Mega Meta-QTLs: A Strategy for the Production of Golden Barley (Hordeum vulgare L.) Tolerant to Abiotic Stresses
Abstract
1. Introduction
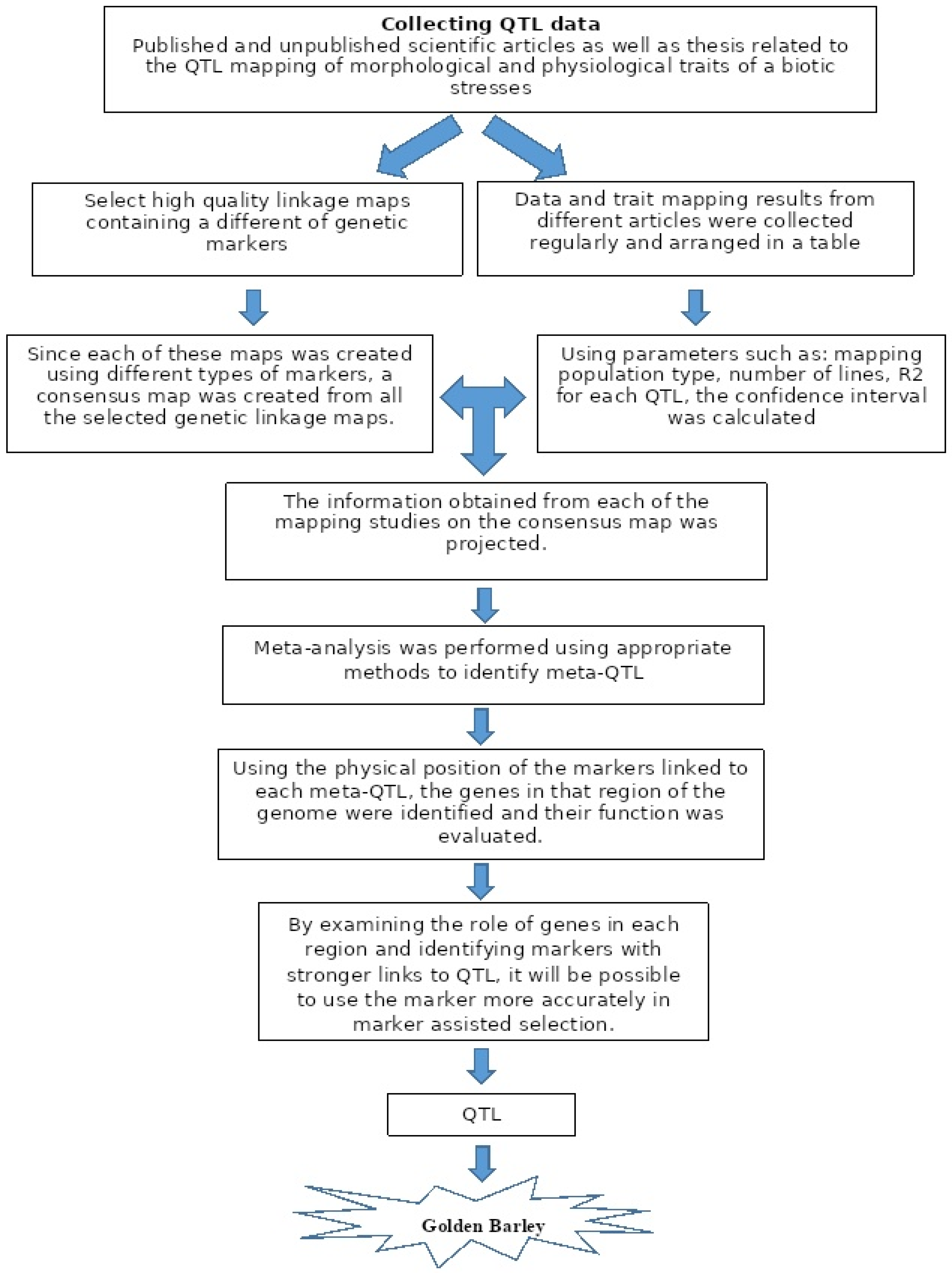
2. Materials and Methods
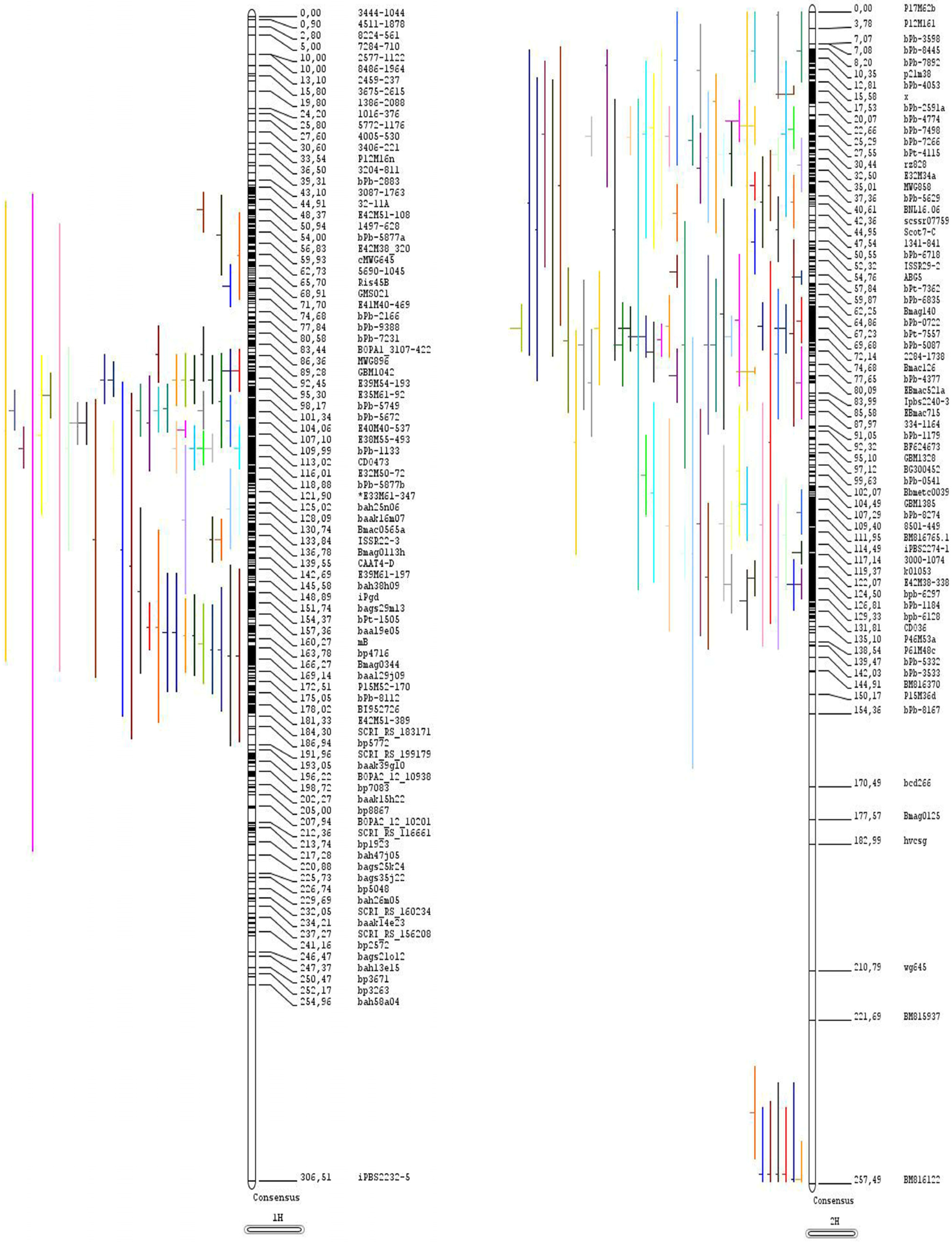
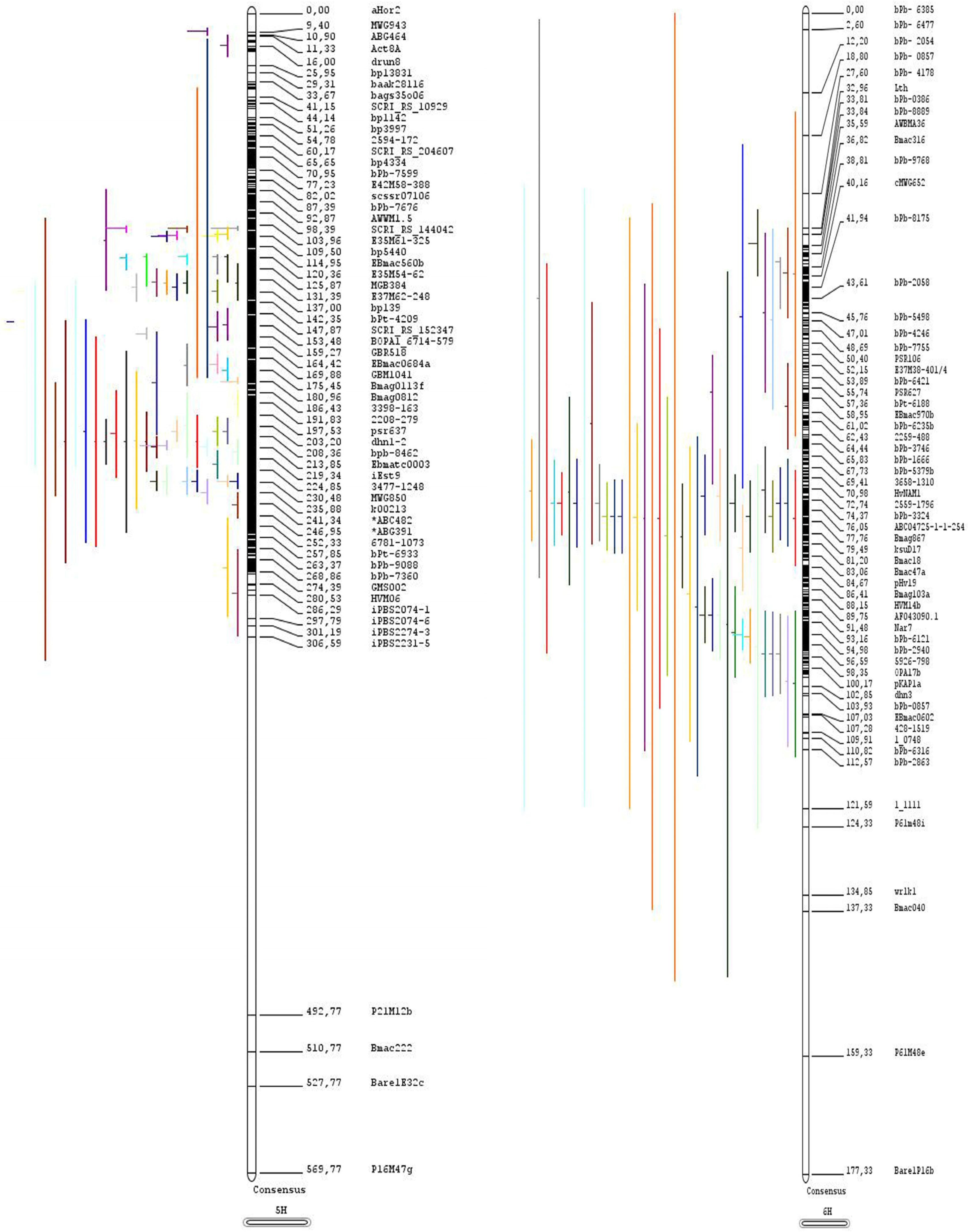
Construction of a Consensus Linkage Map
3. Results
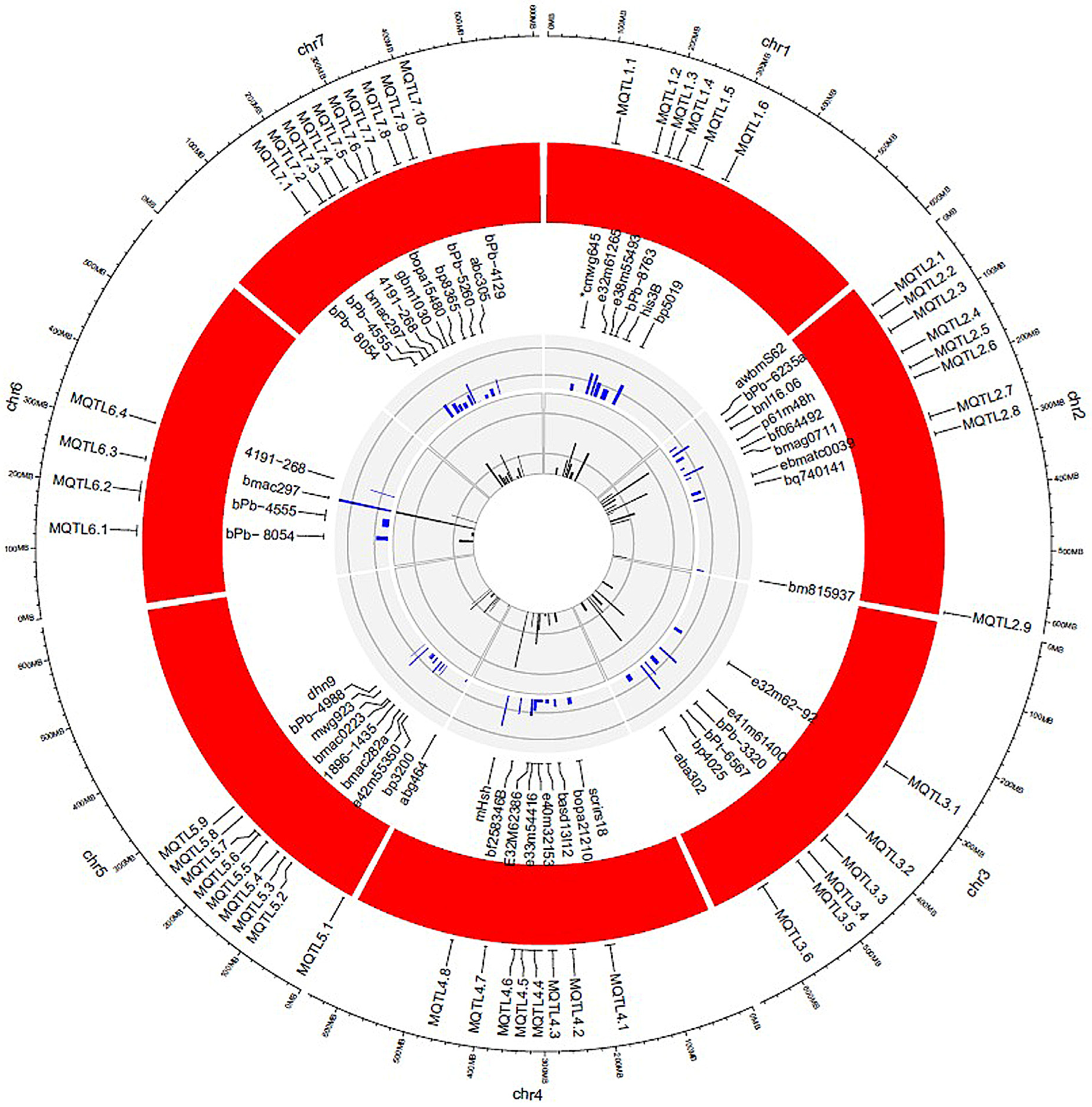
3.1. Distribution of QTLs and MQTLs
3.2. Overlap of QTLs in Meta-QTLs
3.3. Major MQTLs
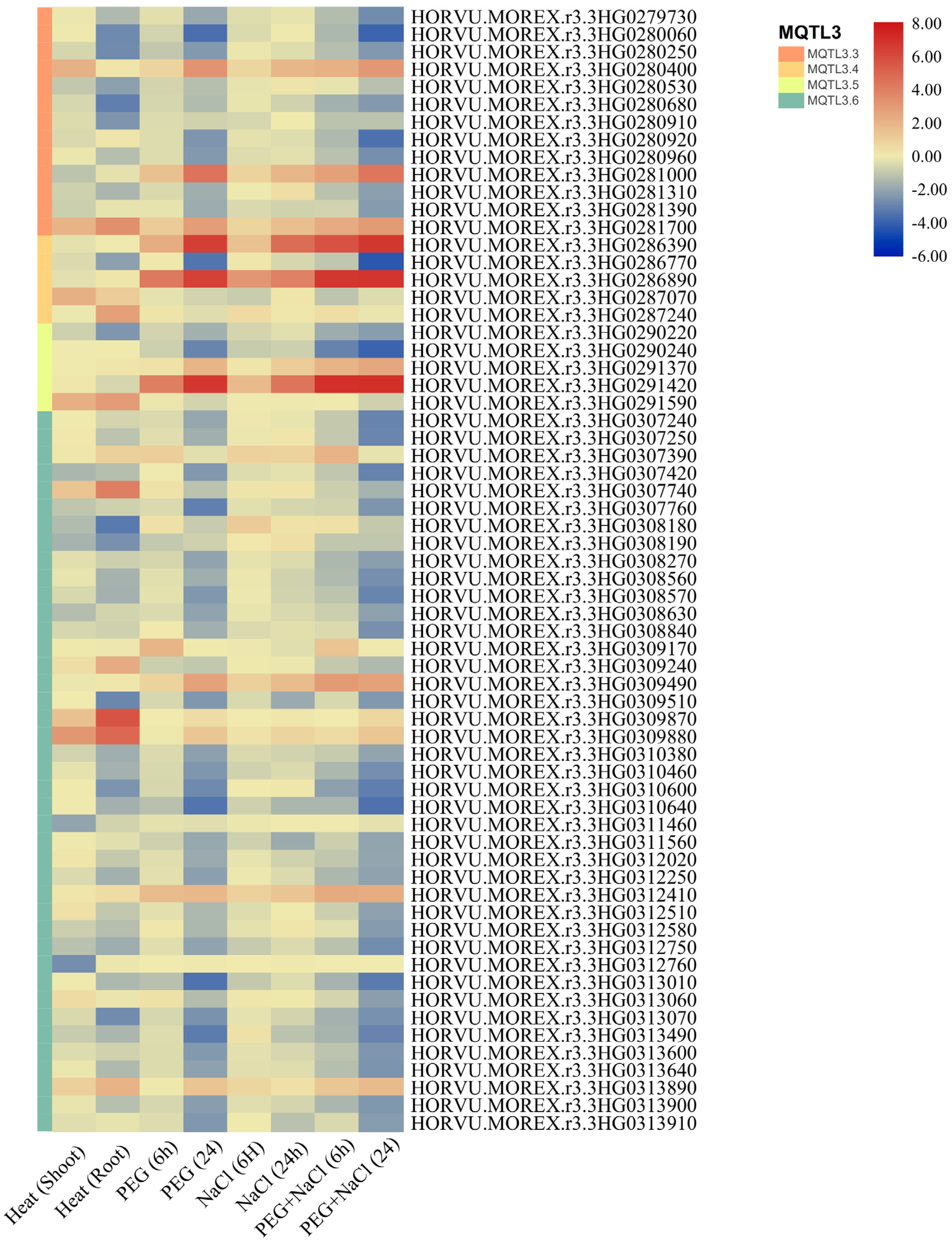
3.4. Candidate Genes
3.5. Evaluation of MQTLs in Geneinvestigator Software
4. Discussion
4.1. Mega MQTLs
4.1.1. Mega MQTL6.3
4.1.2. Mega MQTL4.8
4.1.3. Mega MQTL3.5
4.2. Gene Ontology
4.3. Markers Selection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, M.; Kour, D.; Yadav, A.N.; Saxena, R.; Rai, K.P.; Jyoti, A.; Tomar, R.S. Biodiversity of methylotrophic microbial communities and their potential role in mitigation of abiotic stresses in plants. Biologia 2019, 74, 287–308. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R. Silicon (Si): Review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotoxicol. Environ. Saf. 2018, 147, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and Abiotic Stresses in Plants, 1st ed.; de Oliveira, A.B., Ed.; IntechOpen: London, UK, 2019; pp. 3–8. [Google Scholar]
- Gürel, F.; Öztürk, Z.N.; Uçarlı, C.; Rosellini, D. Barley Genes as Tools to Confer Abiotic Stress Tolerance in Crops. Front. Plant Sci. 2016, 7, 1137. [Google Scholar] [CrossRef] [PubMed]
- Kuczyńska, A.; Cardenia, V.; Ogrodowicz, P.; Kempa, M.; Rodriguez-Estrada, M.T.; Mikołajczak, K. Effects of multiple abiotic stresses on lipids and sterols profile in barley leaves (Hordeum vulgare L.). Plant Physiol. Biochem. 2019, 141, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Makhtoum, S.; Sabouri, H.; Qolizadeh, A.; Ahangar, A.; Katouzi, M.; Gholamalipour-Alamdari, A. Mapping of genes controlling secondry metabolits in barley (Hordeum vulgare L.) under salinity stress. Environ. Stresses Crop Sci. 2021, 14, 771–781. [Google Scholar] [CrossRef]
- Ghaffari-Moghadam, S.; Sabouri, H.; Gholizadeh, A.; Fallahi, H. Genetic structure of some agronomic traits of barley under normal and salinity conditions. Mod. Genet. J. 2019, 13, 489–502. [Google Scholar] [CrossRef]
- Fan, F.; Zhou, G.; Shabala, S.; Chen, Z.H.; Cai, S.; Li, C.; Zhou, M. Genome-wide association study reveals a new QTL for salinity tolerance in barley (Hordeum vulgare L.). Front. Plant Sci. 2016, 7, 946. [Google Scholar] [CrossRef]
- Wójcik-Jagła, M.; Fiust, A.; Kościelniak, J.; Rapacz, M. Association mapping of drought tolerance-related traits in barley to complement a traditional biparental QTL mapping study. Theor. Appl. Genet. 2018, 131, 167–181. [Google Scholar] [CrossRef]
- Derakhshani, B.; Jafary, H.; Maleki-Zanjani, B.; Hasanpur, K.; Mishina, K.; Tanaka, T.; Kawahara, Y.; Oono, Y. Combined QTL mapping and RNA-Seq profiling reveals candidate genes associated with cadmium tolerance in barley. PLoS ONE 2020, 15, e0230820. [Google Scholar] [CrossRef]
- Capo-chichi, L.J.A.; Eldridge, S.; Elakhdar, A.; Kubo, T.; Brueggeman, R.; Anyia, A.O. QTL mapping and phenotypic variation for seedling vigour traits in barley (Hordeum vulgare L.). Plants 2021, 10, 1149. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, S.; Liu, Y.; Liu, Y.; You, J.; Deng, M.; Ma, J.; Chen, G.; Wei, Y.; Liu, C.; et al. Mapping and validation of major quantitative trait loci for kernel length in wild barley (Hordeum vulgare ssp. spontaneum). BMC Genet. 2016, 171, 130. [Google Scholar] [CrossRef] [PubMed]
- Soltani, E.; Soltani, A. Necessity of using meta-analysis in field crops researches. Crop Prod. 2014, 7, 203–216. Available online: https://www.sid.ir/paper/135090/en (accessed on 15 October 2022). (In Persian).
- Azizi, F. Meta-Analysis Articles in Medicine: Capabilities and limitations. Iran. J. Endocrinol. Metab. 2014, 16, 77–80. Available online: http://ijem.sbmu.ac.ir/article-1-1762-fa.html (accessed on 15 October 2022). (In Persian).
- Gurevitch, J.; Koricheva, J.; Nakagawa, S.; Stewart, G. Meta-analysis and the science of research synthesis. Nature 2018, 555, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Li, W.T.; Liu, C.; Liu, Y.X.; Pu, Z.E.; Dai, S.F.; Wang, J.R.; Lan, X.J.; Zheng, Y.L.; Wei, Y.M. Meta-analysis of QTL associated with tolerance to abiotic stresses in barley. Euphytica 2013, 189, 31–49. [Google Scholar] [CrossRef]
- Gudys, K.; Guzy-Wrobelska, J.; Janiak, A.; Dziurka, M.A.; Ostrowska, A.; Hura, K.; Jurczyk, J.; Zmuda, K.; Grzybkowska, D.; Sróbka, J.; et al. Prioritization of candidate genes in QTL regions for physiological and biochemical traits underlying drought response in barley (Hordeum vulgare L.). Front. Plant Sci. 2018, 9, 769. [Google Scholar] [CrossRef]
- Khahani, B.; Tavakol, E.; Shariati, V.; Rossini, L. Meta-QTL and ortho-MQTL analyses identified genomic regions controlling rice yield, yield-related traits and root architecture under water deficit conditions. Sci. Rep. 2021, 11, 6942. [Google Scholar] [CrossRef]
- Ellis, R.P.; Forster, B.P.; Gordon, D.C.; Handley, L.L.; Keith, R.P.; Lawrence, P.; Meyer, R.; Powell, W.; Robinson, D.; Scrimgeour, C.M.; et al. Phenotype/genotype associations for yield and salt tolerance in a barley mapping population segregating for two dwarfing genes. J. Exp. Bot. 2002, 53, 1163–1176. [Google Scholar] [CrossRef]
- Zhou, G.; Johnson, P.; Ryan, P.R.; Delhaize, E.; Zhou, M. Quantitative trait loci for salinity tolerance in barley (Hordeum vulgare L.). Mol. Breed. 2012, 29, 427–436. [Google Scholar] [CrossRef]
