Plastidial Phosphoglucomutase (pPGM) Overexpression Increases the Starch Content of Transgenic Sweet Potato Storage Roots
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Reagents
2.2. Cloning and the Sequence Analysis of IbpPGM
2.3. Expression Analysis of IbpPGM in the Sweet Potato
2.4. Prokaryotic Expression of IbpPGM
2.5. Subcellular Localization
2.6. Production of the Transgenic Plants
2.7. Quantification of the Carbohydrate Contents
2.8. Expression Analysis of the Starch Biosynthetic Genes
3. Results
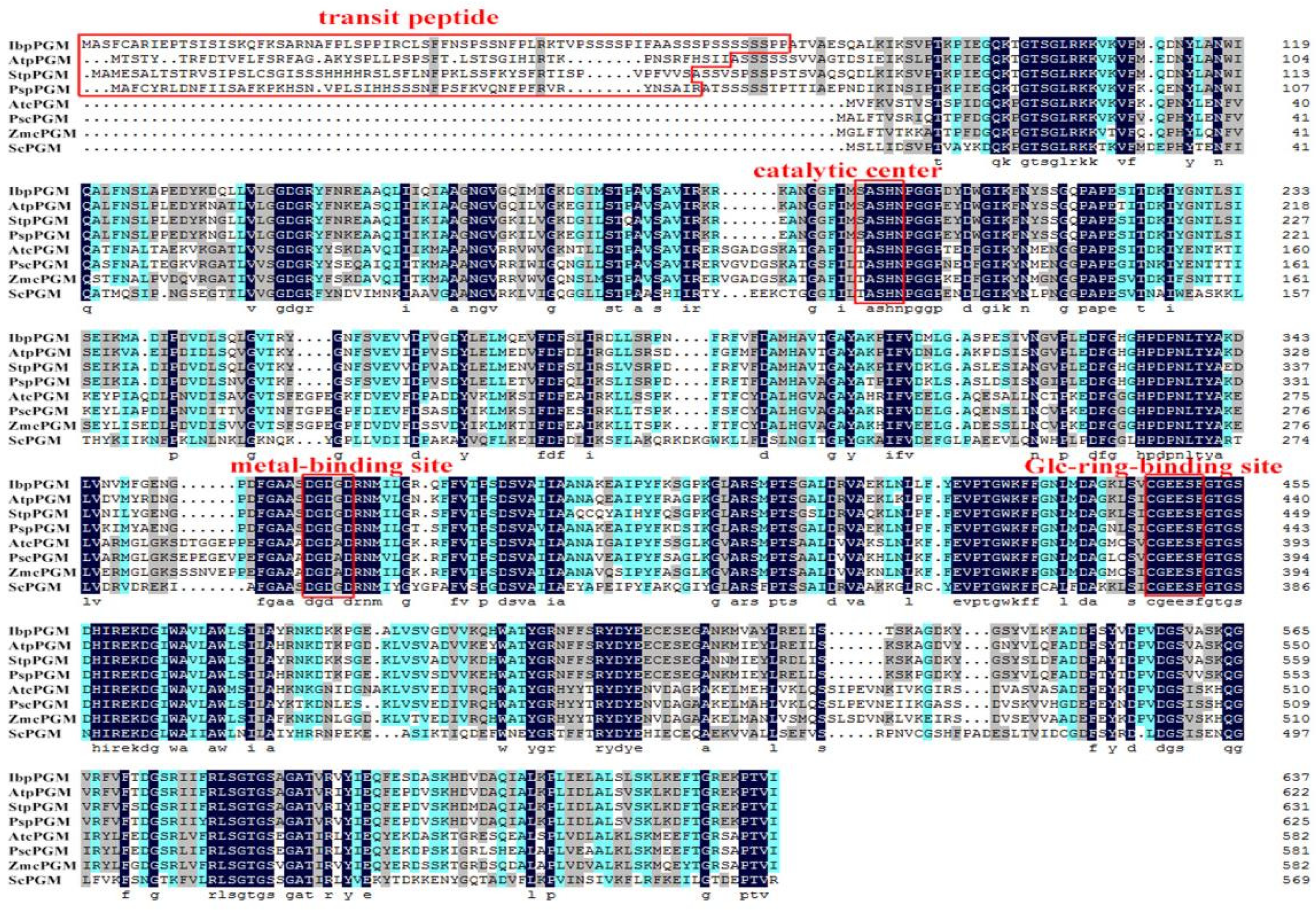
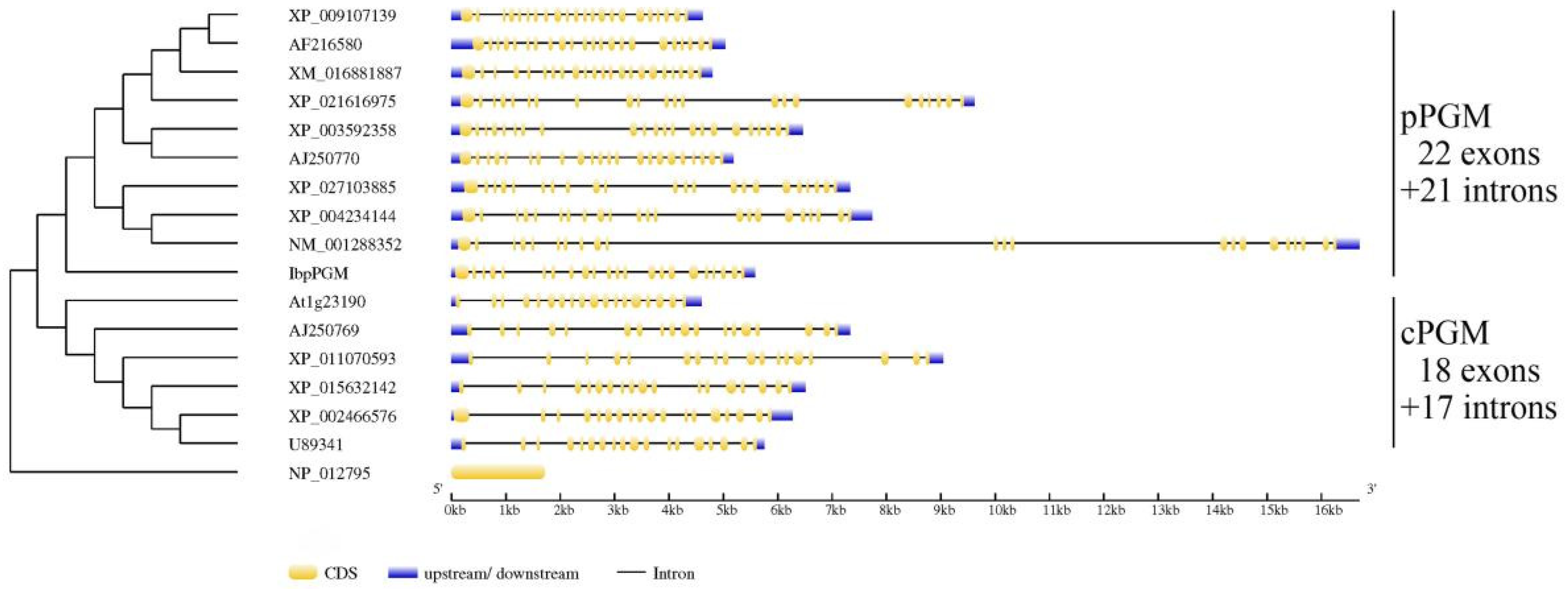
3.1. Cloning and Sequence Analysis of IbpPGM
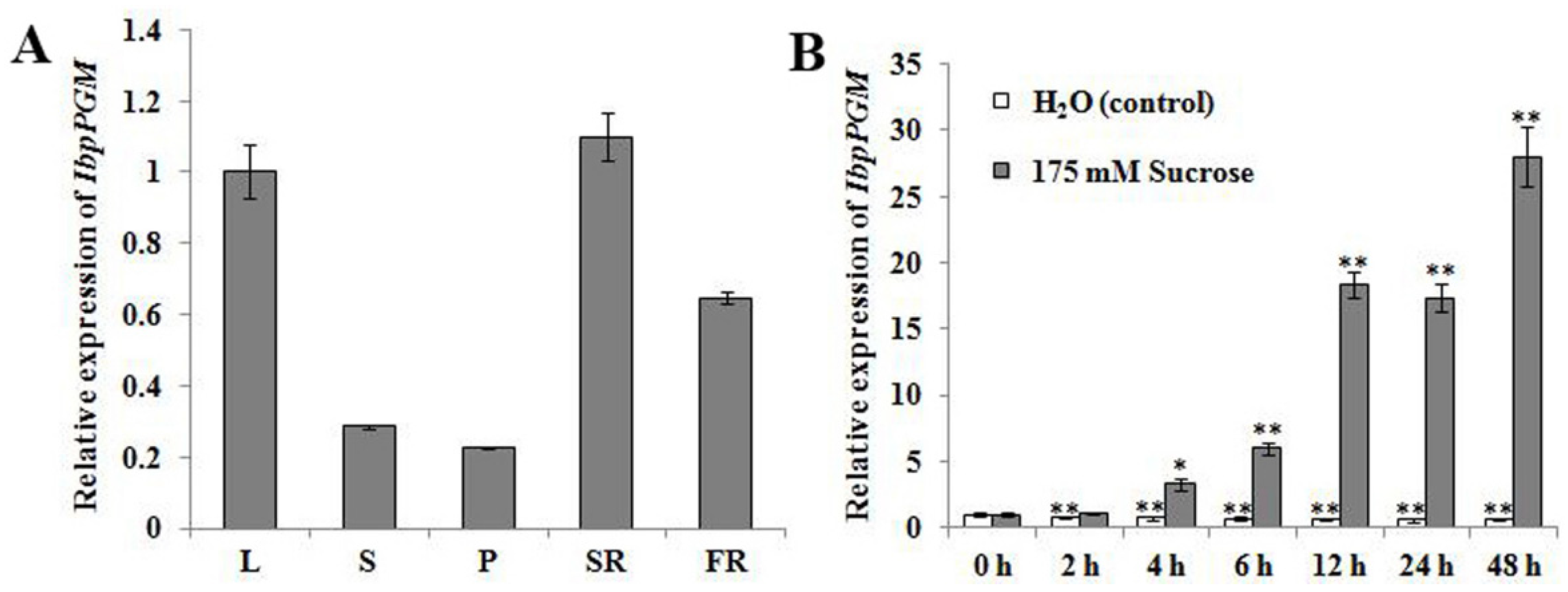
3.2. Expression of IbpPGM in the Sweet Potato
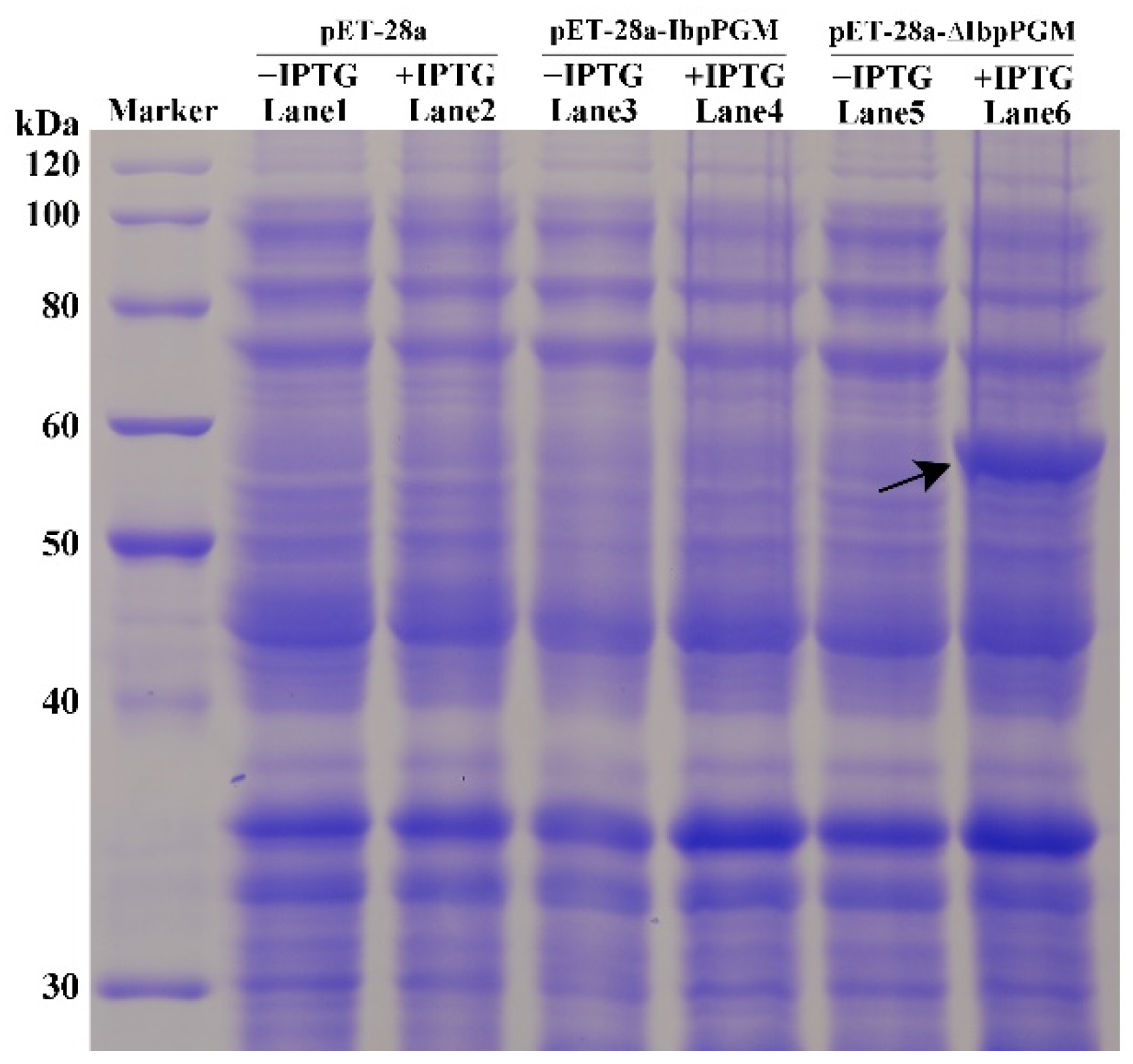
3.3. Expression of IbpPGM in E. coli
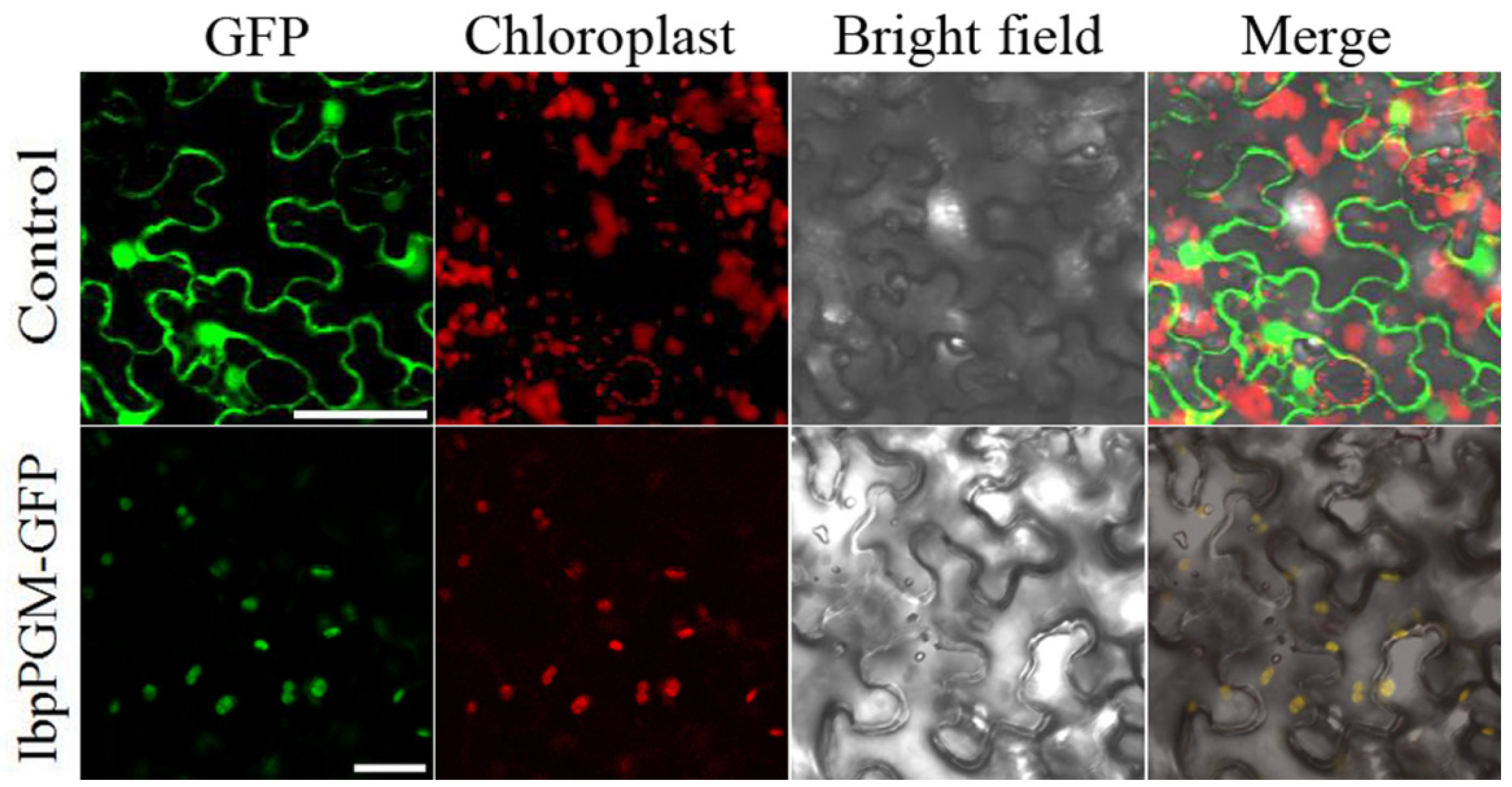
3.4. Subcellular Localization of IbpPGM in N. benthamiana
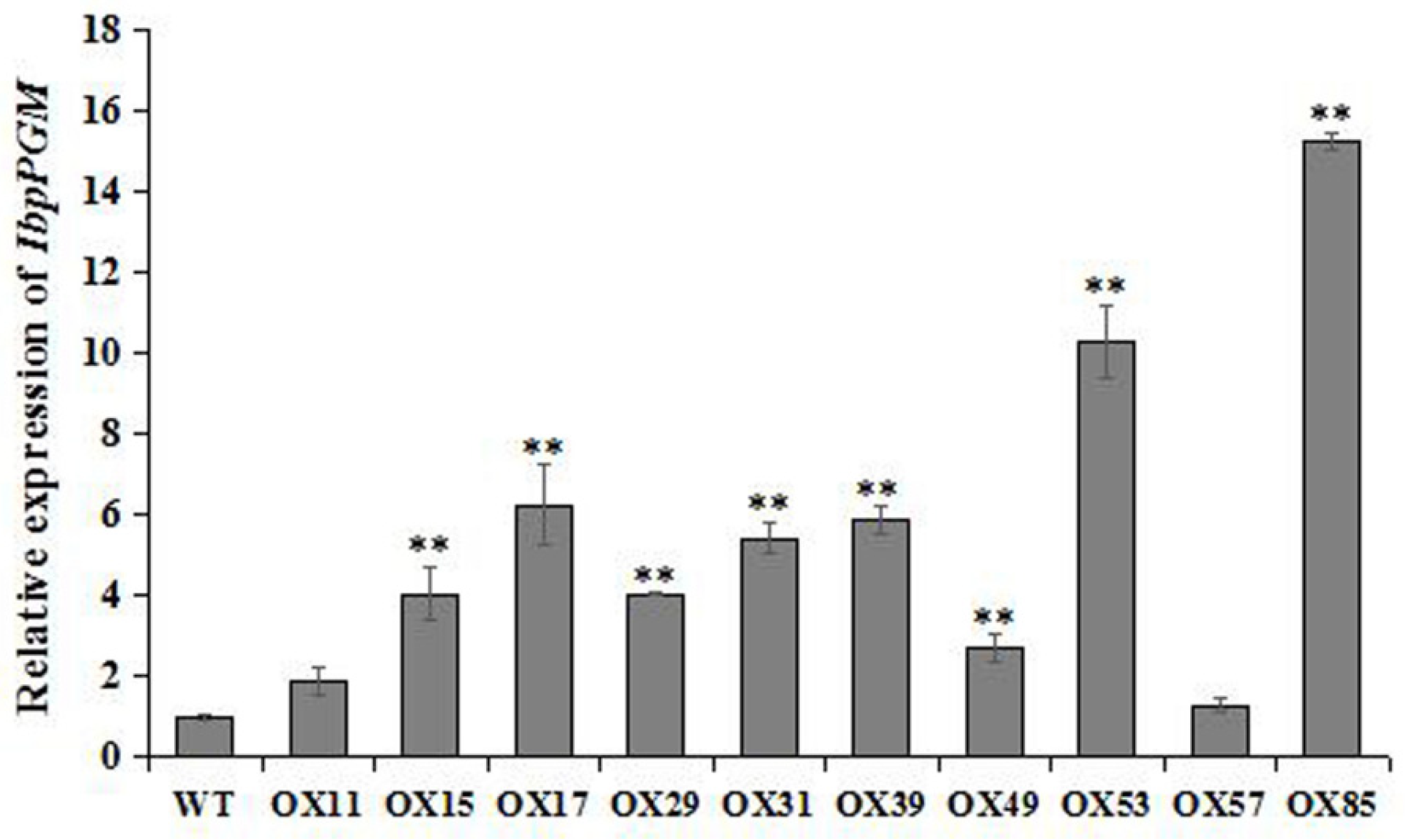
3.5. Overexpression of IbpPGM in the Sweet Potato
3.6. Starch and Sugar Contents in the Transgenic Sweet Potato
3.7. Expression Profiles of the Starch Biosynthetic Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeeman, S.C.; Kossmann, J.; Smith, A.M. Starch: Its metabolism, evolution, and biotechnological modification in plants. Annu. Rev. Plant Biol. 2010, 61, 209–234. [Google Scholar] [CrossRef] [PubMed]
- Bahaji, A.; Baroja-Fernández, E.; Sánchez-Lόpez, A.M.; Muñoz, F.J.; Li, J.; Almagro, G.; Montero, M.; Pujol, P.; Galarza, R.; Kaneko, K.; et al. HPLC-MS/MS analyses show that the near-starchless aps1 and pgm leaves accumulate wild type levels of ADPglucose: Further evidence for the occurrence of important ADPglucose biosynthetic pathway(s) alternative to the pPGI-pPGM-AGP pathway. PLoS ONE 2014, 9, e104997. [Google Scholar] [CrossRef] [PubMed]
