Abstract
Sucrose non-fermentation-related protein kinase 1 (SnRK1) a Ser/Thr protein kinase, is known to play a crucial role in plants during biotic and abiotic stress responses by activating protein phosphorylation pathways. SnRK1 and some members of the plant-specific SnRK2 and SnRK3 sub-families have been studied in different plant species. However, a comprehensive study of the SnRK gene family in Phaseolus vulgaris is not available. Symbiotic associations of P. vulgaris with Rhizobium and/or mycorrhizae are crucial for the growth and productivity of the crop. In the present study, we identified PvSnRK genes and analysed their expression in response to the presence of the symbiont. A total of 42 PvSnRK genes were identified in P. vulgaris and annotated by comparing their sequence homology to Arabidopsis SnRK genes. Phylogenetic analysis classified the three sub-families into individual clades, and PvSnRK3 was subdivided into two groups. Chromosome localization analysis showed an uneven distribution of PvSnRK genes on 10 of the 11 chromosomes. Gene structural analysis revealed great variation in intron number in the PvSnRK3 sub-family, and motif composition is specific and highly conserved in each sub-family of PvSnRKs. Analysis of cis-acting elements suggested that PvSnRK genes respond to hormones, symbiosis and other abiotic stresses. Furthermore, expression data from databases and transcriptomic analyses revealed differential expression patterns for PvSnRK genes under symbiotic conditions. Finally, an in situ gene interaction network of the PvSnRK gene family with symbiosis-related genes showed direct and indirect interactions. Taken together, the present study contributes fundamental information for a better understanding of the role of the PvSnRK gene family not only in symbiosis but also in other biotic and abiotic interactions in P. vulgaris.
1. Introduction
Sucrose nonfermenting 1 (SNF-1)-related protein kinase (SnRK1), animal homologue AMP-activated protein kinase (AMPK) and yeast homologue Sucrose Non-Fermenting1 kinase/plant SNF1-related kinase1 (SNF1) are highly conserved serine/threonine protein kinases that function as cellular energy sensors activating catabolism and repress anabolic processes [1]. These protein kinases function as heterotrimeric complexes, with α, β and γ subunits carrying out catalytic and regulatory functions [2,3,4]. All SnRK proteins have a conserved N-terminal kinase domain and variable regulatory C-terminal domain, which mediates interaction between the β and γ subunits [1]. The prerequisite for AMPK/SNF1/SnRK1 functioning as kinases is phosphorylation of the T-loop at the N-terminal kinase domain [5,6,7].
SnRKs in plants are classified into SnRK1, SnRK2 and SnRK3 sub-families based on sequence similarity and structural organization [1,8]. SnRK1 is the homologue of AMPK, and SNF1, SnRK2 and SnRK3 are unique to plants and evolved from the SnRK1 family via gene duplication during plant evolution, and play a key role in the stress, calcium and ABA signalling pathways with epigenetic and metabolic responses [8].
The SnRK1 sub-family consists of an N-terminal kinase domain, a ubiquitin-associated (UBA) domain and a C-terminal kinase-associated 1 (KA1) domain [7]. The SnRK1 gene family is a comparatively smaller sub-family primarily found to be core sensors of energy deficit in plants. The mode of regulation is found to be through downstream phosphorylation and inhibition of the enzymes HMG-CoA reductase (HMGR), sucrose phosphate synthase (SPS), nitrate reductase (NR) and seaweed phosphate synthase 5 (TPS5) [9,10,11] involved in metabolic regulation and plant developmental processes. SnRK1 genes are pivotal in various signalling pathways, such as meristem development, cell cycle regulation and pathogen responses [12].
The SnRK2 sub-family harbours the regulatory C-terminal domain containing acidic amino acids, either Glu or Asp [7]. The regulatory role of the SnRK2 sub-family involves activation of basic region-leucine zipper (bZIP) transcription factors in association with an epigenetic mechanism [13,14]. The SnRK2 sub-family has been well studied in plants for its involvement in abiotic stress, either ABA mediated or ABA independent. The SnRK2 sub-family in Arabidopsis is classified into three groups: ABA dependent, drought responsive and ABA activated [15,16]. In Arabidopsis and rice, the SnRK2 sub-family is larger than that of SnRK1, with 10 members found to be abiotic stress responsive and activated by ABA and to phosphorylate transcription factors of the ABA-response element-binding protein class (AREBP) [17,18,19,20,21].
The SnRK3 sub-family, also named CIPKs (CBL-interacting protein kinases), contains two conserved domains at the C-terminus, including NAF (named with conserved amino acids N, A and F) and PPI (protein–protein interacting) domains [1]. The SnRK3 sub-family is the largest in all plants studied thus far; these proteins transport Na+, K+ and NO3 ions, along with their binding ability to Ca2+-dependent CBL to regulate downstream genes and enhance abiotic stress tolerance [22,23,24]. SnRK3 responds to abiotic stress factors such as salinity stress, as reported in rice, Arabidopsis, and tomato [25,26,27]. The other important mechanism of the SOS (salt overly sensitive) system is provided by SOS2/AtCIPK24 (salt overly sensitive 2), which is a member of the SnRK3 sub-family in Arabidopsis thaliana. As a Na+/H+ antiporter, it improves plant salt tolerance by maintaining ionic homeostasis [28,29]. Taken together, SnRKs represent one of the larger gene families in plants involved in a variety of regulatory mechanisms including metabolism, growth, development, and abiotic stress response.
Due to the varied regulatory roles played by SnRK genes, the recent years have seen extensive genome-wide analysis in various plant species. Starting form Arabidopsis [1] to economically important crops such as Brassica napus [30], Cucumis sativus [31], Eucalyptus grandis [32], Fragaria ananassa [33], Triticum aestivum [34], Hordeum vulgare [35], Oryza sativa [17] and Brachypodium distachyon [36]. Each of these analyses has revealed similar classification of the SnRK gene family and the highly conserved nature of gene structure and function. Curiously, these studies do not involve any legumes.
Among flowering plants, legumes are the most important agricultural crops after cereals due to their high economic value. Legumes play a central role at the food system level, both for human and animal consumption, as a source of plant proteins [37]. Furthermore, at the cropping system level, legumes are used as diversification crops in agroecosystems to break the cycles of pests and diseases and contribute to balancing the deficit in plant protein production; also at the cropping system level, legumes contribute to low-input cropping systems due to their ability to fix atmospheric nitrogen [38,39,40,41]. Legumes are distinct in their ability to establish a symbiotic interaction between nitrogen-fixing rhizobia and nutrient-transporter mycorrhizae. Although legumes are of such high economic value, some, such as P. vulgaris, are not considered subjects in the exploration of gene families.
A better understanding of the signalling pathways that affect the productivity and sustainability of such crop systems is particularly important. A gene family such as SnRK with diverse regulatory mechanisms might play a pivotal role in biotic and abiotic interactions of legumes, and greater knowledge of these aspects may help in crop improvement. In the present study, we sought to identify and understand the diversity of the SnRK gene family in P. vulgaris and elucidate their differential expression patterns under rhizobial and mycorrhizal symbiotic conditions.
2. Materials and Methods
2.1. Identification and Alignment of PvSnRK Family Genes in P. vulgaris
The amino acid and nucleotide sequences of the A. thaliana SnRK gene family were downloaded from NCBI (https://www.ncbi.nlm.nih.gov/, accessed on 14 July 2021). Homologues of AtSnRKs were searched in the Phytozome (https://phytozome.jgi.doe.gov, accessed on 14 July 2021) and Legume Information System (https://legumeinfo.org, accessed on 25 July 2021) databases using BLASTN and BLASTP methods. The respective nucleic acid and peptide sequences were downloaded from the online tool PhytoMine from the plant comparative genomics portal Phytozome v12.1 for further analysis and annotation. The hidden Markov model (HMM) and BLASTP program were applied for preliminary identification of PvSnRK proteins. Local BLASTP (E-value-20) searches were performed based on hidden Markov model (HMM) profiles of PvSnRK gene domains from the Pfam database (http://pfam.janelia.org/, accessed on 18 August 2021). The SMART database (http://smart.embl-heidelberg.de/, accessed on 18 August 2021) was used to confirm PvSnRK gene sequences [42], as were the Pfam database [43] and the NCBI Conserved Domain database [44].
