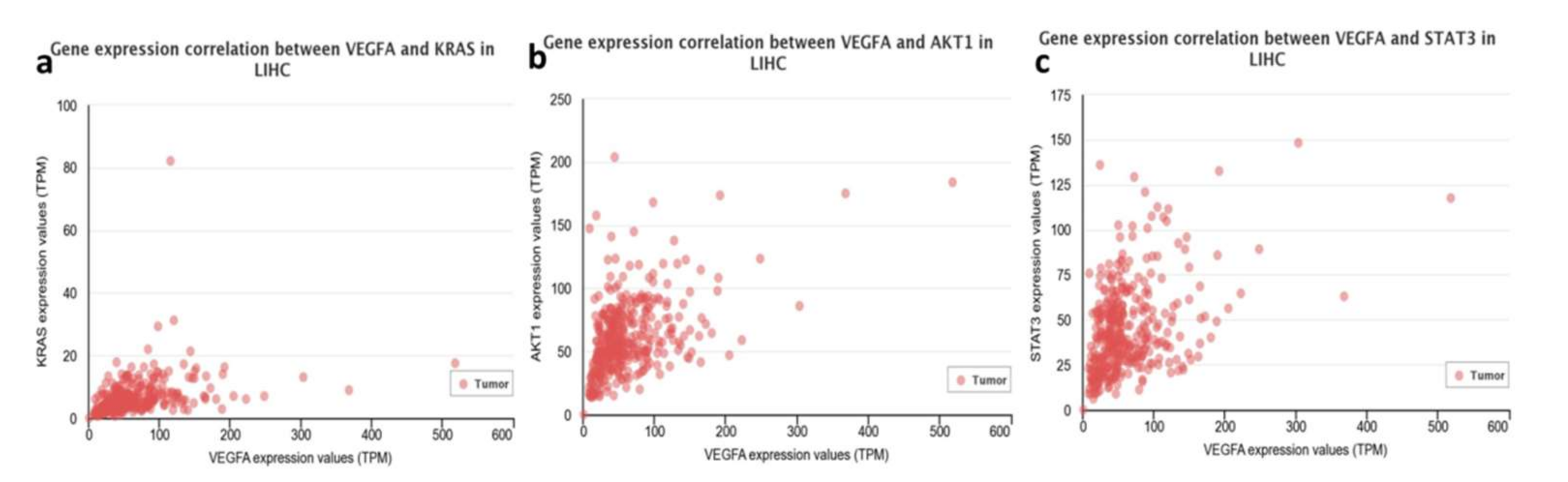
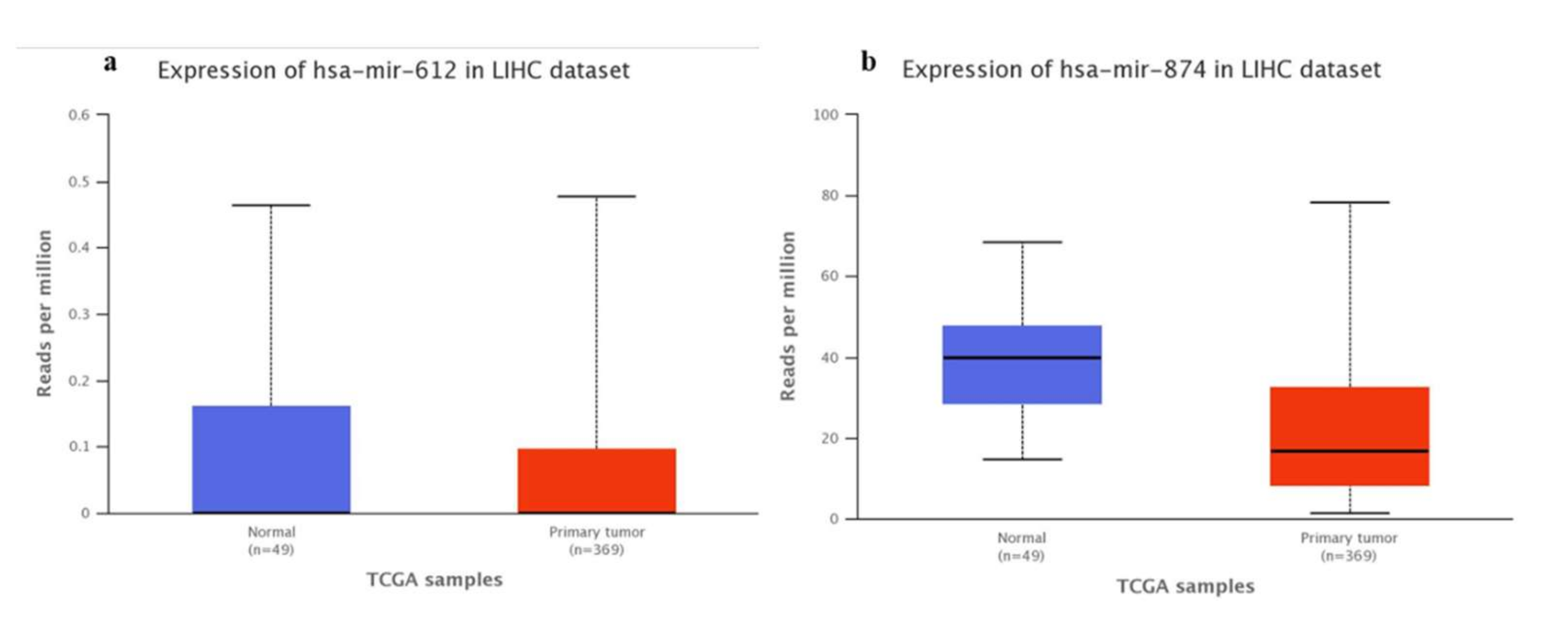
3.1. Bioinformatics-TCGA Analysis
3.2. Transfection Efficiency Test
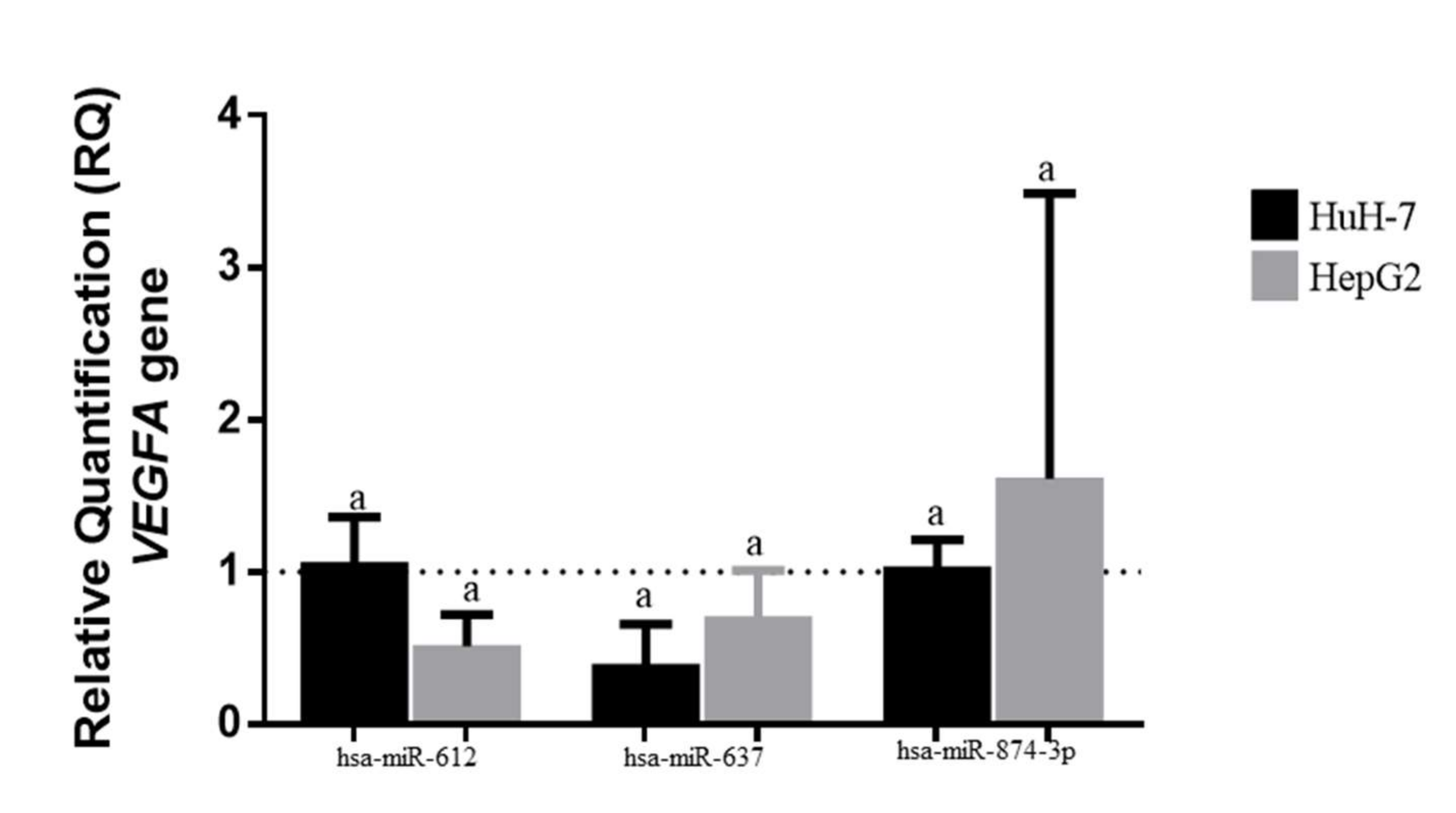
3.3. MiR-874, miR-612, and miR-637 Expression in Transfected HCC Cells
3.4. VEGFA Expression in Transfected HCC Cell Lines
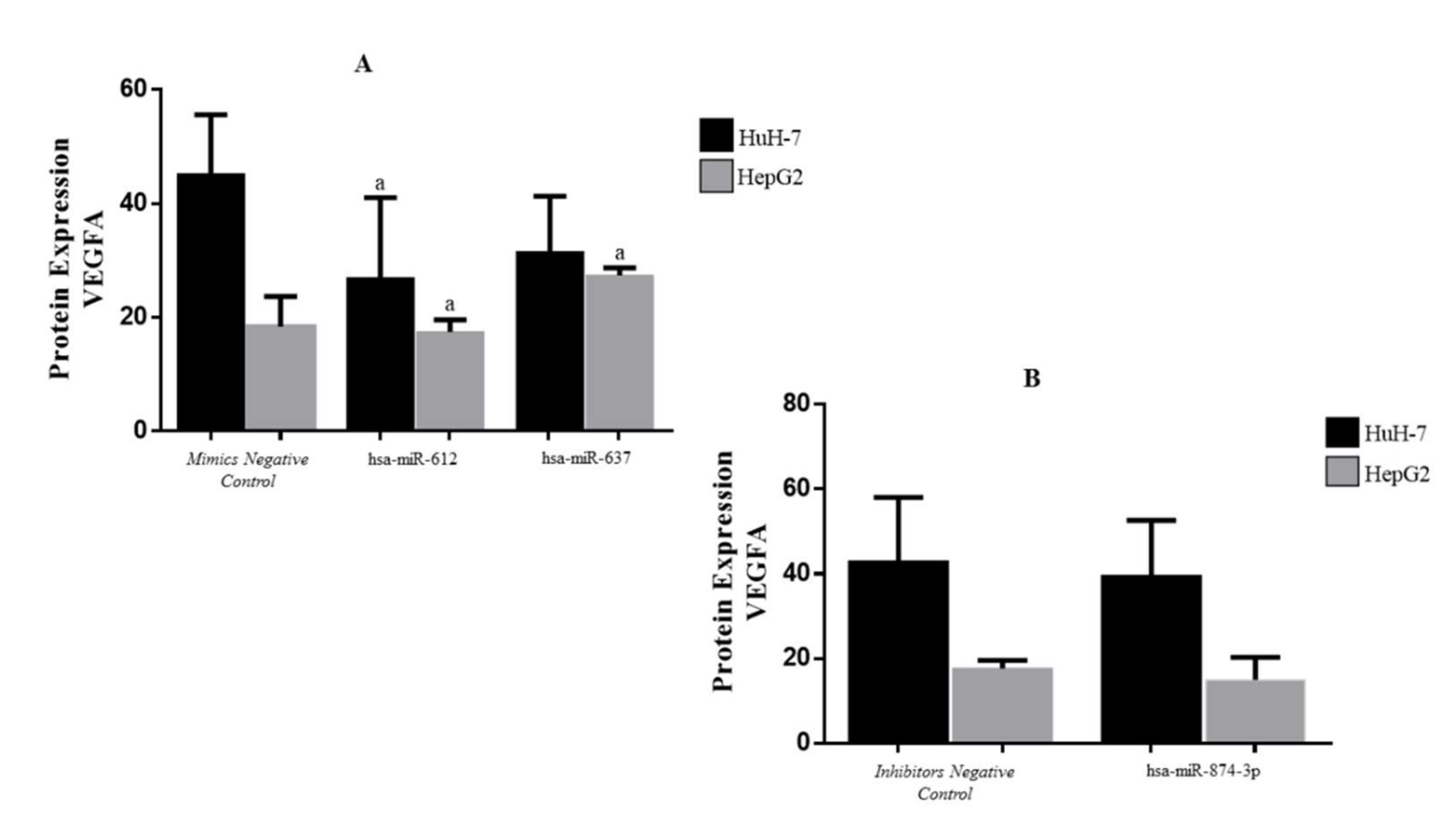
3.5. Protein Expression of VEGFA in Transfected HCC Cell Lines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. 2021. Available online: https://www.cdc.gov/cancer/liver/ (accessed on 22 December 2021).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, methods and major patterns in globocan 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.M.; Tsang, F.H.; Ng, I.O. Non-coding RNAs in hepatocellular carcinoma: Molecular functions and pathological im-plications. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 137–151. [Google Scholar] [CrossRef]
- Kappas, N.C.; Zeng, G.; Chappell, J.C.; Kearney, J.B.; Hazarika, S.; Kallianos, K.G.; Patterson, C.; Annex, B.H.; Bautch, V.L. The VEGF receptor Flt-1 spatially modulates Flk-1 signaling and blood vessel branching. J. Cell Biol. 2008, 181, 847–858. [Google Scholar] [CrossRef]
- Dang, Y.; Luo, D.; Rong, M.; Chen, G. Underexpression of miR-34a in Hepatocellular Carcinoma and Its Contribution towards Enhancement of Proliferating Inhibitory Effects of Agents Targeting c-MET. PLoS ONE 2013, 8, e61054. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Bai, Y.-N.; Luo, L.-X.; Wu, H.; Zeng, Y. Expression of microRNA-150 targeting vascular endothelial growth factor-A is downregulated under hypoxia during liver regeneration. Mol. Med. Rep. 2013, 8, 287–293. [Google Scholar] [CrossRef]