- Streb, S.; Egli, B.; Eicke, S.; Zeeman, S.C. The debate on the pathway of starch synthesis: A closer look at low-starch mutants lacking plastidial phosphoglucomutase supports the chloroplast-localized pathway. Plant Physiol. 2009, 151, 1769–1772. [Google Scholar] [CrossRef]
- Uematsu, K.; Suzuki, N.; Iwamae, T.; Inui, M.; Yukawa, H. Expression of Arabidopsis plastidial phosphoglucomutase in tobacco stimulates photosynthetic carbon flow into starch synthesis. J. Plant Physiol. 2012, 169, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Egli, B.; Kölling, K.; Köhler, C.; Zeeman, S.C.; Streb, S. Loss of cytosolic phosphoglucomutase compromises gametophyte development in Arabidopsis. Plant Physiol. 2010, 154, 1659–1671. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Rees, T.A.P. Carbohydrate breakdown by chloroplasts of Pisum sativum. Biochim. Biophys. Acta 1980, 627, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Heldt, H.W. Physiological rates of starch breakdown in isolated intact spinach chloroplasts. Plant Physiol. 1981, 68, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Malinova, I.; Kunz, H.-H.; Alseekh, S.; Herbst, K.; Fernie, A.R.; Gierth, M.; Fettke, J. Reduction of the cytosolic phosphoglucomutase in Arabidopsis reveals impact on plant growth, seed and root development, and carbohydrate partitioning. PLoS ONE 2014, 9, e112468. [Google Scholar] [CrossRef]
- Mühlbach, H.; Schnarrenberger, C. Properties and intracellular distribution of two phosphoglucomutases from spinach leaves. Planta 1978, 141, 65–70. [Google Scholar] [CrossRef]
- Harrison, C.J.; Mould, R.M.; Leech, M.J.; Johnson, S.A.; Turner, L.; Schreck, S.L.; Baird, K.M.; Jack, P.L.; Rawsthorne, S.; Hedley, C.L.; et al. The rug3 locus of pea encodes plastidial phosphoglucomutase. Plant Physiol. 2000, 122, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Lytovchenko, A.; Fernie, A.R. Photosynthetic metabolism is severely impaired on the parallel reduction of plastidial and cytosolic isoforms of phosphoglucomutase. Plant Physiol. Biochem. 2003, 41, 193–200. [Google Scholar] [CrossRef]
