Can Polymorphisms in NLRP3 Inflammasome Complex Be Associated with Postmenopausal Osteoporosis Severity?
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. SNVs Selection and Genotyping
2.3. Statistical Analysis
3. Results
3.1. Clinical and Biochemical Bone Markers Levels
3.2. Genetic Association Study
3.2.1. SNVs and OP Susceptibility
3.2.2. SNVs and Correlation with BMD
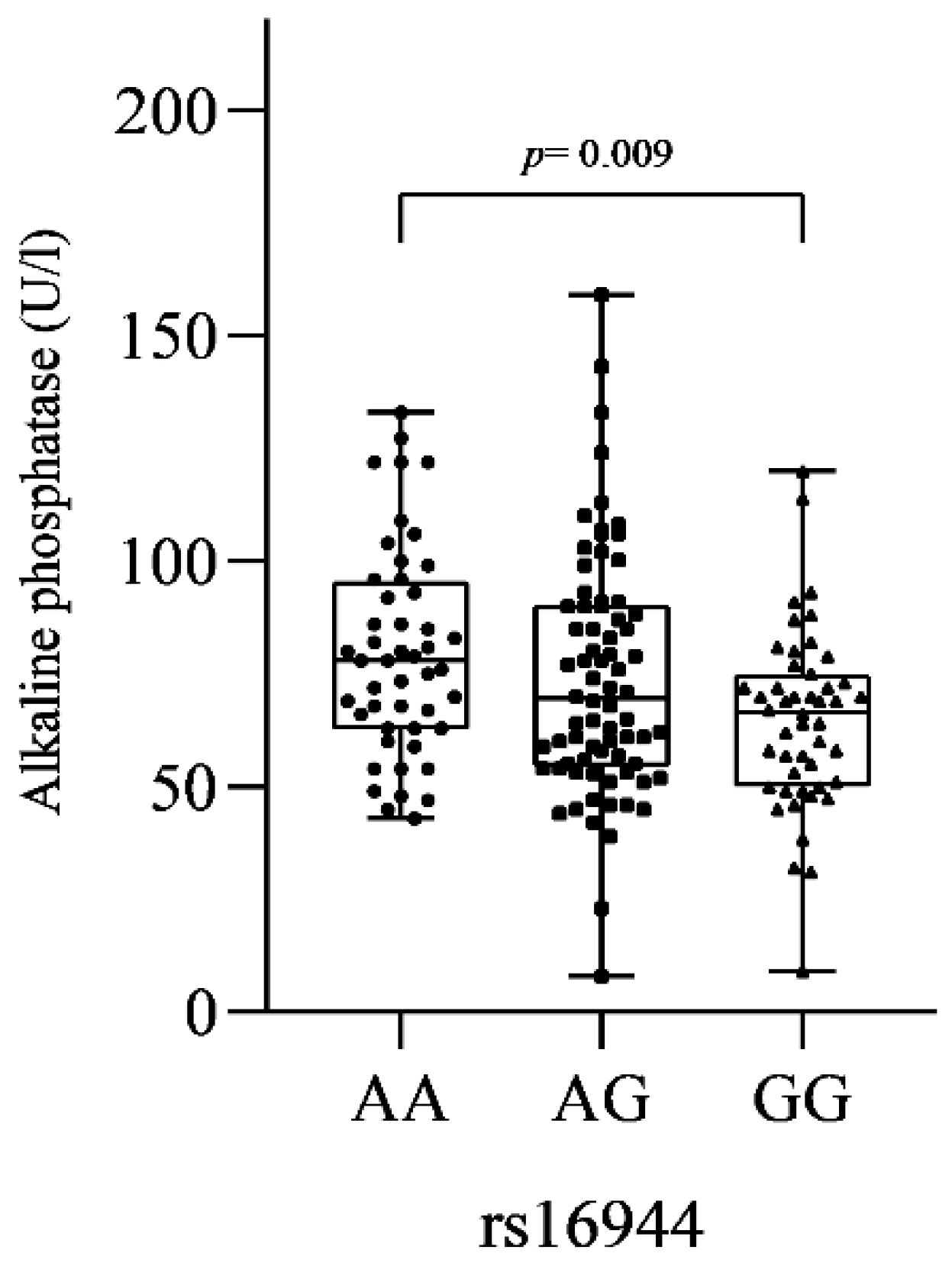
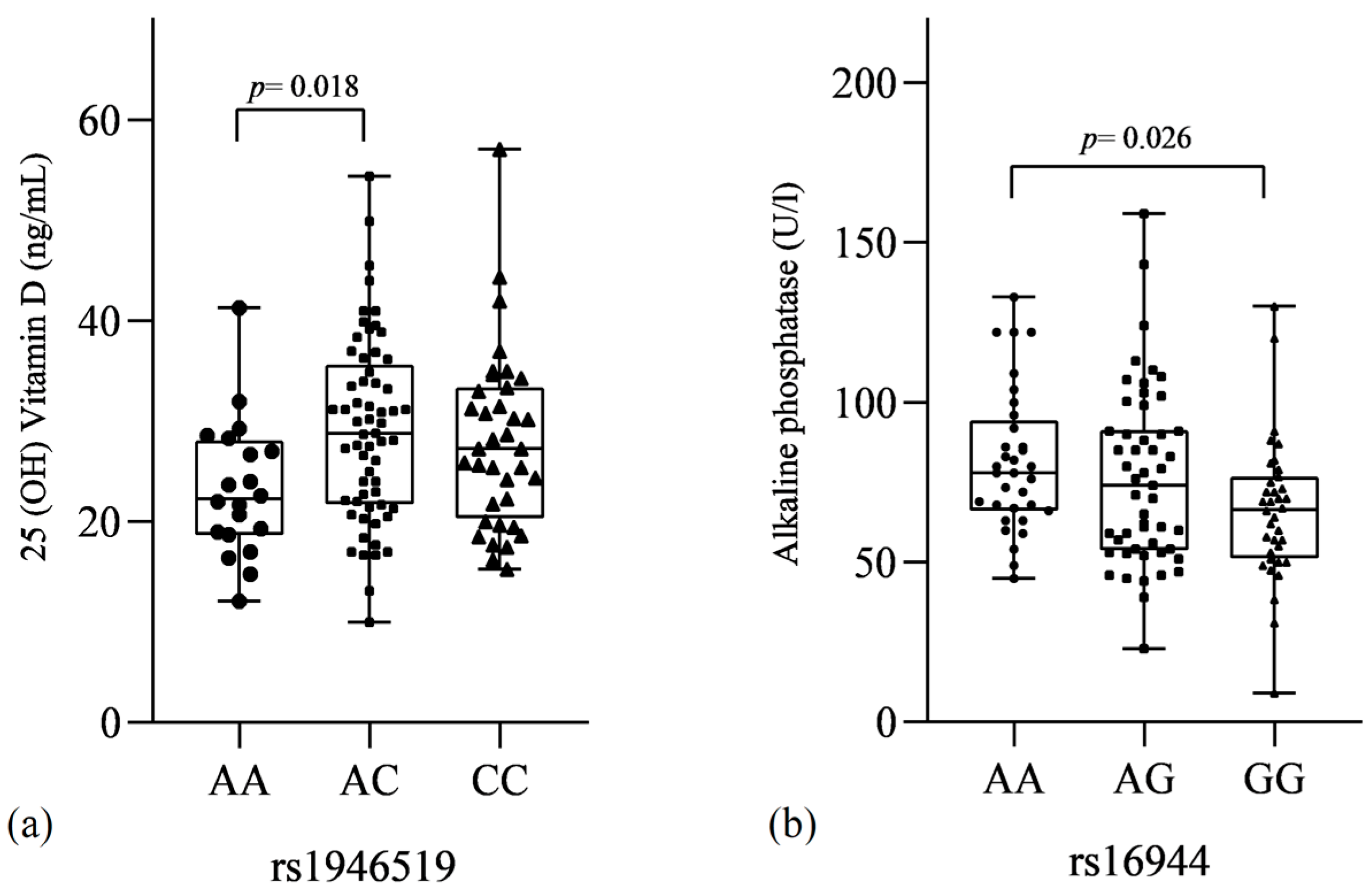
3.2.3. SNVs and Biochemical Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cummings, S.R.; Melton, L.J. Osteoporosis I: Epidemiology and outcomes of osteoporotic fractures. Lancet 2002, 359, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Huang, X.; Wu, J.; Lin, X.; Zhou, X.; Zhu, Z.; Pan, X.; Xu, J.; Qiao, J.; Zhang, T.; et al. The Global Burden of Osteoporosis, Low Bone Mass, and Its Related Fracture in 204 Countries and Territories, 1990–2019. Front. Endocrinol. 2022, 13, 882241. [Google Scholar] [CrossRef] [PubMed]
- Lewiecki, E.M.; Wright, N.C.; Curtis, J.R.; Siris, E.; Gagel, R.F.; Saag, K.G.; Singer, A.J.; Steven, P.M.; Adler, R.A. Hip fracture trends in the United States, 2002 to 2015. Osteoporos. Int. 2017, 29, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Detzen, L.; Cheat, B.; Besbes, A.; Hassan, B.; Marchi, V.; Baroukh, B.; Lesieur, J.; Sadoine, J.; Torrens, C.; Rochefort, G.; et al. NLRP3 is involved in long bone edification and the maturation of osteogenic cells. J. Cell. Physiol. 2020, 236, 4455–4469. [Google Scholar] [CrossRef] [PubMed]
- Gross, O.; Thomas, C.J.; Guarda, G.; Tschopp, J. The inflammasome: An integrated view. Immunol. Rev. 2011, 243, 136–151. [Google Scholar] [CrossRef]
- Evavold, C.L.; Kagan, J.C. Inflammasomes: Threat-Assessment Organelles of the Innate Immune System. Immunity 2019, 51, 609–624. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Man, S.M.; Kanneganti, T.D. Regulation of inflammasome activation. Immunol. Rev. 2015, 265, 6–21. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; Macdonald, K.L.; Speert, D.P.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.; et al. Cutting Edge: NF-κB Activating Pattern Recognition and Cytokine Receptors License NLRP3 Inflammasome Activation by Regulating NLRP3 Expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef]
