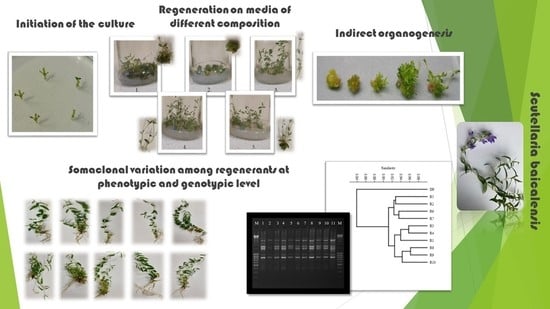
Potential of In Vitro Culture of Scutellaria baicalensis in the Formation of Genetic Variation Confirmed by ScoT Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Procedure for Obtaining Regenerants
2.1.1. Callus Induction and Shoot Regeneration
2.1.2. Shoot Multiplication of Regenerants
2.2. Acclimatization
2.3. Molecular Assays
2.3.1. DNA Extraction
2.3.2. SCoT Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Callus Induction and Shoot Regeneration

3.2. Shoot Multiplication of Regenerants
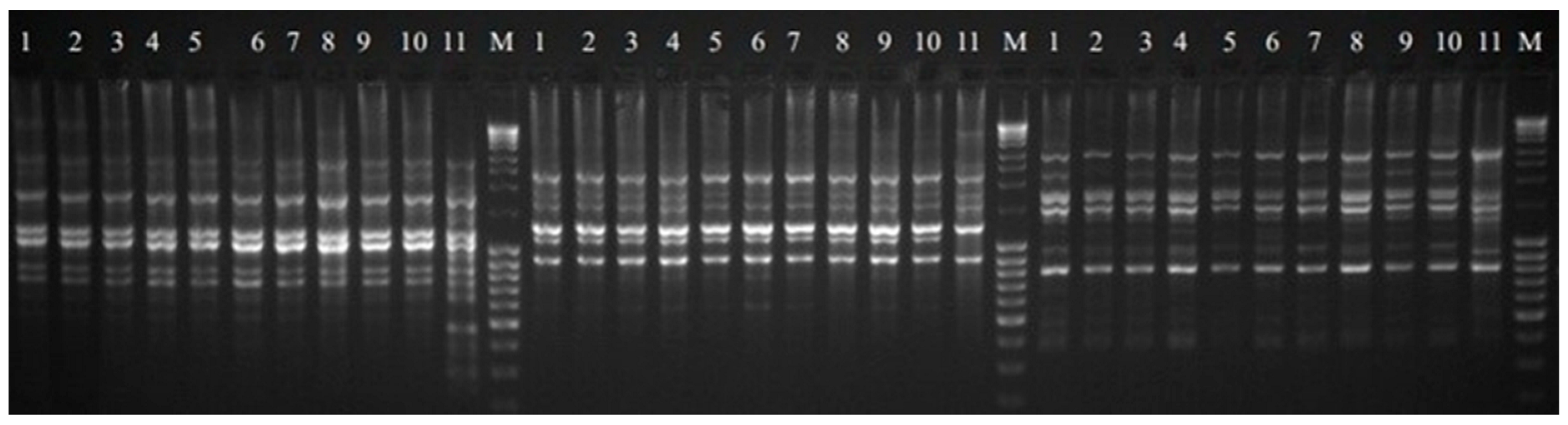
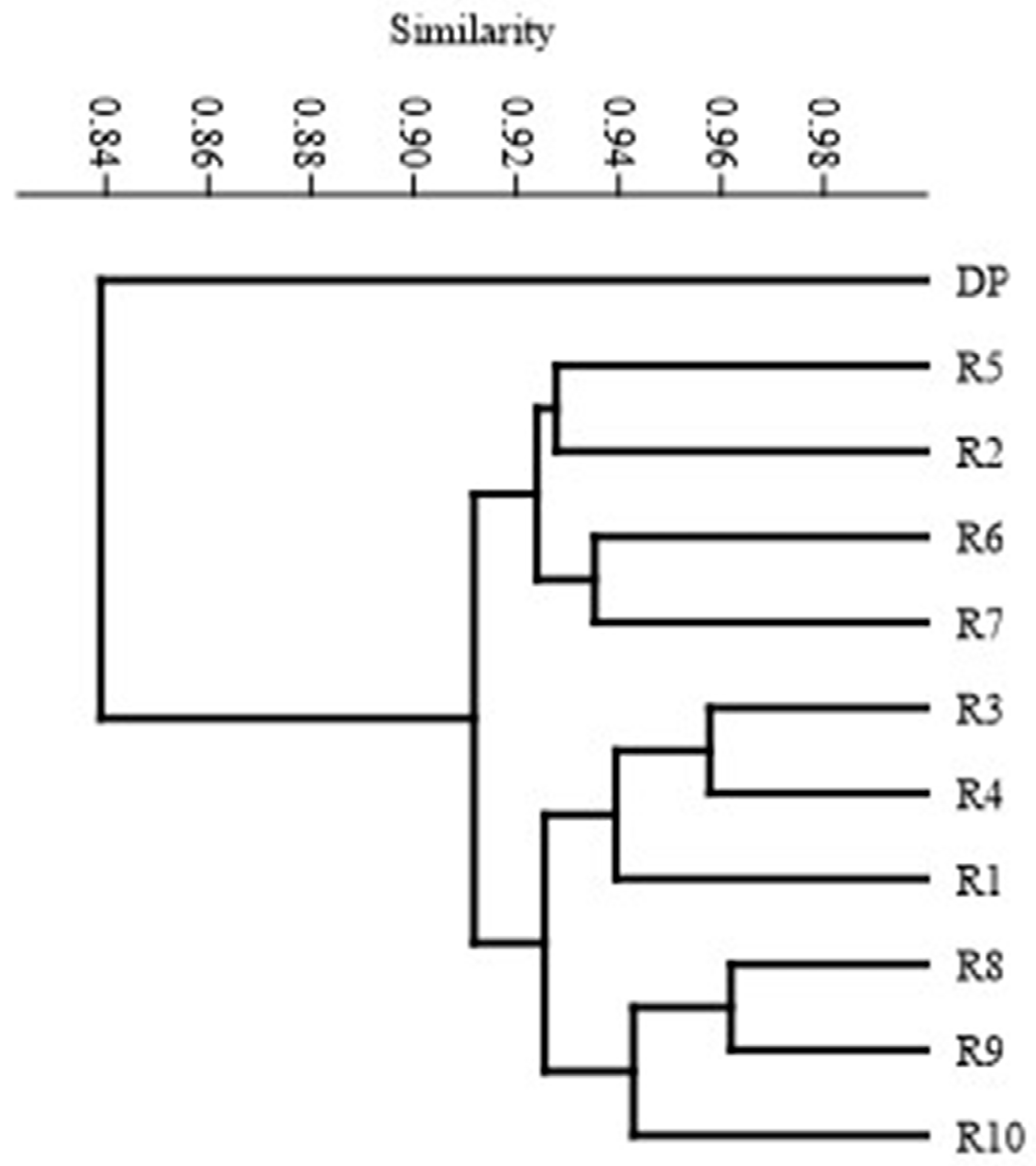
3.3. Molecular Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Z.; Gao, R.; Pu, X.; Xu, R.; Wang, J.; Zheng, S.; Zeng, Y.; Chen, J.; He, C.; Song, J. Comparative Genome Analysis of Scutellaria baicalensis and Scutellaria barbata Reveals the Evolution of Active Flavonoid Biosynthesis. Genom. Proteom. Bioinform. 2000, 18, 230–240. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Z.; He, D.; Wang, W. Pollination biology characters of Scutellaria baicalensis. Acta Hortic. Sin. 2011, 38, 2209–2214. [Google Scholar]
- Ciocarlan, N. Scutellaria baicalensis Georgi in the national botanical garden (institute), Republic of Moldova. In Main, Rare and Non-Traditional Plant Types—From Research to Development (Agricultural and Biological Sciences); National Academy of Agrarian Sciences of Ukraine: Georgia, Ukraine, 2021; Volume 4, pp. 123–127. [Google Scholar]
- Jarosławska, A.; Sokół-Łętowska, A.; Oszmiański, I.J. Use of natural polyphenols to stabilization of sunflower oil. Food 2003, 2, 77–86. [Google Scholar]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, I.X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of itstraditional uses, botany, phytochemistry, pharmacology and toxicology. JPP 2019, 71, 1353–1369. [Google Scholar] [CrossRef]
- Song, J.-W.; Long, J.-Y.; Xie, L.; Zhang, L.-L.; Xie, Q.-X.; Chen, H.-J.; Deng, M.; Li, X.-F. Applications, phytochemistry, pharmacological effects, pharmacokinetics, toxicity of Scutellaria baicalensis Georgi. and its probably potential therapeutic effects on COVID-19: A review. Chin. Med. 2020, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Krishna, H.; Alizadeh, M.; Singh, D.; Singh, U.; Chauhan, N.; Eftekhari, M.; Kishan, R. Somaclonal variations and their applications in horticultural crops improvement. 