Abstract
Small non-coding RNAs are widespread in the biological world and have been extensively explored over the past decades. Their fundamental roles in human health and disease are increasingly appreciated. Furthermore, a growing number of studies have investigated the functions of small non-coding RNAs in cancer initiation and progression. In this review, we provide an overview of the biogenesis of small non-coding RNAs with a focus on microRNAs, PIWI-interacting RNAs, and a new class of tRNA-derived small RNAs. We discuss their biological functions in human cancer and highlight their clinical application as molecular biomarkers or therapeutic targets.
1. Introduction
It is estimated that the human genome contains approximately 20,000 protein-coding genes, accounting for only about 2% of the genome sequence [1,2], while the other ~3000 genes, which do not encode proteins and are only transcribed into RNA, are called non-coding RNA (ncRNAs) [3]. For many years, this part of the human genome that does not encode proteins was considered “junk” DNA [4]. It was not until the early 2000s that ncRNAs began to be regarded as potentially crucial regulators of biological processes [5]. These ncRNAs have been reported to exist in almost all fields of life. Small non-coding RNAs (sncRNAs) are a kind of non-coding RNA with less than 200 nucleotides (nt) that exists widely in various prokaryotes and eukaryotic organisms [6,7,8,9]. sncRNAs include classical small-interfering RNAs (siRNAs), microRNAs (miRNAs), PIWI-interacting RNAs (piRNAs), transfer RNAs (tRNAs), small nucleolar RNA (snoRNA), and small nuclear RNA (snRNA), as well as newly discovered non-canonical sncRNAs in the biological world, including tRNA-derived-small RNAs (tsRNAs), vault RNA-derived-small RNAs (vtRNAs), and Y RNA-derived-small RNAs (ytRNAs) [9]. They can regulate cell differentiation, proliferation, migration, angiogenesis, apoptosis, and other crucial biological processes by regulating gene expression during cancer development [10,11], and their dysregulation can trigger homeostatic imbalances that lead to the occurrence and development of diseases such as cancer. Here, we focus on the biological process and roles in tumorigenesis and progression of sncRNAs with length < 50 nt, mainly including classical microRNAs, PIWI-interacting RNAs, and tRNA-derived-small RNAs.
2. Biogenesis and Classification
2.1. miRNA
microRNA is a class of highly conserved RNAs with 20–25 nt associated with post-transcriptional gene silencing [12]. In 1993, Ambros’ lab discovered the first miRNA from Caenorhabditis elegans, LIN-4, an endogenous regulator of genes that control developmental timing [13]. miRNAs are widely present in eukaryotic cells and are one of the most prominent gene families [14]. According to miRBase database, more than 38,000 hairpin precursors and close to 50,000 mature miRNAs from 271 organisms had been identified [15]. miRNA biogenesis is a multistep process tightly regulated by several steps. miRNA genes in the genome are transcribed into primary transcripts (pri-miRNA) by RNA polymerase II [16,17]. Then, primary transcripts are first sheared into hairpin loop-like precursor miRNAs (pre-miRNA) of approximately 70 nt in length by a microprocessor consisting of RNase III (Drosha) and double-stranded RNA-binding protein (DGCR8) [18,19]. Subsequently, pre-miRNAs with characteristic structures can be recognized by the nuclear export protein Exportin-5 (Exp5), which could transport the pre-miRNAs into the cytoplasm through a Ran GTP-dependent mechanism [20]. Then, pre-miRNAs in the cytoplasm are recognized and captured by the Dicer enzyme, resulting in the cleavage of pre-miRNAs by the RNase III domains of the Dicer and TAR RNA binding protein (TRBP) into a double-stranded RNA of about 22 nt in length [21,22,23]. Finally, the double helix structure is unwound, and the miRNA strand and Argonaute (AGO) protein assemble into an RNA-induced silencing complex (RISC), while the remaining strand is degraded [24,25]. A schematic representation of small RNAs biogenesis is presented in Figure 1.
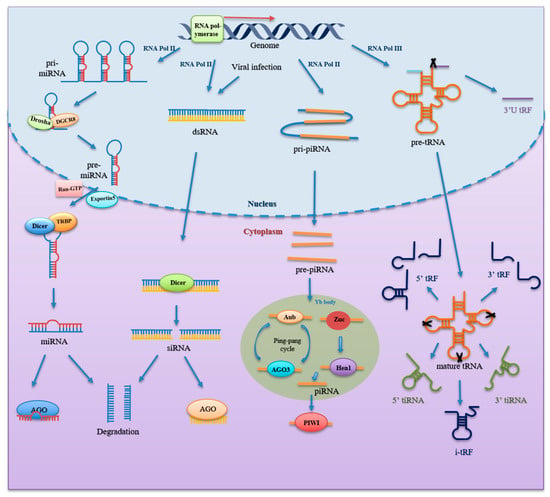
Figure 1.
Formation of small non-coding RNAs. miRNA genes are transcribed by RNA polymerase (Pol) II to generate pri-miRNA, which is then processed by DGCR8 and Drosha to form pre-miRNA inside the nucleus. It is further exported to cytoplasm by Exportin5 and Ran-GTP. Dicer and TRBP in cytoplasm further cleave and process pre-miRNA into mature, short double-stranded miRNA. One strand of the mature miRNA duplex binds with Argonaute protein to form RISC, while another strand is degraded. siRNA is derived from long dsRNA produced by transcription of sense and antisense strands by RNA Pol II and viral infection. Then, dsRNA is processed by Dicer into siRNA duplex, of which one strand binds with Argonaute protein to form the siRNA-induced silencing complexes, while another strand is degraded as well. As for piRNA, piRNA genes are transcribed by RNA Pol II to produce pri-piRNA through the primary procession pathway. pri-piRNA is exported into the cytoplasm and cleaved into pre-piRNA. Then, pre-piRNA is processed by Zuc and Hen1 to form mature piRNA. Mature piRNA in complex with PIWI proteins to work. Additionally, Aub and AGO3 coupled with mature piRNA cleave transcript-bearing sites complementary to the piRNA sequence, thus amplifying mature piRNA species through the “ping-pong” cycle. As for tsRNA, after it is transcribed by RNA Pol III, pre-tRNA undergoes processes and modifications to form mature tRNA. Ribonuclease cleavage in various region of pre-tRNA and mature tRNA generates different types of tsRNAs, including 3′U tRF, 5′tRF, 3′tRF, i-tRF, 5′tiRNA, and 3′tiRNA. Abbreviations: miRNA, microRNA; DGCR8, double-stranded RNA-binding protein; pri-miRNA, primary miRNA; pre-miRNA, precursor miRNA; TRBP, TAR RNA binding protein; RISC, RNA- induced silencing complex; siRNA, small interfering RNA; dsRNA, double-stranded RNA; piRNA, PIWI-interacting RNA; pri-piRNA, primary piRNA; pre-piRNA, precursor miRNA; AGO3, Argonaute 3; tsRNA, tRNA-derived small RNA.
