Pituitary-Gland-Based Genes Participates in Intrauterine Growth Restriction in Piglets
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sampling
2.2. RNA Extraction and Purification
2.3. Library Construction and Sequencing
2.4. Quality Control of Sequencing Data and Comparison with Reference Genomes
2.5. Differential Gene Expression Analysis
2.6. Enrichment Analysis of DEGs
2.7. Detection of Gene Expression
2.8. Statistical Analysis
3. Results
3.1. Statistical Analysis
3.2. Pituitary Transcriptome Sequencing and Reads Mapping
3.3. Expression Characterization in Pituitary Transcriptome
3.4. Functional Annotation and Pathway Enrichment Analysis of DEGs
3.5. PPI Network of the DEGs
3.6. Validation of RNA-seq Results Using RT–PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albu, A.R.; Anca, A.F.; Horhoianu, V.V.; Horhoianu, I.A. Predictive factors for intrauterine growth restriction. J. Med. Life 2014, 7, 165–171. [Google Scholar] [PubMed]
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. Board-invited review: Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, Y.; Feng, C.; Lin, G.; Wu, G.; Li, D.; Wang, J. Innate differences and colostrum-induced alterations of jejunal mucosal proteins in piglets with intra-uterine growth restriction. Br. J. Nutr. 2018, 119, 734–747. [Google Scholar] [CrossRef]
- Sharma, D.; Shastri, S.; Farahbakhsh, N.; Sharma, P. Intrauterine growth restriction-part 1. J. Matern. Neonatal Med. 2016, 29, 3977–3987. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Song, Y.; Tian, Y.; Zhang, H.; Yu, B.; He, J.; Mao, X.; Yu, J.; Luo, Y.; Luo, J.; et al. Dietary Arginine Supplementation Affects Intestinal Function by Enhancing Antioxidant Capacity of a Nitric Oxide-Independent Pathway in Low-Birth-Weight Piglets. J. Nutr. 2018, 148, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, B.; Lv, J.; Dong, L.; Zhang, L.; Wang, T. Choline