- Sbei, H.; Sato, K.; Shehzad, T.; Harrabi, M.; Okuno, K. Detection of QTLs for salt tolerance in Asian barley (Hordeum vulgare L.) by association analysis with SNP markers. Breed. Sci. 2014, 64, 378–388. [Google Scholar] [CrossRef]
- Ghaffari-Moghadam, S.; Sabouri, H.; Gholizadeh, A.; Fallahi, H. Identification of QTLs associated with some (Hordeum vulgare L.) traits in a germination stage under salt stress conditions. Iran. J. Plant Biol. 2019, 11, 79–94. (In Persian) [Google Scholar] [CrossRef]
- Xue, D.; Huang, Y.; Zhang, X.; Wei, K.; Westcott, K.; Li, C.; Chen, M.; Zhang, G.; Lance, L. Identification of QTLs associated with salinity tolerance at late growth stage in barley. Euphytica 2009, 169, 187–196. [Google Scholar] [CrossRef]
- Xue, W.; Yan, J.; Zhao, G.; Jiang, Y.; Cheng, J.; Cattivelli, L.; Tondelli, A. A major QTL on chromosome 7HS controls the response of barley seedling to salt stress in the Nure × Tremois population. BMC Genet. 2017, 18, 79. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, J.; Li, C.; Johnson, P.; Lu, C.; Zhou, M. A single locus is responsible for salinity tolerance in a Chinese landrace barley (Hordeum vulgare L.). PLoS ONE 2012, 7, e43079. [Google Scholar] [CrossRef]
- Shavrukov, Y.; Gupta, N.K.; Miyazaki, J.; Baho, M.N.; Chalmers, K.J.; Tester, M.; Langridge, P.; Collins, N.C. HvNax3—A locus controlling shoot sodium exclusion derived from wild barley (Hordeum vulgare ssp. spontaneum). Funct. Integr. Genom. 2010, 10, 277–291. [Google Scholar] [CrossRef]
- Makhtoum, S.; Sabouri, H.; Gholizadeh, A.; Ahangar, L.; Taliei, F.; Katouzi, M. Important chromosomal regions for genetic control of powdery mildew resistance under control, drought, and saline conditions in barley (Hordeum vulgare L.). Trop. Plant Pathol. 2021, 46, 622–642. [Google Scholar] [CrossRef]
- Mano, Y.; Takeda, K. Mapping quantitative trait loci for salt tolerance at germination and the seedling stage in barley (Hordeum vulgare L.). Euphytica 1997, 94, 263–272. [Google Scholar] [CrossRef]
- Liu, X.; Fan, Y.; Mak, M.; Babla, M.; Holford, P.; Wang, F.; Chen, G.; Scott, G.; Wang, G.; Shabala, S.; et al. QTLs for stomatal and photosynthetic traits related to salinity tolerance in barley. BMC Genom. 2017, 18, 9. [Google Scholar] [CrossRef]
- Fan, Y.; Shabala, S.; Ma, Y.; Xu, R.; Zhou, M. Using QTL mapping to investigate the relationships between abiotic stress tolerance (drought and salinity) and agronomic and physiological traits. BMC Genom. 2015, 16, 43. [Google Scholar] [CrossRef]
- Makhtoum, S.; Sabouri, H.; Gholizadeh, A.; Ahangar, L.; Katouzi, M. QTLs controlling physiological and morphological traits of barley (Hordeum vulgare L.) seedlings under salinity, drought, and normal conditions. BioTech 2022, 11, 26. [Google Scholar] [CrossRef]
- Makhtoum, S.; Sabouri, H.; Gholizadeh, A.; Ahangar, L.; Katouzi, M.; Mastinu, A. Mapping of QTLs controlling barley agronomic traits (Hordeum vulgare L.) under normal conditions and drought and salinity stress at reproductive stage. Plant Gene 2022, 31, 100375. [Google Scholar] [CrossRef]
- Sayed, M.A. QTL Analysis for Drought Tolerance Related to Root and Shoot Traits in Barley (Hordeum vulgare L.). Ph.D. Thesis, Universitäts Bonn, Bonn, Germany, 2011. [Google Scholar]
- Diab, A.A.; Teulat-Merah, B.; This, D.; Ozturk, N.Z.; Benscher, D.; Sorrells, M.E. Identification of drought-inducible genes and differentially expressed sequence tags in barley. Theor. Appl. Genet. 2004, 109, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Arifuzzaman, M.; Sayed, M.A.; Muzammil, S.; Pillen, K.; Schumann, H.; Naz, A.A.; Léon, J. Detection and validation of novel QTL for shoot and root traits in barley (Hordeum vulgare L.). Mol. Breed. 2014, 34, 1373–1387. [Google Scholar] [CrossRef]
- Sayed, M.A.; Schumann, H.; Pillen, K.; Naz, A.A.; Léon, J. AB-QTL analysis reveals new alleles associated to proline accumulation and leaf wilting under drought stress conditions in barley (Hordeum vulgare L.). BMC Genet. 2012, 13, 61. [Google Scholar] [CrossRef]
- Huang, X.; Fan, Y.; Shabala, L.; Rengel, Z.; Shabala, S.; Zhou, M.X. A major QTL controlling the tolerance to manganese toxicity in barley (Hordeum vulgare L.). Mol. Breed. 2018, 38, 16. [Google Scholar] [CrossRef]
- Kindu, G.A.; Tang, J.; Yin, X.; Struik, P.C. Quantitative trait locus analysis of nitrogen use efficiency in barley (Hordeum vulgare L.). Euphytica 2014, 199, 207–221. [Google Scholar] [CrossRef]
- Navakode, S.; Weidner, A.; Varshnez, R.K.; Lohasser, U.; Scholy, U.; Borner, A. A QTL Analysis of Aluminium Tolerance in Barley, Using Gene-Based Markers. Cereal Res. Commun. 2009, 37, 531–540. [Google Scholar] [CrossRef]
- Saal, B.; Von-Korff, M.; Léon, J.; Pillen, K. Advanced-backcross QTL analysis in spring barley: IV. Localization of QTL × nitrogen interaction effects for yield-related traits. Euphytica 2011, 177, 223–239. [Google Scholar] [CrossRef]
- Yang, L.; Mickelson, S.; See, D.; Blake, T.K.; Fischer, A.M. Genetic analysis of the function of major leaf proteases in barley (Hordeum vulgare L.) nitrogen remobilization. J. Exp. Bot. 2004, 55, 2607–2616. [Google Scholar] [CrossRef]
- Li, H.; Vaillancourt, R.; Mendham, N.; Zhou, M. Comparative mapping of quantitative trait loci associated with waterlogging tolerance in barley (Hordeum vulgare L.). BMC Genom. 2008, 9, 401. [Google Scholar] [CrossRef]