- Caspar, T.; Huber, S.C.; Somerville, C. Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiol. 1985, 79, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.R.; McHale, N.A. A starchless mutant of Nicotiana sylvestris containing a modified plastid phosphoglucomutase. Plant Physiol. 1988, 88, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, S.; Lee, C.-H.K.; VanWinkle, P.; Bailey-Serres, J. Molecular and biochemical characterization of cytosolic phosphoglucomutase in maize. Expression during Development and in Response to Oxygen Deprivation. Plant Physiol. 1998, 117, 997–1006. [Google Scholar] [CrossRef]
- Kofler, H.; Häusler, R.E.; Schulz, B.; Gröner, F.; Flügge, U.-I.; Weber, A. Molecular characterization of a new mutant allele of the plastid phosphoglucomutase in Arabidopsis, and complementation of the mutant with the wild-type cDNA. Mol. Gen. Genet. MGG 2000, 263, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.J.; Hedley, C.L.; Wang, T.L. Evidence that the rug3 locus of pea (Pisum sativum L.) encodes plastidial phosphoglucomutase confirms that the imported substrate for starch synthesis in pea amyloplasts is glucose-6-phosphate. Plant J. 1998, 13, 753–762. [Google Scholar] [CrossRef]
- Lytovchenko, A.; Bieberich, K.; Willmitzer, L.; Fernie, A.R. Carbon assimilation and metabolism in potato leaves deficient in plastidial phosphoglucomutase. Planta 2002, 215, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Lytovchenko, A.; Sweetlove, L.; Pauly, M.; Fernie, A.R. The influence of cytosolic phosphoglucomutase on photosynthetic carbohydrate metabolism. Planta 2002, 215, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-K.; Eom, J.