supplementation improves the lipid metabolism of intrauterine-growth-restricted pigs. Asian-Australas J. Anim. Sci. 2018, 31, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Bai, K.; Wang, A.; Ge, X.; Zhao, Y.; Zhang, L.; Wang, T. Effects of dimethylglycine sodium salt supplementation on growth performance, hepatic antioxidant capacity, and mitochondria-related gene expression in weanling piglets born with low birth weight1. J. Anim. Sci. 2018, 96, 3791–3803. [Google Scholar] [CrossRef] [PubMed]
- Redmer, D.A.; Wallace, J.M.; Reynolds, L.P. Effect of nutrient intake during pregnancy on fetal and placental growth and vascular development. Domest. Anim. Endocrinol. 2004, 27, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Lefort, G.; Servien, R.; Quesnel, H.; Billon, Y.; Canario, L.; Iannuccelli, N.; Canlet, C.; Paris, A.; Vialaneix, N.; Liaubet, L. The maturity in fetal pigs using a multi-fluid metabolomic approach. Sci. Rep. 2020, 10, 19912. [Google Scholar] [CrossRef]
- Zhao, Y.; Albrecht, E.; Sciascia, Q.L.; Li, Z.; Görs, S.; Schregel, J.; Metges, C.C.; Maak, S. Effects of Oral Glutamine Supplementation on Early Postnatal Muscle Morphology in Low and Normal Birth Weight Piglets. Animals 2020, 10, 1976. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Tan, B.; Wang, J.; Liao, S.; Li, J.; Liu, Y.; Yin, Y. Post-natal Growth Retardation Associated With Impaired Gut Hormone Profiles, Immune and Antioxidant Function in Pigs. Front. Endocrinol. 2019, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Amdi, C.; Lynegaard, J.C.; Thymann, T.; Williams, A.R. Intrauterine growth restriction in piglets alters blood cell counts and impairs cytokine responses in peripheral mononuclear cells 24 days post-partum. Sci. Rep. 2020, 10, 4683. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.W.; Ellsworth, B.S.; Peréz Millan, M.I.; Gergics, P.; Schade, V.; Foyouzi, N.; Brinkmeier, M.L.; Mortensen, A.H.; Camper, S.A. Pituitary gland development and disease: From stem cell to hormone production. Curr. Top. Dev. Biol. 2013, 106, 1–47. [Google Scholar] [PubMed]