- Xue, D.W.; Zhou, M.X.; Zhang, X.Q.; Chen, S.; Wei, K.; Zeng, F.R.; Mao, Y.; Wu, F.B.; Zhang, G.P. Identification of QTLs for yield and yield components of barley under different growth conditions. J. Zhejiang Univ. Sci. B 2010, 11, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Johnson, P.; Zhou, G.; Li, C.; Lance, R. Quantitative Trait Loci for Waterlogging Tolerance in a Barley Cross of Franklin × YuYaoXiangTian Erleng and the Relationship Between Waterlogging and Salinity Tolerance. J. Crop Sci. 2012, 52, 2082–2088. [Google Scholar] [CrossRef]
- Broughton, S.; Zhou, G.; Teakle, N.L.; Matsuda, R.; Zhou, M.; O’Leary, R.A.; Colmer, T.D.; Li, C. Waterlogging tolerance is associated with root porosity in barley (Hordeum vulgare L.). Mol. Breed. 2015, 35, 27. [Google Scholar] [CrossRef]
- Francia, E.; Rizza, F.; Cattivelli, L.; Stanca, A.M.; Galiba, G.; Tóth, B.; Hayes, P.M.; Skinner, J.S.; Pecchioni, N. Two loci on chromosome 5H determine low-temperature tolerance in a ‘Nure’ (winter) x ‘Tremois’ (spring) barley map. Theor. Appl. Genet. 2004, 108, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Skinner, J.S.; Szucs, P.; Von-Zitzewitz, J.; Marquez-Cedillo, L.; Filichkin, T.; Stockinger, E.J.; Thomashow, M.F.; Chen, T.H.H.; Hayes, P.M. Mapping of barley homologs to genes that regulate low temperature tolerance in Arabidopsis. Theor. Appl. Genet. 2006, 112, 832–842. [Google Scholar] [CrossRef]
- Makhtoum, S.; Sabouri, H.; Qolizadeh, A.; Ahangar, A.; Katouzi, M. Quantitative Genes Controlling Chlorophyll Fluorescence Attributes in Barley (Hordeum vulgare L.). J. Genet. Resour. 2021, 7, 72–86. [Google Scholar] [CrossRef]
- Xue, D.W.; Chen, M.C.; Zhou, M.X.; Chen, S.; Mao, Y.; Zhang, G.P. QTL analysis of flag leaf in barley (Hordeum vulgare L.) for morphological traits and chlorophyll content. J. Zhejiang Univ. Sci. B 2008, 9, 938–943. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, Q.; Tan, C.; Zhang, X.Q.; Li, C. Development of genome-wide InDel markers and their integration with SSR, DArT and SNP markers in single barley map. BMC Genom. 2015, 16, 804. [Google Scholar] [CrossRef]
- Wenzl, P.; Li, H.; Carling, J.; Zhou, M.; Raman, H.; Paul, E.; Hearnden, P.; Maier, C.; Xia, L.; Caig, V.; et al. A high-density consensus map of barley linking DArT markers to SSR, RFLP and STS loci and agricultural traits. BMC Genom. 2006, 7, 206. [Google Scholar] [CrossRef]
- Sosnowski, O.; Charcosset, A.; Joets, J. BioMercator V3: An upgrade of genetic map compilation and quantitative trait loci meta-analysis algorithms. Bioinformatics 2012, 28, 2082–2083. [Google Scholar] [CrossRef]
- Zhang, X.; Shabala, S.; Koutoulis, A.; Shabala, L.; Zhou, M. Meta-analysis of major QTL for abiotic stress tolerance in barley and implications for barley breeding. Planta 2016, 245, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Khahani, B.; Tavakol, E.; Shariati, V. Genome-wide meta-analysis on yield and yield-related QTLs in barley (Hordeum vulgare L.). J. Plant Mol. Breed. 2019, 39, 56. [Google Scholar] [CrossRef]
- Darvasi, A.; Soller, M. A Simple Method to Calculate Resolving Power and Confidence Interval of QTL Map Location. Behav. Genet. 1997, 27, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Gui, B.; Sleper, D.A.; Lu, P.; Shannon, J.G.; Nguyen, H.T.; Arelli, P.R. QTLs Associated with Resistance to Soybean Cyst Nematode in Soybean: Meta-Analysis of QTL Locations. J. Crop Sci. 2006, 46, 595–602. [Google Scholar] [CrossRef]
- Karakousis, A.; Barr, A.R.; Kretschmer, J.M.; Manning, S.; Jefferies, S.P.; Chalmers, K.J.; Islam, A.K.M.; Langridge, P. Mapping and QTL analysis of the barley population Clipper × Sahara. Aust. J. Agric. Res. 2003, 54, 1137–1140. [Google Scholar] [CrossRef]
- Horsley, R.D.; Schmierer, D.; Maier, C.; Kudrna, D.; Urrea, C.A.; Steffenson, B.J.; Schwarz, P.B.; Franckowiak, J.D.; Green, M.J.; Zhang, B.; et al. Identification of QTLs Associated with Fusarium Head Blight Resistance in Barley Accession CIho 4196. J. Crop Sci. 2006, 46, 45–156. [Google Scholar] [CrossRef]
- Mesfin, A.; Smith, K.P.; Dill-Macky, R.; Evans, C.K.; Waugh, R.; Gustus, C.D.; Muehlbauer, G.J. Quantitative Trait Loci for Fusarium Head Blight Resistance in Barley Detected in a Two-Rowed by Six-Rowed Population. J. Crop Sci. 2003, 43, 307–318. [Google Scholar] [CrossRef]
- Kleinhofs, A.; Kilian, A.; Saghai-Maroof, M.A.; Biyashev, R.M.; Hayes, P.; Chen, F.Q.; Lapitan, N.; Fenwick, A.; Blake, T.K.; Kanazin, V.; et al. A molecular, isozyme and morphological map of the barley (Hordeum vulgare) genome. Theor. Appl. Genet. 1993, 86, 705–712. [Google Scholar] [CrossRef]
- Li, H.B.; Zhou, M.X.; Liu, C.J. A major QTL conferring crown rot resistance in barley and its association with plant height. Theor. Appl. Genet. 2009, 118, 903–910. [Google Scholar] [CrossRef]
- Li, J.Z.; Sjakste, T.G.; Röder, M.S.; Ganal, M.W. Development and genetic mapping of 127 new microsatellite markers in barley. Theor. Appl. Genet. 2003, 107, 1021–1027. [Google Scholar] [CrossRef]
- Graner, A.; Jahoor, A.; Schondelmaier, J.; Siedler, H.; Pillen, K.; Fischbeck, G.; Wenzel, G.; Herrmann, R.G. Construction of an RFLP map of barley. Theor. Appl. Genet. 1991, 83, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Salvo-Garrido, H.; Laurie, D.A.; Jaffé, B.; Snape, J.W. An RFLP map of diploid Hordeum bulbosum L. and comparison with maps of barley (H. vulgare L.) and wheat (Triticum aestivum L.). Theor. Appl. Genet. 2001, 103, 869–880. [Google Scholar] [CrossRef]