3 Biotech 2016, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Orbović, V.; Ćalović, M.; Viloria, Z.; Nielsen, B.; Gmitter, F.; Castle, W.; Grosser, J. Analysis of genetic variability in various tissue culture-derived lemon plant populations using RAPD and flow cytometry. Euphytica 2000, 161, 329–335. [Google Scholar] [CrossRef]
- Collard, B.C.Y.; Mackill, D.J. Start Codon Targeted (SCoT) Polymorphism: A Simple, Novel DNA Marker Technique for Generating Gene-Targeted Markers in Plants. Plant Mol. Biol. Rep. 2009, 27, 86–93. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F.A. Revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 9, 11–15. [Google Scholar]
- Nei, M.; Li, W. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 1979, 76, 5269–5273. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Elect. 2001, 4, 9. [Google Scholar]
- Guo, B.; Abbasi, B.H.; Zeb, A.; Xu, L.L.; Wei, Y.H. Thidiazuron: A multi-dimensional plant growth regulator. Afr. J. Biotechnol. 2011, 10, 45. [Google Scholar] [CrossRef]
- Ozdemir, F.; Yildirim, M.; Kahriz, P. Micropropagation of Endemic Scutellaria orientalis L. Subsp. Bicolor Using Modified MS Medium & TDZ. Emir. J. Food Agric. 2015, 27, 818–824. [Google Scholar] [CrossRef]
- Stojakowska, A.; Malarz, J.; Kohlmünzer, J. Micropropagation of Scutellaria baicalensis Georgi. Acta Soc. Bot. Pol. 1999, 68, 103–107. [Google Scholar] [CrossRef]
- Zhang, C.G.; Li, W.; Mao, Y.F.; Zhao, D.L.; Dong, W.; Guo, G.Q. Endogenous Hormonal Levels in Scutellaria baicalensis Calli Induced by Thidiazuron. Russ. J. Plant Physiol. 2005, 52, 345–351. [Google Scholar] [CrossRef]
- Gharari, Z.; Bagheri, K.; Sharafi, A.; Danafar, H. Thidiazuron induced efficient in vitro organogenesis and regeneration of Scutellaria bornmuelleri: An important medicinal plant. Vitr. Cell. Dev. Biol.-Plant 2019, 55, 133–138. [Google Scholar] [CrossRef]
- Gharari, Z.; Bagheri, K.; Sharafi, A. High-frequency adventitious shoot organogenesis from in vitro stem explants of Scutellaria araxensis Grossh. BioTechnologia 2022, 103, 143–151. [Google Scholar] [CrossRef]
- Hwang, I.-T.; Lee, J.-J.; Lee, J.; Paik, S.-W.; Kim, Y.-H. Production of Baicalin, Baicalein, and Wogonin on Plant Tissue Culture of Scutellaria baicalensis. Korean J. Plant Resour. 2015, 28, 526–532. [Google Scholar] [CrossRef]