- Ding, Y.X.; Cui, H. The brain development of infants with intrauterine growth restriction: Role of glucocorticoids. Horm. Mol. Biol. Clin. Investig. 2019, 39. [Google Scholar] [CrossRef]
- Wang, T.; Liu, C.; Feng, C.; Wang, X.; Lin, G.; Zhu, Y.; Yin, J.; Li, D.; Wang, J. IUGR alters muscle fiber development and proteome in fetal pigs. Front. Biosci. (Landmark Ed.) 2013, 18, 598–607. [Google Scholar] [CrossRef]
- Reynolds, L.P.; Caton, J.S.; Redmer, D.A.; Grazul-Bilska, A.T.; Vonnahme, K.A.; Borowicz, P.P.; Luther, J.S.; Wallace, J.M.; Wu, G.; Spencer, T.E. Evidence for altered placental blood flow and vascularity in compromised pregnancies. J. Physiol. 2006, 572 Pt 1, 51–58. [Google Scholar] [CrossRef]
- Hu, C.; Yang, Y.; Deng, M.; Yang, L.; Shu, G.; Jiang, Q.; Zhang, S.; Li, X.; Yin, Y.; Tan, C.; et al. Placentae for Low Birth Weight Piglets Are Vulnerable to Oxidative Stress, Mitochondrial Dysfunction, and Impaired Angiogenesis. Oxid. Med. Cell Longev. 2020, 2020, 8715412. [Google Scholar] [CrossRef]
- Owens, N.D.L.; De Domenico, E.; Gilchrist, M.J. An RNA-Seq Protocol for Differential Expression Analysis. Cold Spring Harb. Protoc. 2019, 6, 098368. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Scully, K.M.; Rosenfeld, M.G. Pituitary development: Regulatory codes in mammalian organogenesis. Science 2002, 295, 2231–2235. [Google Scholar] [CrossRef] [PubMed]