2.2. piRNA
piRNA is a single-stranded RNA with a length of 26–31 nt, discovered by scientists in 2006 when extracting and purifying RNA from mouse testis tissue [26,27,28]. piRNA clusters in the genome encode long single-stranded transcripts, named primary piRNAs (pri-piRNAs) [26,29,30]. The process of mature piRNA formation is complex and, as we currently understand it, involves two main pathways: the primary procession pathway and the “ping-pong” cycle [31]. As for the primary processing pathway: piRNA clusters are transcribed into pri-piRNAs by RNA polymerase II and further processed to precursor piRNAs (pre-piRNAs) [30]. The pre-piRNAs are then transported to the cytoplasmic Yb bodies, where they are cleaved by the Zuc riboendonuclease, giving them a 5′-uracil end [32,33]. Subsequently, the 5′ uracil of the pre-piRNA is recognized and bound by the PIWI protein through the PAZ domain to form a piRNA/PIWI complex. After the 3′ end of the piRNA is cleaved by exonuclease, or Zuc, HEN1, or its homolog methylate, the 2′-hydroxyl group at the 3′ end to form the mature piRNA/PIWI complex [34,35,36]. The “ping-pong” cycle is characterized by piRNA/AGO3 or piRNA/Aub complexes. Aub cleaves sense piRNA precursors by coupling to antisense piRNAs, resulting in sense piRNAs loaded onto AGO3. In contrast, antisense piRNA precursors are cleaved by AGO3 with sense strand piRNAs and generate antisense piRNAs that bind to Aub [36,37]. Ultimately, these piRNAs bind to the PIWI protein to work (Figure 1).
2.3. tsRNA
tsRNA, ~15–40 nt in length, is produced from precursor tRNAs or mature tRNAs by specific endonucleases activities. tsRNAs were first thought to be random degradation products of tRNAs. Researchers did not find that the expression of certain tsRNAs was stress-induced, as opposed to being the product of random degradation until 2008 [38]. Subsequently, Lee et al. found that tRF-1001, which was the first functional tsRNA discovered, was highly expressed in prostate cancer and affected the growth of prostate cancer cells [39]. Then, its structure and function attracted more attention. According to the cleavage positions and length of the tsRNAs, tsRNAs are classified into three main categories [40]: (1) tiRNA (also called tRNA halves, 31–40 nt), including 5′tiRNA and 3′tiRNA, is produced by specific cleavage within the anticodon loop of mature tRNA by specific enzymes including Angiogenin (ANG), RNase T2, and RNase L under stress conditions [38,39,41,42,43,44,45,46]. 5′tiRNA starts with the 5′end, and 3′tiRNA is derived from the 3′end of mature tRNAs. (2) tRFs (14–30 nt) include 5′tRF, 3′tRF and inter tRF (i-tRF) [47,48]. 5′tRF originates from cleavage in in D-loop, while 3′tRF is mainly cleaved in T-loop. The i-tRFs are derived from the internal region of mature tRNAs, around the anticodon. (3) 3′U tRFs (20–40 nt) are derived from cleavage in 3′ end of precursor tRNAs (Figure 1).
2.4. siRNA
Small interfering RNAs are double-stranded RNA molecules 20 to 25 nt in length [12]. In 1999, scientists discovered that transgenic and virus-induced plant silencing is accompanied by the emergence of RNAs of ~20–25 nt that match the sequence of the targeted mRNA [49]. The siRNA was then recorded. It is derived from splicing of long, complementary double-stranded RNA (dsRNA) formed by Dicer [12]. One of the strands binds to the AGO protein to form a functional RISC, which can recognize and bind mRNAs with complementary sequences, while another chain is degraded [12,50]. In many species, target-bound siRNAs can enhance and maintain silencing responses via amplifying by RNA-dependent RNA polymerase (RdRP), while mammalian genomes lack RdRP-encoding potential. Although some genomically derived siRNAs have been identified in mammals [51], most of the characterized siRNAs are either virally encoded or experimentally induced. Therefore, in mammalian cells, including those of humans, the classical RNA interference pathway via siRNA is considered to be mainly used for defense against external threats. A schematic representation of small RNAs biogenesis is presented in Figure 1.
2.5. Other sncRNAs
Aside from the above-mentioned small non-coding RNAs, recently discovered non-canonical sncRNAs derived from structural RNAs are emerging, such as rsRNA (from ribosomal RNA) [52], ysRNA (from Y RNA) [52,53], snsRNA (from small nuclear RNA) [54,55], snosRNA (from small nucleolar RNA) [56], and vtsRNA (from vault tRNA) [57]. Compared with classical small RNAs including siRNA, miRNA, and piRNA, non-canonical small RNAs have distinct features in terms of origin, biogenesis, abundance, and function, which may update our understanding of sncRNAs [9,40]. Nevertheless, the research on the generation and function of these sncRNAs is still in its infancy and needs to be explored.
3. Functions of sncRNAs
As sncRNAs is increasingly studied, its function becomes clearer than before. They regulate various biological processes mainly through transcriptional, post-transcriptional, translational, post-translational, and reverse transcriptional regulation.
3.1. Transcriptional Regulation
After the small RNA matures in the cytoplasm, some small RNAs re-enter the nucleus to regulate the transcription level. The piRNA/PIWI complex can recruit the cofactor Panoramix/Silesio (panx) or the epigenetic factor HP1a and the necessary histone modifying enzymes to the modification site to maintain the inhibitory state of chromatin [58,59]. Another small noncoding RNA member, tsRNA, can regulate genome expression in a PIWI-dependent manner through a mechanism similar to that of piRNA. For example, Zhang et al. found that a piRNA-like tsRNA, 5′tRH-Glu-CTC, could bind to PIWIL4 to form a complex in human monocytes or dendritic cells, methylating H3K9 and repressing CD1A transcription by recruiting H3K9 methyltransferase and heterochromatin protein 1b to the CD1A promoter region [60]. In addition to transcriptional gene silencing (TGS), small RNAs are involved in transcriptional gene activation (TGA). The nuclear-enriched miR-195-5p increased Foxo3 expression by targeting the Foxo3 promoter, which may be engaged in AGO2 recruitment and histone demethylation and acetylation in cells [61]. Apart from the promoter, small RNAs can regulate transcription at enhancers as well. For example, elevation of miR-26a-1 leads to enhanced transcriptional of ITGA9 and VILL genes by targeting their enhancers [62]. Overexpression of miR-24-1 resulted in the increase of RNA polymerase II, p300/CBP, and enhancer RNA [63]. In summary, these studies suggest a regulatory function of small RNAs for transcriptional regulation at the enhancer and promoter level.
3.2. Post-Transcriptional Regulation
In mammals, RNA-induced silencing complex (RISC) gene silencing is a well-known mechanism of cytoplasmic post-transcriptional gene regulation. Typically, small RNAs target specific sites in mRNAs to engage in their post-transcriptional regulation in the form of mRNA degradation or translational inhibition. This process relies on the recognition of target mRNAs by small RNAs. In a typical RNAi pathway, the siRNA guide strand directs RISC to a fully complementary RNA target. Guided by the siRNA, RISC cleaves mRNA in the mRNA-siRNA duplex, and the resulting mRNA is degraded [6]. miRNA binding sites in mammalian mRNAs are located in the 3′UTR region in most cases. Most animal miRNAs bind with mismatches and bulges, although miRNA nucleotides 2–8 form seeds critical for the recognition of target mRNAs [12], leading to translation inhibition. When the miRNA acts in compete complementarity with the target gene, its mode of action and function is very similar to that of siRNA, resulting in the instability and degradation of mRNA. Cytosolic polyadenylate-binding protein (PABPC) is recruited through the functions of AGO protein and glycine-tryptophan protein (GW182), promoting mRNA deadenylation [64,65]. The mRNA is then rapidly degraded after uncapping by the DCP1-DCP2 complex [66]. Additional small non-coding RNAs, piRNAs, and tsRNAs, can also repress target gene function through regulatory post-transcriptional networks similar to miRNAs. In contrast, piRNA interactions require strict base pairing within 2–11 nt and less stringent base pairing within 12–21 nt of the 5′ end of the piRNA [67]. For example, piR-55490 targets the 3′UTR region of mTOR mRNA, resulting in mRNA degradation and the inhibition of cell growth and proliferation in lung cancer [68]. CU1276, a miRNA derived from tRNA-Gly-GCC, could bind to AGO protein and target the 3′UTR of mRNA in normal germinal center B cells to inhibit gene expression [69]. Furthermore, miR-3676 targets three consecutive 28-bp repeats within the 3′UTR of TCL1 mRNA, thereby inhibiting the translation of TCL1 [70]. In addition, small RNAs can also undergo post-transcriptional regulation through other pathways. For example, piR-30840 is crucial to the development of Th2 lymphocytes by suppressing the maturation of interleukin-4 mRNA [71]. tsRNAs can act as protein decoys, sequestering RNA-binding proteins from targeted RNAs, thereby influencing RNA stability [72,73]. The above mechanisms provide new insights into how small non-coding RNAs regulate gene expression in the post-transcriptional level.