MEME and motif discovery tools were employed to filter out PvSnRK homologues based on domain structure [45]. The chromosomal localization of PvSnRK gene family members was verified in the Phytozome v12.1 database, chromosomal images were drawn using the EnsemblPlant tool [46], and the scale was determined based on Wang et al. (2016) [47]. The number of amino acids, molecular weights (MWs) and isoelectric points (pIs) of PvSnRK proteins were calculated using tools from ExPASy (http://www.expasy.ch/tools/pi_tool.html, accessed on 2 September 2021). Subcellular localization of PvSnRK proteins were predicted by ProtCompv.9.0 (http://linux1.softberry.com/berry.phtml, accessed on 10 September 2021), BaCelLo (http://gpcr.biocomp.unibo.it/bacello/pred.htm, accessed on 10 September 2021) [48], LocTree3 (https://rostlab.org/services/loctree3/, accessed on 10 September 2021) [49], WoLF PSORT (https://wolfpsort.hgc.jp/, accessed on 10 September 2021) [50] and Cello (http://cello.life.nctu.edu.tw/, accessed on 10 September 2021) [51].
2.2. Phylogenetic Analysis of PvSnRK Family Genes
Multiple sequence alignment of PvSnRKs and AtSnRKs was performed using ClustalW. Based on the alignments, phylogenetic analysis of the aligned sequences was carried out using Molecular Evolutionary Genetics Analysis (MEGA XI) with the neighbour-joining (NJ) method and the JTT + I + G substitution model with 1000 bootstrap replicates and default parameters [52].
Conserved motifs in the PvSnRK gene family in P. vulgaris were identified using the Multiple Expectation Maximization for Motif Elicitation (MEME) online program (http://meme.sdsc.edu/meme/itro.html, accessed on 2 October 2021) with the following parameters: number of repetitions = any, maximum number of motifs = 20; and optimum motif length = 6 to 100 residues. The gene structure of the PvSnRK gene family was analysed using the Gene Structure Display Server online program (GSDS: http://gsds.cbi.pku.edu.ch, accessed on 10 October 2021) [53].
Sequences 2 kb upstream of PvSnRKs were downloaded from the Phytozome database. The plant transcriptional regulatory map (http://plantregmap.gao-lab.org/, accessed on 25 October 2021) was used to analyse the promoter sequences.
2.3. Calculation of Ka/Ks and Dating of Duplication Events
To identify putative orthologues between two different species (A and B), each sequence from species A was searched against all sequences from species B using BLASTN; each sequence from species B was also searched against all sequences from species A. The two sequences were defined as orthologues when reciprocal best hits were each within ≥300 bp of the two aligned sequences. The duplication period (Million Years ago, MYA) and divergence of each PvSnRK gene were calculated using the following formula: T = Ks/2λ (λ = 6.56 × 10−9) [54]. The calculation of Ka and Ks was performed using TBtools. Graphs were developed with R studio with the R commander package [55].
2.4. Protein Interaction Network and Gene Ontology Analysis
A high-expression candidate gene protein interaction network was analysed using STRING v11.0 (https://stringpreview. org/, accessed on 15 March 2022) and visualized using Cytoscape v3.9.1 (http://www.cytoscape.org/download.php, accessed on 21 March 2022). Finally, enrichment analysis of Gene Ontology (GO) terms was performed using the online tool AgriGO (v2.0) (http://systemsbiology.cau.edu.cn/agriGOv2/, accessed on 6 April 2022) [56].
2.5. Transcriptome Profiling and RT–qPCR Analysis
Data on differential expression of SnRK genes in P. vulgaris tissues under nitrogen treatments and after inoculation with Rhizobium tropici (CIAT899) were obtained from the PvGEA website (https://plantgrn.noble.org/PvGEA/, accessed on 12 January 2022). Previously, we performed global transcriptome profiling in P. vulgaris L. cv. Negro Jamapa roots colonized by Rhizophagus irregularis spores or Rhizobium tropici strain CIAT899 [57]. The present study uses the same transcriptomic data to obtain expression profiles of PvSnRK family genes under both types of symbiotic conditions. Heatmaps were constructed with fold-change values applying the R package (https://www.r-project.org/, accessed on 14 January 2022).
To validate the RNA-seq data, we surface-sterilized P. vulgaris L. cv. Negro Jamapa seeds and germinated them as described by Nanjareddy et al. [57]. Two-day-old germinated seedlings were transplanted into sterile vermiculite and inoculated with R. irregularis or R. tropici according to Nanjareddy et al. [58]. Total RNA preparation and RT–qPCR analysis were carried out according to Quezada et al., 2019 [59].
3. Results
3.1. Identification of PvSnRK Protein Orthologues in P. vulgaris
A BLAST search was carried out using Arabidopsis SnRKs as a reference, and a total of 42 genes were identified based on conserved domains in each sub-family (Table S1). The sub-family PvSnRK1 was identified by the domain KA1 (PF02149), the PvSnRK2 sub-family by the OST domain [60], and the PvSnRK3 sub-family by the NAF/FISL domain (PF02149). The genes were named PvSnRK1.1, PvSnRK1.2, PvSnRK2.1-PvSnRK2.11 and PvSnRK3.1-PvSnRK3.29 based on sequence homology with the Arabidopsis SnRK family. The amino acid length of the 42 PvSnRK gene family members ranged from 310 aa (PvSnRK2.11) to 528 aa (PvSnRK1.2), corresponding to molecular weights of 60.54 to 35.67 kDa (Table 1).

Table 1.
Gene information of Phaseolus vulgaris SnRK gene family. *Phytozome gene ID; bp—base pairs; CDS—coding sequence; aa—amino acids; pI—isoelectric point; MW—molecular weight; kDa—kilodaltons.
The theoretical isoelectric point of PvSnRKs (PI) ranged from 4.7 to 9.24, with PvSnRK1 sub-family members showing a basic PI, the PvSnRK2 sub-family being mostly acidic (4.7–6.65) and the PvSnRK3 sub-family being slightly acidic to highly basic (6.4–9.37). Subcellular localization analysis was carried out using ProtComp v.9.0, and the results showed PvSnRK1s to localize to the extracellular space; PvSnRK2s mostly localized to the nucleus and plasma membrane and PvSnRK3 sub-family members to the plasma membrane (Table 1). However, the subcellular localization analysis through different software showed some variation, as depicted in Table S3.
3.2. Phylogenetic and Structural Analyses
To determine the evolutionary relationship among Arabidopsis and Phaseolus SnRK superfamily genes, a phylogenetic tree was constructed using the protein sequences of 42 PvSnRK genes and 34 AtSnRK genes using the neighbour-joining (NJ) method with 1000 bootstrap replications (Figure 1, Figure S2). The accession numbers or locus IDs of the SnRK genes are listed in Table 1 and Supplementary Table S2. The resulting tree categorized the PvSnRKs and AtSnRKs into four clades, indicating that the ancestral genes of these two clades diverged before Brassicaceae and Fabaceae separated. In the P. vulgaris phylogeny, clade I contains PvSnRK2 sub-family members represented by the OST domain, clade II contains the PvSnRK1 sub-family containing the KA1 domain, and clades III and IV contain the NAF/FISL domain and 3 and 26 members of the PvSnRK3 sub-family, respectively (Figure S1). The combined phylogenetic analysis of Phaseolus and Arabidopsis SnRK proteins also showed a similar distribution, whereby the proteins from two species appear scattered across the branches of the evolutionary tree, suggesting that they experienced duplications after the lineages diverged.
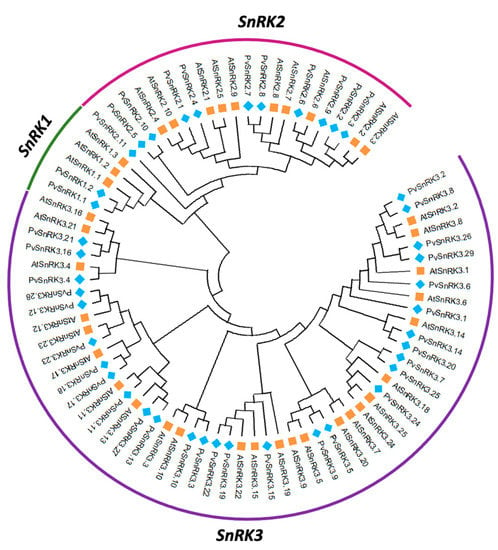
Figure 1.
Phylogeny of the SnRK gene family in Arabidopsis thaliana and Phaseolus vulgaris. Phylogenetic tree constructed with distance and neighbour-joining method using deduced full-length protein sequences of SnRK family genes of two species. The phylogenetic tree was constructed using MEGA XI software with the neighbour-joining tree method with 1000 bootstrap values.
To examine the evolution of Phaseolus SnRK genes, their chromosomal distribution was determined. PvSnRK superfamily genes are distributed across 10 pairs of homologous chromosomes among 11 pairs in the P. vulgaris genome. PvSnRK1 genes are located on chromosomes 4 and 8 and PvSnRK2 genes on chromosomes 2, 3, 6 and 8. PvSnRK3s are located on nine chromosomes, where chromosomes 1 and 11 are the exceptions, and chromosome 3 contains the highest number of PvSnRK genes (nine PvSnRKs), followed by chromosome 8 (eight PvSnRKs). Chromosomes 1, 4, 5 and 7 each have one gene each, as shown in Figure 2 and Table 1.