- Khuwaja, G.; Al-Bratty, M.; Alhazmi, H.A.; Khan, A.; Safhi, M.M.; Ashafaq, M.; Islam, F.; Islam, F.; Taha, M.M. Pharmacological melioration by Selenium on the toxicity of tellurium in neuroendocrine centre (Pituitary Gland) in male wistar rats: A mechanistic approach. Saudi Pharm J. 2020, 28, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ma, J.; Wang, Z.; Yao, X.; Zhao, J.; Zhao, X.; Wang, F.; Zhang, Y. Genome-Wide Analysis and Function Prediction of Long Noncoding RNAs in Sheep Pituitary Gland Associated with Sexual Maturation. Genes 2020, 11, 320. [Google Scholar] [CrossRef]
- Cho, W.K.; Suh, B.K. Catch-up growth and catch-up fat in children born small for gestational age. Korean J. Pediatr. 2016, 59, 1–7. [Google Scholar] [CrossRef] [PubMed]
- McHugh, B.J.; Buttery, R.; Lad, Y.; Banks, S.; Haslett, C.; Sethi, T. Integrin activation by Fam38A uses a novel mechanism of R-Ras targeting to the endoplasmic reticulum. J. Cell Sci. 2010, 123 Pt 1, 51–61. [Google Scholar] [CrossRef] [PubMed]
- McCarty, J.H. αvβ8 integrin adhesion and signaling pathways in development, physiology and disease. J. Cell Sci. 2020, 133, jcs239434. [Google Scholar] [CrossRef]
- Daval, M.; Gurlo, T.; Costes, S.; Huang, C.J.; Butler, P.C. Cyclin-dependent kinase 5 promotes pancreatic β-cell survival via Fak-Akt signaling pathways. Diabetes 2011, 60, 1186–1197. [Google Scholar] [CrossRef]
- Boutahar, N.; Guignandon, A.; Vico, L.; Lafage-Proust, M.H. Mechanical strain on osteoblasts activates autophosphorylation of focal adhesion kinase and proline-rich tyrosine kinase 2 tyrosine sites involved in ERK activation. J. Biol. Chem. 2004, 279, 30588–30599. [Google Scholar] [CrossRef]
- Cromi, A.; Ghezzi, F.; Raffaelli, R.; Bergamini, V.; Siesto, G.; Bolis, P. Ultrasonographic measurement of thymus size in IUGR fetuses: A marker of the fetal immunoendocrine response to malnutrition. Ultrasound Obstet. Gynecol. 2009, 33, 421–426. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, L.; Wang, L.; Liu, X.; Hou, X.; Zhao, F.; Yan, H.; Wang, L. Liver transcriptome profiling and functional analysis of intrauterine growth restriction (IUGR) piglets reveals a genetic correction and sexual-dimorphic gene expression during postnatal development. BMC Genom. 2020, 21, 701. [Google Scholar] [CrossRef]