- Ramsay, L.; Macaulay, M.; Degli-Ivanissevich, S.; MacLean, K.; Cardle, L.; Fuller, J.; Edwards, K.J.; Tuvesson, S.; Morgante, M.; Massari, A.; et al. A simple sequence repeat-based linkage map of barley. J. Genet. 2000, 156, 1997–2005. [Google Scholar] [CrossRef]
- Fan, C.; Zhai, H.; Wang, H.; Yue, Y.; Zhang, M.; Li, J.; Wen, S.; Guo, G.; Zeng, Y.; Ni, Z.; et al. Identification of QTLs controlling grain protein concentration using a high-density SNP and SSR linkage map in barley (Hordeum vulgare L.). BMC Plant Biol. 2017, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Dracatos, P.M.; Haghdoust, R.; Singh, R.P.; Huerta-Espino, J.; Barnes, C.W.; Forrest, K.; Hayden, M.; Niks, R.E.; Park, R.F.; Singh, D. High-Density Mapping of Triple Rust Resistance in Barley Using DArT-Seq Markers. Front. Plant Sci. 2019, 26, 467. [Google Scholar] [CrossRef]
- Marcel, T.C.; Varshney, R.K.; Barbieri, M.; Jafary, H.; de Kock, M.J.D.; Graner, A.; Niks, R.E. A high-density consensus map of barley to compare the distribution of QTLs for partial resistance to Puccinia hordei and of defence gene homologues. Theor. Appl. Genet. 2007, 114, 487–500. [Google Scholar] [CrossRef]
- Ren, X.; Wang, J.; Liu, L.; Sun, G.; Li, C.; Luo, H.; Sun, D. SNP-based high density genetic map and mapping of btwd1 dwarfing gene in barley. Sci. Rep. 2016, 6, 31741. [Google Scholar] [CrossRef]
- Varshney, R.K.; Marcel, T.C.; Ramsay, L.; Russell, J.; Röder, M.S.; Stein, N.; Waugh, R.; Langridge, P.; Niks, R.E.; Graner, A. A high density barley microsatellite consensus map with 775 SSR loci. Theor. Appl. Genet. 2007, 114, 1091–1103. [Google Scholar] [CrossRef]
- Sato, K.; Nankaku, N.; Takeda, K. A high-density transcript linkage map of barley derived from a single population. Heredity 2009, 103, 110–117. [Google Scholar] [CrossRef]
- Dahleen, L.S.; Agrama, H.A.; Horsley, R.D.; Steffenson, B.J.; Schwarz, P.B.; Mesfin, A.; Franckowiak, J.D. Identification of QTLs associated with Fusarium head blight resistance in Zhedar 2 barley. Theor. Appl. Genet. 2003, 108, 95–104. [Google Scholar] [CrossRef]
- Costa, J.M.; Corey, A.; Hayes, P.M.; Jobet, C.; Kleinhofs, A.; Kopisch-Obusch, A.; Kramer, S.F.; Kudrna, D.; Li, M.; Riera-Lizarazu, O.; et al. Molecular mapping of the Oregon Wolfe Barleys: A phenotypically polymorphic doubled-haploid population. Theor. Appl. Genet. 2001, 103, 415–424. [Google Scholar] [CrossRef]
- Han, M.; Wong, J.; Su, T.; Beatty, P.H.; Good, A.G. Identification of Nitrogen Use Efficiency Genes in Barley: Searching for QTLs Controlling Complex Physiological Traits. Front. Plant Sci. 2016, 7, 1587. [Google Scholar] [CrossRef] [PubMed]
- Veyrieras, J.B.; Goffinet, B.; Charcosset, A. MetaQTL: A package of new computational methods for the meta-analysis of QTL mapping experiments. BMC Bioinform. 2007, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Arcade, A.; Labourdette, A.; Falque, M.; Mangin, B.; Chardon, F.; Charcosset, A.; Joets, J. BioMercator: Integrating genetic maps and QTL towards discovery of candidate genes. J. Bioinform. 2004, 20, 2324–2326. [Google Scholar] [CrossRef] [PubMed]
- Khalily, M.; Aharizad, S.; Poraboghadareh, A. Response of Barley Double Haploid Lines to the Grain Yield and Morphological Traits under Water Deficit Stress Conditions. J. Crop Ecophysiol. 2017, 10, 927–943. (In Persian) [Google Scholar]
- Keshavarznia, R.; Shahbazi, M.; Mohammadi, M.; Hosseini-Salekdeh, G.; Ahmadi, A.; Mohseni-Fard, E. The impact of barley root structure and physiological traits on drought response. Iran. J. Field Crop Sci. 2015, 45, 553–563. (In Persian) [Google Scholar] [CrossRef]
- Liu, X.; Mak, M.; Babla, M.; Wang, F.; Chen, G.; Veljanoski, F.; Wang, G.; Shabala, S.; Zhou, M.; Chen, Z.H. Linking stomatal traits and expression of slow anion channel genes HvSLAH1 and HvSLAC1 with grain yield for increasing salinity tolerance in barley. Front. Plant Sci. 2014, 5, 634. [Google Scholar] [CrossRef]
- Parkar, N.; Pirasteh-Anosheh, H.; Emam, Y.; Pesarakli, M. Barley growth, yield, antioxidant enzymes, and ion accumulation affected by PGRs under salinity stress conditions. J. Plant Nutr. 2014, 39, 1372–1379. [Google Scholar] [CrossRef]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.A.; Baenziger, P.S.; Börner, A. Drought Stress Tolerance in Wheat and Barley: Advances in Physiology, Breeding and Genetics Research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef]
- Bandurska, H.; Niedziela, J.; Pietrowska-Borek, M.; Nuc, K.; Chadzinikolau, T.; Radzikowska, D. Regulation of proline biosynthesis and resistance to drought stress in two barley (Hordeum vulgare L.) genotypes of different origin. Plant Physiol. Biochem. 2017, 118, 427–437. [Google Scholar] [CrossRef]
- Zhu, J.; Fan, Y.; Li, C.; Shabala, S.; Zhao, C.; Hong, Y.; Lv, C.; Guo, B.; Xu, R.; Zhou, M. Candidate genes for salinity tolerance in barley revealed by RNA-seq analysis of near-isogenic lines. Plant Growth Regul. 2020, 92, 571–582. [Google Scholar] [CrossRef]
- Nikkhah, H.R.; Mohammadi, V.A.; Naghavi, M.R.; Soltanloo, H. The Effect of Salinity Stress on Morphological and physiological Traits of Recombinant Inbred Lines Population of Barley derived a cross between Arigashar×Igri. Iran. J. Field Crop Sci. 2015, 46, 181–192. (In Persian) [Google Scholar] [CrossRef]
- Wu, H.; Zhu, M.; Shabala, L.; Zhou, M.; Shabala, S. K+ retention in leaf mesophyll, an overlooked component of salinity tolerance mechanism: A case study for barley. J. Integr. Plant Biol. 2015, 57, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Janiak, A.; Kwasniewski, M.; Sowa, M.; Kuczyńska, A.; Mikołajczak, K.; Ogrodowicz, P.; Szarejko, I. Insights into Barley Root Transcriptome under Mild Drought Stress with an Emphasis on Gene Expression Regulatory Mechanisms. Int. J. Mol. Sci. 2019, 20, 6139. [Google Scholar] [CrossRef] [PubMed]
