ABCG2 Polymorphisms and Predictive Fluoroquinolone Phototoxicity in Nondomestic Felids
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Ethics Statement, and Collection of DNA
2.2. Targeted Genotyping
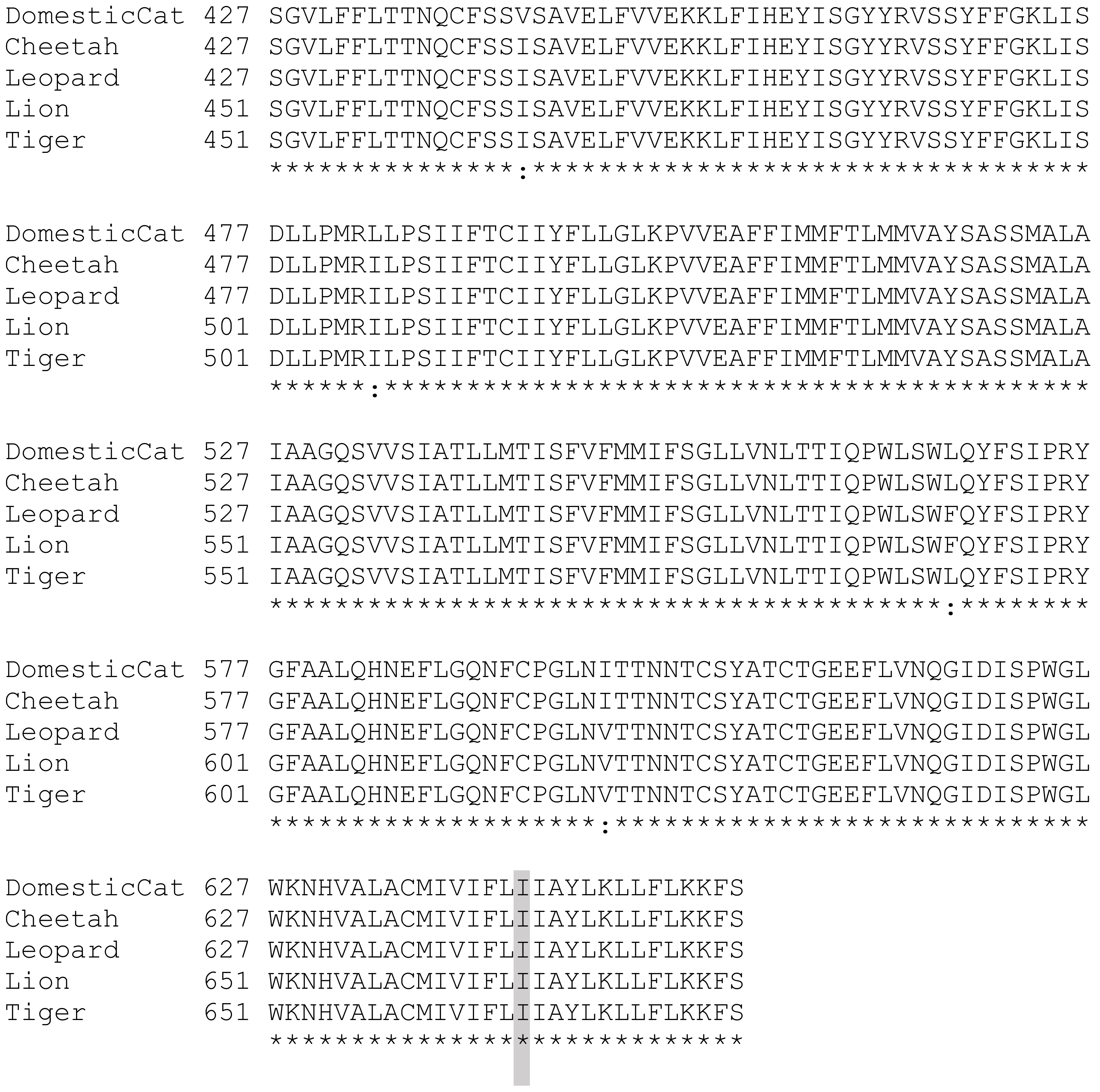
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


| Species | Scientific Name | Assembly Number | Assembly Name | Assembly Level |
|---|---|---|---|---|
| Asian leopard cat | P. bengalensis | GCF_016509475.1 | Fcat_Pben_1.1_paternal_pri | Chromosome |
| Black-footed cat | F. nigripes | GCA_004023925.1 | FelNig_v1_BIUU | Scaffold |
| Caracal | C. caracal | GCA_016801355.1 | CarCar1.0 | Scaffold |
| Cheetah | A. jubatus | GCF_003709585.1 | Aci_jub_2 | Scaffold |
| Cougar | P. concolor | GCF_003327715.1 | PumCon1.0 | Scaffold |
| Fishing cat | P. viverrinus | GCF_022837055.1 | UM_Priviv_1.0 | Chromosome |
| Jaguarundi | P. yagouaroundi | GCF_014898765.1 | PumYag | Scaffold |
| Jungle cat | F. chaus | GCA_019924945.1 | FelChav1.0 | Chromosome |
| Leopard | P. pardus | GCF_001857705.1 | PanPar1.0 | Scaffold |
| Lion | P. leo | GCA_018350215.1 | P.leo_Ple1_pat1.1 | Chromosome |
| Snow leopard | P. uncia | GCF_023721935.1 | Puncia_PCG_1.0 | Chromosome |
| Tiger | P. tigris | GCF_018350195.1 | P.tigris_Pti1_mat1.1 | Chromosome |
References
- WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance. Critically Important Antibacterial Agents for Human Medicine for Risk Management Strategies of Non-Human Use, 5th ed.; World Health Organization: Geneva, Switzerland, 2005; pp. 1–8.
- Plumb’s Veterinary Drugs. Available online: https://www.plumbsveterinarydrugs.com/#!/monograph/ajastQOmuU/ (accessed on 10 September 2022).
- Sheld, W.M. Maintaining Fluoroquinolone Class Efficacy: Review of Influencing Factors. Emerg. Infect. Dis. 2003, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of Quinolone Action and Resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Gelatt, K.N.; van der Woerdt, A.; Ketring, K.L.; Andrew, S.E.; Brooks, D.E.; Biros, D.J.; Denis, H.M.; Cutler, T.J. Enrofloxacin-Associated Retinal Degeneration in Cats. Vet. Ophthalmol. 2001, 4, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, V.; Hamilton, P. Fluoroquinolone-Induced Retinal Degeneration in Cats. J. Am. Vet. 2002, 221, 1568–1571. [Google Scholar] [CrossRef] [PubMed]