- Meng, X.; Yang, D.; Yu, R.; Zhu, H. EPSTI1 Is Involved in IL-28A-Mediated Inhibition of HCV Infection. Mediat. Inflamm. 2015, 2015, 716315. [Google Scholar] [CrossRef] [PubMed]
- de Neergaard, M.; Kim, J.; Villadsen, R.; Fridriksdottir, A.J.; Rank, F.; Timmermans-Wielenga, V.; Langerød, A.; Børresen-Dale, A.L.; Petersen, O.W.; Rønnov-Jessen, L. Epithelial-stromal interaction 1 (EPSTI1) substitutes for peritumoral fibroblasts in the tumor microenvironment. Am. J. Pathol. 2010, 176, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Liu, C.; Feng, C.; Fan, Z.; Dai, Z.; Lai, C.; Li, Z.; Wu, G.; Wang, J. Metabolomic analysis reveals differences in umbilical vein plasma metabolites between normal and growth-restricted fetal pigs during late gestation. J. Nutr. 2012, 142, 990–998. [Google Scholar] [CrossRef]
- Krauss, S.; Zhang, C.Y.; Scorrano, L.; Dalgaard, L.T.; St-Pierre, J.; Grey, S.T.; Lowell, B.B. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic beta cell dysfunction. J. Clin. Invest. 2003, 112, 1831–1842. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.H.; Li, M.L.; Li, N.; Sun, Q.; Zhou, J.; Ma, R.J.; Lin, Y.; Xie, M.; Ge, X.; Chen, L.; et al. Maternal diabetes impairs the initiation of meiosis in murine female germ cells. Mol. Med. Rep. 2017, 16, 5189–5194. [Google Scholar] [CrossRef]
- Saldivar Lemus, Y.; Vielle-Calzada, J.P.; Ritchie, M.G.; Macías Garcia, C. Asymmetric paternal effect on offspring size linked to parent-of-origin expression of an insulin-like growth factor. Ecol. Evol. 2017, 7, 4465–4474. [Google Scholar] [CrossRef]