- Kul, R.; Ekinci, M.; Turan, M.; Ors, S.; Yildirim, E. How Abiotic Stress Conditions Affects Plant Roots, 1st ed.; Ekinci, M., Turan, M., Yildirim, E., Eds.; IntechOpen: London, UK, 2021; pp. 53–76. [Google Scholar]
- Haghpanah, K.; Mirfakhraee, S.; Khodadadi, M.; Shamsifar, S. Study on Genetic Diversity of some Barley (Hordeum vulgare L.) Cultivars using SSR Marker and Physiological Traits Plant Pigments and Proline undar Late Cold Stress. JCB 2020, 12, 199–209. Available online: http://jcb.sanru.ac.ir/article-1-1115-en.html (accessed on 15 October 2022). (In Persian). [CrossRef]
- Malesevic, M.; Glamoclija, D.; Przulj, N.; Popovic, V.; Stankovic, S.; Zivanovic, T.; Tapanarova, A. Production characteristics of different malting barley genotypes in intensive nitrogen fertilization. Genetika 2010, 42, 323–330. [Google Scholar] [CrossRef]
- Tong, C.; Hill, C.B.; Zhou, G.; Zhang, X.Q.; Jia, Y.; Li, C. Opportunities for Improving Waterlogging Tolerance in Cereal Crops—Physiological Traits and Genetic Mechanisms. Plants 2021, 10, 1560. [Google Scholar] [CrossRef]
- Liu, K.; Harrison, M.; Ibrahim, A.; Manik, S.M.N.; Johnson, P.; Tian, X.; Meinke, H.; Zhou, M. Evaluating Genetic Contribution to Mitigation of Barley Grain Yield Penalty Caused by Soil Waterlogging. Authorea 2020. [Google Scholar] [CrossRef]
- de San Celedonio, R.P.; Abeledo, L.G.; Mantese, A.I.; Miralles, D.J. Differential root and shoot biomass recovery in wheat and barley with transient waterlogging during preflowering. Plant Soil 2017, 417, 481–498. [Google Scholar] [CrossRef]
- Rajaie, M.; Tahmasbi, S.; Attarzadeh, M. Evaluation of yield and salinity tolerance indices in productive barley lines under saline condition. J. Cereal Res. 2017, 7, 143–153. (In Persian) [Google Scholar] [CrossRef]
- Kebedea, A.; Kang, M.S.; Bekele, E. Advances in mechanisms of drought tolerance in crops, with emphasis on barley. Adv. Agron. 2019, 156, 265–314. [Google Scholar] [CrossRef]
- Honsdorf, N.; March, T.J.; Pillen, K. QTL controlling grain filling under terminal drought stress in a set of wild barley introgression lines. PLoS ONE 2017, 12, e0185983. [Google Scholar] [CrossRef] [PubMed]
- Bian, M.; Jin, X.; Broughton, S.; Zhang, X.Q.; Zhou, G.; Zhou, M.; Zhang, G.; Sun, D.; Li, C. A new allele of acid soil tolerance gene from a malting barley variety. BMC Genet. 2015, 16, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Raman, H.; Zhang, G.P.; Mendham, N.; Zhou, M.X. Aluminium tolerance in barley (Hordeum vulgare L.): Physiological mechanisms, genetics and screening methods. J. Zhejiang Univ. Sci. B 2006, 7, 769–787. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Maurer, A.; Pillen, K. Detection of nitrogen deficiency QTL in juvenile wild barley introgression linesgrowing in a hydroponic system. BMC Genet. 2012, 13, 88. [Google Scholar] [CrossRef]
- Zare-Kohan, M.; Babaeian-Jelodar, N.; Aghnoum, R.; Tabatabaee, S.A.; Kazemi-Tabar, S.K.; Ghasemi-Nezhad-Raeini, M.R.; Shahriaripour, R. Genetic diversity among barley genotypes and path analysis of several agronomic and physiological characters at normal and salinity stress conditions. J. Plant Physiol. Breed. 2021, 11, 121–134. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, K.; Singh, J. A root-specific wall-associated kinase gene, HvWAK1, regulates root growth and is highly divergent in barley and other cereals. Funct. Integr. Genom. 2013, 13, 167–177. [Google Scholar] [CrossRef]
- Wu, F.; Sheng, P.; Tan, J.; Chen, X.; Lu, G.; Ma, W.; Heng, Y.; Lin, Q.; Zhu, S.; Wang, J.; et al. Plasma membrane receptor-like kinase leaf panicle 2 acts downstream of the Drought and salt tolerance transcription factor to regulate drought sensitivity in rice. J. Exp. Bot. 2015, 66, 271–281. [Google Scholar] [CrossRef]
- Tripathi, R.K.; Aguirre, J.A.; Singh, J. Genome-wide analysis of wall associated kinase (WAK) gene family in barley. Genomics 2020, 113, 523–530. [Google Scholar] [CrossRef]
- Kidwai, M.; Ahmad, I.Z.; Chakrabarty, D. Class III peroxidase: An indispensable enzyme for biotic/abiotic stress tolerance and a potent candidate for crop improvement. Plant Cell Rep. 2020, 39, 1381–1393. [Google Scholar] [CrossRef]
- Vicuna-Requesens, D.; Malone, R.P.; Dix, P.J. Expression of a Barley Peroxidase in Transgenic Apple (Malus domestica L.) Results in Altered Growth, Xylem Formation and Tolerance to Heat Stress. J. Plant Sci. 2014, 9, 58–66. [Google Scholar] [CrossRef][Green Version]
- Wang, M.; Yuan, J.; Qin, L.; Shi, W.; Xia, G.; Liu, S. TaCYP81D5, one member in a wheat cytochrome P450 gene cluster, confers salinity tolerance via reactive oxygen species scavenging. Plant Biotechnol. J. 2020, 18, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Caitar, V.S.; de Carvalho, M.C.; Darben, L.M.; Kuwahara, M.K.; Nepomuceno, A.L.; Dias, W.P.; Abdelnoor, R.V.; Marcelino-Guimarães, F.C. Genome-wide analysis of the Hsp20 gene family in soybean: Comprehensive sequence, genomic organization and expression profile analysis under abiotic and biotic stresses. BMC Genom. 2013, 14, 577. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cheng, Y.; Feng, K.; Ruan, M.; Ye, Q.; Wang, R.; Li, Z.; Zhou, G.; Yao, Z.; Yang, Y.; et al. Genome-Wide Identification and Expression Profiling of Tomato Hsp20 Gene Family in Response to Biotic and Abiotic Stresse. Front. Plant Sci. 2016, 7, 1215. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Liu, J.H.; Lu, J.P.; Zhai, Y.F.; Wang, H.; Gong, Z.H.; Wang, S.B.; Liu, M.H. Genome-wide analysis of the CaHsp20 gene family in pepper: Comprehensive sequence and expression profile analysis under heat stress. Front. Plant Sci. 2015, 6, 806. [Google Scholar] [CrossRef]