- Murakami, T.; Nakaminami, Y.; Takahata, Y.; Hata, K.; Nishimura, R. Activation and Function of NLRP3 Inflammasome in Bone and Joint-Related Diseases. Int. J. Mol. Sci. 2022, 23, 5365. [Google Scholar] [CrossRef]
- Netea, M.G.; Nold-Petry, C.A.; Nold, M.F.; Joosten, L.A.B.; Opitz, B.; van der Meer, J.H.M.; van de Veerdonk, F.L.; Ferwerda, G.; Heinhuis, B.; Devesa, I.; et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1β in monocytes and macrophages. Blood 2009, 113, 2324–2335. [Google Scholar] [CrossRef]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; Di Paola, R. Focus on the Role of NLRP3 Inflammasome in Diseases. Int. J. Mol. Sci. 2020, 21, 4223. [Google Scholar] [CrossRef]
- Valencia, B.M.; Cvejic, E.; Vollmer-Conna, U.; Hickie, I.B.; Wakefield, D.; Li, H.; Pedergnana, V.; Rodrigo, C.; Lloyd, A.R. The severity of the pathogen-induced acute sickness response is affected by polymorphisms in genes of the NLRP3 inflammasome pathway. Brain Behav. Immun. 2021, 93, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Särndahl, E.; Andersson, H.; Eriksson, P.; Fredrikson, M.; Jönsson, J.-I.; Lerm, M.; Söderkvist, P. The Q705K Polymorphism in NLRP3 Is a Gain-of-Function Alteration Leading to Excessive Interleukin-1β and IL-18 Production. PLoS ONE 2012, 7, e34977. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, S.; Liang, T. Association of NLRP3 rs35829419 and rs10754558 Polymorphisms with Risks of Autoimmune Diseases: A Systematic Review and Meta-Analysis. Front. Genet. 2021, 12, 690860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fan, H.; Zhang, J.; Wang, Y.; Xing, H. NLRP3 rs35829419 polymorphism is associated with increased susceptibility to multiple diseases in humans. Genet. Mol. Res. 2015, 14, 13968–13980. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, C.; Kuang, Z.; Zheng, Q. The Role of NLRP3 Inflammasome Activities in Bone Diseases and Vascular Calcification. Inflammation 2021, 44, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Snouwaert, J.N.; Nguyen, M.; Repenning, P.W.; Dye, R.; Livingston, E.W.; Kovarova, M.; Moy, S.S.; Brigman, B.E.; Bateman, T.A.; Ting, J.P.-Y.; et al. An NLRP3 Mutation Causes Arthropathy and Osteoporosis in Humanized Mice. Cell Rep. 2016, 17, 3077–3088. [Google Scholar] [CrossRef]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Diab, D.L.; Eldeiry, L.S.; Farooki, A.; Harris, S.T.; Hurley, D.L.; Kelly, J.; Lewiecki, E.M.; et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis—2020 Update. Endocr. Pract. 2020, 26, 1–46. [Google Scholar] [CrossRef]
- Kanis, J.A. Assessment of Osteoporosis at the Primary Health Care Level; WHO Collaborating Centre for Metabolic Bone Diseases, University of Sheffield Medical School: Sheffield, UK, 2008. [Google Scholar]
- Pontillo, A.; Brandao, L.; Guimaraes, R.; Segat, L.; Araujo, J.; Crovella, S. Two SNPs in NLRP3 gene are involved in the predisposition to type-1 diabetes and celiac disease in a pediatric population from northeast Brazil. Autoimmunity 2010, 43, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Addobbati, C.; da Cruz, H.L.A.; Adelino, J.E.; Ramos, A.L.M.T.; Fragoso, T.S.; Domingues, A.; Duarte, L.B.P.; Oliveira, R.D.R.; Louzada-Júnior, P.; Donadi, E.A.; et al. Polymorphisms and expression of inflammasome genes are associated with the development and severity of rheumatoid arthritis in Brazilian patients. Inflamm. Res. 2017, 67, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Theodoropoulou, K.; Spel, L.; Zaffalon, L.; Delacrétaz, M.; Martinon, F. NLRP3 leucine-rich repeats control induced and spontaneous inflammasome activation in cryopyrin-associated periodic syndrome. J. Allergy Clin. Immunol. 2022; in press. [Google Scholar] [CrossRef]
- Jiang, N.; An, J.; Yang, K.; Liu, J.; Guan, C.; Ma, C.; Tang, X. NLRP3 Inflammasome: A New Target for Prevention and Control of Osteoporosis? Front. Endocrinol. 2021, 12, 752546. [Google Scholar] [CrossRef]