- Liu, H.B.; Muhammad, T.; Guo, Y.; Li, M.J.; Sha, Q.Q.; Zhang, C.X.; Liu, H.; Zhao, S.G.; Zhao, H.; Zhang, H.; et al. RNA-Binding Protein IGF2BP2/IMP2 is a Critical Maternal Activator in Early Zygotic Genome Activation. Adv. Sci. 2019, 6, 1900295. [Google Scholar] [CrossRef]
- Lien, Y.C.; Wang, P.Z.; Lu, X.M.; Simmons, R.A. Altered Transcription Factor Binding and Gene Bivalency in Islets of Intrauterine Growth Retarded Rats. Cells 2020, 9, 1435. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, D.; Zhou, T.; Song, H.; Hulsurkar, M.; Su, N.; Liu, Y.; Wang, Z.; Shao, L.; Ittmann, M.; et al. Androgen deprivation promotes neuroendocrine differentiation and angiogenesis through CREB-EZH2-TSP1 pathway in prostate cancers. Nat. Commun. 2018, 9, 4080. [Google Scholar] [CrossRef]
- Yin, C.; Li, K.; Yu, Y.; Huang, H.; Yu, Y.; Wang, Z.; Yan, J.; Pu, Y.; Li, Z.; Li, D.; et al. Genome-wide association study identifies loci and candidate genes for non-idiopathic pulmonary hypertension in Eastern Chinese Han population. BMC Pulm. Med. 2018, 18, 158. [Google Scholar] [CrossRef]
- Lu, J.; Xu, D.; Jiang, Y.; Kong, S.; Shen, Z.; Xia, S.; Lu, L. Integrated analysis of mRNA and viral miRNAs in the kidney of Carassius auratus gibelio response to cyprinid herpesvirus 2. Sci. Rep. 2017, 7, 13787. [Google Scholar] [CrossRef] [PubMed]
- Malek, M.H.; Olfert, I.M. Global deletion of thrombospondin-1 increases cardiac and skeletal muscle capillarity and exercise capacity in mice. Exp. Physiol. 2009, 94, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Poon, R.T.; Chung, K.K.; Cheung, S.T.; Lau, C.P.; Tong, S.W.; Leung, K.L.; Yu, W.C.; Tuszynski, G.P.; Fan, S.T. Clinical significance of thrombospondin 1 expression in hepatocellular carcinoma. Clin. Cancer Res. 2004, 10 Pt 1, 4150–4157. [Google Scholar] [CrossRef] [PubMed]