- Ford, M.; Dubielzig, R.; Giuliano, E.; Moore, C.; Narfstrom, K. Ocular and Systemic Manifestations After Oral Administration of a High Dose of Enrofloxacin in Cats. Am. J. Vet. Res. 2007, 68, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, K.; Yoshida, M.; Wagai, N.; Takayama, S.; Kato, M. Phototoxic Lesions Induced by Quinolone Antibacterial Agents in Auricular Skin and Retina of Albino Mice. Toxicol. Pathol. 1993, 21, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.I.; Pérez, M.; Prieto, J.G.; Molina, A.J.; Real, R.; Merino, G. Fluoroquinolone Efflux Mediated by ABC Transporters. J Pharm. Sci. 2008, 97, 3483–3493. [Google Scholar] [CrossRef] [PubMed]
- Mealey, K.L. (Ed.) Pharmacotherapeutics for Veterinary Dispensing, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 90–99. [Google Scholar]
- Ramirez, C.J.; Minch, J.D.; Gay, J.M.; Lahmers, S.M.; Guerra, D.J.; Haldorson, G.J.; Schneider, T.; Mealey, K.L. Molecular Genetic Basis for Fluoroquinolone-Induced Retinal Degeneration in Cats. Pharmacogenet. Genom. 2011, 21, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Mealey, K.L.; Waiting, D.; Raunig, D.L.; Schmidt, K.R.; Nelson, F.R. Oral Bioavailability of P-glycoprotein Substrate Drugs do not Differ Between ABCB1-1Δ and ABCB1 Wild Type Dogs. J. Vet. Pharmacol. Ther. 2010, 33, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.; Zhang, J.-T. Human ABCG2: Structure, Function, and its Role in Multidrug Resistance. Int. J. Biochem. Mol. Biol. 2012, 3, 1–27. [Google Scholar] [PubMed]
