The Diverse Role of CUB and Sushi Multiple Domains 1 (CSMD1) in Human Diseases
Abstract
1. Introduction
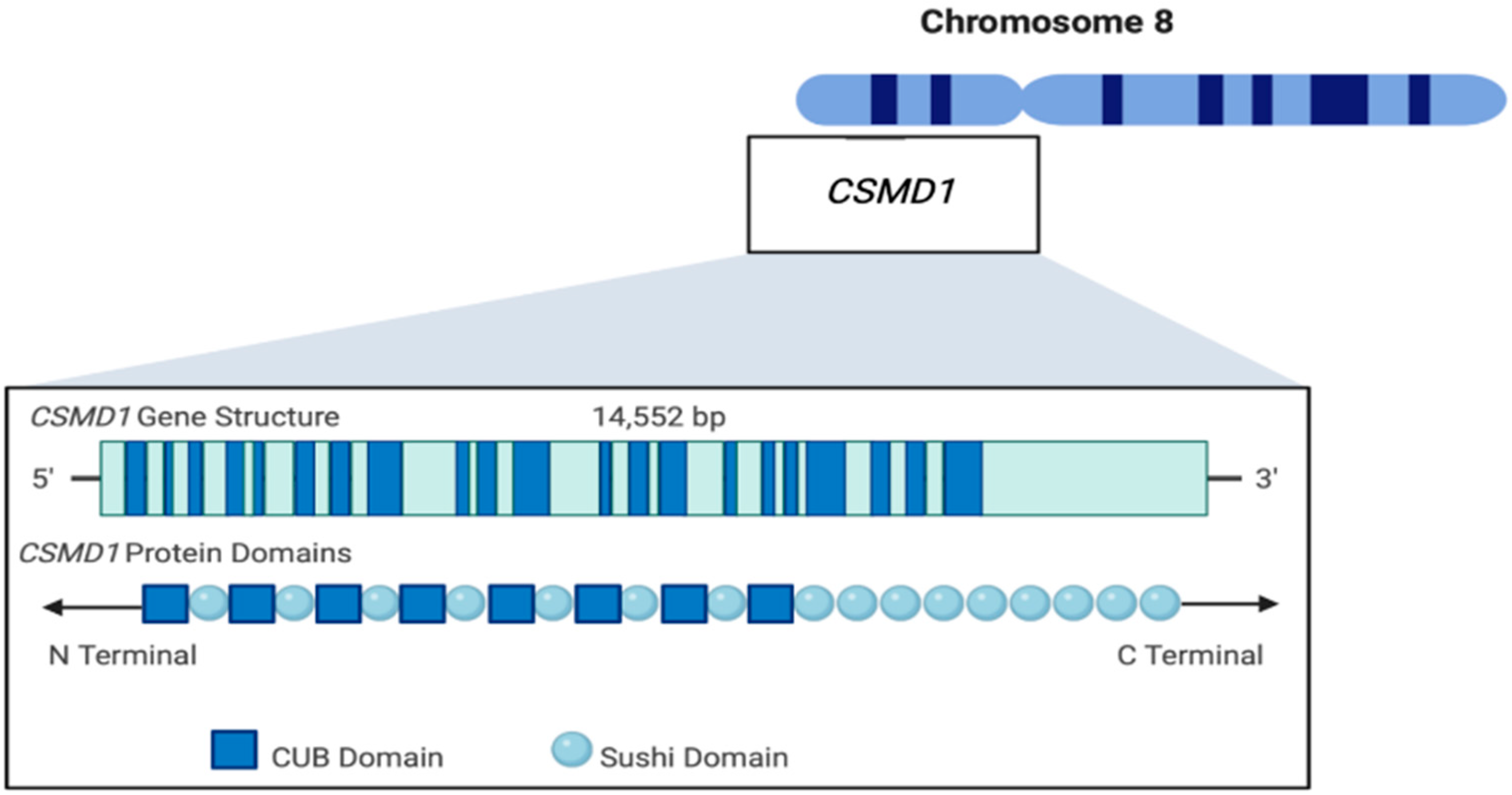
2. CSMD1 Structure
3. CSMD Gene Family
4. CSMD1 Function
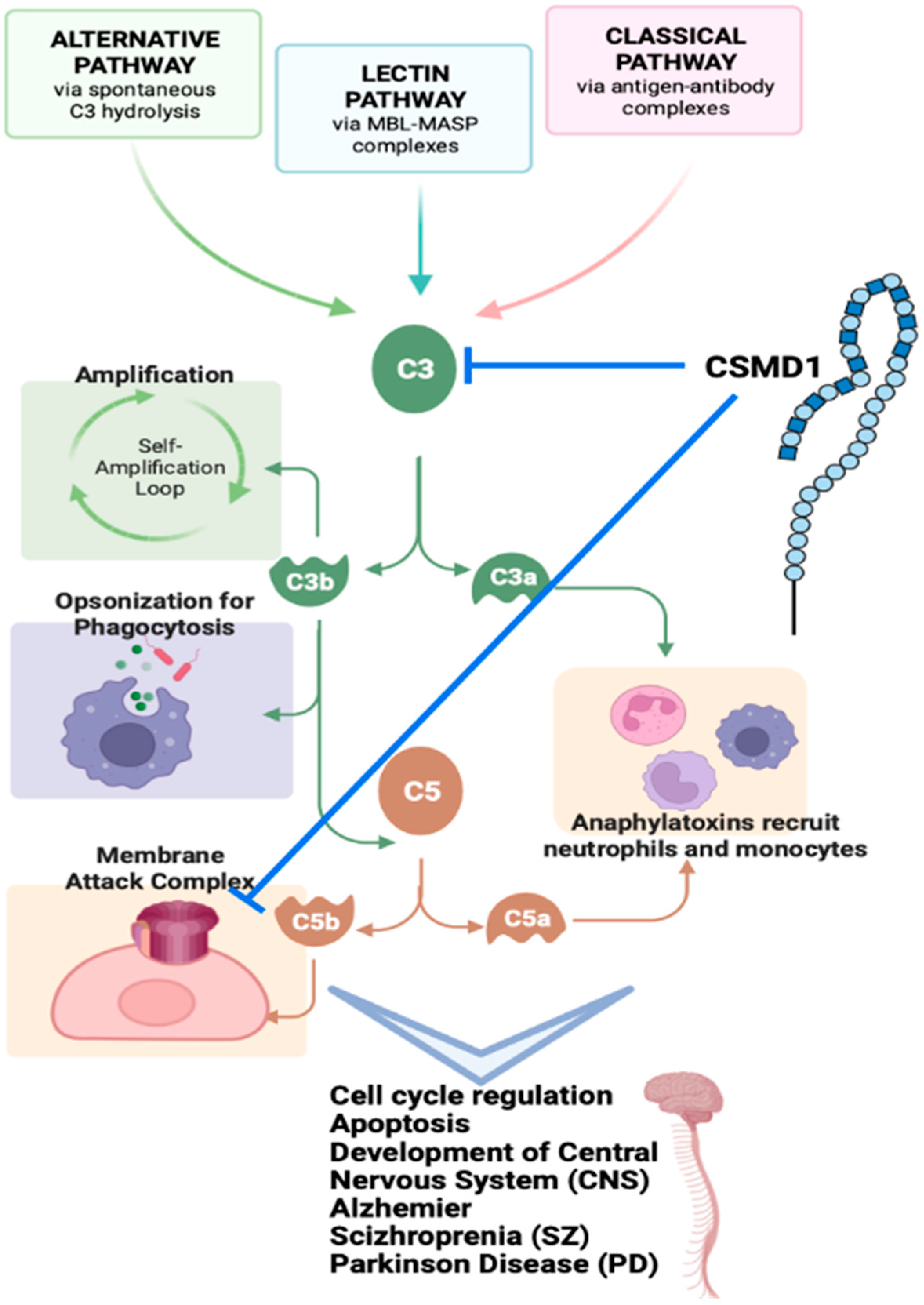
4.1. CSMD1 in Complement System
4.2. CSMD1 in Neurodevelopment Diseases
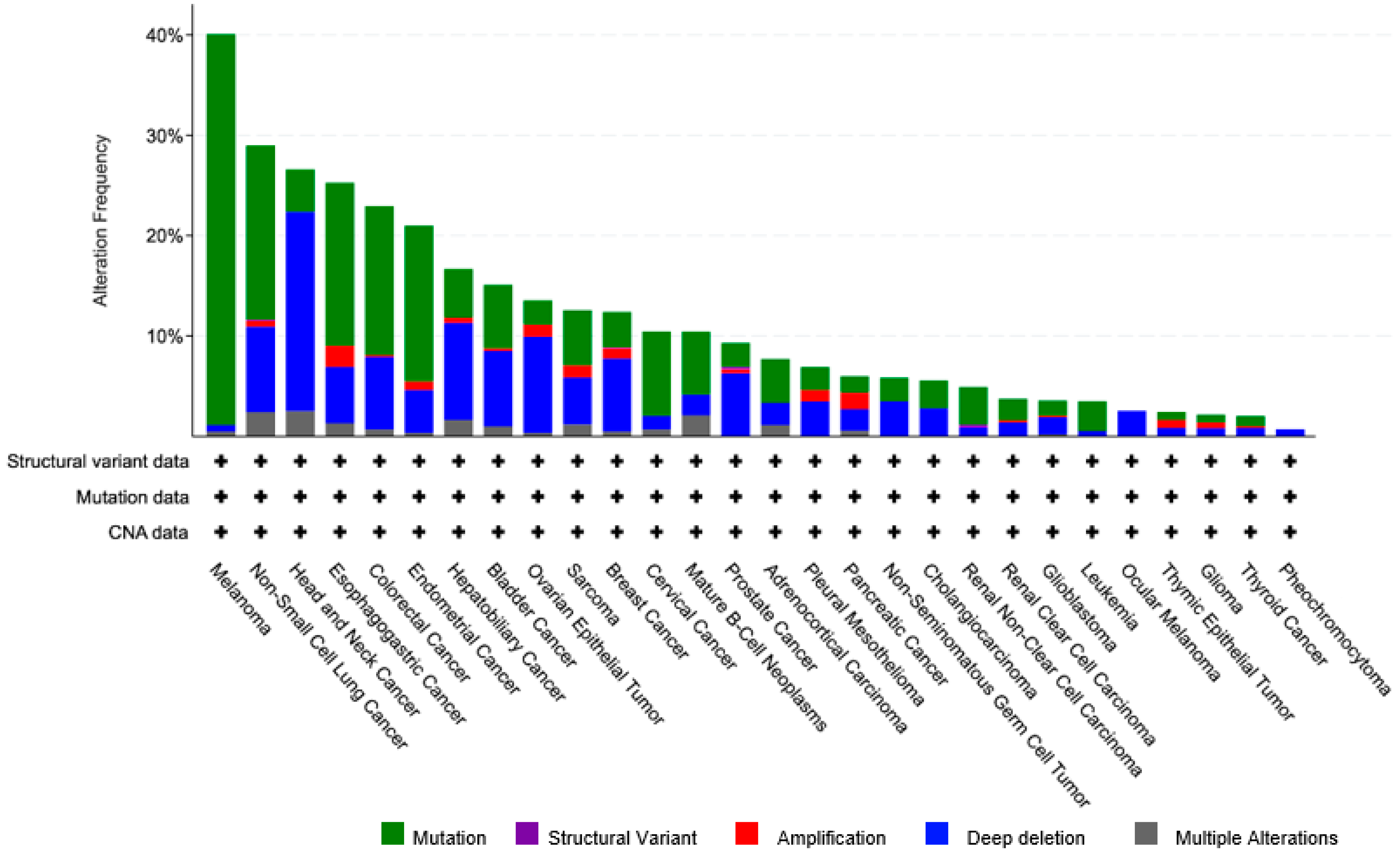
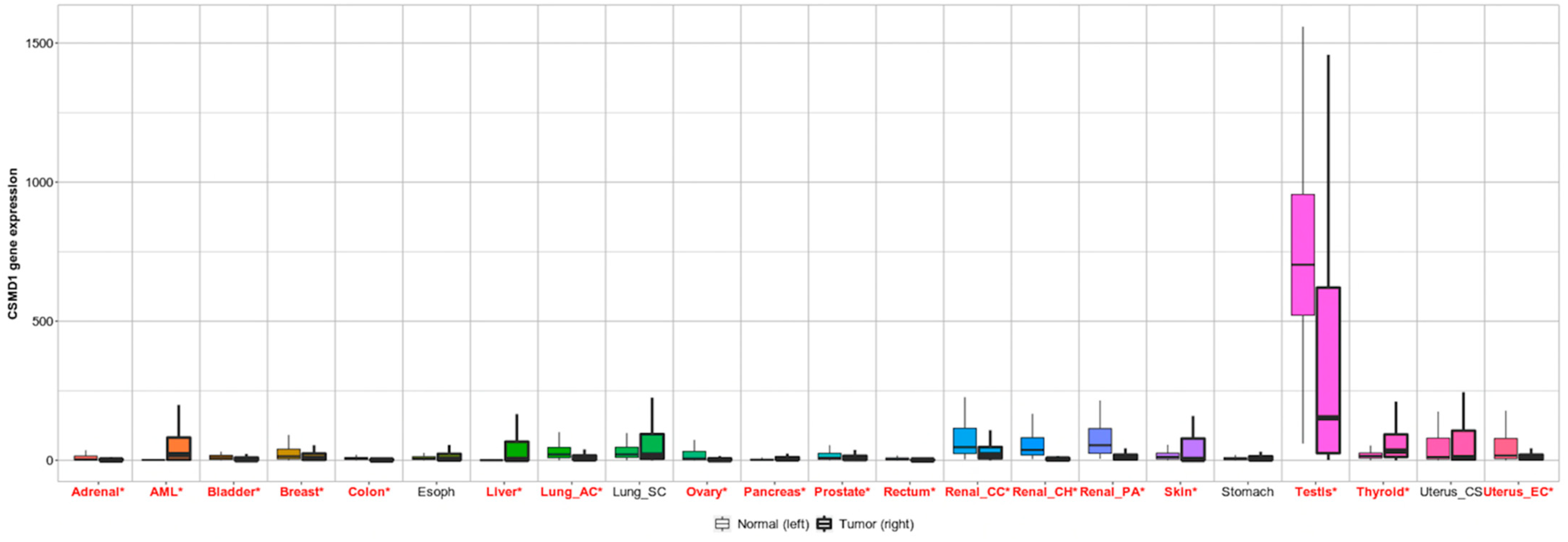
4.3. CSMD1 in Cancer
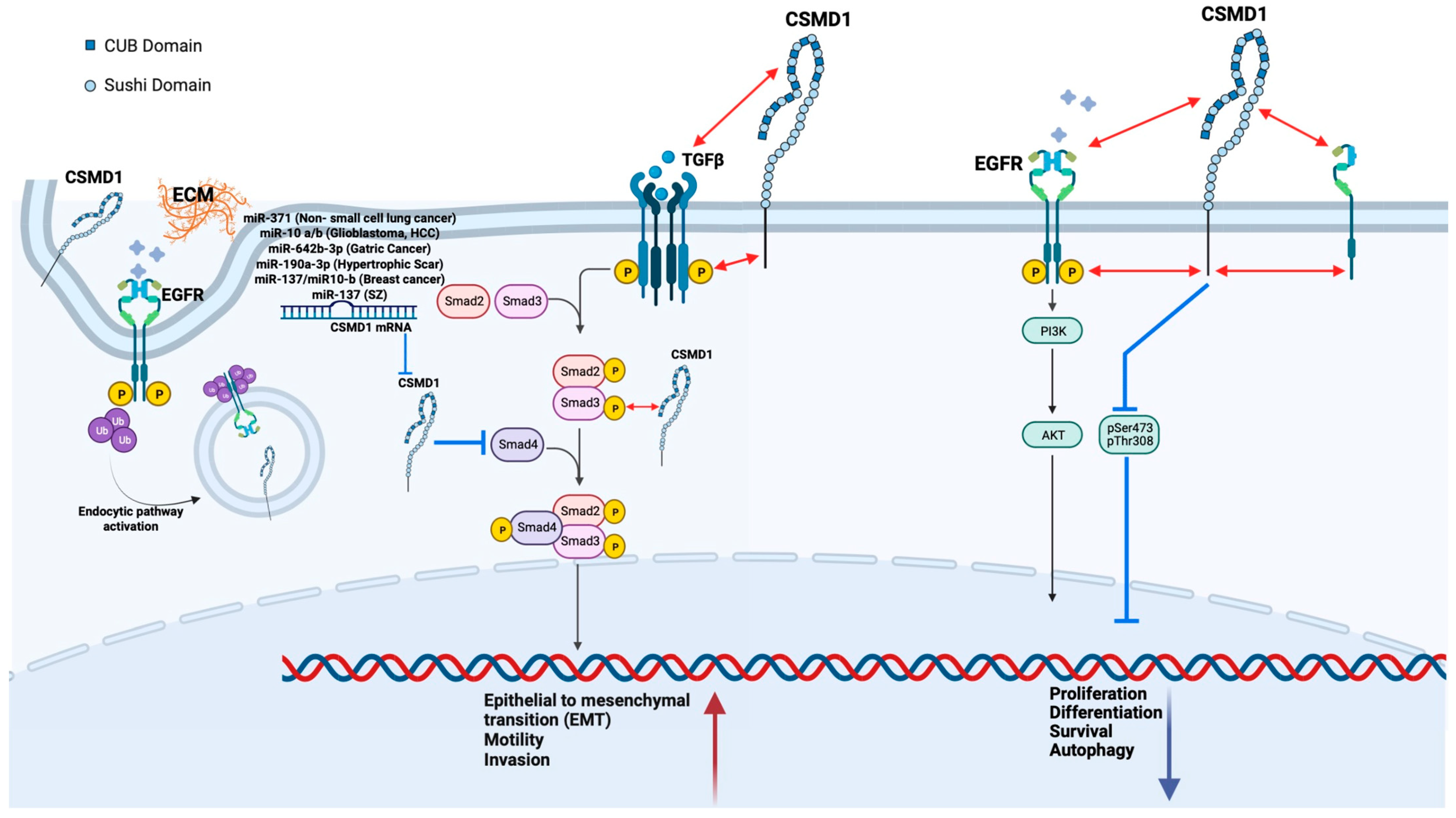