- Liu, L.; Bi, N.; Wu, L.; Ding, X.; Men, Y.; Zhou, W.; Li, L.; Zhang, W.; Shi, S.; Song, Y.; et al. MicroRNA-29c functions as a tumor suppressor by targeting VEGFA in lung adenocarcinoma. Mol. Cancer 2017, 16, 50. [Google Scholar] [CrossRef]
- Tunissiolli, N.M.; Castanhole-Nunes, M.; Biselli-Chicote, P.M.; Pavarino, É.C.; Da Silva, R.F.; Da Silva, R.D.; Goloni-Bertollo, E.M. Hepatocellular Carcinoma: A Comprehensive Review of Biomarkers, Clinical Aspects, and Therapy. Asian Pac. J. Cancer Prev. 2017, 18, 863–872. [Google Scholar]
- Thurnherr, T.; Mah, W.-C.; Lei, Z.; Jin, Y.; Rozen, S.G.; Lee, C.G. Differentially Expressed miRNAs in Hepatocellular Carcinoma Target Genes in the Genetic Information Processing and Metabolism Pathways. Sci. Rep. 2016, 6, 20065. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.-H.; Wei, W.; Krawczyk, M.; Wang, W.; Luo, H.; Flagg, K.; Yi, S.; Shi, W.; Quan, Q.; Li, K.; et al. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat. Mater. 2017, 16, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Zou, H.; Liao, W. Prospect of Circular RNA in Hepatocellular Carcinoma: A Novel Potential Biomarker and Thera-peutic Target. Front. Oncol. 2018, 8, 332. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Ceci, C.; Atzori, M.G.; Lacal, P.M.; Graziani, G. Role of VEGFs/VEGFR-1 Signaling and its Inhibition in Modulating Tumor In-vasion: Experimental Evidence in Different Metastatic Cancer Models. Int. J. Mol. Sci. 2020, 21, 1388. [Google Scholar] [CrossRef]
- Knowles, B.B.; Aden, D.P. Human Hepatoma Derived Cell Line, Process for Preparation Thereof, and Uses Therefor. U.S. Patent 4393133, 12 July 1983. [Google Scholar]
- Nakabayashi, H.; Taketa, K.; Miyano, K.; Yamane, T.; Sato, J. Growth of human hepatoma cells lines with differentiated func-tions in chemically defined medium. Cancer Res. 1982, 42, 3858–3863. [Google Scholar] [PubMed]
- De Oliveira, A.R.; Castanhole-Nunes, M.M.; Biselli-Chicote, P.M.; Pavarino, É.C.; Da Silva, R.D.; Da Silva, R.F.; Goloni-Bertollo, E.M. Differential expression of angiogenesis-related miRNAs and VEGFA in cirrhosis and hepatocellular carcinoma. Arch. Med. Sci. 2020, 16, 1150–1157. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.; Varambally, S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Tao, Z.-H.; Wan, J.-L.; Zeng, L.-Y.; Xie, L.; Sun, H.-C.; Qin, L.-X.; Wang, L.; Zhou, J.; Ren, Z.-G.; Li, Y.-X.; et al. miR-612 suppresses the invasive-metastatic cascade in hepatocellular carcinoma. J. Exp. Med. 2013, 210, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; He, M.L.; Fu, W.M.; Wang, H.; Chen, L.Z.; Zhu, X.; Chen, Y.; Xie, D.; Lai, P.; Chen, G.; et al. Pri-mate-specific microRNA-637 inhibits tumorigenesis in hepatocellular carcinoma by disrupting signal transducer and ac-tivator of transcription 3 signaling. Hepatology 2011, 54, 2137–2148. [Google Scholar] [CrossRef]