- Youm, Y.-H.; Grant, R.W.; McCabe, L.R.; Albarado, D.C.; Nguyen, K.Y.; Ravussin, A.; Pistell, P.; Newman, S.; Carter, R.; Laque, A.; et al. Canonical Nlrp3 Inflammasome Links Systemic Low-Grade Inflammation to Functional Decline in Aging. Cell Metab. 2013, 18, 519–532. [Google Scholar] [CrossRef]
- Pietschmann, P.; Mechtcheriakova, D.; Meshcheryakova, A.; Föger-Samwald, U.; Ellinger, I. Immunology of Osteoporosis: A Mini-Review. Gerontology 2015, 62, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Iacoviello, L.; Di Castelnuovo, A.; Gattone, M.; Pezzini, A.; Assanelli, D.; Lorenzet, R.; Del Zotto, E.; Colombo, M.; Napoleone, E.; Amore, C.; et al. Polymorphisms of the interleukin-1β gene affect the risk of myocardial infarction and ischemic stroke at young age and the response of mononuclear cells to stimulation in vitro. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 222–227. [Google Scholar] [CrossRef]
- Rogus, J.; Beck, J.D.; Offenbacher, S.; Huttner, K.; Iacoviello, L.; Latella, M.C.; De Gaetano, M.; Wang, H.-Y.; Kornman, K.S.; Duff, G.W. IL1B gene promoter haplotype pairs predict clinical levels of interleukin-1β and C-reactive protein. Qual. Life Res. 2008, 123, 387–398. [Google Scholar] [CrossRef]
- He, Z.; Sun, Y.; Wu, J.; Xiong, Z.; Zhang, S.; Liu, J.; Liu, Y.; Li, H.; Jin, T.; Yang, Y.; et al. Evaluation of genetic variants in IL-1B and its interaction with the predisposition of osteoporosis in the northwestern Chinese Han population. J. Gene Med. 2020, 22, e3214. [Google Scholar] [CrossRef]
- Roudsari, J.M.; Mahjoub, S. Quantification and comparison of bone-specific alkaline phosphatase with two methods in normal and paget’s specimens. Casp. J. Intern. Med. 2012, 3, 478–483. [Google Scholar]