- Leow, M.K.S.; Dogra, S.; Ge, X.; Chuah, K.L.; Liew, H.; Loke, K.S.H.; McFarlane, C. Paraneoplastic Secretion of Multiple Phosphatonins From a Deep Fibrous Histiocytoma Causing Oncogenic Osteomalacia. J. Clin. Endocrinol. Metab. 2021, 106, e2299–e2308. [Google Scholar] [CrossRef]
- Sabatakos, G.; Sims, N.A.; Chen, J.; Aoki, K.; Kelz, M.B.; Amling, M.; Bouali, Y.; Mukhopadhyay, K.; Ford, K.; Nestler, E.J.; et al. Overexpression of DeltaFosB transcription factor(s) increases bone formation and inhibits adipogenesis. Nat. Med. 2000, 6, 985–990. [Google Scholar] [CrossRef]
- Eagle, A.L.; Williams, E.S.; Beatty, J.A.; Cox, C.L.; Robison, A.J. ΔFosB Decreases Excitability of Dorsal Hippocampal CA1 Neurons. eNeuro 2018, 5. [Google Scholar] [CrossRef]
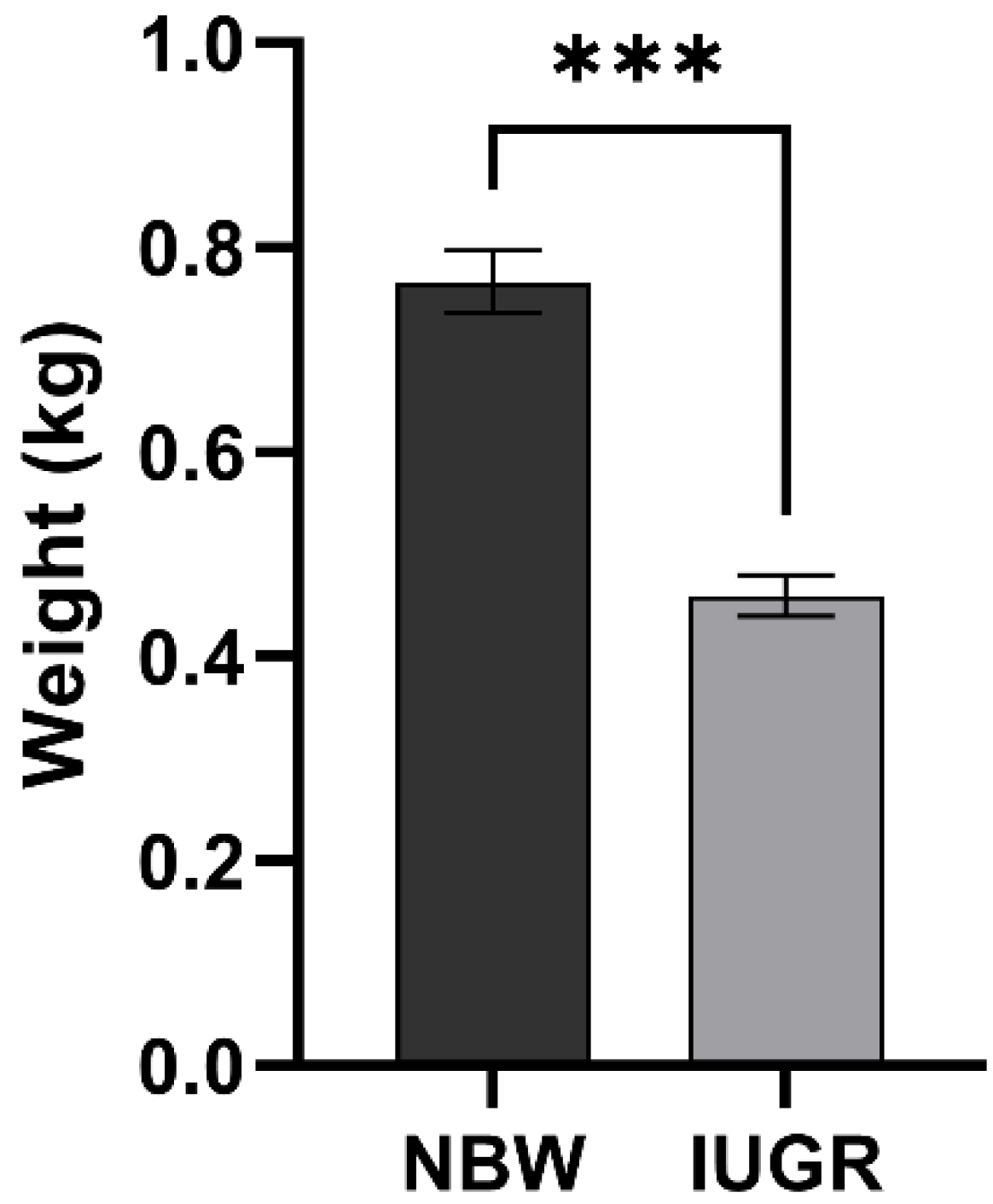
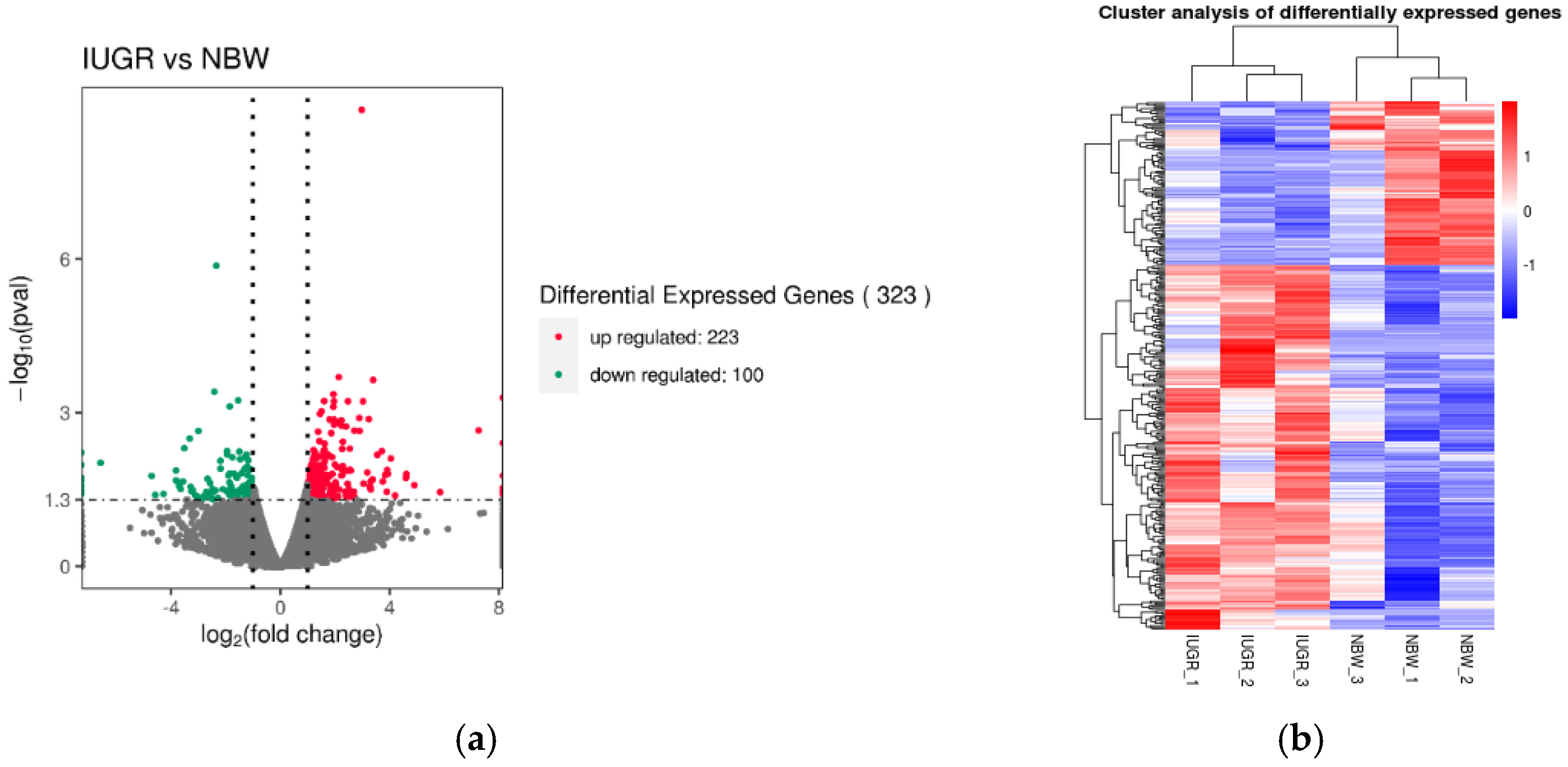

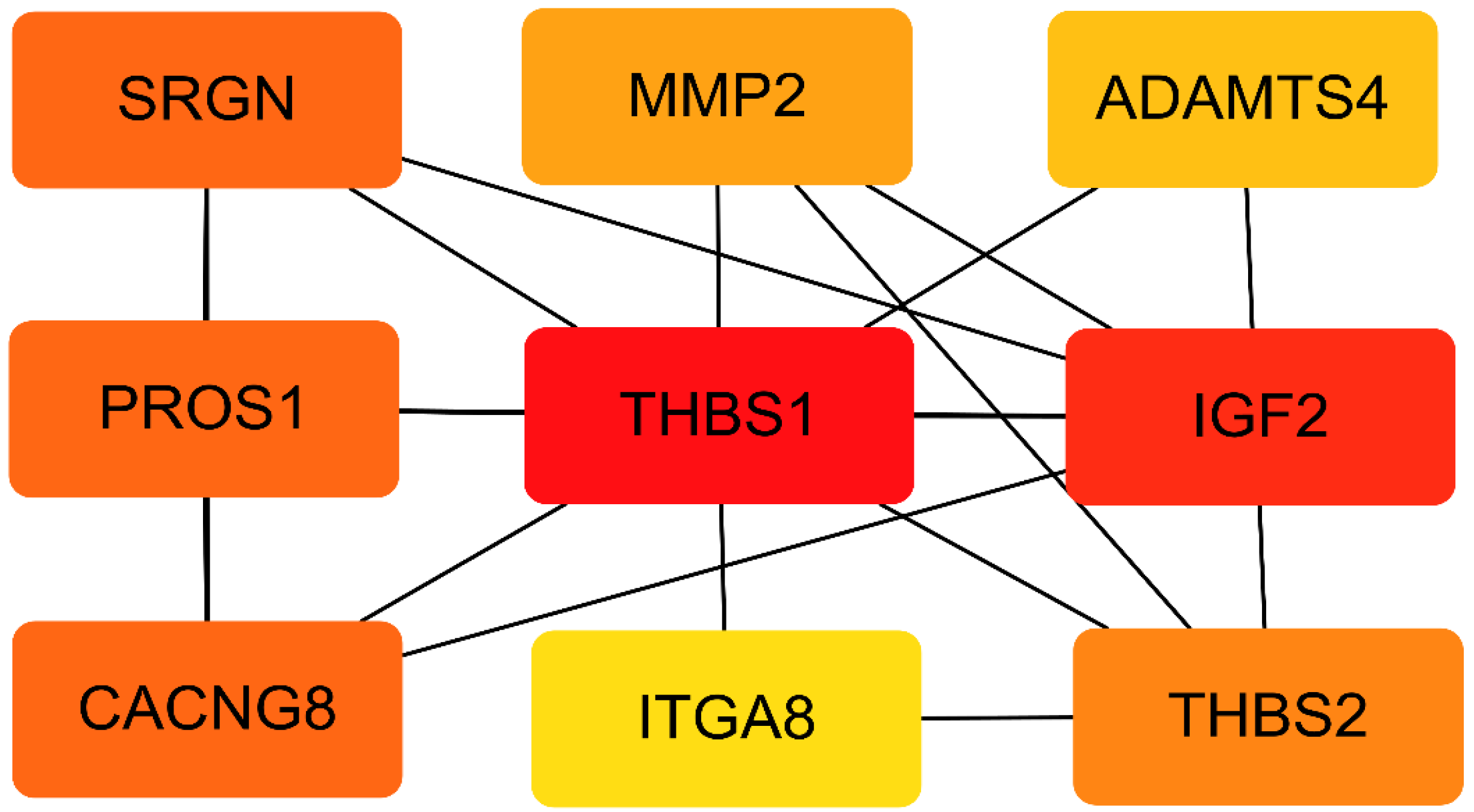

| Group | Sample ID | Body Weight (kg) | Mean of Litter Weight (kg) | SD | Number of Litter | Number of Alive Litter |
|---|---|---|---|---|---|---|
| NBW | NBW1 | 0.76 | 0.68 | 0.09 | 12 | 10 |
| NBW2 | 0.80 | 0.69 | 0.10 | 14 | 11 | |
| NBW3 | 0.74 | 0.67 | 0.09 | 11 | 9 | |
| IUGR | IUGR1 | 0.44 | 0.69 | 0.09 | 12 | 11 |
| IUGR2 | 0.48 | 0.70 | 0.10 | 10 | 9 | |
| IUGR3 | 0.46 | 0.68 | 0.08 | 13 | 10 |
| Sample Name | Raw Reads | Clean Reads | Total Mapped | Multiple Mapped | Uniquely Mapped | Q30 (%) |
|---|---|---|---|---|---|---|
| IUGR1 | 49,413,660 | 49,049,218 | 42,890,986 | 567,530 | 42,323,456 | 93.77 |
| IUGR2 | 55,880,062 | 55,364,908 | 46,921,638 | 679,036 | 46,242,602 | 93.67 |
| IUGR3 | 52,301,590 | 51,877,426 | 45,486,562 | 644,576 | 44,841,986 | 93.75 |
| NBW1 | 54,197,428 | 53,609,282 | 47,021,570 | 700,368 | 46,321,202 | 94.50 |
| NBW2 | 54,158,458 | 53,521,394 | 47,632,404 | 643,780 | 46,988,624 | 93.97 |
| NBW3 | 56,278,682 | 55,660,888 | 47,666,648 | 751,188 | 46,915,460 | 94.46 |