- Gao, P.; Niu, N.; Wei, T.; Tozawa, H.; Chen, X.; Zhang, C.; Zhang, J.; Wada, Y.; Kapron, C.M.; Liu, J. The roles of signal transducer and activator of transcription factor 3 in tumor angiogenesis. Oncotarget 2017, 8, 69139–69161. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Yada, N.; Hagiwara, S.; Arizumi, T.; Minaga, K.; Kamata, K.; Takenaka, M.; Minami, Y.; Watanabe, T.; Nishida, N.; et al. Gankyrin induces STAT3 activation in tumor microenvironment and sorafenib resistance in hepatocellular car-cinoma. Cancer Sci. 2017, 108, 1996–2003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, J.; Zhi, X.; Xie, K.; Wang, W.; Li, Z.; Zhu, Y.; Yang, L.; Xu, H.; Xu, Z. miR-874 functions as a tumor suppressor by inhibiting angiogenesis through STAT3/VEGF-A pathway in gastric cancer. Oncotarget 2015, 6, 1605–1617. [Google Scholar] [CrossRef]
- Barbosa, A.S.; Lin, C.J. Gene silencing with RNA interference: A novel tool for the study of physiology and pathophysiology of adrenal cortex. Arq. Bras. De Endocrinol. Metabol. 2004, 48, 612–619. [Google Scholar] [CrossRef]
- Beeghly-Fadiel, A.; Shu, X.O.; Lu, W.; Long, J.; Cai, Q.; Xiang, Y.B.; Zheng, Y.; Zhao, Z.; Gu, K.; Gao, Y.T.; et al. Genetic variation in VEGF family genes and breast cancer risk: A report from the Shanghai Breast Cancer Genetics Study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 33–41. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castanhole-Nunes, M.M.U.; Tunissiolli, N.M.; Oliveira, A.R.C.P.; Mattos, M.F.; Galbiatti-Dias, A.L.S.; Kawasaki-Oyama, R.S.; Pavarino, E.C.; da Silva, R.F.; Goloni-Bertollo, E.M. MiR-612, miR-637, and miR-874 can Regulate VEGFA Expression in Hepatocellular Carcinoma Cell Lines. Genes 2022, 13, 282. https://doi.org/10.3390/genes13020282
Castanhole-Nunes MMU, Tunissiolli NM, Oliveira ARCP, Mattos MF, Galbiatti-Dias ALS, Kawasaki-Oyama RS, Pavarino EC, da Silva RF, Goloni-Bertollo EM. MiR-612, miR-637, and miR-874 can Regulate VEGFA Expression in Hepatocellular Carcinoma Cell Lines. Genes. 2022; 13(2):282. https://doi.org/10.3390/genes13020282
Chicago/Turabian StyleCastanhole-Nunes, Márcia Maria U., Nathalia M. Tunissiolli, André R. C. P. Oliveira, Marlon F. Mattos, Ana Lívia S. Galbiatti-Dias, Rosa S. Kawasaki-Oyama, Erika C. Pavarino, Renato F. da Silva, and Eny M. Goloni-Bertollo. 2022. "MiR-612, miR-637, and miR-874 can Regulate VEGFA Expression in Hepatocellular Carcinoma Cell Lines" Genes 13, no. 2: 282. https://doi.org/10.3390/genes13020282
APA StyleCastanhole-Nunes, M. M. U., Tunissiolli, N. M., Oliveira, A. R. C. P., Mattos, M. F., Galbiatti-Dias, A. L. S., Kawasaki-Oyama, R. S., Pavarino, E. C., da Silva, R. F., & Goloni-Bertollo, E. M. (2022). MiR-612, miR-637, and miR-874 can Regulate VEGFA Expression in Hepatocellular Carcinoma Cell Lines. Genes, 13(2), 282. https://doi.org/10.3390/genes13020282