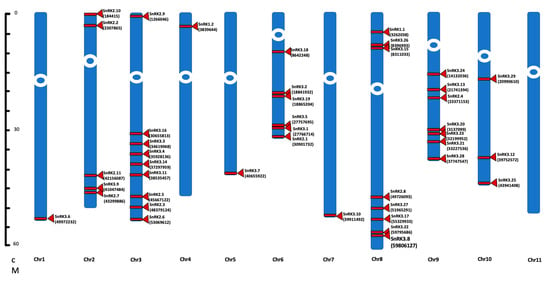
Figure 2.
Chromosomal localization of the SnRK genes. The sequences of 42 genes of SnRK genes were identified on P. vulgaris genome. The chromosomes are represented by the numerically distributed blue bars. Red bands and triangles indicate the location of each gene on the chromosome. The numbers in the parenthesis represent the start site of the individual SnRK gene location on the P. vulgaris genome.
Intron–exon analysis was carried out to obtain better insight into the structure of PvSnRK genes. PvSnRK gene family members exhibited a great variation, from 1 to 14 introns, as shown in Figure 3 and Table 1. In the PvSnRK1 sub-family, PvSnRK1.1 and PvSnRK1.2 have 9 and 10 introns, respectively, and all members of the PvSnRK2 sub-family have 8 introns each. A great variety in introns numbers was found in the sub-family PvSnRK3, with 16 PvSnRK3 members having no introns, PvSnRK3.19 having 1 intron, PvSnRK3.21 and 3.28 having 11 introns, and PvSnRK3.4 having 14 introns; the remaining eight PvSnRK3 sub-family members have 13 introns. This divergence in introns numbers indicates that exon gain, and loss occurred during evolution of the PvSnRK gene family. These findings are corroborated by the clades in the phylogenetic analysis, where clade I contains all PvSnRK2 members, clade II has PvSnRK1 individuals, and PvSNRK3 members are divided into clade III, with genes comprising 11 and 14 introns. Finally, clade IV includes all the remaining members of PvSNRK3 (Figure S1).
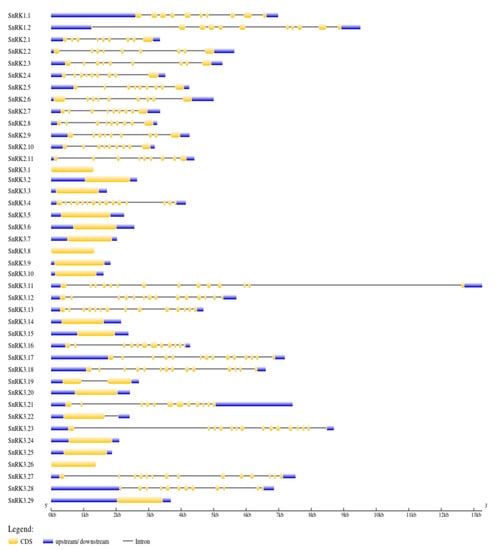
Figure 3.
The exon–intron organization of the corresponding SnRK genes. The exons are represented by the yellow boxes, introns by black lines, and the untranslated regions (UTRs) are indicated by the blue boxes. The sizes of the exons and introns can be estimated using the scale detailed at the bottom.
A search for conserved motifs in all 42 PvSnRK proteins using the MEME program revealed a total of 25 conserved motifs, named from 1 to 25. The identified motifs were annotated in Pfam, and the details of the putative motifs are shown in Table S4. Motifs 1-4 are designated protein kinase domains and are found in all three sub-families. Motif 8, a kinase domain associated with kinase1 and KA1, and motifs 10, 11, and 20, which encode an NAF domain, are only present in PvSnRK3. Ubiquitin-associated domain 20 was found in PvSnRK1 and PvSnRK3. Furthermore, motifs 5 and 13, designated protein superfamily kinase domains, were found only in the PvSnRK1 and PvSnRK3 sub-families (Figure 4). The remaining motifs were not annotated functionally.
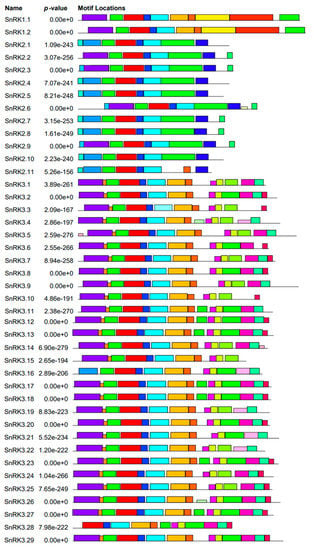
Figure 4.
Schematic representation of putative conserved motifs identified MEME in Phaseolus SnRK proteins. Putative conserved motifs shared by Phaseolus SnRK proteins were mined in MEME program. Twenty-five motifs are indicated by differently coloured boxes and the regular motif sequences are shown in the Table S4.
3.3. Ka/Ks and Gene Duplication
To further explore evolutionary constraints on Phaseolus PvSnRK genes, synonymous (Ks) and nonsynonymous (Ka) substitutions per site and their ratio (Ka/Ks) and divergence time of paralogous and orthologous SnRK family genes were calculated for AtSnRK orthologues of PvSnRKs (Table 2 and Table S5). The Ka/Ks ratio among all SnRK sequences was lower than 1, indicating purifying selection. These Ka/Ks ratios suggest the conservation of SnRK homologues in terms of both sequence and biological function [61].

Table 2.
One-to-one orthologous Ka/Ks relationships between P. vulgaris and A. thaliana.
3.4. Cis-Elements in Promoter Regions of PvSnRKs
To determine the gene expression pattern of PvSnRKs, the 2 kb region upstream of the CDS was analysed using the PlantRegMap database. Among all transcription factors recorded, ERF and MYB were found to be the most abundant. PvSnRK3.7 contained the greatest number of cis-elements (607) in the examined regulatory region, with 286 ERF binding sites and 49 MYB and 44 C2H2 sites. PvSnRK 3.20 has 338 TF sites; this was followed by PvSnRK 3.19 with 318 TFs, with 111 TFs being ERF TFs and 56 being bHLH TFs, and PvSnRK 1.2, with 312 TFs, with 100 being NAC TFs, 45 being MYB TFs and 40 being ERF TFs (Figure 5, Table S6, Figure S3). The most abundant TFs, ERF, C2H2, bHLH, NAC and MYB identified in PvSnRKs were also found by symbiosis related studies in other species such as M. truncatula and L. japonicus (Table 3).
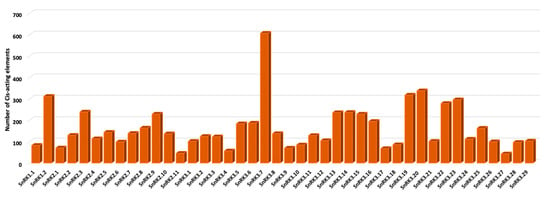
Figure 5.
Frequency of PvSnRK cis-acting elements in P. vulgaris.

Table 3.
Putative symbiosis cis-acting elements of SnRK gene family genes in P. vulgaris, based on studies on symbiotic transcription factors performed in different legumes.
MYB transcription factors promote expression of genes involved in cell proliferation and differentiation. ERFs are involved in regulation of developmental processes in response to stimuli, and NAC, C2H2 and bHLH are involved in the pathogen response, cell proliferation and development.
3.5. GO Analysis
Gene Ontology analysis of all PvSnRK genes showed them to be involved in biological processes and molecular functions, but not cellular components. The biological processes involved related to PvSnRKs are cellular signalling, phosphorylation, and metabolic processes such as protein, macromolecule, and phosphorous metabolism. Among molecular functions, the majority are associated with binding and catalytic activities, followed by transferase and nucleotide-binding functions (Figure 6a).
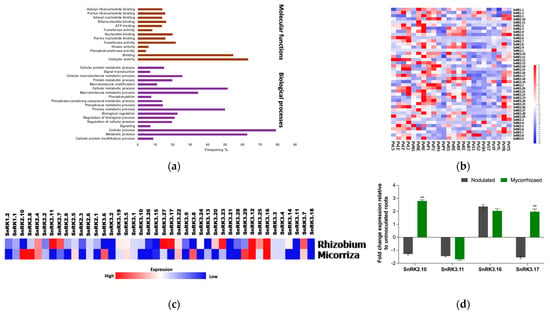
Figure 6.