- Li, J.; Liu, X. Genome-wide identification and expression profile analysis of the Hsp20 gene family in Barley (Hordeum vulgare L.). PeerJ 2019, 7, e6832. [Google Scholar] [CrossRef]
- Peng, Z.; Dittmer, N.T.; Lang, M.; Brummett, L.M.; Braun, C.L.; Davis, L.C.; Kanost, M.R.; Gorman, M.J. Multicopper oxidase-1 orthologs from diverse insect species have ascorbate oxidase activity. Insect Biochem. Mol. Biol. 2015, 59, 58–71. [Google Scholar] [CrossRef]
- Sakurai, T.; Kataoka, K. Basic and applied features of multicopper oxidases, CueO, bilirubin oxidase, and laccase. Chem. Rec. 2007, 7, 220–229. [Google Scholar] [CrossRef]
- Hoegger, P.J.; Kilaru, S.; James, T.Y.; Thacker, J.R.; Kües, U. Phylogenetic comparison and classification of laccase and related multicopper oxidase protein sequences. FEBS Lett. 2006, 273, 2308–2326. [Google Scholar] [CrossRef]
- Kong, W.; An, B.; Zhang, Y.; Yang, J.; Li, S.; Sun, T.; Li, Y. Sugar Transporter Proteins (STPs) in Gramineae Crops: Comparative Analysis, Phylogeny, Evolution, and Expression Profiling. Cells 2019, 8, 560. [Google Scholar] [CrossRef]





| Reference | Marker | Population | Parents | Population Size | No. of Marker |
|---|---|---|---|---|---|
| [6] | SSR, ISSR, iPBS, Scot, IRAP, CAAT | RIL | Badia × Kavir | 106 | 392 |
| [7] | SSR, ISSR, iPBS | F3 | Badia × Comino | 100 | 128 |
| [17] | SNP, SSR | RILs | Maresi × Cam/B1/CI08887//CI05761 | 100 | 819 |
| [19] | AFLP, SSAP, SSR, | DH | Derkado × B83-12/21/5 | 156 | 241 |
| [20] | DArT, SSR | DH | Yuyaoxiangtian Erleng × Franklin | 172 | 858 |
| [22] | SSR, ISSR, iPBS | F3 | Badia × Comino | 100 | 128 |
| [23] | SSR, DArT | DH | CM72 × Gairdner | 93 | 332 |
| [24] | SSR, SNP, DArT, STS, CAPS, dCAPS, | DH | Nure × Tremois | 118 | 162 |
| [25] | SSR, DArT | DH | TX9425 × Naso Nijo | 188 | 626 |
| [26] | SSR, DArT, CAPS | DH | Barque-73 × CPI-71284-48 | 72 | 1180 |
| [27] | SSR, ISSR, iPBS, Scot, CAAT, IRAP, | RILs | Badia × Kavir | 106 | 302 |
| [28] | SSR | DH | Steptoe × Morex, Harrington × TR306 | 149 146 | 103 |
| [29] | DArT, SSR | DH | CM72 × Gairdner | 108 | 886 |
| [30] | DArT, AFLP, SSR | DH | TX9425 ×Franklin | 72 | 520 |
| [33] | SSR, DArT, gene-specific marker | DH | Scarlett × ISR42-8 | 76 | 371 |
| [34] | RFLP | RILs | Tadmor × Er/Apm | 167 | 77 |
| [35] | SSR, DArT | DH | Scarlett × ISR42-8 | 301 | 371 |
| [36] | SSR, DArT, gene-specific marker | DH | Scarlett × ISR42-8 | 76 | 371 |
| [37] | SSR, DArT | DH | Yerong × Franklin TX9425 ×Naso Nijo | 177 188 | 2500 524 |
| [38] | AFLP | RILs | Prisma × Apex R | 94 | 191 |
| [39] | EST, BR, GBM, GBS, RFLP, SSR, SNP | DH | OWBDOM × OWBREC | 94 | 650 |
| [40] | SSR | DH | ISR42-8 × Scarlett | 301 | 98 |
| [41] | SSR | RIL | Lewis(CI15856) × Karl(CI15487) | 146 | 104 |
| [42] | SSR, AFLP, DArT | DH | TX9425 × Franklin, Yerong × Franklin | 92 177 | 520 524 |
| [43] | DArT, AFLP, microsatellite markers | DH | Yerong × Franklin | 156 | 604 |
| [44] | SSR, DArT | DH | YuYaoXiangTian Erleng × Franklin | 172 | 2223 |
| [45] | DArT, SSR | DH | Franklin × YuYaoXiangTian Erleng | 126 | 858 |
| [46] | SSR, RAPD, RFLP, CAPS, AFLP, STS, | DH | Nure × Tremois | 136 | 127 |
| [47] | SSR | DH | Dicktoo × Morex | 91 | 94 |
| [51] | DArT, SSR, RFLP, STS | DH, RIL | Barque73 × CPI71284-48, Clipper × Sahara, Dayton × Zhepi2, Foster × CI4196, Steptoe × Morex, TX9425 × Franklin, Yerong × Franklin | 707 | 2935 |
| [57] | AFLP, SSR, RFLP | DH | Clipper × Sahara 3771 | 150 | 211 |
| [58] | RFLP, SSR | F8–9, RIL | Foster × CIho 4196 | 250 | 206 |
| [59] | SSR | F4–6, RIL | Fredrickson × Stander | 116 | 143 |
| [60] | RFLP, RAPD, SAP | DH | Steptoe × Morex | 150 | 295 |
| [61] | DArT, AFLP, SSR | DH | TX9425 × Franklin | 92 | 520 |
| [62] | SSR | DH | Steptoe × Morex, Igri × Franka | 133 | 133 |
| [63] | RFLP | DH, F2/F3 | IGRI × FRANKA, VADA × H. spontaneum | 206 | 251 |
| [64] | RFLP | DH | PB1 × PB11 | 111 | 136 |
| [65] | SSR | DH | Lina × H. spontaneum Canada Park | 86 | 325 |
| [66] | SSR, SNP | RILs | ZGMLEL × Schooner | 190 | 1011 |
| [67] | DArT, SNP | RIL | Pompadour × Biosaline-19 | 98 | 8610 |
| [68] | RFLP, AFLP, SSR | DH RIL | Steptoe × Morex, Dom × Rec Igri × Franka, L94 ×Vada | 317 | 3258 |
| [69] | SNP, SSR | DH | Huadamai 6 × Huaai 11 | 122 | 1962 |
| [70] | SSR | RIL DH | Igri × Franka, Steptoe × Morex, OWBRec × OWBDom, Lina × Canada Park, L94 × Vada, SusPtrit × Vada | 645 | 775 |
| [71] | EST, CAPS, STS, SNP, SSR | DH | Haruna Nijo × H602 | 93 | 2948 |
| [72] | RFLP, SSR, AFLP, RGA | DH | Foster × ND9712 × Zhedar | 75 | 214 |
| [73] | RFLP, RAPD, STS, IFLP, SSR, AFLP | DH | Oregon Wolfe Barley | 94 | 830 725 |
| [74] | SNP | RIL | Morex × Barke | 81 | 195 |
| Stress | Traits |
|---|---|