-S.; Hwang, S.-K.; Shin, D.J.; An, G.; Okita, T.W.; Jeon, J.-S. Plastidic phosphoglucomutase and ADP-glucose pyrophosphorylase mutants impair starch synthesis in rice pollen grains and cause male sterility. J. Exp. Bot. 2016, 67, 5557–5569. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Z.; Fox, T.W.; Trimnell, M.R.; Wang, L.J.; Xu, R.-J.; Cigan, A.M.; Huffman, G.A.; Garnaat, C.W.; Hershey, H.; Albertsen, M.C. Development of a novel recessive genetic male sterility system for hybrid seed production in maize and other cross-pollinating crops. Plant Biotechnol. J. 2016, 14, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Chamusco, K.C.; Chourey, P.S. Starch biosynthesis during pollen maturation is associated with altered patterns of gene expression in maize. Plant Physiol. 2002, 130, 1645–1656. [Google Scholar] [CrossRef] [PubMed]
- Baroja-Fernández, E.; Muñoz, F.J.; Zandueta-Criado, A.; Morán-Zorzano, M.T.; Viale, A.M.; Alonso-Casajús, N.; Pozueta-Romero, J. Most of ADP·glucose linked to starch biosynthesis occurs outside the chloroplast in source leaves. Proc. Natl. Acad. Sci. USA 2004, 101, 13080–13085. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, F.J.; Baroja-Fernández, E.; Morán-Zorzano, M.T.; Viale, A.M.; Etxeberria, E.; Alonso-Casajús, N.; Pozueta-Romero, J. Sucrose synthase controls both intracellular ADP glucose levels and transitory starch biosynthesis in source leaves. Plant Cell Physiol. 2005, 46, 1366–1376. [Google Scholar] [CrossRef] [PubMed]
- Tauberger, E.; Fernie, A.R.; Emmermann, M.; Renz, A.; Kossmann, J.; Willmitzer, L.; Trethewey, R.N. Antisense inhibition of plastidial phosphoglucomutase provides compelling evidence that potato tuber amyloplasts import carbon from the cytosol in the form of glucose-6-phosphate. Plant J. 2000, 23, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Swiedrych, A.; Tauberger, E.; Lytovchenko, A.; Trethewey, R.N.; Willmitzer, L. Potato plants exhibiting combined antisense repression of cytosolic and plastidial isoforms of phosphoglucomutase surprisingly approximate wild type with respect to the rate of starch synthesis. Plant Physiol. Biochem. 2002, 40, 921–927. [Google Scholar] [CrossRef]
- Bae, J.M.; Liu, J.R. Molecular cloning and characterization of two novel isoforms of the small subunit of ADPglucose pyrophosphorylase from sweet potato. Mol. Gen. Genet. 1997, 254, 179–185. [Google Scholar] [CrossRef]
- Wang, S.-J.; Yeh, K.-W.; Tsai, C.-Y. Molecular characterization and expression of starch granule-bound starch synthase in the sink and source tissues of sweet potato. Physiol. Plant. 1999, 106, 253–261. [Google Scholar] [CrossRef]
- Kim, S.-H.; Hamada, T.; Otani, M.; Shimada, T. Cloning and characterization of sweetpotato isoamylase gene (IbIsa1) isolated from tuberous root. Breed. Sci. 2005, 55, 453–458. [Google Scholar] [CrossRef][Green Version]
- Kitahara, K.; Hamasuna, K.; Nozuma, K.; Otani, M.; Hamada, T.; Shimada, T.; Fujita, K.; Suganuma, T. Physicochemical properties of amylose-free and high-amylose starches from transgenic sweetpotatoes modified by RNA interference. Carbohydr. Polym. 2007, 69, 233–240. [Google Scholar] [CrossRef]
- Takahata, Y.; Tanaka, M.; Otani, M.; Katayama, K.; Kitahara, K.; Nakayachi, O.; Nakayama, H.; Yoshinaga, M. Inhibition of the expression of the starch synthase II gene leads to lower pasting temperature in sweetpotato starch. Plant Cell Rep. 2010, 29, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, K.; Takahata, Y.; Otani, M.; Tanaka, M.; Katayama, K.; Yoshinaga, M.; Fujita, K.; Suganuma, T. Starch properties of transgenic sweetpotato plants modified by RNA interference of the starch synthase II gene. J. Appl. Glycosci. 2011, 58, 85–90. [Google Scholar] [CrossRef]