Gene Ontology and in silico expression analysis of SnRK genes. (a) Gene Ontology enrichment analysis of molecular analysis and biological processes for the members of Phaseolus SnRK genes. (b) Tissue-specific expression profiles of Phaseolus SnRKs. Heat map expression profiles of SnRK genes in various Phaseolus tissues. The transcriptome data across different tissues were extracted from the P. vulgaris gene expression atlas (PvGEA). (c) Heat maps showing PvSnRK gene expression patterns specific to AM and rhizobial colonization. Colour bar shows the fold-change range, with red and blue representing upregulation and downregulation, respectively. The heat map was generated by R using the Fragments per kilobase of exon model per million reads mapped (FPKM) values of each SnRK gene. (d) RT-qPCR analysis showing relative expression of Phaseolus SnRK2.10, SnRK3.11, SnRK3.16, and SnRK3.17 genes. Candidate genes were selected and corresponding transcript accumulation under mycorrhized and nodulated conditions was quantified by RT-qPCR. RT-qPCR data are the averages of three biological replicates (n > 9). The statistical significance of differences between mycorrhized and nodulated roots was determined using an unpaired two-tailed Student’s t-test (** p < 0.01). Error bars represent means ± Standard error mean (SEM).
3.6. Expression Profiles of PvSnRK Genes in Different Tissues
Differential expression data for PvSnRK genes were obtained from PvGEA: Common Bean Gene Expression Atlas and Network Analysis (https://plantgrn.noble.org/PvGEA/, accessed on 16 November 2021). The expression patterns of all 42 PvSnRK members were analysed in 25 different tissues, including leaves, stems, shoots, pods, seeds, roots (inoculated and uninoculated with Rhizobium) and nodules, at different developmental stages and treatments (Figure 6b, Table S7). The lowest expression of all PvSnRK genes was found in young flower, seed and shoot tissues; at least one of the 42 genes was expressed in the remaining tissues. Among nodulation-related tissues, most of the PvSnRK genes showed high expression, except for the PvSnRK1 sub-family, in whole roots separated from fix+ nodules collected at 21 days after inoculation (PvRE). Pre-fixing (effective) nodules collected at 5 days after inoculation (PvN5) showed very high expression of only PvSnRK3.5 and PvSnRK3.29 (Figure 6b).
3.7. Expression Patterns of PvSnRK Genes under Symbiotic Conditions
Additionally, differential expression patterns of PvSnRKs under rhizobial and mycorrhizal symbiotic conditions were analysed by transcriptomics at the early infection stages 5 dpi and 7 dpi, respectively. Most of the genes encoding PvSnRK1s and PvSnRK3s were found to be upregulated under both symbiotic conditions, though PvSnRK2 was less induced during symbiosis with rhizobia. It was particularly interesting to find highly induced PvSnRK1.2 in comparison to PvSnRK1.1, and among the PvSnRK3 sub-families, PvSnRK3.10 was highly induced, followed by PvSnRK3.7, PvSnRK3.20, and PvSnRK3.22. Under mycorrhizal inoculation conditions, PvSnRK1s and PvSnRK3s were all induced, and some PvSnRK2 genes were also induced. In the SnRK1 sub-family, PvSnRK1.2 was more induced; among PvSnRK2 genes, PvSnRK2.6, PvSnRK2.7 and PvSnRK2.11 were least induced. In the PvSnRK3 sub-family, PvSnRK3.1, PvSnRK3.2, PvSnRK3.3, PvSnRK3.4, PvSnRK3.5 and PvSnRK3.6 were less induced than other members (Figure 6c,d).
3.8. PvSnRK Protein–Protein Interaction Network Prediction
A protein interaction network was constructed for PvSnRK proteins based on orthologues in Arabidopsis using the STRING database with the highest bit score (0.9). While predicting interactions among the PvSnRK proteins, we used experimentally proven, co-expressed and co-occurring protein interactions, revealing a total of 40 interacting proteins. The majority of them are phosphoprotein phosphatase (PPP) family proteins, PP2A (protein phosphatase PP1-α catalytic subunit), PP2CA/PP2CB (protein phosphatase 2A catalytic subunit α/β isoform), PPP2RA/PPP2RB (protein phosphatase 2A 65 kDa regulatory subunit A β isoform) and PPP1C (protein phosphatase PP1-α catalytic subunit)-type proteins. Phosphatases are proteins involved in substrate recognition, plant signalling pathways such as stress regulation, light, pathogen defence and hormonal signalling, the cell cycle, differentiation, metabolism, and plant growth.
The next important interactions were with cell cycle proteins and cyclin B proteins. Cyclin B proteins have been implicated in plant growth and development. All PvSnRK protein sub-families were found to interact with PPP family members and cyclin B proteins through PKGII, indicating their involvement in various cellular and developmental processes. SnRK2 sub-family members were found to specifically interact with PP2C proteins (Figure 7).
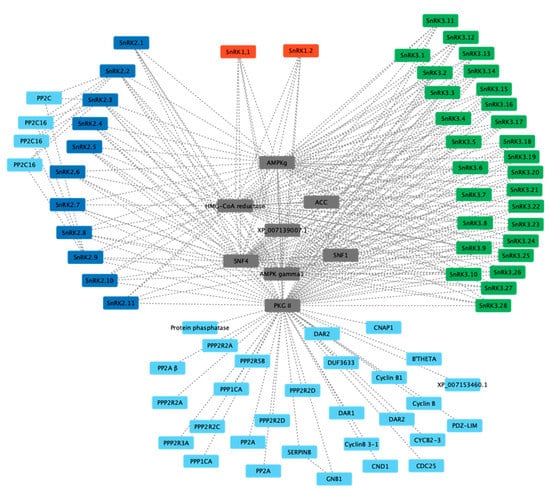
Figure 7.
In silico prediction of protein–protein interactions among SnRK1s, SnRK2s and SnRK3s using the Cytoscape tool based on the Pearson correlation coefficients of the relative expression of the gene. Each node represents a protein, and each edge refers an interaction. The red-coloured box represents SnRK1s, blue represents SnRK2s, and green represents SnRK3s.
3.9. Prediction of Coregulatory and Interaction Networks of PvSnRK and Symbiotic Genes
The symbiotic interaction between legumes and rhizobia is unique and involves a set of common symbiotic genes that regulate root nodule symbiosis and mycorrhizal symbiosis. Another 190 genes have been implicated in regulating symbiotic interactions. A total of 200 genes known to be involved in symbiosis were chosen to predict an interaction network with each of the PvSnRK sub-families in the STRING database. Interaction between PvSnRK1 sub-family members and symbiosis-related proteins shows that PvSnRK1.1 and PvSnRK1.2 do not interact directly with any of the symbiosis-related genes. However, they do interact with TOR, PI3K and ATG-RP, which interact with other symbiotic genes represented by 54 nodes and 119 edges. The network mostly involves nucleoporins (NUPs), RAB proteins, auxin-responsive ARP proteins and many proteins involved in vesicle transport (Figure 8a).
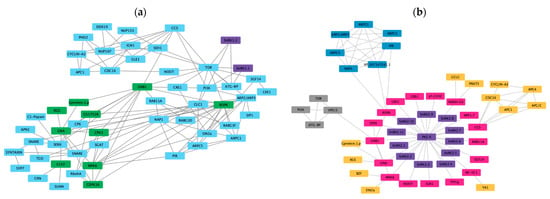
Figure 8.
In silico prediction of protein–protein interactions among (a) SnRK1s and (b) SnRK2s with Scheme 11. PvSnRK2 sub-family proteins with symbiosis-related proteins interact through 52 nodes and 81 edges. All PvSnRK2 proteins interact with PKGII, which interacts with all other proteins shown in the network. Through CCS (Cell division Cycle 20 like protein) it interacts with cell cycle-related proteins such as Cyclin A2, CDC16 and APC (Anaphase-promoting complex) and through ROP6 interacted with ARP (auxin-related proteins). The prediction showed that few PvSnRK2 sub-family proteins interact with symbiosis-related proteins (Figure 8b).
The largest PvSnRK3 sub-family, with 29 members, interacts with TOR, PI3K and ATG-RP at the first level, similar to PvSnRK1 members. The predictions showed 102 nodes and 319 edges, indicating a larger network. CCS interacts with nucleoporins (NUPs), and through GNBI, they interact with several RPSs (ribosomal proteins) and a variety of symbiosis-related proteins. Network prediction showed that PvSnRK3 sub-family members may be involved in regulating symbiosis in Phaseolus via different signalling pathways (Figure 9).