| Drought | Root–shoot ratio, Root dry weight, Plant height, Harvest Index, Leaf weight, Pm.SEVAD, Stomata number, Thousand kernel weight, Leaf wilting score, Leaf osmotic potential, Free proline content, Ethylene content, Light absorption flux (ABS) per PSII reaction center, Water content, Trapped energy flux per PSII reaction center, Root Length, Drought tolerance score, Relative water content, Pm.AUDPCAD, Plant number, No. of Kernels/spike, Number of spikes/plant, Shoot dry weight, Water-soluble carbohydrate concentration at full turgor, Drought water-soluble carbohydrate concentration, Grain yield, Electron transport flux per PSII reaction center (RC), Osmotic adjustment, Osmotic potential under irrigation, Flag leaf weight, Leaf number, Plumule weight, Peduncle length, Flag leaf width, Water loss rate, The average fraction of open RC during the time needed to complete the closure of all RCs, Flag leaf length, Peduncle diameter, Internode length, Water-soluble carbohydrate concentration, REo/RC, DIo/CSm, TRo/RC, ABS/CSo, Fo, TRo/CSo, ABS/CSm, Fm, Sm, DIo/RC, DIo/CSo, ABS/RC |
| Salinity | GY grain yield, Phenol, Salinity score, Root length, Stomata length, Leaf number, Sugar content, Plumule length, Spike diameter, Peroxidase, Shoot dry weight, Leaf injury score, Stomatal pore area, Spikes per plant, Shoot weight, Biomass, T/C ratio, TR transpiration rate, GS stomatal conductance, Leaf weight, Flag leaf length, Grain weight, Seed dormancy, Catalase, Dry weight per plant, Grain number per plant, Flag leaf width, Plumule weight, Dry weight of roots, Green dry weight of shoots, Green fresh weight of shoots, Fresh weight of roots per plant, Pm.SEVAS, Proline content, Stomata width, Shoot diameter, Tiller number, Seedling weight, Seedling gibberellic acid response, Root dry weight, Leaf length, Flag leaf weight, Na+:K+ ratio, Awn weight, Seminal roots, Peduncle length, REo/RC, DIo/CSm, TRo/RC, ABS/CSo, Fo, TRo/CSo, ABS/CSm, Fm, Sm, DIo/RC, DIo/CSo, ABS/RC |
| Waterlogging | Longest root length, Shoot dry weight, Shoot fresh weight, Root fresh weight, Leaf chlorosis, Waterlogging index, Grain yield, Spike length, Plant survival, Tiller number, Leaf yellowing proportion, Porosity, Plant biomass reduction, Grains per spike |
| Cold stress | Cold score, TMC-Ap3 accumulation, Frost tolerance, Fv/Fm value, plant survival, COR14b accumulation, Vernalization requirement |
| Nitrogen Deficiency | Thousand-grain weight, Plant survival, Plant height, Number of ears, Grain yield, Days until heading, Thousand kernel weight, Sheath weight, Stem weight, Straw weight, Above-ground nitrogen uptake N, Nitrogen use efficiency of biomass, Above-ground biomass, Leaf weight, CP at maturity carboxypeptidase, Leaf chlorosis, N remobilization efficiency, Grain protein content |
| Aluminum toxicity | Aluminum tolerance score |
| Mn toxicity | Leaf chlorosis, Plant survival |
| Chr | Meta-QTL | AIC | R2 Meta | Meta-QTL Peak Position (bp) | Meta-QTL CI Range bp | Closest Markers |
|---|---|---|---|---|---|---|
| 1 | MQTL1.1 | 546.03 | 0.07 | 120604292 | 113763968–128399829 | * Cmwg645(59.938)-bPb-0395(60.740) |
| MQTL1.2 | 0.22 | 191424829 | 187288939–198521321 | E32M61-265(94.97)-SCRI_RS_113745(95.12) | ||
| MQTL1.3 | 0.22 | 215895490 | 209987507–219651209 | E38M55-493(107.09)-0501A(107.11) | ||
| MQTL1.4 | 0.15 | 227122225 | 224396043–241295755 | bPb-8763(113.65)-E40M32-654(113.84) | ||
| MQTL1.5 | 0.11 | 274117654 | 257758192–281940383 | His3B(135.24)-ABC152F(135.81) | ||
| MQTL1.6 | 0.22 | 328366571 | 317252081–331517042 | bp5019(162.71)-bPt-5002(163.17) | ||
| 2 | MQTL2.1 | 966.53 | 0.09 | 42367561 | 39815442–44919679 | AWBMS62(17.55)-WG516(17.79) |
| MQTL2.2 | 0.2 | 68439906 | 64629701–72250111 | bPb-6235a(28.42)-bPb-6128(28.76) | ||
| MQTL2.3 | 0.09 | 97387878 | 91468880–103306875 | BNL16.06(40.61)-bPb-3574(41.08) | ||
| MQTL2.4 | 0.04 | 139419949 | 135909288–142930609 | P61M48h(58.13)-bPb-3575(59.69) | ||
| MQTL2.5 | 0.22 | 170213114 | 166570654–173855574 | BF064492(71.03)-AWBMA33(71.19) | ||
| MQTL2.6 | 0.06 | 189767373 | 187766416–191768330 | Bmag0711(49.14)-EBmac521a(80.08) | ||
| MQTL2.7 | 0.1 | 263383407 | 256445958–270320855 | EBmatc0039(109.90)-AWBMA21(109.95) | ||
| MQTL2.8 | 0.12 | 294464135 | 290701857–298226412 | BQ740141(122.82)-3179-497(122.96) | ||
| MQTL2.9 | 0.07 | 611525900 | 608806036–614245763 | BM815937(221.69)-BM816122(257.49) | ||
| 3 | MQTL3.1 | 775.59 | 0.09 | 268408330 | 261707356–275109306 | E32M62-92(101.28)-E35M48-250(101.83) |
| MQTL3.2 | 0.23 | 379792248 | 376613242–384250770 | E41M61-400(143.95)-E42M51-442(143.96) | ||
| MQTL3.3 | 0.1 | 436460332 | 428189641–444731023 | bPb-3320(165.22)-basd27g02(165.47) | ||
| MQTL3.4 | 0.17 | 473526748 | 471178768–475874727 | bPt-6567(179.18)-bPt-5150(179.52) | ||
| MQTL3.5 | 0.31 | 498826884 | 495476397–502177372 | bp4025(189.03)-7241-553(189.17) | ||
| MQTL3.6 | 0.07 | 573909459 | 563449078–584369838 | ABA302(217.25)-P15M47-91(217.59) | ||
| 4 | MQTL4.1 | 889.8 | 0.07 | 182695125 | 175965050–193845575 | SCRI_RS_180891(80.50)-SCRI_RS_119628(83.45) |
| MQTL4.2 | 0.08 | 250437750 | 245089075–255786425 | BOPA2_12_10063(113.09)-E36M62-78(113.51) | ||
| MQTL4.3 | 0.04 | 285624125 | 277921575–293326675 | basd13l12(128.85)-E33M60--5.5(129.43) | ||
| MQTL4.4 | 0.03 | 317672025 | 304090350–331253725 | E40M32-153(143.70)-E36M59-94(143.91) | ||
| MQTL4.5 | 0.12 | 339354100 | 332668250–346039950 | E33M54-416(153.49)-bPb-6872(153.58) | ||
| MQTL4.6 | 0.17 | 352946825 | 348117550–357776125 | E32M62-386(158.61)-E38M55-139(158.80) | ||
| MQTL4.7 | 0.17 | 403538300 | 400996575–406080025 | BF258346B(182.46)-bPb-7395(182.61) | ||
| MQTL4.8 | 0.32 | 462329625 | 460130475–465799650 | mHsh(209.15)-ABC305(209.24) | ||
| 5 | MQTL5.1 | 710.31 | 0.03 | 11389965 | 9196970–13582960 | ABG464(10.90)-Act8A(11.32) |
| MQTL5.2 | 0.12 | 121232310 | 120331310–122133305 | bp3200(106.34)-E40M40-354(107.08) | ||
| MQTL5.3 | 0.09 | 135954265 | 133744270–138164260 | E42M55-350(119.80)-E37M50-70(120.07) | ||