- Wang, Y.N.; Li, Y.; Zhang, H.; Zhai, H.; Liu, Q.C.; He, S.Z. A plastidic ATP/ADP transporter gene, IbAATP, increases starch and amylose contents and alters starch structure in transgenic sweetpotato. J. Integr. Agri. 2016, 15, 1968–1982. [Google Scholar] [CrossRef]
- Wang, Y.N.; Li, Y.; Zhang, H.; Zhai, H.; Liu, Q.C.; He, S.Z. A soluble starch synthase I gene, IbSSI, alters the content, composition, granule size and structure of starch in transgenic sweet potato. Sci. Rep. 2017, 7, 2315. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.C.; Zhai, H.; Wang, Y.; Zhang, D.P. Efficient plant regeneration from embryogenic suspension cultures of sweetpotato. In Vitro Cell. Dev. Biol.-Plant 2001, 37, 564–567. [Google Scholar] [CrossRef]
- Smith, A.M.; Zeeman, S.C. Quantification of starch in plant tissues. Nat. Protoc. 2006, 1, 1342–1345. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.T.; He, S.Z.; Zhao, N.; Zhai, H.; Liu, Q.C. A sucrose non-fermenting-1-related protein kinase-1 gene, IbSnRK1, improves starch content, composition, granule size, degree of crystallinity and gelatinization in transgenic sweet potato. Plant Biotechnol. J. 2018, 17, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Koßmann, J.; Visser, R.G.F.; Müller-Röber, B.; Willmitzer, L.; Sonnewald, U. Cloning and expression analysis of a potato cDNA that encodes branching enzyme: Evidence for co-expression of starch biosynthetic genes. Mol. Gen. Genet. 1991, 230, 39–44. [Google Scholar] [CrossRef]
- Rook, F.; Corke, F.; Card, R.; Munz, G.; Smith, C.; Bevan, M.W. Impaired sucrose-induction mutants reveal the modulation of sugar-induced starch biosynthetic gene expression by abscisic acid signaling. Plant J. 2001, 26, 421–433. [Google Scholar] [CrossRef]