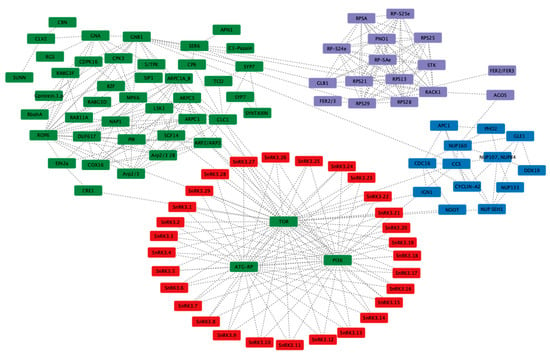
Figure 9.
In silico prediction of protein–protein interactions among SnRK3 subfamily proteins with symbiosis-related proteins using the Cytoscape tool based on the Pearson correlation coefficients of the relative expression of the gene. Each node represents a protein, and each edge refers an interaction. The different colours represent the different gene families.
4. Discussion
The SnRK gene family, serine/threonine kinases, and its orthologues in animals and yeast are highly conserved. In plants, they have been identified as regulators of abiotic and biotic stress [80,81,82]. In recent years, genome-wide analysis of this gene family in an array of both model and economically important plant species has focused primarily on abiotic stress. Phaseolus vulgaris is the most important legume consumed by humans worldwide, as it is an affordable source of proteins, vitamins, minerals, antioxidants, and bioactive compounds [83]. Climate change has impacted the world’s crop yield and quality, leading to socioeconomic and food insecurity [84]. The yield quantity and quality of N2-fixing crops can be improved by several agronomic practices, such as irrigation, sowing density and Rhizobium application. Since common bean does not need exogenous N fertilizer application, productivity is cost effective. Any efforts towards the betterment of rhizobial association to improve N fixation in crops such as Phaseolus should be undertaken. The present investigation encompasses the identification, classification, and analysis of the expression patterns of the SnRK gene family under symbiotic conditions.
Genome-wide identification studies have been carried out, with early reports documenting the presence of various numbers of members in both monocots and dicots. A total of 39, 114, 30, 34, 26, 149, 46, 48 and 44 SnRK genes have been identified in A. thaliana [1], Brassica napus [30], C. sativus [31], E. grandis [32], F. ananassa [33], T. aestivum [34], H. vulgare [35], O. sativa [17] and B. distachyon [36], respectively. In Phaseolus, we identified 42 SnRK genes with 2 SnRK1s, 11 SnRK2s and 29 SnRK3s with the characteristic domains of each sub-family. As in any other species, the SnRK2 and SnRK3 sub-families in Phaseolus are larger than SnRK1, supporting the view that these two sub-families originated from duplication of SnRK1 [35]. The nonmotile nature of plants exposes them to biotic and abiotic factors, and plants adopt such changes by expanding their genes and gene families. Gene and genome duplications are important events that contribute to polyploidy and genome evolution. One or multiple polyploidies are prevalent in angiosperms [85,86] and explain the large number of SnRK2 and SnRK3 sub-family members in all reported plant species.
In Phaseolus, the SnRK gene family is distributed on 10 of 11 chromosomes. This distribution is unlike in other species, in which all chromosomes in the genome contain at least one of these genes [1,17,30,31,32,33,34,35,36]. Phaseolus chr3, chr6, chr8 and chr9 show clustering of genes, mostly of the SnRK3 sub-family.
Each of the SnRK sub-families has a characteristic domain; however, the N-terminal kinase domain is highly conserved across species and sub-families. Exon–intron structural diversification and motif composition play an important role in the evolution and function of many gene families [87]. PvSnRK1 sub-family members have 10–11 exons, such as BdSnRK1s, EgrSnRK1s and CsSnRK1s. All PvSnRK2s have nine exons, similar to most HvSnRKs, HcSnRK2s, EgrSnRK2s, VvSnRK2s, AtSnRK2s and BdSnRK2s, indicating the conserved nature of these sub-families. The sub-family PvSnRK3 can also be subdivided into exon-rich and exon-poor types, as can all other species reported thus far. Reports suggest the origin of the SnRK3 sub-family from green algae, and the intron-poor group first appeared in seed plants [88]. These results are consistent with phylogenetic tree studies showing that SnRK exon–intron numbers are highly conserved during the evolution of each sub-family. The phylogenetic tree and exon–intron structure showed that most paralogous gene pairs contain the same exon number, though some gene pairs have different exon numbers. Motif analysis revealed a close evolutionary relationship within sub-groups due to the conserved nature of motif composition among sub-families. The motif structure of each sub-family might define the biological function in which they are involved. The gene structure and sequence conservation were similar to most of the previously studied species [1,17,30,31,32,33,34,35,36].
Gene expression is regulated by external factors that are perceived through signalling mechanisms. Such signals activate specific transcription factors that, when combined with cis-acting elements in the promoter regions of genes, alter gene expression. In most genome-wide analysis studies of SnRK gene families, detection of cis-acting elements has focused on abiotic stress conditions in which the presence of hormone-, salt-, temperature-, and drought-specific cis-elements [1,17,30,31,32,33,34,35,36]. As the aim of our investigation was to predict the possible role of the PvSnRK gene family in symbiosis, we analysed symbiosis-related cis-elements as demonstrated in Medicago and L. japonicus. All PvSnRK gene sub-families contain symbiosis-related cis-elements. Among all cis-elements, ERF and MYB are the most abundant, followed by C2H2 and bHLH.
Ka/Ks analysis showed that PvSnRK gene family duplications either occurred slowly or are highly conserved [89]. Gene Ontology studies revealed that the PvSnRK genes function mostly in molecular and biological processes, specifically in cellular and metabolic processes followed by nucleotide binding activity. When we analysed expression of PvSnRK gene family members in different tissues, the lowest expression of any PvSnRK was found in aerial tissues such as young pods, flowers, seeds, and shoots. Most of the SnRKs were found to be expressed in root or root nodules at some stage of their development. Furthermore, transcriptomic data under early symbiotic conditions showed elevated expression levels of PvSnRK1 s and PvSnRK3s, with some members being more highly induced than others. For some genes, these expression patterns were found under both root nodule and mycorrhizal symbiotic conditions, suggesting a possible role for PvSnRKs in the establishment of symbiotic relationships.
To predict possible interaction networks among the PvSnRKs and PvSnRKs with symbiosis-related genes, we carried out in situ interaction network building using the STRING database and Cytoscape. The results were interesting, as most of the PvSnRKs interact among themselves, and interaction is mediated by master regulators of cellular processes such as TOR and PKGII. Through these proteins, PvSnRKs interact with several protein phosphatases and cell cycle-regulating cyclins. We chose symbiosis-related genes based on a previously published article and identified a total of 200 genes in the Phaseolus genome. PvSnRK1s mostly interact with other major proteins, such as TOR and PI3Ks, which are connected to the NUPs, RAB proteins, ARPs and proteins involved in vesicle transport. On the other hand, PvSnRK2s interact with PKGII, which interact with the cell cycle regulatory proteins cyclins, CDC, and APC. In contrast, PvSNRK2s shows very few symbiotic gene interactions. Of the largest PvSnRK sub-families, PvSnRK3 interacts with NUPs, RPSs and many symbiotic genes.
Taken together, our extensive analysis of the PvSnRK gene family revealed structural conservation of genes across species and possible functional conservation as well. Furthermore, the principle aim of this study was to understand the putative role of PvSnRKs in regulating symbiotic interactions between Phaseolus and Rhizobium or Phaseolus; mycorrhiza are promising, as some genes contain specific cis-elements and showed transcript upregulation in response to symbionts. Finally, the identified in situ protein–protein interactions may help in predicting candidate genes for functional characterization to obtain a clear picture of the regulatory mechanisms involved.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes13112107/s1, Figure S1: Phylogeny of the SnRK family genes in Phaseolus vulgaris. (A) Phylogenetic tree constructed with distance and neighbour-joining method using deduced full-length protein sequences of SnRK family genes. The phylogenetic tree was constructed using MEGA XI software with the neighbour-joining tree method with 1000 bootstrap values; Figure S2: Multiple sequence alignment of kinase domains of Phaseolus SnRK gene family. The alignment was generated using Clustal Omega bioinformatic program.; Figure S3: Cis-acting element analysis of the promoter regions of SnRK family genes in Phaseolus. Percent of various Cis-acting elements in 2 Kb promoter region upstream to the start codon was used to analyse the Cis-acting elements using Plant PAN 3.0 tool. Table S1: Arabidopsis SnRK gene family sequences; Table S2: Phaseolus vulgaris SnRK gene family information; Table S3: PvSnRK in situ subcellular protein localization analysis using different software; Table S4: Putative conserved motifs identified MEME in Phaseolus SnRK proteins. Table S5: Ka/Ks relationship of all SnRK gene family members of P. vulgaris against all A. thaliana SnRK members; Table S6: PvSnRK cis-acting elements related to rhizobial and mycorrhizal symbiosis; Table S7: PvGEA key for samples in the heat map showing the expression profiles of SnRK genes in various Phaseolus tissues.