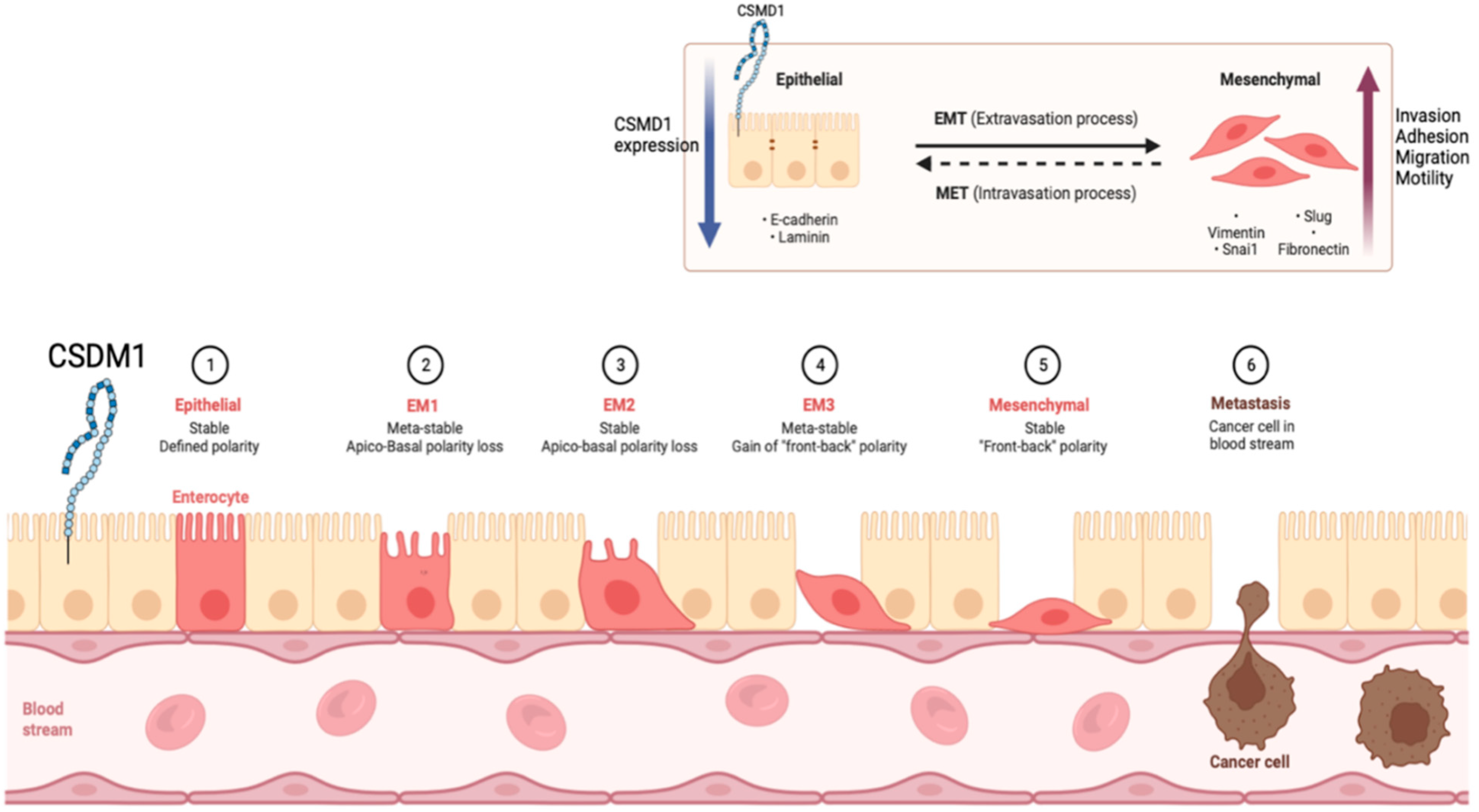
4.4. CSMD1 in Metastasis and EMT
4.5. CSMD1 Inactivation
4.6. CSMD1 and Immunotherapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toomes, C.; Jackson, A.; Maguire, K.; Wood, J.; Gollin, S.; Ishwad, C.; Paterson, I.; Prime, S.; Parkinson, K.; Bell, S. The presence of multiple regions of homozygous deletion at the CSMD1 locus in oral squamous cell carcinoma question the role of CSMD1 in head and neck carcinogenesis. Genes Chromosomes Cancer 2003, 37, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Baum, M.L.; Wilton, D.K.; Muthukumar, A.; Fox, R.G.; Carey, A.; Crotty, W.; Scott-Hewitt, N.; Bien, E.; Sabatini, D.A.; Lanser, T. CUB and Sushi Multiple Domains 1 (CSMD1) opposes the complement cascade in neural tissues. bioRxiv 2020. [Google Scholar] [CrossRef]
- Blom, A. The role of complement inhibitors beyond controlling inflammation. J. Intern. Med. 2017, 282, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.C.; Uppaluri, R.; Schmidt, A.P.; Pashia, M.E.; Quant, E.C.; Sunwoo, J.B.; Gollin, S.M.; Scholnick, S.B. Transcript map of the 8p23 putative tumor suppressor region. Genomics 2001, 75, 17–25. [Google Scholar] [CrossRef]
- Created with BioRender.com. 2022. Available online: https://app.biorender.com (accessed on 6 June 2022).