3.3. Translation and Post-Translation Regulation
tsRNA can interact with ribosomal subunits in a similar manner to its precursor, mature tRNA, thereby affecting translation [74]. Gebetsberger et al. reported that Val-tRF is able to compete with mRNA rather than tRNA for ribosomal 30S subunits, affecting translation initiation to inhibit translation [75]. Ivanov et al. found that tiRNAs directly or indirectly bind to eIF4G, eIF4A, or eIF4G/A complexes, thereby inhibiting translation. In addition, tiRNAs bind to YB-1, which together prevent eIF4G/A from initiating translation [76]. Shi et al. illustrated that 5′tsRNA-Ala and –Cys with a terminal oligo g motif could form into G-quadruplex-like structure, which can competitively bind to translation-initiation factor eIF4E/G/A and then suppress mRNA translation [77]. In addition, studies have shown that small RNAs can also facilitate translational processes. For example, tRNA-Leu-CAG derived small RNA binds to the coding region CDS and the non-coding 3′UTR region of ribosomal protein S28 (RPS28), altering the secondary structure to increase its translation, while inhibiting this tsRNA reduces RPS28 protein levels, suggesting that 3′ tsRNA-Leu-CAG may maintain ribosome biosynthesis through a conserved gene regulatory mechanism [78,79]. Furthermore, Fricker et al. showed that the tRNA-Thr 3′half produced by Trypanosoma brucei during nutrient deprivation could bind to ribosomes and multimers, stimulated by promoting mRNA loading during stress recovery after starvation conditions have ceased. Translation, blocking or depletion of endogenous tRNA-Thr halves alleviated this stimulatory effect in vivo and in vitro [80].
Small RNAs can modulate the structure and function of interacting proteins by binding to translation-independent proteins. For example, 21 nt i-tRF-Gly interacts with hnRNPL and then prevents phosphorylation of hnRNPL by AKT2, thereby reducing the AKT2-hnRNAPL-DDX17 axis and attenuating the malignant phenotype in pancreatic cancer cells [81]. Zhao et al. found that piRNAs regulate the ubiquitination of the PIWI protein Miwi by enhancing the interaction of Miwi with the substrate-binding subunit of APC/C during late mouse spermatogenesis [82]. Yin et al. found that piR-823 could upregulate the transcriptional activity of the heat shock factor 1(HSF1) by interacting with HSF1 and promoting its phosphorylation at Ser326 [83]. Mai et al. reported that piR-54265/PIWIL2 could recruit STAT3 and phosphorylated-SRC to form a complex through the PIWIL2 PAZ domain, facilitating phosphorylated-SRC-mediated STAT3 phosphorylation and activation of related signaling pathways [84]. The direct binding of piRNAs/PIWI complexes to specific proteins via piRNAs or the PIWI protein PAZ domain can alter their subcellular localization [85].
3.4. Reverse Transcription Regulation
Viruses rely on the host cell’s translation machinery to efficiently synthesize their own proteins. Many RNA viruses and retrotransposons can self-replicate by using the 3′ end of mature tRNA as a primer for their reverse transcription (RT). Emerging evidence suggests that specific host tRNA fragments can be used as primers for reverse transcription and packaged into retroviruses [86]. For example, Ruggero et al. demonstrated that tRF-3019 has good sequence complementarity with the primer binding site of human T-cell leukemia virus type 1 (HTLV-1), and the results of in vitro reverse transcriptase tests confirmed that tRF-3019 can trigger HTLV-1 reverse transcription [87]. This suggests that tRF-3019, as a reverse transcription primer, plays an important role in initiating reverse transcription of HTLV-1 and may become a new target for controlling HTLV-1 infection. Additionally, Schorn et al. showed that exogenous 18 nt 3′ tsRNA could target the tRNA primer binding sites necessary for endogenous retroviral reverse transcription, IAP and MusD/ETn, resulting in strong reverse transcription inhibition [88]. These studies show that the viral world adapts to specific tsRNAs that can block or mimic the function of tRNAs and thus can be potential therapeutic targets.
4. Functions of Small Non-Coding RNAs in Cancer
Cancer is thought to be characterized by a series of functional capabilities that human cells acquire during the transition from a normal state to a tumor-growing state that is critical to the ability to form malignancies. In 2002, Calin et al. found that miR-15 and miR-16 were absent and downregulated frequently in chronic lymphocytic leukemia, presenting evidence for the involvement of miRNA genes in human cancer progression [89]. The importance of tsRNA in the progression of human cancer was first illustrated in prostate cancer. In 2009, Lee et al. created and sequenced a small RNAs library in prostate cancer cell lines, finding that Trf-1001, a tsRNA derived from pre-tRNA-Ser, could accelerate the growth of prostate cancer [39]. In past studies, almost all known cancer occurrence or development has been associated with small RNAs (Table 1). These small RNAs are closely associated with cancer hallmarks reported by Hanahan [90,91,92], including maintaining proliferative signaling, resisting cell death, inducing or accessing angiogenesis, tumor-promoting inflammation, activating invasion and metastasis, etc. Here, we mainly focus on four recently proposed cancer hallmarks, which encourage us to study small RNAs in terms of other features of cancer.

Table 1.
Functions of small non-coding RNAs related to human cancer.
4.1. Unlocking Phenotypic Plasticity
Usually, cellular differentiation leads to a distinct barrier to continued proliferation necessary for tumorigenesis. Growing evidence suggests that unlocking the restricted ability of phenotypic plasticity to avoid a terminally differentiated state is significant for tumor development [91,114]. Overexpression of miR-4423 induced a differentiation-like pattern of gene expression in airway epithelial cells and reversed the expression of some genes that were altered in lung cancer, i.e., ablation of miR-4423 function contributed to the development of lung cancer [115]. Overexpression of miR-17-92 cluster in normal thyroid follicular cells could impair thyroid differentiation and promote cancer-related effects by inhibiting TGF-β signaling [116]. Upregulation of miR-34a expression was observed at the same time as the induction of osteosarcoma cells into a stem cell-like phenotype. Further study found that plasminogen activator inhibitor-1 (PAI-1) was the downstream of miR-34a, and suppressing PAI-1 could inhibit osteosarcoma dedifferentiation into cancer stem-like cells by blocking Sox2 expression [117]. miR-215 could promote the self-renewal of colorectal cancer stem cells via the targeted gene BMI1 [118]. miRNA-302a/d could downregulate the self-renewal ability of liver cancer stem cells and proliferation of liver cancer cells by inhibiting E2F7/AKT/β-catenin/CCND1 signaling pathway [119]. These studies suggest that small RNAs play an important role in unlocking tumor phenotypic plasticity.