- Trivedi, M.; Trivedi, R.K.; Guag, Z.C.; Guo, G.; Zheng, G. Hormone-Induced Indirect Regeneration Protocol for Scutellaria baicalensis Georgi (Huang-qin). J. Crop Improv. 2011, 25, 550–559. [Google Scholar] [CrossRef]
- Ozdemir, F.A.; Kilic, O.; Atalan, E. In vitro callus propagation and antibacterial activities of callus an edible endemic and medicinal plant Scutellaria orientalis L. subsp. bicolor. Prog. Nutr. 2016, 18, 81–86. [Google Scholar]
- Ngezahayo, F.; Liu, B.Y. Axillary Bud Proliferation Approach for Plant Biodiversity Conservation and Restoration. Int. J. Biodivers. 2014, 2014, 727025. [Google Scholar] [CrossRef]
- Zakaria, I.A.; Sherman, S.; Vaidya, B.; Joshee, N. In vitro propagation and synseed mediated short-term conservation of Scutellaria alpine L. and Scutellaria altissima L. Plant Cell Cult. Micropropag. 2020, 16, e163. [Google Scholar] [CrossRef]
- Brearley, T.; Vaidya, B.; Joshee, N. Cytokinin, Carbon Source, and Acclimatization Requirements for In Vitro Propagation of Scutellaria barbata D. Don and Scutellaria racemose Pers. Am. J. Plant Sci. 2014, 5, 3662–3672. [Google Scholar] [CrossRef]
- Dyduch-Siemińska, M. A fast and effective protocol for obtaining genetically diverse stevia (Stevia rebaudiana Bertoni) regenerants through indirect organogenesis. Agron. Sci. 2021, 76, 47–62. [Google Scholar] [CrossRef]
- Bairu, M.W.; Aremu, A.O.; Van Staden, J. Somaclonal variation in plants: Causes and detection methods. Plant Growth Regul. 2011, 63, 147–173. [Google Scholar] [CrossRef]
- Govindaraju, S.; Arulselvi, P.I. Effect of cytokinin combined elicitors (l-phenylalanine, salicylic acid and chitosan) on in vitro propagation, secondary metabolites and molecular characterization of medicinal herb—Coleus aromaticus Benth (L.). J. Saudi Soc. Agric. Sci. 2018, 17, 435–444. [Google Scholar] [CrossRef]
- Su, S.; He, C.M.; Li, L.C.; Chen, K.J.; Zhou, T.S. Genetic characterization and phytochemical analysis of wild and cultivated populations of Scutellaria baicalensis. Chem. Biodivers. 2008, 5, 1353–1363. [Google Scholar] [CrossRef]
- Hosokawa, K.; Minami, M.; Kawahara, K.; Nakamura, I.; Shibata, T. Discrimination among three species of medicinal Scutellaria plants using RAPD markers. Planta Med. 2000, 66, 270–272. [Google Scholar] [CrossRef]