- Silva, B.C.; Bilezikian, J.P. Parathyroid hormone: Anabolic and catabolic actions on the skeleton. Curr. Opin. Pharmacol. 2015, 22, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Giedraitis, V.; He, B.; Huang, W.-X.; Hillert, J. Cloning and mutation analysis of the human IL-18 promoter: A possible role of polymorphisms in expression regulation. J. Neuroimmunol. 2001, 112, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Suganthan, N.; Kumanan, T.; Kesavan, V.; Aravinthan, M.; Rajeshkannan, N. Vitamin D status among postmenopausal osteoporotic women: A hospital based cross-sectional study from Northern Sri Lanka. BMC Nutr. 2020, 6, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Mangin, M.; Sinha, R.; Fincher, K. Inflammation and vitamin D: The infection connection. Inflamm. Res. 2014, 63, 803–819. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, E.S.; Desai, M.; Perrin, N.; Wactawski-Wende, J.; Manson, J.A.E.; Cauley, J.A.; Michael, Y.L.; Tang, J.; Womack, C.; Song, Y.; et al. Vitamin D levels and menopause-related symptoms. Menopause 2014, 21, 1197–1203. [Google Scholar] [CrossRef]
- Khundmiri, S.J.; Murray, R.D.; Lederer, E. PTH and Vitamin, D. Compr. Physiol. 2016, 6, 561–601. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.S.; Brockwell, S.E.; Mehta, V.; Greendale, G.A.; Sowers, M.R.; Ettinger, B.; Lo, J.C.; Johnston, J.M.A.; Cauley, J.; Danielson, M.E.; et al. Bone Mineral Density Changes during the Menopause Transition in a Multiethnic Cohort of Women. J. Clin. Endocrinol. Metab. 2008, 93, 861–868. [Google Scholar] [CrossRef]

| Characteristics | OP | Control | p |
|---|---|---|---|
| Demographic | |||
| Age (range) | 62 (51–72) | 61 (53–73) | 0.06 |
| Mean of years since menopause (range) | 16 (3–36) | 13 (2–35) | 0.019 * |
| Clinical | |||
| Vitamin D (ng/mL) | 28.15 ± 8.68 | 32.75 ± 10.72 | 0.001 * |
| Calcium (mg/dL) | 9.45 ± 0.67 | 9.48 ± 0.78 | 0.79 |
| Alkaline phosphatase (U/L) | 75.48 ± 28.38 | 70.16 ± 23.70 | 0.33 |
| Phosphorus (mg/dL) | 3.47 ± 0.56 | 3.61 ± 0.58 | 0.11 |
| Parathyroid hormone (pg/mL) | 56.39 ± 27.25 | 48.33 ± 27.05 | 0.043 * |
| Magnesium (mg/dL) | 2.06 ± 0.26 | 2.02 ± 0.47 | 0.74 |
| SNVs | Model | Alleles/Genotypes | Controls, n (%) | OP, n (%) | p | OR | 95% CI |
|---|---|---|---|---|---|---|---|
| NLRP3 rs35829419 | C | 196 (97) | 364 (97) | 1 | |||