- Bork, P.; Beckmann, G. The CUB domain: A widespread module in developmentally regulated proteins. J. Mol. Biol. 1993, 231, 539–545. [Google Scholar] [CrossRef]
- Gaboriaud, C.; Gregory-Pauron, L.; Teillet, F.; Thielens, N.M.; Bally, I.; Arlaud, G.J. Structure and properties of the Ca2+-binding CUB domain, a widespread ligand-recognition unit involved in major biological functions. Biochem. J. 2011, 439, 185–193. [Google Scholar] [CrossRef]
- Ichinose, A.; Bottenus, R.E.; Davie, E.W. Structure of transglutaminase. J. Biol. Chem. 1990, 265, 13411–13414. [Google Scholar] [CrossRef]
- Kirkitadze, M.D.; Barlow, P.N. Structure and flexibility of the multiple domain proteins that regulate complement activation. Immunol. Rev. 2001, 180, 146–161. [Google Scholar] [CrossRef]
- Reid, K.B.; Day, A.J. Structure-function relationships of the complement components. Immunol. Today 1989, 10, 177–180. [Google Scholar] [CrossRef]
- Blom, N.; Gammeltoft, S.; Brunak, S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 1999, 294, 1351–1362. [Google Scholar] [CrossRef]
- Jung, A.R.; Eun, Y.-G.; Lee, Y.C.; Noh, J.K.; Kwon, K.H. Clinical significance of CUB and sushi multiple domains 1 inactivation in head and neck squamous cell carcinoma. Int. J. Mol. Sci. 2018, 19, 3996. [Google Scholar] [CrossRef]
- Tang, M.-R.; Wang, Y.-X.; Guo, S.; Han, S.-Y.; Wang, D. CSMD1 exhibits antitumor activity in A375 melanoma cells through activation of the Smad pathway. Apoptosis 2012, 17, 927–937. [Google Scholar] [CrossRef] [PubMed]
- The Human Protein Atlas. CSMD1. Available online: https://www.proteinatlas.org/ENSG00000183117-CSMD1/tissue (accessed on 6 June 2022).
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.L.; Scholnick, S.B. Identification of two new members of the CSMD gene family☆. Genomics 2003, 82, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Song, C. Loss of CSMD1 or 2 may contribute to the poor prognosis of colorectal cancer patients. Tumor Biol. 2014, 35, 4419–4423. [Google Scholar] [CrossRef]
- Yang, P.-S.; Hsu, H.-H.; Hsu, T.-C.; Chen, M.-J.; Wang, C.-D.; Yu, S.-L.; Hsu, Y.-C.; Li, K.-C. Genome-wide scan for copy number alteration association with relapse-free survival in colorectal cancer with liver metastasis patients. J. Clin. Med. 2018, 7, 446. [Google Scholar] [CrossRef]
- Yan, J.; Li, S.; Xi, W.; Chen, D.; Xiao, M. Immunoregulation related gene of CSMD2 and RNA activity as potential predicter of 2-year survival in pancreatic cancer: A DNA and RNA sequencing analysis. J. Clin. Oncol. 2021, 39, e16212. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Zhang, Y.; Zhao, C.; Wang, H.; Lin, J.; Liu, C.; Wang, X.; Wang, H. Genomic Variations and Immune-Related Features of TMB, PD-L1 Expression and CD8+ T Cell Infiltration in Chinese Pulmonary Sarcomatoid Carcinoma. Int. J. Gen. Med. 2022, 15, 4209. [Google Scholar] [CrossRef]
- Gutierrez, M.A.; Dwyer, B.E.; Franco, S.J. Csmd2 Is a Synaptic Transmembrane Protein that Interacts with PSD-95 and Is Required for Neuronal Maturation. Eneuro 2019, 6. [Google Scholar] [CrossRef]
- Athanasiu, L.; Giddaluru, S.; Fernandes, C.; Christoforou, A.; Reinvang, I.; Lundervold, A.J.; Nilsson, L.-G.; Kauppi, K.; Adolfsson, R.; Eriksson, E. A genetic association study of CSMD1 and CSMD2 with cognitive function. Brain Behav. Immun. 2017, 61, 209–216. [Google Scholar] [CrossRef]
- Shimizu, A.; Asakawa, S.; Sasaki, T.; Yamazaki, S.; Yamagata, H.; Kudoh, J.; Minoshima, S.; Kondo, I.; Shimizu, N. A novel giant gene CSMD3 encoding a protein with CUB and sushi multiple domains: A candidate gene for benign adult familial myoclonic epilepsy on human chromosome 8q23. 3–q24. 1. Biochem. Biophys. Res. Commun. 2003, 309, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yu, P.; Jin, X.; Xu, X.; Li, J.; Li, Z.; Wang, M.; Wang, T.; Wu, X.; Jiang, Y. Genomic landscapes of Chinese sporadic autism spectrum disorders revealed by whole-genome sequencing. J. Genet. Genom. 2018, 45, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Curran, S.; Ahn, J.W.; Grayton, H.; Collier, D.A.; Ogilvie, C.M. NRXN1 deletions identified by array comparative genome hybridisation in a clinical case series–further understanding of the relevance of NRXN1 to neurodevelopmental disorders. J. Mol. Psychiatry 2013, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Magri, C.; Sacchetti, E.; Traversa, M.; Valsecchi, P.; Gardella, R.; Bonvicini, C.; Minelli, A.; Gennarelli, M.; Barlati, S. New copy number variations in schizophrenia. PLoS ONE 2010, 5, e13422. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, D.; McCarthy, S.; Michaelson, J.J.; Vacic, V.; Burdick, K.E.; Yoon, S.; Cichon, S.; Corvin, A.; Gary, S.; Gershon, E.S. High frequencies of de novo CNVs in bipolar disorder and schizophrenia. Neuron 2011, 72, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, T.; Kohno, T.; Hattori, M. CUB and Sushi multiple domains 3 regulates dendrite development. Neurosci. Res. 2016, 110, 11–17. [Google Scholar] [CrossRef]