- Mealey, K.L.; Bentjen, S.A.; Gay, J.M.; Cantor, G.H. Ivermectin Sensitivity in Collies is Associated with a Deletion Mutation of the MDR1 Gene. Pharmacogenetics 2001, 11, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Mealey, K.L. ABCG2 Transporter: Therapeutic and Physiologic Implications in Veterinary Species. J. Vet. Pharmacol. Ther. 2012, 35, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Stafford, E.G.; Harrison, T.; Kortum, A.; Mealey, K.L. Prevalence of the ABCB1-1Δ Gene in Nondomestic Species of the Canidae Family. J. Zoo Wildl. Med. 2021, 51, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Newkirk, K.M.; Beard, L.K.; Sun, X.; Ramsay, E.C. Investigation of Enrofloxacin-Associated Retinal Toxicity in Nondomestic Felids. J. Zoo Wildl. Med. 2017, 48, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A. Zoo and Wild Mammal Formulary, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 169–194. [Google Scholar]
- Di Tommaso, P.; Moretti, S.; Xenarios, I.; Orobitg, M.; Montanyola, A.; Chang, J.; Taly, J.; Notredame, C. T-Coffee: A Web Server for the Multiple Sequence Alignment of Protein and RNA Sequences Using Structural Information and Homology Extension. Nucleic Acids Res. 2011, 39, W13–W17. [Google Scholar] [CrossRef] [PubMed]
- Notredame, C.; Higgins, D.G.; Heringa, J. T-Coffee: A Novel Method for Fast and Accurate Multiple Sequence Alignment. J. Mol. Biol. 2000, 302, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, K.; Robey, R.W.; Özvegy-Laczka, C.; Honjo, Y.; Polgar, O.; Steadman, K.; Sarkadi, B.; Bates, S.E. Single Nucleotide Polymorphisms Modify the Transporter Activity of ABCG2. Cancer Chemother. Pharmacol. 2005, 56, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Zoetis: Zeniquin Package Insert. Available online: https://www2.zoetisus.com/content/_assets/docs/vmips/package-inserts/zeniquin.pdf (accessed on 10 September 2022).
- Lees, P. Pharmacokinetics, Pharmacodynamics and Therapeutics of Pradofloxacin in the Dog and Cat. J. Vet. Pharmacol. Ther. 2013, 36, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Freedom of Information Summary NADA 141-081 Orbifloxacin Tablets. Available online: https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/615 (accessed on 10 September 2022).