- Shao, A.J.; Li, X.; Huang, L.Q.; Lin, S.F.; Chen, J. RAPD analysis of Scutellaria baicalensis from different germplasms. Zhongguo Zhong Yao Za Zhi 2006, 31, 452–455. [Google Scholar]
- Bai, C.; Wen, M.; Zhang, L.; Li, G. Genetic diversity and sampling strategy of Scutellaria baicalensis germplasm resources based on ISSR. Genet. Resour. Crop Evol. 2013, 60, 1673–1685. [Google Scholar] [CrossRef]
- Muraseva, D.S.; Guseva, A.A. ISSR primer screening for analysis of genetic diversity among Scutellaria tuvensis (Lamiaceae) populations. BIO Web Conf. 2021, 38, 00082. [Google Scholar] [CrossRef]
- Yuan, Y.; Long, P.; Jiang, C.; Li, M.; Huang, L. Development and characterization of simple sequence repeat (SSR) markers based on a full-length cDNA library of Scutellaria baicalensis. Genomics 2015, 105, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Jameel, A.; Abbasi, B.H.; Manir, F.; Naqvi, S.M.S. In vitro callogenesis and detection of somaclonal variations in Plantago ovata L. J. Crop Sci. Biotechnol. 2012, 15, 289–295. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Khadivi-Khub, A. Somaclonal variation in callus samples of Plantago major using inter-simple sequence repeat marker. Caryologia 2015, 68, 19–24. [Google Scholar] [CrossRef]
- Vitamvas, J.; Viehmannova, I.; Cepkova, P.H.; Mrhalova, H.; Eliasova, K. Assessment of somaclonal variation in indirect morphogenesis-derived plants of Arracacia xanthorrhiza. Pesqui. Agropecuária Bras. 2019, 54, e00301. [Google Scholar] [CrossRef]
- Chávez-Cortazar, A.; Mata-Rosas, M.; Oyama, K.; Samain, M.S.; Quesada, M. Induction of somatic embryogenesis and evaluation of genetic stability in regenerated plants of Magnolia dealbata. Biol. Plant. 2020, 64, 224–233. [Google Scholar] [CrossRef]
- Etminan, A.; Pour-Aboughadareh, A.; Noori, A.; Ahmadi-Rad, A.; Shooshtari, L.; Mahdavian, Z.; Yousefiazar-Khanian, M. Genetic relationships and diversity among wild Salvia accessions revealed by ISSR and SCoT markers. Biotechnol. Biotechnol. Equip. 2018, 32, 610–617. [Google Scholar] [CrossRef]
- Shahlaei, A.; Torabi, S.; Khosroshahli, M. Efficacy of SCoT and ISSR marekers in assessment of tomato (Lycopersicum esculentum Mill.) genetic diversity. Int. J. Biosci. 2014, 5, 14–22. [Google Scholar]