- Song, W.; Li, Q.; Wang, T.; Li, Y.; Fan, T.; Zhang, J.; Wang, Q.; Pan, J.; Dong, Q.; Sun, Z.S. Putative complement control protein CSMD3 dysfunction impairs synaptogenesis and induces neurodevelopmental disorders. Brain Behav. Immun. 2022, 102, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Liu, J.; Xu, M.; Liang, J.; Wang, Y.; Wu, Z.; Xing, Y.; Diao, F. CSMD3 is Associated with Tumor Mutation Burden and Immune Infiltration in Ovarian Cancer Patients. Int. J. Gen. Med. 2021, 14, 7647. [Google Scholar] [CrossRef]
- Li, F.; Hu, S.; Kong, K.; Cao, P.; Han, P.; Deng, Y.; Zhao, B. Next-generation sequencing analysis identified genomic alterations in pathological morphologies of 3 cases of pulmonary carcinosarcoma. OncoTargets Ther. 2020, 13, 7963. [Google Scholar] [CrossRef]
- Yang, H.-S.; Liu, W.; Zheng, S.-Y.; Cai, H.-Y.; Luo, H.-H.; Feng, Y.-F.; Lei, Y.-Y. A Novel Ras--Related Signature Improves Prognostic Capacity in Oesophageal Squamous Cell Carcinoma. Front. Genet. 2022, 13, 822966. [Google Scholar] [CrossRef]
- Burgess, S.J.; Gibbs, H.; Toomes, C.; Coletta, P.L.; Bell, S.M. The role of Csmd1 during mammary gland development. Genes 2021, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Rusch, J.; Lima, A.C.; Usmani, A.; Huang, N.; Lepamets, M.; Vigh-Conrad, K.A.; Worthington, R.E.; Mägi, R.; Wu, X. Rare mutations in the complement regulatory gene CSMD1 are associated with male and female infertility. Nat. Commun. 2019, 10, 4626. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Holliday, D.L.; Morrison, E.E.; Speirs, V.; Toomes, C.; Bell, S.M. Loss of CSMD1 expression disrupts mammary duct formation while enhancing proliferation, migration and invasion. Oncol. Rep. 2017, 38, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, G.; Walters, J.; Hargreaves, A.; Rose, E.J.; Morris, D.; Fahey, C.; Bellini, S.; Cummins, E.; Giegling, I.; Hartmann, A.M. Neuropsychological effects of the CSMD1 genome-wide associated schizophrenia risk variant rs10503253. Genes Brain Behav. 2013, 12, 203–209. [Google Scholar] [CrossRef]
- Escudero-Esparza, A.; Kalchishkova, N.; Kurbasic, E.; Jiang, W.G.; Blom, A.M. The novel complement inhibitor human CUB and Sushi multiple domains 1 (CSMD1) protein promotes factor I-mediated degradation of C4b and C3b and inhibits the membrane attack complex assembly. FASEB J. 2013, 27, 5083–5093. [Google Scholar] [CrossRef]
- Ma, C.; Quesnelle, K.M.; Sparano, A.; Rao, S.; Park, M.S.; Cohen, M.A.; Wang, Y.; Samanta, M.; Kumar, M.S.; Aziz, M.U. Characterization CSMD1 in a large set of primary lung, head and neck, breast and skin cancer tissues. Cancer Biol. Ther. 2009, 8, 907–916. [Google Scholar] [CrossRef]
- Gialeli, C.; Gungor, B.; Blom, A.M. Novel potential inhibitors of complement system and their roles in complement regulation and beyond. Mol. Immunol. 2018, 102, 73–83. [Google Scholar] [CrossRef]
- Kraus, D.M.; Elliott, G.S.; Chute, H.; Horan, T.; Pfenninger, K.H.; Sanford, S.D.; Foster, S.; Scully, S.; Welcher, A.A.; Holers, V.M. CSMD1 is a novel multiple domain complement-regulatory protein highly expressed in the central nervous system and epithelial tissues. J. Immunol. 2006, 176, 4419–4430. [Google Scholar] [CrossRef]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef]
- Martin, M.; Blom, A.M. Complement in removal of the dead–balancing inflammation. Immunol. Rev. 2016, 274, 218–232. [Google Scholar] [CrossRef]
- Sarma, J.V.; Ward, P.A. The complement system. Cell Tissue Res. 2011, 343, 227–235. [Google Scholar] [PubMed]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement system part I–molecular mechanisms of activation and regulation. Front. Immunol. 2015, 6, 262. [Google Scholar] [CrossRef] [PubMed]
- Garcia, B.L.; Zwarthoff, S.A.; Rooijakkers, S.H.; Geisbrecht, B.V. Novel evasion mechanisms of the classical complement pathway. J. Immunol. 2016, 197, 2051–2060. [Google Scholar] [CrossRef] [PubMed]
- Håvik, B.; Le Hellard, S.; Rietschel, M.; Lybæk, H.; Djurovic, S.; Mattheisen, M.; Mühleisen, T.W.; Degenhardt, F.; Priebe, L.; Maier, W. The complement control-related genes CSMD1 and CSMD2 associate to schizophrenia. Biol. Psychiatry 2011, 70, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.S.; Marion, M.C.; Langefeld, C.D.; Genetics, I.C.o.S.L.E.; Buyon, J.P.; Clancy, R.M. Brief report: Enrichment of associations in genes with fibrosis, apoptosis, and innate immunity functions with cardiac manifestations of neonatal lupus. Arthritis Rheum. 2012, 64, 4060–4065. [Google Scholar] [CrossRef]
- Schafer, D.; Lehrman, E.; Kautzman, A.; Koyama, R.; Mardinly, A.; Yamasaki, R.; Ransohoff, R.; Greenberg, M.; Barres, B.; Stevens, B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Stevens, B. The “quad-partite” synapse: Microglia-synapse interactions in the developing and mature CNS. Glia 2013, 61, 24–36. [Google Scholar] [CrossRef]