| MQTL5.4 | 0.14 | 152138215 | 149316225–154960210 | Bmac282a(134.20)-E37M62-231(134.43) | ||
| MQTL5.5 | 0.07 | 177842140 | 174833150–180851130 | 1896-1435(156.80)-bags4p07(157.03) | ||
| MQTL5.6 | 0.06 | 197188085 | 192853095–201523070 | Bmac0223(173.52)-CDO57B(174.12) | ||
| MQTL5.7 | 0.06 | 206322725 | 204237395–208408050 | MWG923(181.91)-GBM1227(182.46) | ||
| MQTL5.8 | 0.26 | 239937290 | 238061630–241812955 | bPb-4988(211.63)-7523-440(211.80) | ||
| MQTL5.9 | 0.19 | 262479225 | 262054225–262904225 | dhn9(231.4)-bPb-6367(231.8) | ||
| 6 | MQTL6.1 | 438.51 | 0.12 | 129309961 | 120005985–138613926 | bPb- 8054(38.32)-bPb-9768(38.80) |
| MQTL6.2 | 0.07 | 198777337 | 181047449–216507225 | bPb-4555(58.80)-GBM1049(58.86) | ||
| MQTL6.3 | 0.55 | 256188394 | 251612394–260764394 | Bmac297(75.84)-bPb-1116(75.92) | ||
| MQTL6.4 | 0.26 | 320961575 | 319965327–321957823 | 4191-268(95.03)-E45M48e(93.05) | ||
| 7 | MQTL7.1 | 640.38 | 0.15 | 172041612 | 165726420–178356810 | bPb- 8054(38.32)-bPb-9768(38.80) |
| MQTL7.2 | 0.11 | 205841892 | 197894802–213788982 | bPb-4555(58.80)-GBM1049(58.86) | ||
| MQTL7.3 | 0.07 | 224776302 | 218058726–231493884 | Bmac297(75.84)-bPb-1116(75.92) | ||
| MQTL7.4 | 0.06 | 248606394 | 240290454–256922334 | 4191-268(95.03)-E45M48e(93.05) | ||
| MQTL7.5 | 0.11 | 278695794 | 273308316–284083272 | GBM1030(124.47)-bPb-1039(124.83) | ||
| MQTL7.6 | 0.21 | 293069850 | 289839600–296300106 | BOPA1_5480-826(131.10)-BOPA1_ABC11989-1-2-148(131.14) | ||
| MQTL7.7 | 0.01 | 311177142 | 306538548–319839582 | bp8365(138.93)-SCRI_RS_122512(139.52) | ||
| MQTL7.8 | 0.04 | 352488594 | 345402156–359575026 | bPb-5260(157.53)-Bmag0174b(157.88) | ||
| MQTL7.9 | 0.08 | 381929706 | 374642082–389217330 | ABC305(170.76)-bPb-9269(171.07) | ||
| MQTL7.10 | 0.16 | 413807478 | 411996750-415618206 | bPb-4129(185.06)-bp5141(185.26) |
| Chr | Drought | Low Temperature | Water-Logging | Salinity | Mineral Toxicity and Deficiency | Total |
|---|---|---|---|---|---|---|
| 1H | 18(4) | 2(2) | 11(4) | 21(6) | 12(4) | 64(20) |
| 2H | 54(6) | 1(1) | 10(4) | 43(8) | 12(4) | 120(23) |
| 3H | 43(6) | 0 | 8(4) | 23(5) | 25(2) | 99(17) |
| 4H | 32(4) | 2(1) | 8(3) | 39(8) | 15(4) | 96(20) |
| 5H | 16(4) | 9(1) | 3(2) | 23(6) | 7(2) | 58(15) |
| 6H | 21(2) | 2(1) | 2(2) | 22(4) | 12(3) | 59(12) |
| 7H | 26(6) | 0 | 5(4) | 35(7) | 23(4) | 89(21) |
| Total | 210(32) | 16(6) | 47(23) | 206(44) | 106(23) | 585(128) |
| Meta-QTL. | Stress | Meta-QTL | Stress | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low Temperature | Mineral Toxicity and Deficiency | Waterlogging Tolerance | Salinity | Drought | Low Temperature | Mineral Toxicity and Deficiency | Waterlogging Tolerance | Salinity | Drought | ||
| MQTL1.1 | 1 | 3 | 2 | 1 | 0 | MQTL4.6 | 0 | 0 | 0 | 15 | 3 |
| MQTL1.2 | 0 | 0 | 5 | 5 | 4 | MQTL4.7 | 0 | 9 | 4 | 1 | 0 |
| MQTL1.3 | 0 | 1 | 3 | 10 | 4 | MQTL4.8 | 2 | 3 | 3 | 10 | 18 |
| MQTL1.4 | 0 | 0 | 0 | 1 | 2 | MQTL5.1 | 0 | 0 | 0 | 2 | 0 |
| MQTL1.5 | 1 | 1 | 0 | 2 | 0 | MQTL5.2 | 9 | 0 | 0 | 8 | 0 |
| MQTL1.6 | 0 | 7 | 1 | 2 | 8 | MQTL5.3 | 0 | 0 | 0 | 5 | 0 |
| MQTL2.1 | 1 | 0 | 0 | 14 | 0 | MQTL5.4 | 0 | 5 | 0 | 2 | 2 |
| MQTL2.2 | 0 | 7 | 0 | 7 | 18 | MQTL5.5 | 0 | 0 | 0 | 0 | 1 |
| MQTL2.3 | 0 | 1 | 0 | 0 | 8 | MQTL5.6 | 0 | 0 | 2 | 0 | 0 |
| MQTL2.4 | 0 | 2 | 2 | 1 | 1 | MQTL5.7 | 0 | 0 | 1 | 0 | 0 |
| MQTL2.5 | 0 | 0 | 5 | 4 | 14 | MQTL5.8 | 0 | 0 | 0 | 1 | 11 |
| MQTL2.6 | 0 | 2 | 0 | 2 | 0 | MQTL5.9 | 0 | 2 | 0 | 5 | 2 |
| MQTL2.7 | 0 | 0 | 2 | 5 | 12 | MQTL6.1 | 2 | 0 | 1 | 3 | 0 |
| MQTL2.8 | 0 | 0 | 1 | 4 | 1 | MQTL6.2 | 0 | 1 | 0 | 1 | 0 |
| MQTL2.9 | 0 | 0 | 0 | 6 | 0 | MQTL6.3 | 0 | 9 | 1 | 9 | 19 |
| MQTL3.1 | 0 | 0 | 1 | 2 | 5 | MQTL6.4 | 0 | 2 | 0 | 9 | 2 |
| MQTL3.2 | 0 | 7 | 4 | 8 | 5 | MQTL7.1 | 0 | 11 | 2 | 10 | 2 |
| MQTL3.3 | 0 | 0 | 2 | 3 | 8 | MQTL7.2 | 0 | 0 | 0 | 0 | 6 |
| MQTL3.4 | 0 | 0 | 1 | 0 | 14 | MQTL7.3 | 0 | 0 | 1 | 2 | 0 |
| MQTL3.5 | 0 | 18 | 0 | 8 | 5 | MQTL7.4 | 0 | 7 | 1 | 0 | 0 |
| MQTL3.6 | 0 | 0 | 0 | 2 | 6 | MQTL7.5 | 0 | 0 | 0 | 7 | 0 |
| MQTL4.1 | 0 | 0 | 0 | 5 | 0 | MQTL7.6 | 0 | 0 | 0 | 6 | 6 |
| MQTL4.2 | 0 | 0 | 1 | 1 | 6 | MQTL7.7 | 0 | 3 | 0 | 0 | 0 |
| MQTL4.3 | 0 | 2 | 0 | 1 | 0 | MQTL7.8 | 0 | 0 | 0 | 1 | 2 |
| MQTL4.4 | 0 | 0 | 0 | 1 | 0 | MQTL7.9 | 0 | 0 | 1 | 5 | 1 |
| MQTL4.5 | 0 | 1 | 0 | 5 | 5 | MQTL7.10 | 0 | 2 | 0 | 4 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbari, M.; Sabouri, H.; Sajadi, S.J.; Yarahmadi, S.; Ahangar, L.; Abedi, A.; Katouzi, M. Mega Meta-QTLs: A Strategy for the Production of Golden Barley (Hordeum vulgare L.) Tolerant to Abiotic Stresses. Genes 2022, 13, 2087. https://doi.org/10.3390/genes13112087
Akbari M, Sabouri H, Sajadi SJ, Yarahmadi S, Ahangar L, Abedi A, Katouzi M. Mega Meta-QTLs: A Strategy for the Production of Golden Barley (Hordeum vulgare L.) Tolerant to Abiotic Stresses. Genes. 2022; 13(11):2087. https://doi.org/10.3390/genes13112087
Chicago/Turabian StyleAkbari, Mahjoubeh, Hossein Sabouri, Sayed Javad Sajadi, Saeed Yarahmadi, Leila Ahangar, Amin Abedi, and Mahnaz Katouzi. 2022. "Mega Meta-QTLs: A Strategy for the Production of Golden Barley (Hordeum vulgare L.) Tolerant to Abiotic Stresses" Genes 13, no. 11: 2087. https://doi.org/10.3390/genes13112087
APA StyleAkbari, M., Sabouri, H., Sajadi, S. J., Yarahmadi, S., Ahangar, L., Abedi, A., & Katouzi, M. (2022). Mega Meta-QTLs: A Strategy for the Production of Golden Barley (Hordeum vulgare L.) Tolerant to Abiotic Stresses. Genes, 13(11), 2087. https://doi.org/10.3390/genes13112087