- Rathore, M.S.; Chikara, J.; Mastan, S.G.; Rahman, H.; Anand, K.G.V.; Shekhawat, N.S. Assessment of genetic stability and instability of tissue culture-propagated plantlets of Aloe vera L. by RAPD and ISSR markers. Appl. Biochem. Biotechnol. 2011, 165, 1356–1365. [Google Scholar] [CrossRef]
- Rahmani, M.S.; Pijut, P.M.; Shabanian, N.; Nasri, M. Genetic fidelity assessment of in vitro-regenerated plants of Albizia julibrissin using SCoT and IRAP fingerprinting. Vitr. Cell. Dev. Biol.-Plant. 2015, 51, 407–419. [Google Scholar] [CrossRef]
- Soares, D.M.M.; Sattler, M.C.; Ferreira, M.F.D.S.; Praça-Fontes, M.M. Assessment of genetic stability in three generations of in vitro propagated Jatropha curcas L. plantlets using ISSR markers. Trop. Plant Biol. 2016, 9, 229–238. [Google Scholar] [CrossRef]


| Medium Number | Plant Growth Regulator Concentration and Combination (mg × dm−3) | Mean Number of Shoot per Explant | Mean Shoot Length (cm) | Mean Number of Nodes per Shoot | Calli Formation | ||||
|---|---|---|---|---|---|---|---|---|---|
| KIN | BAP | TDZ | GA3 | 2iP | |||||
| 1. | 0.5 | 0.5 | - | - | - | 7.4 a * (I) | 1.7 b | 3.0 b | +++ |
| 2. | - | - | 0.5 | - | - | - | - | - | +++ |
| 3. | - | - | - | 0.5 | - | 2.1 b (D) | 6.3 a | 4.4 a | - |
| 4. | - | - | - | - | 0.5 | 6.0 ab (I) | 5.5 a | 3.5 ab | −/+ |
| 5. | - | - | - | 0.5 | 0.5 | 4.8 ab (I) | 4.8 a | 3.2 ab | + |
| Plant Number | Mean Number of Shoot | Mean Shoot Length | Mean Number of Nodes per Shoot | Root System Development Stage 1 |
|---|---|---|---|---|
| R1 | 3.4 bc * | 4.1 d | 5.7 de | 1 |
| R2 | 4.6 b | 6.0 c | 8.4 c | 3 |
| R3 | 2.0 c | 7.8 bc | 7.0 cd | 1 |
| R4 | 3.4 bc | 7.7 bc | 6.7 cd | 3 |
| R5 | 2.0 c | 13.3 a | 16.5 a | 2 |
| R6 | 7.4 a | 2.8 e | 4.6 e | 2 |
| R7 | 9.4 a | 3.2 de | 4.0 e | 3 |
| R8 | 8.6 a | 5.0 cd | 5.4 de | 2 |
| R9 | 2.4 bc | 8.7 b | 10.5 b | 3 |
| R10 | 3.6 bc | 8.4 b | 6.9 cd | 2 |
| Mean | 4.6 | 6.7 | 7.5 | - |
| Coefficient of variation (%) | 56.8 | 44.8 | 45.2 | - |
| Primer | Primer Sequence 5′-3′ | Number of Products | % of Polymorphism | Size Range (bp) | |||
|---|---|---|---|---|---|---|---|
| Total | Polymorphic | Specific | Monomorphic | ||||
| SCoT 4 | CAACAATGGCTACCACCT | 10 | 2 | 1 | 7 | 20.00 | 600–6000 |
| SCoT 9 | CAACAATGGCTACCAGCA | 9 | 4 | 0 | 5 | 44.44 | 1200–5800 |
| SCoT 14 | ACGACATGGCGACCACGC | 13 | 3 | 2 | 8 | 23.08 | 420–3200 |