| A | 6 (3) | 10 (3) | 0.83 | 0.90 | 0.30–2.46 | ||
| Codominant | CC | 95 (94) | 177 (95) | 1 | |||
| CA | 6 (6) | 10 (5) | 0.83 | 0.89 | 0.33–2.52 | ||
| AA | 0 (0) | 0 (0) | ND | ND | ND | ||
| HWE | 1 | 1 | |||||
| NLRP3 rs10754558 | G | 70 (36) | 136 (36) | 1 | |||
| C | 126 (64) | 246 (64) | 0.97 | 1.00 | 0.70–1.44 | ||
| Codominant | GG | 9 (9) | 25 (13) | 1 | |||
| GC | 52 (53) | 86 (45) | 0.22 | 0.59 | 0.24–1.35 | ||
| CC | 37 (38) | 80 (42) | 0.56 | 0.77 | 0.54–3.09 | ||
| Dominant | GG | 9 (9) | 25 (13) | 1 | |||
| GC-CC | 89 (81) | 166 (87) | 0.75 | 0.67 | 0.29–1.52 | ||
| Recessive | GG-GC | 61 (62) | 111 (58) | 1 | |||
| CC | 37 (38) | 80 (42) | 1.12 | 1.18 | 0.71–1.96 | ||
| Log-additive | --- | --- | --- | 0.98 | 0.99 | 0.69–1.44 | |
| HWE | 0.19 | 0.87 | |||||
| CASP1 rs61751523 | T | 140 (92) | 283 (94) | 1 | |||
| C | 12 (8) | 17 (6) | 0.36 | 0.70 | 0.33–1.50 | ||
| Codominant | TT | 64 (84) | 134 (89) | 1 | |||
| TC | 12 (16) | 15 (10) | 0.21 | 0.59 | 0.27–1.39 | ||
| CC | 0 (0) | 1 (1) | 0.49 | ND | ND | ||
| Dominant | TT | 64 (84) | 134 (89) | 1 | |||
| TC-CC | 12 (16) | 16 (11) | 0.28 | 0.63 | 0.29–1.46 | ||
| Recessive | TT-TC | 76 (100) | 149 (99) | 1 | |||
| CC | 0(0) | 1 (1) | >0.99 | ND | ND | ||
| Log-additive | --- | --- | --- | 0.37 | 0.70 | 0.33–1.51 | |
| HWE | 1 | 0.38 | |||||
| IL-18 rs1946519 | A | 79 (40) | 144 (46) | 1 | |||
| C | 121 (60) | 172 (54) | 0.17 | 0.77 | 0.54–1.12 | ||
| Codominant | AA | 15 (15) | 33 (21) | 1 | |||
| AC | 49 (49) | 78 (49) | 0.36 | 0.72 | 0.35–1.42 | ||
| CC | 36 (36) | 47 (30) | 0.17 | 0.59 | 0.28–1.28 | ||
| Dominant | AA | 15 (15) | 33 (21) | 1 | |||
| AC-CC | 85 (85) | 125 (79) | 0.25 | 0.66 | 0.34–1.28 | ||
| Recessive | AA-AC | 64 (64) | 111 (70) | 1 | |||
| CC | 36 (36) | 47 (30) | 0.33 | 0.75 | 0.45–1.27 | ||
| Log-additive | --- | --- | --- | 0.17 | 1.28 | 0.89–1.84 | |
| HWE | 1 | 1 | |||||
| IL-1β rs16944 | A | 96 (47) | 189 (50) | 1 | |||
| G | 110 (53) | 189 (50) | 0.43 | 0.87 | 0.62–1.23 | ||
| Codominant | AA | 20 (20) | 54 (29) | 1 | |||