- Kay-Mugford, P.; Ramsey, D.; Dublielzig, R.; Tuomari, D.; Turck, P. Ocular Effects of Orally Administered Orbifloxacin in Cats. In Proceedings of the American College of Veterinary Ophthalmologists 32nd Annual Meeting, Sarasota, FL, USA, 9–13 October 2001. [Google Scholar]
| Species | Animals |
|---|---|
| Asian leopard cat (Prionailurus bengalensis) | 1 |
| Black-footed cat (Felis nigripes) | 1 |
| Caracal (Caracal caracal) | 1 |
| Cheetah (Acinonyx jubatus) | 1 |
| Clouded leopard (Neofelis nebulosi) | 1 |
| Cougar (Puma concolor) | 4 |
| Fishing cat (Prionailurus viverrinus) | 1 |
| Asian golden cat (Catopuma temminckii) | 1 |
| Jaguarundi (Herpailurus yagouaroundi) | 1 |
| Jungle cat (Felis chaus) | 1 |
| Leopard (Panthera pardus) | 1 |
| Lion (Panthera leo) | 2 |
| Marbled cat (Pardofelis marmorata) | 1 |
| Margay (Leopardus wiedii) | 1 |
| Pallas’s cat (Otocolobus manul) | 1 |
| Serval (Leptailurus serval) | 1 |
| Snow leopard (Panthera uncia) | 1 |
| Tiger (Panthera tigris) | 10 |
| Tigrina (Oncilla) (Leopardus tigrinus) | 1 |
| Total | 32 |
| Variant | Primer | Sequence | Product Size (BP) |
|---|---|---|---|
| E159M | SNP1F | ATGTCGTGATGGGCACTCTG | 156 |
| SNP1R | CACATTACCTTGGAATCCGCC | ||
| S279L | SNP2F | TCTGAACAGGGACGAACAATCA | 221 |
| SNP2R | TCCTATAGACCCCTTTTACTCTCT | ||
| H283Q | SNP3F | TCACCTCACTCACCCCTGTA | 190 |
| SNP3R | TCATACCTTCACCTGCCTGG | ||
| T664I | SNP4F | TGTTGAATTTCAGATGTACTGGCG | 193 |
| SNP4R | TGTGCACATAAATCATGTAAATTCAAG |
| Species | E159M AA a = (ATG) | S279L AA = (TTA) | H283Q AA = (CAG) | T664I AA = (ATA) |
|---|---|---|---|---|
| Asian leopard cat | X b | X | # c | X |
| Black-footed cat | X | X | X | X |
| Caracal | X | X | # c | X |
| Cheetah | X | X | X | X |
| Clouded leopard | X | X | X | X |
| Cougar (n = 4) | X | X | X | X |
| Fishing cat | X | X | # c | X |
| Asian golden cat | X | X | X | X |
| Jaguarundi | X | X | X | X |
| Jungle cat | X | X | X | X |
| Leopard | ATA (=I) | X | X | X |
| Lion (n = 2) | ATA (=I) | X | X | X |
| Marbled cat | X | X | X | X |
| Margay | X | X | X | ACA (=T) |
| Pallas’s cat | X | X | ? d | X |
| Serval | ATA (=I) | X | X | X |
| Snow leopard | ATA (=I) | X | X | X |
| Tiger (n = 10) | ATA (=I) | X | X | X |
| Tigrina | X | X | ? d | X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gochenauer, A.E.; Dreger, D.L.; Davis, B.W.; Cook, S.; Barber, K.E.; Ekenstedt, K.J. ABCG2 Polymorphisms and Predictive Fluoroquinolone Phototoxicity in Nondomestic Felids. Genes 2022, 13, 2178. https://doi.org/10.3390/genes13122178
Gochenauer AE, Dreger DL, Davis BW, Cook S, Barber KE, Ekenstedt KJ. ABCG2 Polymorphisms and Predictive Fluoroquinolone Phototoxicity in Nondomestic Felids. Genes. 2022; 13(12):2178. https://doi.org/10.3390/genes13122178
Chicago/Turabian StyleGochenauer, Alexandria E., Dayna L. Dreger, Brian W. Davis, Shawna Cook, Katie E. Barber, and Kari J. Ekenstedt. 2022. "ABCG2 Polymorphisms and Predictive Fluoroquinolone Phototoxicity in Nondomestic Felids" Genes 13, no. 12: 2178. https://doi.org/10.3390/genes13122178
APA StyleGochenauer, A. E., Dreger, D. L., Davis, B. W., Cook, S., Barber, K. E., & Ekenstedt, K. J. (2022). ABCG2 Polymorphisms and Predictive Fluoroquinolone Phototoxicity in Nondomestic Felids. Genes, 13(12), 2178. https://doi.org/10.3390/genes13122178