- Wang, S.-J.; Yeh, K.-W.; Tsai, C.-Y. Regulation of starch granule-bound starch synthase I gene expression by circadian clock and sucrose in the source tissue of sweet potato. Plant Sci. 2001, 161, 635–644. [Google Scholar] [CrossRef]
- Kammerer, B.; Fischer, K.; Hilpert, B.; Schubert, S.; Gutensohn, M.; Weber, A.; Flügge, U.-I. Molecular characterization of a carbon transporter in plastids from heterotrophic tissues: The glucose 6-phosphate/phosphate antiporter. Plant Cell 1998, 10, 105–117. [Google Scholar] [CrossRef]

| Name | Sequence (5′-3′) | Name | Sequence (5′-3′) |
|---|---|---|---|
| DF | AGCCAYAAYCCWGGTGGWCC | DR | CCRTAWGTVGCCCARTRCT |
| GF | ACACAACACAACACACACTCTTCT | GR | GCGCAGACAAAATTATACAGATT |
| qPGM-F | TCAAGCACTCAAGATTAAATCGGTT | qPGM-R | ATCACCTCCGAGAACCAACAAC |
| Actin-F | AGCAGCATGAAGATTAAGGTTGTAGCAC | Actin-R | TGGAAAATTAGAAGCACTTCCTGTGAAC |
| pET-F (Nco I) | CATGCCATGGGGGCGTCGTTTTGTGCG | pET-R (Xho I) | CCCTCGAGTGTTATGACAGTTGGCTTCTC |
| pET-ΔF (Nco I) | CATGCCATGGGGGCTACCGTCGCCGAATC | ||
| 83-F (Pac I) | CCTTAATTAAATGGCGTCGTTTTGTGC | 83-R (Asc I) | AGGCGCGCCATGTTATGACAGTTGGCTTCTCTCT |
| qIbAGP-sTL1-F | AGAGAATTGACGGTGATGTTAGCA | qIbAGP-sTL1-R | ATGAACGGAGCAGTCCGAAC |
| qIbAGP-sTL2-F | CCAAAAGGAGAACAGTTGAAAGCTA | qIbAGP-sTL2-R | CTCCAGGGAACTTTTCTCGAAGTA |
| qIbAGP-TLI-F | GAGATATCCCACATCCAACGACTT | qIbAGP-TLI-R | TAGGGCCAAGTTAGCGTCGTAG |
| qIbGBSSI-F | TGGCAACTATAACTGCCTCACAC | qIbGBSSI-R | GGCACTGGTTCTCAATTGTAACAT |
| qIbSSI-F | GCTGCAGACCGTCTTTGTGC | qIbSSI-R | GAGCCATCCCTCTGTGCTCC |
| qIbSSII-F | AGACTGTGGGATCTACTGAAAGGC | qIbSSII-R | GTGAATCCACGTCCAGTGGC |
| qIbSSIII-F | TCTGTTATCCTGAGGAGGTAAAACC | qIbSSIII-R | CTCCCATGATCAATACATCAGGC |
| qIbSSIV-F | CTGCTTTCTCATTTCTGTCATCGT | qIbSSIV-R | GCTCAACTTCCACTTGACTCAGAG |
| qIbSBEI-F | ATTCTTGGCCTAGACCAAGGG | qIbSBEI-R | ACAATGCAGCCTTCTTCTTTGTTA |