4.2. Non-Mutational Epigenetic Reprogramming
In 2012, Huang proposed the conception of mutation-less cancer evolution and purely epigenetic programming of hallmark cancer phenotypes [91,120] and is increasingly concerned subsequently [121,122,123]. A growing body of research supports small RNAs as novel epigenetic regulators, contributing to the acquisition of multiple hallmark abilities during cancer initiation and progression. piRNAs have been reported to be epigenetic effectors in human tumors [124]. piRNA-30473 is involved in tumorigenesis in diffuse large B-cell lymphoma by upregulating m6A mRNA methylase WTAP [110]. In addition, sperm tsRNA can be an epigenetic factor that mediates the intergenerational inheritance of diet-induced metabolic disorders [125]. Zygote injection of individual tsRNAs or a combination of tsRNAs in early embryo stages could induce alterations gene expression, indicating that sperm tsRNAs transmit paternally acquired traits via remodeling early embryonic development [125,126].
4.3. Polymorphic Microbiomes
Polymorphic variation in microbial communities between individuals has noticeable influences on malignant behaviors [127,128]. Numerous researchers have demonstrated an association between gut microbiomes and cancer [129,130], especially colorectal cancer. Several studies have shown that various subtypes of colorectal cancer (CRC) are linked to distinct microbiome “fingerprints” [131]. Other studies have reported a specific accumulation of seemingly cancer-related microorganisms in CRC. MicroRNAs (miRNAs) influence key cellular processes and are closely related to CRC progression. A growing body of research has found that miRNAs can mediate bidirectional interactions between microbiome and host. Of note, the gut microbiome can regulate miRNA expression to alter the host transcriptome, affecting the progression of CRC [132]. One microorganism closely related to colorectal cancer is Fusobacterium nucleatum. This bacterium upregulates miR-21 in cancer by activating the tlr4-myd88 signaling pathway, decreasing protein levels of tumor suppressor genes RASA1 and PDCD4, thereby promoting CRC growth [133]. Helicobacterpylori ((H. pylori) infection also can affect host miRNAs expression. Chang et al. reported that miR99b-3p, miR-564, and miR-638 significantly upregulated, while miR-204-5p, miR-338-5p, miR-375, and miR-548c-3p downregulated in H. pylori-positive gastric cancer patients, compared with H. pylori-negative patients [134]. Furthermore, the relationship between vaginal microbiota, miRNAs and ovarian cancer was confirmed as well. For example, vaginal isolated Lactococcus lactis can decrease TLR-4, miR-21, and miR-200b expression [135], among which the miRNA-21 and miR-200 family were found to be connected with cancer metastasis, and overall survival rate in ovarian cancer [136,137].
4.4. Senescent Cells
Cellular senescence is a typical irreversible proliferation arrest that may evolve into a protective mechanism to maintain tissue homeostasis [91]. Senescence has long been protective in limiting malignant progression. That is, cancer cells are induced to undergo senescence to inhibit tumor progression [138]. For example, miR-130b~301b overexpression effectively induces prostate cancer cell growth arrest through activation of cellular senescence [139]. miR-30 could decrease cell senescence and induce cancer progression by inhibiting CHD7 and TNRC6A [140]. Epstein–Barr virus-encoded miR-BART3-3p has been reported to accelerate tumorigenesis by inhibiting the senescence process in gastric cancer [141]. miR-7 could repress the gemcitabine-induced senescence by targeting PARP1/NF-κB pathway in pancreatic cancer [142]. However, in some cases, senescent cells promote cancer progression differently [143,144]. Its primary mechanism is senescence-associated secretory phenotype (SASP), involving the release of a large number of biologically active proteins containing prominent pro-inflammatory cytokines and chemokines [145], which can transmit signals in a paracrine manner to nearby living cancer cells and other cells in the TME, thereby promoting tumor progression [146,147].For example, upregulated miR-335 in senescent normal fibroblasts and cancer-associated fibroblasts regulates SASP factor secretion and induces cancer cell motility in co-cultures by inhibiting PTEN expression [148]. However, the mechanism of this small RNA-related senescence-promoting tumor still needs more research to support it.
5. Clinical Significances of Small Non-Coding RNAs in Cancer
Increasing evidence indicates that small non-coding RNAs are differentially expressed in tumor tissue or the circulating blood of tumor patients and play a crucial regulatory role in tumor initiation and progression. It can reflect the presence of the tumor, pathological grading, clinical staging, and clinical outcome of the patient. This suggests that small non-coding RNAs have great potential to serve as cancer diagnosis, prognosis biomarkers, and therapeutic targets.
5.1. Diagnostic and Prognostic Biomarker
The miR-1290 is significantly upregulated in the blood of colorectal adenoma and colorectal adenocarcinoma patients. Blood miR-1290 levels can distinguish adenomas, CRC patients and normal subjects, suggesting that serum miR-1290 is a potential diagnostic biomarker for colorectal cancer. At the same time, the high expression of miR-1290 is closely linked to cancer aggressiveness and poor outcome. It is an independent factor for prognosis of patients with colorectal cancer [149]. Serum tRF-Pro-AGG-004 and tRF-Leu-CAG-002 is significantly elevated in pancreatic cancer patients compared with healthy people, suggesting that they are promising diagnostic biomarkers. Additionally, in situ hybridization (ISH) scores in tumor tissues suggest that tRF-Pro-AGG-004 and tRF-Leu-CAG-002 can predict postoperative survival time of pancreatic cancer patients [113]. Furthermore, small RNAs can be novel diagnostic and prognostic biomarkers in other cancer types, including ovarian cancer [150], breast cancer [151], prostate cancer [152], etc. Notably, exosome-derived small non-coding RNAs can also serve as useful diagnostic and prognostic biomarkers [153,154,155,156]. For example, tRNA-GlyGCC-5 and another small non-coding RNA, sRESE, are enriched in saliva-derived exosomes from esophageal squamous cell carcinoma patients compared to healthy controls, and the bi-signature containing these two small non-coding RNAs can distinguish esophageal carcinoma patients from the controls with a high sensitivity of 90.50% and specificity of 94.20%. Furthermore, patients with a high bi-signature have both poorer overall survival and progression-free survival [155]. In endometrial carcinoma, a plasma-derived exosomal microRNA, miR-15a-5p, can distinguish cancer patients from controls with an Area Under Curve (AUC) value of 0.813. Of note, the combination of miR-15a-5p with serum CEA and CA125 could achieve a higher AUC value of 0.899 [156]. These studies provide unique insights and more dynamic perspectives of cancer diagnosis and prognosis. Research on the use of small non-coding RNAs as molecular biomarkers for cancer diagnosis or prognosis is presented in Table 2.

Table 2.
Small non-coding RNAs as molecular biomarkers for cancer diagnosis or prognosis.
5.2. Therapy
Some studies have suggested that small non-coding RNAs can serve as functional molecules linked to cancer initiation and progression, as well as functioning as promising therapeutic targets or agents. For example, miR-1293 can inhibit tumor cell growth in vitro by inhibiting DNA repair genes and BRD4, a member of the Bromodomain and Extra-terminal Domain (BET) protein family, is recognized as a promising target for cancer therapy [198]. miRNAs can also be combined with existing treatments, such as chemotherapy [199,200], radiotherapy [201,202], and immunotherapy [203,204] to treat tumors synergistically. For example, Temozolomide (TMZ)-resistant glioblastoma cells confer TMZ chemoresistance to receptor TMZ-sensitive cells in an exosomal miR-151a loss-dependent manner, so miR-151a mimics transfected into glioblastoma cell lines can sensitize TMZ-resistant GBM cells via inhibiting XRCC4-mediated DNA repair [199]. miR-205 is highly downregulated in gemcitabine (GEM)-resistant pancreatic cancer cells and can resensitize GEM-resistant pancreatic cancer cells to GEM [200]. In addition, miRNAs can also be used for “replacement therapy”, which aims to restore the expression of repressor miRNAs [205]. For example, the treatment of miR-34a and let-7b in neuroblastomas significantly reduces cell division, proliferation, neo-angiogenesis, and induces apoptosis in orthotopic xenografts [206]. Furthermore, other small RNAs also show good therapeutic prospects. For example, inhibition of 3′tsRNA-Leu-CAG promotes apoptosis in rapidly dividing cells in vitro and in vivo [78]. piRNA-36712 has synergistic anti-cancer effects with paclitaxel and doxorubicin in breast cancer [103]. However, the application of these small RNAs to clinical practice still requires a lot of effort.