| AG | 56 (54) | 81 (43) | 0.05 | 0.53 | 0.29–0.98 | ||
| GG | 27 (26) | 54 (28) | 0.39 | 0.74 | 0.36–1.45 | ||
| Dominant | AA | 20 (20) | 54 (29) | 1 | |||
| AG-GG | 83 (80) | 135 (71) | 0.09 | 0.60 | 0.33–1.07 | ||
| Recessive | AA-AG | 76 (74) | 135 (72) | 1 | |||
| GG | 27 (26) | 54 (28) | 0.68 | 1.12 | 0.64–1.89 | ||
| Log-additive | --- | --- | --- | 0.45 | 1.14 | 0.82–1.58 | |
| HWE | 0.43 | 0.06 | |||||
| CARD8 rs2043211 | A | 145 (72) | 275 (72) | 1 | |||
| T | 55 (28) | 105 (28) | 0.97 | 1.00 | 0.69–1.46 | ||
| Codominant | AA | 51 (51) | 99 (52) | 1 | |||
| AT | 43 (43) | 77 (41) | 0.75 | 0.92 | 0.56–1.52 | ||
| TT | 6 (6) | 14 (7) | 0.72 | 1.20 | 0.45–3.28 | ||
| Dominant | AA | 51 (51) | 99 (52) | 1 | |||
| AT-TT | 49 (49) | 91 (48) | 0.90 | 0.95 | |||
| Recessive | AA-AT | 94 (94) | 176 (93) | 1 | |||
| TT | 6 (6) | 14 (7) | 0.80 | 1.24 | 0.49–3.26 | ||
| Log-additive | --- | --- | --- | 0.97 | 1.01 | 0.68–1.49 | |
| HWE | 0.62 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guaraná, W.L.; Lima, C.A.D.; Barbosa, A.D.; Crovella, S.; Sandrin-Garcia, P. Can Polymorphisms in NLRP3 Inflammasome Complex Be Associated with Postmenopausal Osteoporosis Severity? Genes 2022, 13, 2271. https://doi.org/10.3390/genes13122271
Guaraná WL, Lima CAD, Barbosa AD, Crovella S, Sandrin-Garcia P. Can Polymorphisms in NLRP3 Inflammasome Complex Be Associated with Postmenopausal Osteoporosis Severity? Genes. 2022; 13(12):2271. https://doi.org/10.3390/genes13122271
Chicago/Turabian StyleGuaraná, Werbson Lima, Camilla Albertina Dantas Lima, Alexandre Domingues Barbosa, Sergio Crovella, and Paula Sandrin-Garcia. 2022. "Can Polymorphisms in NLRP3 Inflammasome Complex Be Associated with Postmenopausal Osteoporosis Severity?" Genes 13, no. 12: 2271. https://doi.org/10.3390/genes13122271
APA StyleGuaraná, W. L., Lima, C. A. D., Barbosa, A. D., Crovella, S., & Sandrin-Garcia, P. (2022). Can Polymorphisms in NLRP3 Inflammasome Complex Be Associated with Postmenopausal Osteoporosis Severity? Genes, 13(12), 2271. https://doi.org/10.3390/genes13122271