- Ripke, S.; Sanders, A.; Kendler, K.; Levinson, D.; Sklar, P.; Holmans, P.; Lin, D.; Duan, J.; Ophoff, R.; Andreassen, O. Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium Genome-wide association study identifies five new schizophrenia loci. Nat. Genet. 2011, 43, 969–976. [Google Scholar]
- Bai, X.; Jin, J.; Li, S.; Wang, H.; Xie, A. CUB and sushi Multiple domains (CSMD1) gene polymorphisms and susceptibilities to idiopathic Parkinson’s disease in northern Chinese Han population: A case-control study. Parkinson’s Dis. 2021, 2021, 6661162. [Google Scholar] [CrossRef]
- Steen, V.M.; Nepal, C.; Ersland, K.M.; Holdhus, R.; Nævdal, M.; Ratvik, S.M.; Skrede, S.; Håvik, B. Neuropsychological deficits in mice depleted of the schizophrenia susceptibility gene CSMD1. PLoS ONE 2013, 8, e79501. [Google Scholar] [CrossRef]
- Bickart, K.; Napolioni, V.; Khan, R.; Kim, Y.; Altmann, A.; Richiardi, J.; Newsom, M.; Sadaghiani, S.; Banaschewski, T.; Bokde, A. Genetic variation in CSMD1 affects amygdala connectivity and prosocial behavior. bioRxiv 2020. [Google Scholar] [CrossRef]
- Hatzimanolis, A.; Foteli, S.; Stefanatou, P.; Ntigrintaki, A.-A.; Ralli, I.; Kollias, K.; Nikolaou, C.; Gazouli, M.; Stefanis, N.C. Deregulation of complement components C4A and CSMD1 peripheral expression in first-episode psychosis and links to cognitive ability. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Koiliari, E.; Roussos, P.; Pasparakis, E.; Lencz, T.; Malhotra, A.; Siever, L.J.; Giakoumaki, S.G.; Bitsios, P. The CSMD1 genome-wide associated schizophrenia risk variant rs10503253 affects general cognitive ability and executive function in healthy males. Schizophr. Res. 2014, 154, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Rose, E.J.; Morris, D.W.; Hargreaves, A.; Fahey, C.; Greene, C.; Garavan, H.; Gill, M.; Corvin, A.; Donohoe, G. Neural effects of the CSMD 1 genome-wide associated schizophrenia risk variant rs10503253. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2013, 162, 530–537. [Google Scholar] [CrossRef]
- Abd El Gayed, E.M.; Rizk, M.S.; Ramadan, A.N.; Bayomy, N.R. mRNA Expression of the CUB and Sushi Multiple Domains 1 (CSMD1) and Its Serum Protein Level as Predictors for Psychosis in the Familial High-Risk Children and Young Adults. ACS Omega 2021, 6, 24128–24138. [Google Scholar] [CrossRef]
- Ruiz-Martínez, J.; Azcona, L.J.; Bergareche, A.; Martí-Massó, J.F.; Paisán-Ruiz, C. Whole-exome sequencing associates novel CSMD1 gene mutations with familial Parkinson disease. Neurol. Genet. 2017, 3, e177. [Google Scholar] [CrossRef]
- Shahmohammadibeni, N.; Rahimi-Aliabadi, S.; Jamshidi, J.; Emamalizadeh, B.; Shahmohammadibeni, H.A.; Zare Bidoki, A.; Akhavan-Niaki, H.; Eftekhari, H.; Abdollahi, S.; Shekari Khaniani, M. The analysis of association between SNCA, HUSEYO and CSMD1 gene variants and Parkinson’s disease in Iranian population. Neurol. Sci. 2016, 37, 731–736. [Google Scholar] [CrossRef]
- Mitelman, F.; Mertens, F.; Johansson, B. A breakpoint map of recurrent chromosomal rearrangements in human neoplasia. Nat. Genet. 1997, 15, 417–474. [Google Scholar] [CrossRef]
- Sunwoo, J.B.; Holt, M.S.; Radford, D.M.; Deeker, C.; Scholnick, S.B. Evidence for multiple tumor suppressor genes on chromosome arm 8p in supraglottic laryngeal cancer. Genes Chromosomes Cancer 1996, 16, 164–169. [Google Scholar] [CrossRef]
- Wu, C.L.; Roz, L.; Sloan, P.; Read, A.P.; Holland, S.; Porter, S.; Scully, C.; Speight, P.M.; Thakker, N. Deletion mapping defines three discrete areas of allelic imbalance on chromosome arm 8p in oral and oropharyngeal squamous cell carcinomas. Genes Chromosomes Cancer 1997, 20, 347–353. [Google Scholar] [CrossRef]
- Ishwad, C.S.; Shuster, M.; Bockmühl, U.; Thakker, N.; Shah, P.; Toomes, C.; Dixon, M.; Ferrell, R.E.; Gollin, S.M. Frequent allelic loss and homozygous deletion in chromosome band 8p23 in oral cancer. Int. J. Cancer 1999, 80, 25–31. [Google Scholar] [CrossRef]
- Zhu, Q.; Gong, L.; Wang, J.; Tu, Q.; Yao, L.; Zhang, J.-R.; Han, X.-J.; Zhu, S.-J.; Wang, S.-M.; Li, Y.-H. miR-10b exerts oncogenic activity in human hepatocellular carcinoma cells by targeting expression of CUB and sushi multiple domains 1 (CSMD1). BMC Cancer 2016, 16, 806. [Google Scholar] [CrossRef] [PubMed]