6. Conclusions and Prospects
With the development of high-throughput sequencing technology, more and more sncRNAs have been discovered. With the deepening of sncRNA function research, its essential role in cancer has gradually emerged. Aberrant expression of various sncRNAs is an important regulator of activating or affecting signaling pathways during cancer development, making sncRNAs’ expression a diagnostic and prognostic marker and a potential therapeutic target. However, there is still much to learn surrounding the exact roles of various sncRNAs in cancer. The mechanism of action of sncRNAs in tumors needs more relevant studies to fully understand their mechanism of action and promote the development of this field of cancer biology.
Author Contributions
Conceptualization, Q.X. and Y.Z.; writing—original draft preparation, Q.X. and Y.Z.; writing—review and editing, J.L. and Q.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sichuan Science and Technology Programme (2019YFS0042) and 1.3.5 Project for Disciplines of Excellence, West China Hospital (ZYJC21042), Sichuan University for Qing Zhu.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no competing interest.
References
- Frith, M.C.; Pheasant, M.; Mattick, J.S. The amazing complexity of the human transcriptome. Eur. J. Hum. Genet 2005, 13, 894–897. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, T.; Zhang, G.; He, Q.Y. Understanding the proteome encoded by “non-coding RNAs”: New insights into human genome. Sci. China Life Sci. 2020, 63, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Elgar, G.; Vavouri, T. Tuning in to the signals: Noncoding sequence conservation in vertebrate genomes. Trends. Genet 2008, 24, 344–352. [Google Scholar] [CrossRef]
- Cech, T.R.; Steitz, J.A. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 2014, 157, 77–94. [Google Scholar] [CrossRef]
- Grosshans, H.; Filipowicz, W. Molecular biology: The expanding world of small RNAs. Nature 2008, 451, 414–416. [Google Scholar] [CrossRef]
- Storz, G.; Vogel, J.; Wassarman, K.M. Regulation by small RNAs in bacteria: Expanding frontiers. Mol. Cell 2011, 43, 880–891. [Google Scholar] [CrossRef]
- Babski, J.; Maier, L.K.; Heyer, R.; Jaschinski, K.; Prasse, D.; Jager, D.; Randau, L.; Schmitz, R.A.; Marchfelder, A.; Soppa, J. Small regulatory RNAs in Archaea. RNA Biol. 2014, 11, 484–493. [Google Scholar] [CrossRef]
- Shi, J.; Zhou, T.; Chen, Q. Exploring the expanding universe of small RNAs. Nat. Cell Biol. 2022, 24, 415–423. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, J.; Ju, S.; Cui, M.; Jing, R. Disorders and roles of tsRNA, snoRNA, snRNA and piRNA in cancer. J. Med. Genet 2022, 59, 623–631. [Google Scholar] [CrossRef]
- Xu, D.; Qiao, D.; Lei, Y.; Zhang, C.; Bu, Y.; Zhang, Y. Transfer RNA-derived small RNAs (tsRNAs): Versatile regulators in cancer. Cancer Lett. 2022, 546, 215842. [Google Scholar] [CrossRef]
- Carthew, R.W.; Sontheimer, E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Kim, V.N.; Nam, J.W. Genomics of microRNA. Trends. Genet 2006, 22, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef]
- Cai, X.; Hagedorn, C.H.; Cullen, B.R. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA 2004, 10, 1957–1966. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Jo, M.H.; Choi, Y.G.; Park, J.; Kwon, S.C.; Hohng, S.; Kim, V.N.; Woo, J.S. Functional Anatomy of the Human Microprocessor. Cell 2015, 161, 1374–1387. [Google Scholar] [CrossRef]
- Lee, Y.; Ahn, C.; Han, J.; Choi, H.; Kim, J.; Yim, J.; Lee, J.; Provost, P.; Radmark, O.; Kim, S.; et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003, 425, 415–419. [Google Scholar] [CrossRef]
- Bohnsack, M.T.; Czaplinski, K.; Gorlich, D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA 2004, 10, 185–191. [Google Scholar] [CrossRef]
- Park, J.E.; Heo, I.; Tian, Y.; Simanshu, D.K.; Chang, H.; Jee, D.; Patel, D.J.; Kim, V.N. Dicer recognizes the 5’ end of RNA for efficient and accurate processing. Nature 2011, 475, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kolb, F.A.; Jaskiewicz, L.; Westhof, E.; Filipowicz, W. Single processing center models for human Dicer and bacterial RNase III. Cell 2004, 118, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.C.; Tambe, A.; Kidwell, M.A.; Noland, C.L.; Schneider, C.P.; Doudna, J.A. Dicer-TRBP complex formation ensures accurate mammalian microRNA biogenesis. Mol. Cell 2015, 57, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Kwak, P.B.; Tomari, Y. The N domain of Argonaute drives duplex unwinding during RISC assembly. Nat. Struct. Mol. Biol. 2012, 19, 145–151. [Google Scholar] [CrossRef]
- Gu, S.; Jin, L.; Zhang, F.; Huang, Y.; Grimm, D.; Rossi, J.J.; Kay, M.A. Thermodynamic stability of small hairpin RNAs highly influences the loading process of different mammalian Argonautes. Proc. Natl. Acad. Sci. USA 2011, 108, 9208–9213. [Google Scholar] [CrossRef]
- Girard, A.; Sachidanandam, R.; Hannon, G.J.; Carmell, M.A. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 2006, 442, 199–202. [Google Scholar] [CrossRef]
- Aravin, A.; Gaidatzis, D.; Pfeffer, S.; Lagos-Quintana, M.; Landgraf, P.; Iovino, N.; Morris, P.; Brownstein, M.J.; Kuramochi-Miyagawa, S.; Nakano, T.; et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature 2006, 442, 203–207. [Google Scholar] [CrossRef]
- Grivna, S.T.; Beyret, E.; Wang, Z.; Lin, H. A novel class of small RNAs in mouse spermatogenic cells. Genes Dev. 2006, 20, 1709–1714. [Google Scholar] [CrossRef]
- Li, X.Z.; Roy, C.K.; Dong, X.; Bolcun-Filas, E.; Wang, J.; Han, B.W.; Xu, J.; Moore, M.J.; Schimenti, J.C.; Weng, Z.; et al. An ancient transcription factor initiates the burst of piRNA production during early meiosis in mouse testes. Mol. Cell 2013, 50, 67–81. [Google Scholar] [CrossRef]
- Ray, R.; Pandey, P. piRNA analysis framework from small RNA-Seq data by a novel cluster prediction tool—PILFER. Genomics 2018, 110, 355–365. [Google Scholar] [CrossRef]