| Gene ID | Gene Name | Log2 Fold-Change | p-Value |
|---|---|---|---|
| ENSSSCG00000007805 | ATP2A1 | −3.32 | 0.00319 |
| ENSSSCG00000025535 | TFAP2B | −2.19 | 0.00869 |
| ENSSSCG00000001036 | TFAP2A | −1.95 | 0.00654 |
| ENSSSCG00000014833 | UCP2 | −1.54 | 0.00058 |
| ENSSSCG00000036814 | CLEC11A | −1.50 | 0.00576 |
| ENSSSCG00000010727 | GPR26 | 1.51 | 0.00094 |
| ENSSSCG00000004464 | ITGA1 | 1.61 | 0.00060 |
| ENSSSCG00000011973 | COL8A1 | 1.94 | 0.00043 |
| ENSSSCG00000005498 | PAPPA | 1.94 | 0.00076 |
| ENSSSCG00000029311 | MYPN | 2.23 | 0.00141 |
| ENSSSCG00000015268 | FMO1 | 2.25 | 0.00181 |
| ENSSSCG00000024514 | SHISAL2B | 2.89 | 0.00127 |
| ENSSSCG00000031616 | FOSB | 2.98 | 1.22 × 10−9 |
| ENSSSCG00000003259 | OSCAR | 3.24 | 0.00134 |
| ENSSSCG00000037572 | EPSTI1 | 3.40 | 0.00023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, X.; Shen, Q.; Wu, P.; Chen, H.; Wang, S.; Chen, D.; Yu, Y.; Guo, Z.; Wang, J.; Tang, G. Pituitary-Gland-Based Genes Participates in Intrauterine Growth Restriction in Piglets. Genes 2022, 13, 2141. https://doi.org/10.3390/genes13112141
Ji X, Shen Q, Wu P, Chen H, Wang S, Chen D, Yu Y, Guo Z, Wang J, Tang G. Pituitary-Gland-Based Genes Participates in Intrauterine Growth Restriction in Piglets. Genes. 2022; 13(11):2141. https://doi.org/10.3390/genes13112141
Chicago/Turabian StyleJi, Xiang, Qi Shen, Pingxian Wu, Hongyue Chen, Shujie Wang, Dong Chen, Yang Yu, Zongyi Guo, Jinyong Wang, and Guoqing Tang. 2022. "Pituitary-Gland-Based Genes Participates in Intrauterine Growth Restriction in Piglets" Genes 13, no. 11: 2141. https://doi.org/10.3390/genes13112141
APA StyleJi, X., Shen, Q., Wu, P., Chen, H., Wang, S., Chen, D., Yu, Y., Guo, Z., Wang, J., & Tang, G. (2022). Pituitary-Gland-Based Genes Participates in Intrauterine Growth Restriction in Piglets. Genes, 13(11), 2141. https://doi.org/10.3390/genes13112141