- Bartha, Á.; Győrffy, B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol. Sci. 2021, 22, 2622. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Shaaban, A.M.; Zhang, L.; Walker, C.; Gray, S.; Thakker, N.; Toomes, C.; Speirs, V.; Bell, S.M. Loss of CSMD1 expression is associated with high tumour grade and poor survival in invasive ductal breast carcinoma. Breast Cancer Res. Treat. 2010, 121, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Esparza, A.; Bartoschek, M.; Gialeli, C.; Okroj, M.; Owen, S.; Jirström, K.; Orimo, A.; Jiang, W.G.; Pietras, K.; Blom, A.M. Complement inhibitor CSMD1 acts as tumor suppressor in human breast cancer. Oncotarget 2016, 7, 76920. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Minoche, A.; Harvey, K.; Li, M.; Winkler, J.; Goga, A.; Swarbrick, A. Deep whole genome sequencing identifies recurrent genomic alterations in commonly used breast cancer cell lines and patient-derived xenograft models. Breast Cancer Res. 2022, 24, 63. [Google Scholar] [CrossRef]
- Gialeli, C.; Tuysuz, E.C.; Staaf, J.; Guleed, S.; Paciorek, V.; Mörgelin, M.; Papadakos, K.S.; Blom, A.M. Complement inhibitor CSMD1 modulates epidermal growth factor receptor oncogenic signaling and sensitizes breast cancer cells to chemotherapy. J. Exp. Clin. Cancer Res. 2021, 40, 258. [Google Scholar] [CrossRef]
- Paris, P.L.; Andaya, A.; Fridlyand, J.; Jain, A.N.; Weinberg, V.; Kowbel, D.; Brebner, J.H.; Simko, J.; Watson, J.V.; Volik, S. Whole genome scanning identifies genotypes associated with recurrence and metastasis in prostate tumors. Hum. Mol. Genet. 2004, 13, 1303–1313. [Google Scholar] [CrossRef]
- Fan, X.; Song, J.; Fan, Y.; Li, J.; Chen, Y.; Zhu, H.; Zhang, Z. CSMD1 Mutation Related to Immunity Can Be Used as a Marker to Evaluate the Clinical Therapeutic Effect and Prognosis of Patients with Esophageal Cancer. Int. J. Gen. Med. 2021, 14, 8689. [Google Scholar] [CrossRef]
- Chen, X.-L.; Hong, L.-L.; Wang, K.-L.; Liu, X.; Wang, J.-L.; Lei, L.; Xu, Z.-Y.; Cheng, X.-D.; Ling, Z.-Q. Deregulation of CSMD1 targeted by microRNA-10b drives gastric cancer progression through the NF-κB pathway. Int. J. Biol. Sci. 2019, 15, 2075. [Google Scholar] [CrossRef]
- Huang, T.; Liang, Y.; Zhang, H.; Chen, X.; Wei, H.; Sun, W.; Wang, Y. CSMD1 mutations are associated with increased mutational burden, favorable prognosis, and anti-tumor immunity in gastric cancer. Genes 2021, 12, 1715. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, S.; Han, Y.; Xu, M.; Li, P.; Ke, M.; Teng, Z.; Huang, P.; Diao, Z.; Yan, Y. Association of CSMD1 with Tumor Mutation Burden and Other Clinical Outcomes in Gastric Cancer. Int. J. Gen. Med. 2021, 14, 8293. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Zhou, L.; Jiang, X.; Zhou, X. MicroRNA-642b-3p functions as an oncomiR in gastric cancer by down-regulating the CUB and sushi multiple domains protein 1/smad axis. Bioengineered 2022, 13, 9614–9628. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, R.; Mai, S.-J.; Ma, Y.-S.; Zhang, M.-Y.; Cao, P.-S.; Weng, N.-Q.; Wang, R.-Q.; Cao, D.; Wei, W. LncRNA CSMD1-1 promotes the progression of hepatocellular carcinoma by activating MYC signaling. Theranostics 2020, 10, 7527. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, B.; Chen, D.; Zhou, X.; Wang, M.; Jiang, J.; Wei, L.; Chen, Z. Combined identification of ARID1A, CSMD1, and SENP3 as effective prognostic biomarkers for hepatocellular carcinoma. Aging 2021, 13, 4696. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef]
- Qin, L.-X. Chromosomal aberrations related to metastasis of human solid tumors. World J. Gastroenterol. 2002, 8, 769. [Google Scholar] [CrossRef]
- Lang, M.-F.; Yang, S.; Zhao, C.; Sun, G.; Murai, K.; Wu, X.; Wang, J.; Gao, H.; Brown, C.E.; Liu, X. Genome-wide profiling identified a set of miRNAs that are differentially expressed in glioblastoma stem cells and normal neural stem cells. PLoS ONE 2012, 7, e36248. [Google Scholar] [CrossRef]
- Kwon, E.; Wang, W.; Tsai, L. Validation of schizophrenia-associated genes CSMD1, C10orf26, CACNA1C and TCF4 as miR-137 targets. Mol. Psychiatry 2013, 18, 11–12. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Liu, Y.; Huang, S.; Ding, J.; Wang, B.; Dong, P.; Sun, Z.; Chen, L. CSMD1 suppresses cancer progression by inhibiting proliferation, epithelial-mesenchymal transition, chemotherapy-resistance and inducing immunosuppression in esophageal squamous cell carcinoma. Exp. Cell Res. 2022, 417, 113220. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ermis Akyuz, E.; Bell, S.M. The Diverse Role of CUB and Sushi Multiple Domains 1 (CSMD1) in Human Diseases. Genes 2022, 13, 2332. https://doi.org/10.3390/genes13122332
Ermis Akyuz E, Bell SM. The Diverse Role of CUB and Sushi Multiple Domains 1 (CSMD1) in Human Diseases. Genes. 2022; 13(12):2332. https://doi.org/10.3390/genes13122332
Chicago/Turabian StyleErmis Akyuz, Esra, and Sandra M. Bell. 2022. "The Diverse Role of CUB and Sushi Multiple Domains 1 (CSMD1) in Human Diseases" Genes 13, no. 12: 2332. https://doi.org/10.3390/genes13122332
APA StyleErmis Akyuz, E., & Bell, S. M. (2022). The Diverse Role of CUB and Sushi Multiple Domains 1 (CSMD1) in Human Diseases. Genes, 13(12), 2332. https://doi.org/10.3390/genes13122332