- Huang, Y.; Bai, J.Y.; Ren, H.T. PiRNAs biogenesis and its functions. Bioorg. Khim. 2014, 40, 320–326. [Google Scholar] [PubMed]
- Olivieri, D.; Sykora, M.M.; Sachidanandam, R.; Mechtler, K.; Brennecke, J. An in vivo RNAi assay identifies major genetic and cellular requirements for primary piRNA biogenesis in Drosophila. EMBO J. 2010, 29, 3301–3317. [Google Scholar] [CrossRef] [PubMed]
- Nishimasu, H.; Ishizu, H.; Saito, K.; Fukuhara, S.; Kamatani, M.K.; Bonnefond, L.; Matsumoto, N.; Nishizawa, T.; Nakanaga, K.; Aoki, J.; et al. Structure and function of Zucchini endoribonuclease in piRNA biogenesis. Nature 2012, 491, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Han, B.W.; Wang, W.; Li, C.; Weng, Z.; Zamore, P.D. Noncoding RNA. piRNA-guided transposon cleavage initiates Zucchini-dependent, phased piRNA production. Science 2015, 348, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.W.; Siomi, M.C.; Siomi, H. PIWI-Interacting RNA: Its Biogenesis and Functions. Annu. Rev. Biochem. 2015, 84, 405–433. [Google Scholar] [CrossRef] [PubMed]
- Czech, B.; Hannon, G.J. One Loop to Rule Them All: The Ping-Pong Cycle and piRNA-Guided Silencing. Trends. Biochem. Sci. 2016, 41, 324–337. [Google Scholar] [CrossRef]
- Brennecke, J.; Aravin, A.A.; Stark, A.; Dus, M.; Kellis, M.; Sachidanandam, R.; Hannon, G.J. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 2007, 128, 1089–1103. [Google Scholar] [CrossRef]
- Li, Y.; Luo, J.; Zhou, H.; Liao, J.Y.; Ma, L.M.; Chen, Y.Q.; Qu, L.H. Stress-induced tRNA-derived RNAs: A novel class of small RNAs in the primitive eukaryote Giardia lamblia. Nucleic Acids Res. 2008, 36, 6048–6055. [Google Scholar] [CrossRef]
- Lee, Y.S.; Shibata, Y.; Malhotra, A.; Dutta, A. A novel class of small RNAs: tRNA-derived RNA fragments (tRFs). Genes Dev. 2009, 23, 2639–2649. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.; Shi, J.; Yan, M.; Zhou, T. Origins and evolving functionalities of tRNA-derived small RNAs. Trends. Biochem. Sci. 2021, 46, 790–804. [Google Scholar] [CrossRef]
- Yamasaki, S.; Ivanov, P.; Hu, G.F.; Anderson, P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J. Cell Biol. 2009, 185, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Feng, J.; Liu, Q.; Sun, F.; Tie, Y.; Zhu, J.; Xing, R.; Sun, Z.; Zheng, X. Stress induces tRNA cleavage by angiogenin in mammalian cells. FEBS Lett. 2009, 583, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Collins, K. Starvation-induced cleavage of the tRNA anticodon loop in Tetrahymena thermophila. J. Biol. Chem. 2005, 280, 42744–42749. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.M.; Lu, C.; Green, P.J.; Parker, R. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA 2008, 14, 2095–2103. [Google Scholar] [CrossRef]
- Jochl, C.; Rederstorff, M.; Hertel, J.; Stadler, P.F.; Hofacker, I.L.; Schrettl, M.; Haas, H.; Huttenhofer, A. Small ncRNA transcriptome analysis from Aspergillus fumigatus suggests a novel mechanism for regulation of protein synthesis. Nucleic Acids Res. 2008, 36, 2677–2689. [Google Scholar] [CrossRef]
- Donovan, J.; Rath, S.; Kolet-Mandrikov, D.; Korennykh, A. Rapid RNase L-driven arrest of protein synthesis in the dsRNA response without degradation of translation machinery. RNA 2017, 23, 1660–1671. [Google Scholar] [CrossRef]
- Kumar, P.; Anaya, J.; Mudunuri, S.B.; Dutta, A. Meta-analysis of tRNA derived RNA fragments reveals that they are evolutionarily conserved and associate with AGO proteins to recognize specific RNA targets. BMC Biol. 2014, 12, 78. [Google Scholar] [CrossRef]
- Su, Z.; Wilson, B.; Kumar, P.; Dutta, A. Noncanonical Roles of tRNAs: tRNA Fragments and Beyond. Annu. Rev. Genet 2020, 54, 47–69. [Google Scholar] [CrossRef]
- Hamilton, A.J.; Baulcombe, D.C. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science 1999, 286, 950–952. [Google Scholar] [CrossRef]
- Czech, B.; Hannon, G.J. Small RNA sorting: Matchmaking for Argonautes. Nat. Rev. Genet 2011, 12, 19–31. [Google Scholar] [CrossRef]
- Tam, O.H.; Aravin, A.A.; Stein, P.; Girard, A.; Murchison, E.P.; Cheloufi, S.; Hodges, E.; Anger, M.; Sachidanandam, R.; Schultz, R.M.; et al. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature 2008, 453, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Shi, J.; Liu, H.; Zhang, X.; Zhou, J.J.; Li, M.; Zhou, D.; Li, R.; Lv, J.; Wen, G.; et al. Peripheral blood non-canonical small non-coding RNAs as novel biomarkers in lung cancer. Mol. Cancer 2020, 19, 159. [Google Scholar] [CrossRef] [PubMed]
- Cambier, L.; de Couto, G.; Ibrahim, A.; Echavez, A.K.; Valle, J.; Liu, W.; Kreke, M.; Smith, R.R.; Marban, L.; Marban, E. Y RNA fragment in extracellular vesicles confers cardioprotection via modulation of IL-10 expression and secretion. EMBO Mol. Med. 2017, 9, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Heard, E. Small RNAs derived from structural non-coding RNAs. Methods 2013, 63, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, R.; Li, L.; Zhu, J.; Li, Z.; Peng, C.; Zhuang, X.; Lin, H.; Shi, S.; Huang, P. CPA-seq reveals small ncRNAs with methylated nucleosides and diverse termini. Cell Discov. 2021, 7, 25. [Google Scholar] [CrossRef]
- Ender, C.; Krek, A.; Friedlander, M.R.; Beitzinger, M.; Weinmann, L.; Chen, W.; Pfeffer, S.; Rajewsky, N.; Meister, G. A human snoRNA with microRNA-like functions. Mol. Cell 2008, 32, 519–528. [Google Scholar] [CrossRef]
- Hussain, S.; Sajini, A.A.; Blanco, S.; Dietmann, S.; Lombard, P.; Sugimoto, Y.; Paramor, M.; Gleeson, J.G.; Odom, D.T.; Ule, J.; et al. NSun2-mediated cytosine-5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell Rep. 2013, 4, 255–261. [Google Scholar] [CrossRef]
- Huang, X.A.; Yin, H.; Sweeney, S.; Raha, D.; Snyder, M.; Lin, H. A major epigenetic programming mechanism guided by piRNAs. Dev. Cell 2013, 24, 502–516. [Google Scholar] [CrossRef]
- Yu, Y.; Gu, J.; Jin, Y.; Luo, Y.; Preall, J.B.; Ma, J.; Czech, B.; Hannon, G.J. Panoramix enforces piRNA-dependent cotranscriptional silencing. Science 2015, 350, 339–342. [Google Scholar] [CrossRef]