| SCoT 19 | ACCATGGCTACCACCGGC | 15 | 5 | 2 | 8 | 33.33 | 310–9300 |
| SCoT 24 | CACCATGGCTACCACCAT | 11 | 5 | 1 | 5 | 45.45 | 520–9000 |
| SCoT 25 | ACCATGGCTACCACCGGG | 10 | 3 | 1 | 6 | 30.00 | 370–6000 |
| SCoT 31 | CCATGGCTACCACCGCCT | 12 | 5 | 0 | 7 | 41.67 | 470–8000 |
| SCoT 35 | CATGGCTACCACCGGCCC | 8 | 3 | 1 | 4 | 37.50 | 380–9800 |
| SCoT 36 | GCAACAATGGCTACCACC | 13 | 5 | 0 | 8 | 38.46 | 480–5500 |
| SCoT 46 | ACAATGGCTACCACTGAG | 15 | 4 | 1 | 10 | 26.67 | 500–8200 |
| SCoT 50 | ACAATGGCTACCACTGGG | 14 | 6 | 2 | 6 | 42.86 | 380–5000 |
| Mean per primer | 11.82 | 4.09 | 1.00 | 6.73 | 34.86 | - | |
| Total | 130 | 45 | 11 | 74 | - | 310–9800 | |
| R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 | DP | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| R1 | 1.00 | 0.86 | 0.94 | 0.94 | 0.92 | 0.90 | 0.90 | 0.92 | 0.92 | 0.92 | 0.85 |
| R2 | 1.00 | 0.90 | 0.90 | 0.93 | 0.93 | 0.92 | 0.89 | 0.89 | 0.90 | 0.83 | |
| R3 | 1.00 | 0.96 | 0.93 | 0.91 | 0.94 | 0.95 | 0.93 | 0.93 | 0.86 | ||
| R4 | 1.00 | 0.92 | 0.92 | 0.91 | 0.93 | 0.92 | 0.90 | 0.84 | |||
| R5 | 1.00 | 0.92 | 0.92 | 0.92 | 0.92 | 0.92 | 0.83 | ||||
| R6 | 1.00 | 0.94 | 0.91 | 0.91 | 0.90 | 0.83 | |||||
| R7 | 1.00 | 0.95 | 0.93 | 0.93 | 0.82 | ||||||
| R8 | 1.00 | 0.96 | 0.94 | 0.86 | |||||||
| R9 | 1.00 | 0.95 | 0.84 | ||||||||
| R10 | 1.00 | 0.83 | |||||||||
| DP | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawroński, J.; Dyduch-Siemińska, M. Potential of In Vitro Culture of Scutellaria baicalensis in the Formation of Genetic Variation Confirmed by ScoT Markers. Genes 2022, 13, 2114. https://doi.org/10.3390/genes13112114
Gawroński J, Dyduch-Siemińska M. Potential of In Vitro Culture of Scutellaria baicalensis in the Formation of Genetic Variation Confirmed by ScoT Markers. Genes. 2022; 13(11):2114. https://doi.org/10.3390/genes13112114
Chicago/Turabian StyleGawroński, Jacek, and Magdalena Dyduch-Siemińska. 2022. "Potential of In Vitro Culture of Scutellaria baicalensis in the Formation of Genetic Variation Confirmed by ScoT Markers" Genes 13, no. 11: 2114. https://doi.org/10.3390/genes13112114
APA StyleGawroński, J., & Dyduch-Siemińska, M. (2022). Potential of In Vitro Culture of Scutellaria baicalensis in the Formation of Genetic Variation Confirmed by ScoT Markers. Genes, 13(11), 2114. https://doi.org/10.3390/genes13112114