| qIbSBEII-F | AGTCCGCTGTTTGGAGGCTT | qIbSBEII-R | CCTCAACTGGTTTTGCTTCGTC |
| qIbISA1-F | GGAACGAGGTGGTTATCGGTG | qIbISA1-R | TCTGGGCATAGCAACAGAATTATG |
| qIbPUL-F | GCTGCTCGACGATGCCTCT | qIbPUL-R | CATCCTCAACGTCCACATTCC |
| Lines | Starch (% of DW) | Sucrose (mg 100 g−1 DW) | Glucose (mg 100 g−1 DW) | Fructose (mg 100 g−1 DW) |
|---|---|---|---|---|
| WT | 58.07 + 0.32 | 15.14 + 0.11 | 0.39 + 0.05 | 0.18 + 0.03 |
| OX17 | 60.69 + 1.69 * | 14.19 + 0.09 ** | 1.22 + 0.02 ** | 0.63 + 0.01 ** |
| OX53 | 61.25 + 1.75 * | 13.71 + 0.13 ** | 0.81 + 0.03 ** | 1.39 + 0.02 ** |
| OX85 | 65.05 + 0.97 ** | 11.95 + 0.04 ** | 0.57 + 0.01 ** | 0.24 + 0.02 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, H.; Li, Y.; Zhang, Q.; Liu, Q.; Zhai, H.; Zhao, N.; Yang, Y.; He, S. Plastidial Phosphoglucomutase (pPGM) Overexpression Increases the Starch Content of Transgenic Sweet Potato Storage Roots. Genes 2022, 13, 2234. https://doi.org/10.3390/genes13122234
Wang Y, Zhang H, Li Y, Zhang Q, Liu Q, Zhai H, Zhao N, Yang Y, He S. Plastidial Phosphoglucomutase (pPGM) Overexpression Increases the Starch Content of Transgenic Sweet Potato Storage Roots. Genes. 2022; 13(12):2234. https://doi.org/10.3390/genes13122234
Chicago/Turabian StyleWang, Yannan, Huan Zhang, Yan Li, Qian Zhang, Qingchang Liu, Hong Zhai, Ning Zhao, Yufeng Yang, and Shaozhen He. 2022. "Plastidial Phosphoglucomutase (pPGM) Overexpression Increases the Starch Content of Transgenic Sweet Potato Storage Roots" Genes 13, no. 12: 2234. https://doi.org/10.3390/genes13122234
APA StyleWang, Y., Zhang, H., Li, Y., Zhang, Q., Liu, Q., Zhai, H., Zhao, N., Yang, Y., & He, S. (2022). Plastidial Phosphoglucomutase (pPGM) Overexpression Increases the Starch Content of Transgenic Sweet Potato Storage Roots. Genes, 13(12), 2234. https://doi.org/10.3390/genes13122234