- Zhang, X.; He, X.; Liu, C.; Liu, J.; Hu, Q.; Pan, T.; Duan, X.; Liu, B.; Zhang, Y.; Chen, J.; et al. IL-4 Inhibits the Biogenesis of an Epigenetically Suppressive PIWI-Interacting RNA To Upregulate CD1a Molecules on Monocytes/Dendritic Cells. J. Immunol 2016, 196, 1591–1603. [Google Scholar] [CrossRef]
- Bai, Y.; Pan, B.; Zhan, X.; Silver, H.; Li, J. MicroRNA 195-5p Targets Foxo3 Promoter Region to Regulate Its Expression in Granulosa Cells. Int. J. Mol. Sci. 2021, 22, 6721. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lei, C.; He, Q.; Pan, Z.; Xiao, D.; Tao, Y. Nuclear functions of mammalian MicroRNAs in gene regulation, immunity and cancer. Mol. Cancer 2018, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Li, J.; Li, W.; Wang, Y.; Wu, F.; Xi, Y.; Zhang, L.; Ding, C.; Luo, H.; Li, Y.; et al. MicroRNAs activate gene transcription epigenetically as an enhancer trigger. RNA Biol. 2017, 14, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Elkayam, E.; Faehnle, C.R.; Morales, M.; Sun, J.; Li, H.; Joshua-Tor, L. Multivalent Recruitment of Human Argonaute by GW182. Mol. Cell 2017, 67, 646–658.e3. [Google Scholar] [CrossRef]
- Fabian, M.R.; Cieplak, M.K.; Frank, F.; Morita, M.; Green, J.; Srikumar, T.; Nagar, B.; Yamamoto, T.; Raught, B.; Duchaine, T.F.; et al. miRNA-mediated deadenylation is orchestrated by GW182 through two conserved motifs that interact with CCR4-NOT. Nat. Struct. Mol. Biol. 2011, 18, 1211–1217. [Google Scholar] [CrossRef]
- Braun, J.E.; Truffault, V.; Boland, A.; Huntzinger, E.; Chang, C.T.; Haas, G.; Weichenrieder, O.; Coles, M.; Izaurralde, E. A direct interaction between DCP1 and XRN1 couples mRNA decapping to 5’ exonucleolytic degradation. Nat. Struct. Mol. Biol. 2012, 19, 1324–1331. [Google Scholar] [CrossRef]
- Goh, W.S.; Falciatori, I.; Tam, O.H.; Burgess, R.; Meikar, O.; Kotaja, N.; Hammell, M.; Hannon, G.J. piRNA-directed cleavage of meiotic transcripts regulates spermatogenesis. Genes Dev. 2015, 29, 1032–1044. [Google Scholar] [CrossRef]
- Peng, L.; Song, L.; Liu, C.; Lv, X.; Li, X.; Jie, J.; Zhao, D.; Li, D. piR-55490 inhibits the growth of lung carcinoma by suppressing mTOR signaling. Tumour. Biol. 2016, 37, 2749–2756. [Google Scholar] [CrossRef]
- Maute, R.L.; Schneider, C.; Sumazin, P.; Holmes, A.; Califano, A.; Basso, K.; Dalla-Favera, R. tRNA-derived microRNA modulates proliferation and the DNA damage response and is down-regulated in B cell lymphoma. Proc. Natl. Acad. Sci. USA 2013, 110, 1404–1409. [Google Scholar] [CrossRef]
- Balatti, V.; Rizzotto, L.; Miller, C.; Palamarchuk, A.; Fadda, P.; Pandolfo, R.; Rassenti, L.Z.; Hertlein, E.; Ruppert, A.S.; Lozanski, A.; et al. TCL1 targeting miR-3676 is codeleted with tumor protein p53 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2015, 112, 2169–2174. [Google Scholar] [CrossRef]
- Zhong, F.; Zhou, N.; Wu, K.; Guo, Y.; Tan, W.; Zhang, H.; Zhang, X.; Geng, G.; Pan, T.; Luo, H.; et al. A SnoRNA-derived piRNA interacts with human interleukin-4 pre-mRNA and induces its decay in nuclear exosomes. Nucleic Acids Res. 2015, 43, 10474–10491. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Yim, D.G.; Lakshmanan, V.; Tirumalai, V.; Koh, J.L.; Park, J.E.; Cheong, J.K.; Low, J.L.; Lim, M.J.; Sze, S.K.; et al. Dynamic expression of tRNA-derived small RNAs define cellular states. EMBO Rep. 2019, 20, e47789. [Google Scholar] [CrossRef]
- Goodarzi, H.; Liu, X.; Nguyen, H.C.; Zhang, S.; Fish, L.; Tavazoie, S.F. Endogenous tRNA-Derived Fragments Suppress Breast Cancer Progression via YBX1 Displacement. Cell 2015, 161, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Rudland, P.S.; Dube, S.K. Specific interaction of an initiator tRNA fragment with 30 s ribosomal subunits. J. Mol. Biol. 1969, 43, 273–280. [Google Scholar] [CrossRef]
- Gebetsberger, J.; Wyss, L.; Mleczko, A.M.; Reuther, J.; Polacek, N. A tRNA-derived fragment competes with mRNA for ribosome binding and regulates translation during stress. RNA Biol. 2017, 14, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, P.; Emara, M.M.; Villen, J.; Gygi, S.P.; Anderson, P. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol. Cell 2011, 43, 613–623. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Y.; Zhou, T.; Chen, Q. tsRNAs: The Swiss Army Knife for Translational Regulation. Trends. Biochem. Sci. 2019, 44, 185–189. [Google Scholar] [CrossRef]
- Kim, H.K.; Fuchs, G.; Wang, S.; Wei, W.; Zhang, Y.; Park, H.; Roy-Chaudhuri, B.; Li, P.; Xu, J.; Chu, K.; et al. A transfer-RNA-derived small RNA regulates ribosome biogenesis. Nature 2017, 552, 57–62. [Google Scholar] [CrossRef]
- Kim, H.K.; Xu, J.; Chu, K.; Park, H.; Jang, H.; Li, P.; Valdmanis, P.N.; Zhang, Q.C.; Kay, M.A. A tRNA-Derived Small RNA Regulates Ribosomal Protein S28 Protein Levels after Translation Initiation in Humans and Mice. Cell Rep. 2019, 29, 3816–3824.e3814. [Google Scholar] [CrossRef]
- Fricker, R.; Brogli, R.; Luidalepp, H.; Wyss, L.; Fasnacht, M.; Joss, O.; Zywicki, M.; Helm, M.; Schneider, A.; Cristodero, M.; et al. A tRNA half modulates translation as stress response in Trypanosoma brucei. Nat. Commun. 2019, 10, 118. [Google Scholar] [CrossRef]
- Pan, L.; Huang, X.; Liu, Z.X.; Ye, Y.; Li, R.; Zhang, J.; Wu, G.; Bai, R.; Zhuang, L.; Wei, L.; et al. Inflammatory cytokine-regulated tRNA-derived fragment tRF-21 suppresses pancreatic ductal adenocarcinoma progression. J. Clin. Investig. 2021, 131, e148130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gou, L.T.; Zhang, M.; Zu, L.D.; Hua, M.M.; Hua, Y.; Shi, H.J.; Li, Y.; Li, J.; Li, D.; et al. piRNA-triggered MIWI ubiquitination and removal by APC/C in late spermatogenesis. Dev. Cell 2013, 24, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Jiang, X.Y.; Qi, W.; Ji, C.G.; Xie, X.L.; Zhang, D.X.; Cui, Z.J.; Wang, C.K.; Bai, Y.; Wang, J.; et al. piR-823 contributes to colorectal tumorigenesis by enhancing the transcriptional activity of HSF1. Cancer Sci. 2017, 108, 1746–1756. [Google Scholar] [CrossRef]