Author Contributions
Conceptualization, K.N. and M.-K.A.; methodology, K.N., M.-K.A., M.L. and C.C.-T.; software, C.C.-T. and K.N.; validation, K.N., M.-K.A., L.B. and M.L.; formal analysis, C.C.-T. and K.N.; investigation, K.N.; C.C.-T. and L.B.; resources, C.C.-T.; M.-K.A. and K.N.; writing—original draft preparation, K.N.; writing—review and editing, K.N., M.-K.A.; L.B. and M.L.; visualization, K.N., M.-K.A. and M.L.; supervision, K.N.; project administration, K.N.; funding acquisition, K.N., M.-K.A. and M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Dirección General de Asuntos del Personal Académico, DGAPA/PAPIIT-UNAM grant no. IN216321 to K.N Partially supported by CONACyT project CF-MI-20191017134234199/316538 and DGAPA/PAPIIT-UNAM grant no. IN213221 to M.-K.A.
Data Availability Statement
The data reported in this study are available in the supplementary information provided in the supplementary data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hrabak, E.M.; Chan, C.W.M.; Gribskov, M.; Harper, J.F.; Choi, J.H.; Halford, N.; Kudla, J.; Luan, S.; Nimmo, H.G.; Sussman, M.R.; et al. The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol. 2003, 132, 666–680. [Google Scholar] [PubMed]
- Hedbacker, K.; Carlson, M. SNF1/AMPK pathways in yeast. Front. Biosci. 2008, 13, 2408–2420. [Google Scholar] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar]
- Broeckx, T.; Hulsmans, S.; Rolland, F. The plant energy sensor: Evolutionary conservation and divergence of SnRK1 structure, regulation, and function. J. Exp. Bot. 2016, 67, 6215–6252. [Google Scholar] [PubMed]
- Estruch, F.; Treitel, M.A.; Yang, X.; Carlson, M. N-terminal mutations modulate yeast SNF1 protein kinase function. Genetics 1992, 132, 639–650. [Google Scholar]
- Hawley, S.A.; Davison, M.; Woods, A.; Davies, S.P.; Beri, R.K.; Carling, D.; Hardie, D.G. Characterization of the AMP activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMPactivated protein kinase. J. Biol. Chem. 1996, 271, 27879–27887. [Google Scholar]
- Baena-González, E.; Rolland, F.; Thevelein, J.M.; Sheen, J. A central integrator of transcription networks in plant stress and energy signalling. Nature 2007, 448, 938–942. [Google Scholar]
- Halford, N.G.; Hardie, D.G. SNF1-related protein kinases: Global regulators of carbon metabolism in plants? Plant Mol. Biol. 1998, 37, 735–748. [Google Scholar]
- Dale, S.; Wilson, W.A.; Edelman, A.M.; Hardie, D.G. Similar substrate recognition motifs for mammalian AMP-activated protein kinase, higher plant HMG-CoA reductase kinase-A, yeast SNF1, and mammalian calmodulin-dependent protein kinase I. FEBS Lett. 1995, 361, 191–195. [Google Scholar] [PubMed]
- Christopher, S.; Paul, G.D.; Halford, N.G.; Hardie, D.G. Two SNF1-related protein kinases from spinach leaf phosphorylate and inactive at 3-hydroxy-3-methylglutaryl-coenzyme a reductase, nitrate reductase, and sucrose phosphate synthase in vitro. Plant Physiol. 1999, 120, 257–274. [Google Scholar]
- Harthill, J.E.; Meek, S.E.; Morrice, N.; Peggie, M.W.; Borch, J.; Wong, B.H.; Mackintosh, C. Phosphorylation and 14-3-3 binding of Arabidopsis trehalose-phosphate synthase 5 in response to 2-deoxyglucose. Plant J. 2006, 47, 211–223. [Google Scholar] [PubMed]
- Halford, N.G.; Hey, S.J. Snf1-related protein kinases (SnRKs) act within an intricate network that links metabolic and stress signalling in plants. Biochem. J. 2009, 419, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Baena-González, E.; Sheen, J. Convergent energy and stress signaling. Trends Plant Sci. 2008, 13, 474–482. [Google Scholar] [PubMed]
- Fujii, H.; Verslues, P.E.; Zhu, J.K. Arabidopsis decuple mutant reveals the importance of SnRK2 kinases in osmotic stress responses in vivo. Proc. Natl. Acad. Sci. USA 2001, 1108, 1717–1722. [Google Scholar] [CrossRef]
- Kulik, A.; Wawer, I.; Krzywińska, E.; Bucholc, M.; Dobrowolska, G. SnRK2 protein kinases—Key regulators of plant response to abiotic stresses. OMICS J. Integr. Biol. 2011, 15, 859–872. [Google Scholar]
- Kawa, D.; Meyer, A.J.; Dekker, H.L.; Abd-El-Haliem, A.M.; Gevaert, K.; Van De Slijke, E.; Maszkowska, J.; Bucholc, M.; Dobrowolska, G.; Geert, J.; et al. SnRK2 protein kinases and mRNA decapping machinery control root development and response to salt. Plant Physiol. 2020, 182, 361–371. [Google Scholar]
- Kobayashi, Y.; Yamamoto, S.; Minami, H.; Kagaya, Y.; Hattori, T. Differential activation of the rice sucrose nonfermenting1-related protein kinase2 family by hyperosmotic stress and abscisic acid. Plant Cell 2004, 16, 1163–1177. [Google Scholar] [PubMed]
- Cutler, S.R.; Rodriguez, P.L.; Finklestein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar]
- Kobayashi, Y.; Murata, M.; Minami, H.; Yamamoto, S.; Kagaya, Y.; Hobo, T.; Yamamoto, A.; Hattori, T. Abscisic acid-activated SnRK2 protein kinases function in the gene-regulation pathway of ABA signal transduction by phosphorylating ABA response element-binding factors. Plant J. 2005, 44, 939–949. [Google Scholar]
- Umezawa, T.; Nakashima, K.; Miyakawa, T.; Kuromori, T.; Tanokura, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Molecular basis of the core regulatory network in ABA responses: Sensing, signaling and transport. Plant Cell Physiol. 2010, 51, 1821–1839. [Google Scholar]
- Soon, F.F.; Ng, L.M.; Zhou, X.E.; West, G.M.; Kovach, A.; Tan, M.H.E.; Suino-Powell, K.M.; He, Y.; Xu, Y.; Chalmers, M.J.; et al. Molecular mimicry regulates ABA signaling by SnRK2 kinases and PP2C phosphatases. Science 2012, 335, 85–88. [Google Scholar]
- Kim, K.N.; Cheong, Y.H.; Grant, J.J.; Pandey, G.K.; Luan, S. CIPK3, a Calcium sensor-associated protein kinase that regulates abscisic acid and cold signal transduction in Arabidopsis. Plant Cell 2003, 15, 411–423. [Google Scholar]
- Batistic, O.; Kudla, J. Integration and channeling of calcium signaling through the CBL calcium sensor/CIPK protein kinase network. Planta 2004, 219, 915–924. [Google Scholar] [PubMed]
- Zhu, S.Y.; Yu, X.C.; Wang, X.J.; Zhao, R.; Li, Y.; Fan, R.C.; Shang, Y.; Du, S.Y.; Wu, F.Q.; Xu, Y.H.; et al. Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell 2007, 19, 3019–3036. [Google Scholar] [PubMed]
- Piao, H.L.; Xuan, Y.H.; Park, S.H.; Je, B.I.; Park, S.J.; Park, S.H.; Kim, C.M.; Huang, J.; Wang, G.K.; Kim, M.J.; et al. OsCIPK31, a CBL-interacting protein kinase is involved in germination and seedling growth under abiotic stress conditions in rice plants. Mol. Cells 2010, 30, 19–27. [Google Scholar] [PubMed]
- Tripathi, V.; Parasuraman, B.; Laxmi, A.; Chattopadhyay, D. CIPK6, a CBL-interacting protein kinase is required for development and salt tolerance in plants. Plant J. 2009, 58, 778–790. [Google Scholar]
- Huertas, R.; Olias, R.; Eljakaoui, Z.; Gálvez, F.J.; Li, J.; de Morales, P.A.; Belver, A.; Rodríguez-Rosales, M. Overexpression of SlSOS2 (SlCIPK24) confers salt tolerance to transgenic tomato. Plant Cell Environ. 2012, 35, 1467–1482. [Google Scholar]
- Guo, Y.; Xiong, L.; Song, C.P.; Gong, D.; Halfter, U.; Zhu, J.K. A Calcium Sensor and Its Interacting Protein Kinase Are Global Regulators of Abscisic Acid Signaling in Arabidopsis. Dev. Cell 2002, 3, 233–244. [Google Scholar]
- Liu, J.; Ishitani, M.; Halfter, U.; Kim, C.S.; Zhu, J.K. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Natl. Acad. Sci. USA 2000, 97, 3730–3734. [Google Scholar]
- Zhu, W.Z.; Wu, D.Z.; Jiang, L.X.; Ye, L.Z. Genome-wide identification and characterization of SnRK family genes in Brassica napus. BMC Plant Biol. 2020, 20, 287. [Google Scholar]
- Luo, Y.; Niu, Y.; Gao, R.; Wang, C.; Liao, W. Genome-Wide Identification and Expression Analysis of SnRK Gene Family under Abiotic Stress in Cucumber (Cucumis sativus L.). Agronomy 2022, 12, 1550. [Google Scholar]
- Wang, Y.; Yan, H.; Qiu, Z.; Hu, B.; Zeng, B.; Zhong, C.; Fan, C. Comprehensive analysis of SNRK gene family and their responses to salt stress in eucalyptus grandis. Int. J. Mol. Sci. 2019, 20, 2786. [Google Scholar]
- Zhang, Y.; Ye, Y.; Jiang, L.; Lin, Y.; Gu, X.; Chen, Q.; Sun, B.; Zhang, Y.; Luo, Y.; Wang, Y.; et al. Genome-Wide Characterization of Snf1-Related Protein Kinases (SnRKs) and Expression analysis of SnRK1.1 in Strawberry. Genes 2020, 11, 427. [Google Scholar]
- Jiang, B.; Liu, Y.; Niu, H.; He, Y.; Ma, D.; Li, Y. Mining the Roles of Wheat (Triticum aestivum) SnRK Genes in Biotic and Abiotic Responses. Front. Plant Sci. 2022, 13, 934226. [Google Scholar]
- Xiong, J.; Chen, D.; Su, T.; Shen, Q.; Wu, D.; Zhang, G. Genome-Wide Identification, Expression Pattern and Sequence Variation Analysis of SnRK Family Genes in Barley. Plants 2022, 11, 975. [Google Scholar]
- Wang, L.; Hu, W.; Sun, J.; Liang, X.; Yang, X.; Wei, S.; Wang, X.; Zhou, Y.; Xiao, Q.; Yang, G.; et al. Genome-wide analysis of SnRK gene family in Brachypodium distachyon and functional characterization of BdSnRK29. Plant Sci. 2015, 237, 33–45. [Google Scholar]
- Westhoek, H.; Rood, T.; van den Berg, M.; Janse, J.; Nijdam, D.; Reudink, M.; Stehfest, E. The Protein Puzzle: The Consumption and Production of Meat, Dairy and Fish in the European Union; Netherlands Environmental Assessment Agency (PBL): The Hague, The Netherlands, 2011. [Google Scholar]
- Lemke, R.L.; Zhong, Z.; Campbell, C.A.; Zentner, R.P. Can pulse crops play a role in mitigating greenhouse gases from North American agriculture? Agron. J. 2007, 99, 1719–1725. [Google Scholar]
- Nemecek, T.; von Richthofen, J.S.; Dubois, G.; Casta, P.; Charles, R.; Pahl, H. Environmental impacts of introducing grain legumes into European crop rotations. Eur. J. Agron. 2008, 28, 380–393. [Google Scholar]
- Peoples, M.; Hauggaard-Nielsen, H.; Jensen, E.S. The potential environmental benefits and risks derived from legumes in rotations. In Nitrogen Fixation in Crop Production; Emerich, D., Krishnan, H., Eds.; American Society of Agronomy; Crop Science Society of America; Soil Science Society of America: Baltimore, MD, USA, 2009; Volume 52, pp. 349–385. [Google Scholar]
- Tharanathan, R.N.; Mahadevamma, S. Grain legumes—A boon to human nutrition. Trends Food Sci. Tech. 2003, 14, 507–518. [Google Scholar]
- Letunic, I.; Doerks, T.; Bork, P. SMART 7: Recent updates to the protein domain annotation resource. Nucleic Acids Res. 2012, 40, 302–305. [Google Scholar]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangradorvegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [PubMed]
- Marchler-Bauer, A.; Lu, S.; Anderson, J.B.; Chitsaz, F.; Derbyshire, M.K.; DeWeese-Scott, C.; Fong, J.H.; Geer, L.Y.; Geer, R.C.; Gonzales, N.R.; et al. CDD: A conserved domain database for the functional annotation of proteins. Nucleic Acids Res. 2011, 39, D225–D229. [Google Scholar] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [PubMed]
- Bolser, D.M.; Staines, D.M.; Perry, E.; Kersey, P.J. Ensembl Plants: Integrating tools for visualizing, mining, and analyzing plant genomic data. In Plant Bioinformatics, 2nd ed.; Edwards, D., Ed.; Humana Press: New York, NY, USA, 2017; Volume 1533, pp. 115–140. [Google Scholar]
- Wang, S.; Su, J.H.; Beliveau, B.J.; Bintu, B.; Moffitt, J.R.; Wu, C.; Zhuang, X. Spatial organization of chromatin domains and compartments in single chromosomes. Science 2016, 353, 598–602. [Google Scholar] [CrossRef]
- Pierleoni, A.; Martelli, P.L.; Fariselli, P.; Casadio, R. BaCelLo: A balanced subcellular localization predictor. Bioinformatics 2006, 22, 408–416. [Google Scholar]
- Goldberg, T.; Hecht, M.; Hamp, T.; Karl, T.; Yachdav, G.; Ahmed, N.; Altermann, U.; Angerer, P.; Ansorge, S.; Balasz, K.; et al. LocTree3 prediction of localization. Nucleic Acids Res. 2014, 42, W350–W355. [Google Scholar]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, 585–587. [Google Scholar]
- CELLO. Available online: http://e093.life.nctu.edu.tw/ (accessed on 9 October 2021).
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [PubMed]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [PubMed]
- Yang, Z.; Nielsen, R. Estimating synonymous and nonsynonymous substitution rates under realistic evolutionary models. Mol. Biol. Evol. 2000, 17, 32–43. [Google Scholar] [PubMed]
- Rstudio Team. RStudio: Integrated Development for R; Rstudio PBC: Boston, MA, USA, 2022. [Google Scholar]
- Du, Z.; Zhou, X.; Ling, Y.; Zhang, Z.; Su, Z. agriGO: A GO analysis toolkit for the agricultural community. Nucleic Acids Res. 2010, 38, W64–W70. [Google Scholar] [PubMed]
- Nanjareddy, K.; Arthikala, M.K.; Gómez, B.M.; Blanco, L.; Lara, M. Differentially expressed genes in mycorrhized and nodulated roots of common bean are associated with defense, cell wall architecture, N metabolism, and P metabolism. PLoS ONE 2017, 12, 0182328. [Google Scholar]
- Nanjareddy, K.; Arthikala, M.K.; Aguirre, A.L.; Gómez, B.M.; Lara, M. Plant promoter analysis: Identification and characterization of root nodule specific promoter in the common bean. J. Vis. Exp. 2017, 130, 56140. [Google Scholar]
- Quezada-Rodríguez, E.H.; Gómez-Velasco, H.; Arthikala, M.K.; Lara, M.; Hernández-López, A.; Nanjareddy, K. Exploration of Autophagy Families in Legumes and Dissection of the ATG18 Family with a Special Focus on Phaseolus vulgaris. Plants 2021, 10, 2619. [Google Scholar] [PubMed]
- Mustilli, A.C.; Merlot, S.; Vavasseur, A.; Fenzi, F.; Giraudat, J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 2002, 14, 3089–3099. [Google Scholar] [PubMed]
- Chen, F.-C.; Chen, C.-J.; Li, W.-H.; Chuang, T.-J. Gene Family Size Conservation Is a Good Indicator of Evolutionary Rates. Mol. Biol. Evol. 2010, 27, 1750–1758. [Google Scholar] [CrossRef]
- Wang, Y.; Li, K.; Chen, L.; Zou, Y.; Liu, H.; Tian, Y.; Li, D.; Wang, R.; Zhao, F.; Ferguson, B.J.; et al. MicroRNA167-Directed Regulation of the Auxin Response Factors GmARF8a and GmARF8b Is Required for Soybean Nodulation and Lateral Root Development. Plant Physiol. 2015, 168, 984–999. [Google Scholar] [PubMed]
- Yan, Q.; Wang, L.; Li, X. GmBEHL1, a BES1/BZR1 Family Protein, Negatively Regulates Soybean Nodulation. Sci. Rep. 2018, 8, 7614. [Google Scholar]
- Ariel, F.; Brault-Hernandez, M.; Laffont, C.; Huault, E.; Brault, M.; Plet, J.; Moison, M.; Blanchet, S.; Ichanté, J.L.; Chabaud, M.; et al. Two Direct Targets of Cytokinin Signaling Regulate Symbiotic Nodulation in Medicago Truncatula. Plant Cell 2012, 24, 3838–3852. [Google Scholar]
- Nishimura, R.; Hayashi, M.; Wu, G.J.; Kouchi, H.; Imaizumi-Anraku, H.; Murakami, Y.; Kawasaki, S.; Akao, S.; Ohmori, M.; Nagasawa, M.; et al. HAR1 mediates systemic regulation of symbiotic organ development. Nature 2002, 420, 426–429. [Google Scholar] [CrossRef]
- Sinharoy, S.; Torres-Jerez, I.; Bandyopadhyay, K.; Kereszt, A.; Pislariu, C.I.; Nakashima, J.; Benedito, V.A.; Kondorosi, E.; Udvardi, M.K. The C2H2 Transcription Factor Regulator of Symbiosome Differentiation Represses Transcription of the Secretory Pathway Gene VAMP721a and Promotes Symbiosome Development in Medicago truncatula. Plant Cell 2013, 25, 3584–3601. [Google Scholar] [CrossRef] [PubMed]
- Middleton, P.H.; Jakab, J.; Penmetsa, R.V.; Starker, C.G.; Doll, J.; Kaló, P.; Prabhu, R.; Marsh, J.F.; Mitra, R.M.; Kereszt, A.; et al. An ERF Transcription Factor in Medicago truncatula That Is Essential for Nod Factor Signal Transduction. Plant Cell 2007, 19, 1221–1234. [Google Scholar]
- Devers, E.A.; Teply, J.; Reinert, A.; Gaude, N.; Krajinski, F. An Endogenous Artificial MicroRNA System for Unraveling the Function of Root Endosymbioses Related Genes in Medicago Truncatula. BMC Plant Biol. 2013, 13, 82. [Google Scholar]
- Floss, D.S.; Gomez, S.K.; Park, H.J.; MacLean, A.M.; Müller, L.M.; Bhattarai, K.K.; Lévesque-Tremblay, V.; Maldonado-Mendoza, I.E.; Harrison, M.J. A Transcriptional Program for Arbuscule Degeneration during AM Symbiosis Is Regulated by MYB1. Curr. Biol. 2017, 27, 1206–1212. [Google Scholar] [PubMed]
- Combier, J.P.; Küster, H.; Journet, E.P.; Hohnjec, N.; Gamas, P.; Niebel, A. Evidence for the Involvement in Nodulation of the Two Small Putative Regulatory Peptide-Encoding Genes MtRALFL1 and MtDVL1. Mol. Plant-Microbe Interact. 2008, 21, 1118–1127. [Google Scholar] [PubMed]
- de Zélicourt, A.; Diet, A.; Marion, J.; Laffont, C.; Ariel, F.; Moison, M.; Zahaf, O.; Crespi, M.; Gruber, V.; Frugier, F. Dual Involvement of a Medicago Truncatula NAC Transcription Factor in Root Abiotic Stress Response and Symbiotic Nodule Senescence. Plant J. 2012, 70, 220–230. [Google Scholar] [PubMed]
- Yano, K.; Yoshida, S.; Müller, J.; Singh, S.; Banba, M.; Vickers, K.; Markmann, K.; White, C.; Schuller, B.; Sato, S.; et al. CYCLOPS, a Mediator of Symbiotic Intracellular Accommodation. Proc. Natl. Acad. Sci. USA 2008, 105, 20540–20545. [Google Scholar] [CrossRef]
- Liu, C.W.; Breakspear, A.; Guan, D.; Cerri, M.R.; Jackson, K.; Jiang, S.; Robson, F.; Radhakrishnan, G.V.; Roy, S.; Bone, C.; et al. NIN Acts as a Network Hub Controlling a Growth Module Required for Rhizobial Infection. Plant Physiol. 2019, 179, 1704–1722. [Google Scholar]
- Guillotin, B.; Couzigou, J.M.; Combier, J.P. NIN Is Involved in the Regulation of Arbuscular Mycorrhizal Symbiosis. Front. Plant Sci. 2016, 7, 1704. [Google Scholar]
- Osipova, M.A.; Dolgikh, E.A.; Lutova, L.A. Peculiarities of Meristem-Specific WOX5 Gene Expression during Nodule Organogenesis in Legumes. Russ. J. Dev. Biol. 2011, 42, 226. [Google Scholar]
- Gallou, A.; Declerck, S.; Cranenbrouck, S. Transcriptional Regulation of Defence Genes and Involvement of the WRKY Transcription Factor in Arbuscular Mycorrhizal Potato Root Colonization. Funct. Integr. Genom. 2012, 12, 183–198. [Google Scholar]
- Yang, Y.; Sun, T.; Xu, L.; Pi, E.; Wang, S.; Wang, H.; Shen, C. Genome-Wide Identification of CAMTA Gene Family Members in Medicago Truncatula and Their Expression during Root Nodule Symbiosis and Hormone Treatments. Front. Plant Sci. 2015, 6, 459. [Google Scholar] [PubMed]
- Rieko, N.; Ohmori, M.; Fujita, H.; Kawaguchi, M. A Lotus basic leucine zipper protein with a RING-finger motif negatively regulates the developmental program of nodulation. Proc. Natl. Acad. Sci. USA 2002, 99, 15206–15210. [Google Scholar]
- Duangkhet, M.; Thepsukhon, A.; Widyastuti, R.; Santosa, D.A.; Tajima, S.; Nomura, M. A MYB-Related Transcription Factor Affects Nodule Formation in Lotus japonicus. Plant Biotechnol. 2016, 33, 187–194. [Google Scholar]
- Tsai, A.Y.L.; Gazzarrini, S. Overlapping and distinct roles of AKIN10 and FUSCA3 in ABA and sugar signaling during seed germination. Plant Signal. Behav. 2012, 7, 1238–1242. [Google Scholar] [CrossRef]
- Perochon, A.; Jianguang, J.; Kahla, A.; Arunachalam, C.; Scofield, S.R.; Bowden, S.; Wallington, E.; Doohan, F.M. TaFROG encodes a pooideae orphan protein that interacts with SnRK1 and enhances resistance to the mycotoxigenic fungus Fusarium graminearum. Plant Physiol. 2015, 169, 2895–2906. [Google Scholar]
- Nukarinen, E.; Nägele, T.; Pedrotti, L.; Wurzinger, B.; Mair, A.; Landgraf, R.; Börnke, F.; Hanson, J.; Teige, M.; Baena-González, E.; et al. Quantitative phosphoproteomics reveals the role of the ampk plant ortholog SnRK1 as a metabolic master regulator under energy deprivation. Sci. Rep. 2016, 6, 31697. [Google Scholar] [CrossRef]
- Karavidas, I.; Ntatsi, G.; Vougeleka, V.; Karkanis, A.; Ntanasi, T.; Saitanis, C.; Agathokleous, E.; Ropokis, A.; Sabatino, L.; Tran, F.; et al. Agronomic Practices to Increase the Yield and Quality of Common Bean (Phaseolus vulgaris L.): A Systematic Review. Agronomy 2022, 12, 271. [Google Scholar]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global Warming, Climate Change, and Environmental Pollution: Recipe for a Multifactorial Stress Combination Disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar]
- Soltis, D.E.; Visger, C.J.; Soltis, P.S. The polyploidy revolution then...and now: Stebbins revisited. Am. J. Bot. 2014, 101, 1057–1078. [Google Scholar]
- Severin, A.J.; Cannon, S.B.; Graham, M.M.; Grant, D.; Shoemaker, R.C. Changes in Twelve Homoeologous Genomic Regions in Soybean following Three Rounds of Polyploidy. Plant Cell 2011, 23, 3129–3136. [Google Scholar] [PubMed]
- Jo, B.S.; Choi, S.S. Introns: The functional benefits of introns in genomes. Genom. Inform. 2015, 13, 112–118. [Google Scholar]
- Colina, F.; Amaral, J.; Carbó, M.; Pinto, G.; Soares, A.; Cañal, M.J.; Valledor, L. Genome-wide identification and characterization of CKIN/SnRK gene family in Chlamydomonas reinhardtii. Sci. Rep. 2019, 9, 350. [Google Scholar]
- Shan, Z.; Jiang, Y.; Li, H.; Guo, J.; Dong, M.; Zhang, J.; Liu, G. Genome-wide analysis of the NAC transcription factor family in broomcorn millet (Panicum miliaceum L.) and expression analysis under drought stress. BMC Genom. 2020, 21, 96. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).