- Mai, D.; Ding, P.; Tan, L.; Zhang, J.; Pan, Z.; Bai, R.; Li, C.; Li, M.; Zhou, Y.; Tan, W.; et al. PIWI-interacting RNA-54265 is oncogenic and a potential therapeutic target in colorectal adenocarcinoma. Theranostics 2018, 8, 5213–5230. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dou, M.; Song, X.; Dong, Y.; Liu, S.; Liu, H.; Tao, J.; Li, W.; Yin, X.; Xu, W. The emerging role of the piRNA/piwi complex in cancer. Mol. Cancer 2019, 18, 123. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.; Ribeiro, D.R.; Marques, M.; Santos, M.A.S.; Ribeiro, D.; Soares, A.R. Emerging Roles of tRNAs in RNA Virus Infections. Trends. Biochem. Sci. 2020, 45, 794–805. [Google Scholar] [CrossRef]
- Ruggero, K.; Guffanti, A.; Corradin, A.; Sharma, V.K.; De Bellis, G.; Corti, G.; Grassi, A.; Zanovello, P.; Bronte, V.; Ciminale, V.; et al. Small noncoding RNAs in cells transformed by human T-cell leukemia virus type 1: A role for a tRNA fragment as a primer for reverse transcriptase. J. Virol. 2014, 88, 3612–3622. [Google Scholar] [CrossRef]
- Schorn, A.J.; Gutbrod, M.J.; LeBlanc, C.; Martienssen, R. LTR-Retrotransposon Control by tRNA-Derived Small RNAs. Cell 2017, 170, 61–71.e11. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Dumitru, C.D.; Shimizu, M.; Bichi, R.; Zupo, S.; Noch, E.; Aldler, H.; Rattan, S.; Keating, M.; Rai, K.; et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ji, F.; Zhao, X.; Yang, X.; He, J.; Huang, L.; Zhang, Y. MicroRNA-371a-3p promotes progression of gastric cancer by targeting TOB1. Cancer Lett. 2019, 443, 179–188. [Google Scholar] [CrossRef]
- Cheng, J.; Deng, H.; Xiao, B.; Zhou, H.; Zhou, F.; Shen, Z.; Guo, J. piR-823, a novel non-coding small RNA, demonstrates in vitro and in vivo tumor suppressive activity in human gastric cancer cells. Cancer Lett. 2012, 315, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, Z.; Yu, X.; Ruan, Y.; Shen, Y.; Shao, Y.; Zhang, X.; Ye, G.; Guo, J. The tRNA-derived fragment 5026a inhibits the proliferation of gastric cancer cells by regulating the PTEN/PI3K/AKT signaling pathway. Stem. Cell Res. Ther. 2021, 12, 418. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Zhao, Y.; Zhang, W.J.; Jiang, Y.J.; Fu, H.; Huang, F.; Li, D.J.; Shen, F.M. microRNA-802 accelerates hepatocellular carcinoma growth by targeting RUNX3. J. Cell Physiol. 2020, 235, 7128–7135. [Google Scholar] [CrossRef]
- Law, P.T.; Qin, H.; Ching, A.K.; Lai, K.P.; Co, N.N.; He, M.; Lung, R.W.; Chan, A.W.; Chan, T.F.; Wong, N. Deep sequencing of small RNA transcriptome reveals novel non-coding RNAs in hepatocellular carcinoma. J. Hepatol. 2013, 58, 1165–1173. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, J.; Liu, L.; Yan, M.; Zhang, Q.; Song, X.; Lin, Y.; Zhu, D.; Wei, Y.; Fu, Z.; et al. Gly-tRF enhances LCSC-like properties and promotes HCC cells migration by targeting NDFIP2. Cancer Cell Int. 2021, 21, 502. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Y.; Li, Z.; Zhao, X.; Xi, Z.; Chen, H.; Shi, H.; Xin, T.; Shen, R.; Wang, T. MiR-4319 suppresses colorectal cancer progression by targeting ABTB1. United Eur. Gastroenterol. J. 2019, 7, 517–528. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, X.; Xie, X.; Yin, J.; Zhang, J.; Liu, T.; Chen, S.; Wang, Y.; Zhou, X.; Wang, Y.; et al. piR-823 inhibits cell apoptosis via modulating mitophagy by binding to PINK1 in colorectal cancer. Cell Death Dis. 2022, 13, 465. [Google Scholar] [CrossRef]
- Tao, E.W.; Wang, H.L.; Cheng, W.Y.; Liu, Q.Q.; Chen, Y.X.; Gao, Q.Y. A specific tRNA half, 5’tiRNA-His-GTG, responds to hypoxia via the HIF1alpha/ANG axis and promotes colorectal cancer progression by regulating LATS2. J. Exp. Clin. Cancer Res. 2021, 40, 67. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tan, Z.Q.; Hu, H.; Liu, H.Z.; Wu, T.W.; Zheng, C.; Wang, X.L.; Luo, Z.Z.; Wang, J.; Liu, S.Y.; et al. microRNA-21 promotes breast cancer proliferation and metastasis by targeting LZTFL1. BMC Cancer 2019, 19, 738. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Mai, D.; Zhang, B.; Jiang, X.; Zhang, J.; Bai, R.; Ye, Y.; Li, M.; Pan, L.; Su, J.; et al. PIWI-interacting RNA-36712 restrains breast cancer progression and chemoresistance by interaction with SEPW1 pseudogene SEPW1P RNA. Mol. Cancer 2019, 18, 9. [Google Scholar] [CrossRef]
- Mo, D.; Jiang, P.; Yang, Y.; Mao, X.; Tan, X.; Tang, X.; Wei, D.; Li, B.; Wang, X.; Tang, L.; et al. A tRNA fragment, 5′-tiRNA(Val), suppresses the Wnt/β-catenin signaling pathway by targeting FZD3 in breast cancer. Cancer Lett. 2019, 457, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, T.; Shang, D.; Sun, Z. miR-1254 promotes lung cancer cell proliferation by targeting SFRP1. BioMed Pharmacother. 2017, 92, 913–918. [Google Scholar] [CrossRef]
- Hu, F.; Niu, Y.J.; Mao, X.W.; Cui, J.T.; Wu, X.T.; Ii, C.B.S.; Kang, H.S.; Qin, W.X.; Jiang, L.Y. tsRNA-5001a promotes proliferation of lung adenocarcinoma cells and is associated with postoperative recurrence in lung adenocarcinoma patients. Transl. Lung Cancer Res. 2021, 10, 3957. [Google Scholar] [CrossRef]
- Wu, H.; Liu, C.; Yang, Q.; Xin, C.; Du, J.; Sun, F.; Zhou, L. MIR145-3p promotes autophagy and enhances bortezomib sensitivity in multiple myeloma by targeting HDAC4. Autophagy 2020, 16, 683–697. [Google Scholar] [CrossRef]
- Yan, H.; Wu, Q.L.; Sun, C.Y.; Ai, L.S.; Deng, J.; Zhang, L.; Chen, L.; Chu, Z.B.; Tang, B.; Wang, K.; et al. piRNA-823 contributes to tumorigenesis by regulating de novo DNA methylation and angiogenesis in multiple myeloma. Leukemia 2015, 29, 196–206. [Google Scholar] [CrossRef]
- Hu, T.; Chong, Y.; Lu, S.; Wang, R.; Qin, H.; Silva, J.; Kitamura, E.; Chang, C.S.; Hawthorn, L.; Cowell, J.K. miR-339 Promotes Development of Stem Cell Leukemia/Lymphoma Syndrome via Downregulation of the BCL2L11 and BAX Proapoptotic Genes. Cancer Res. 2018, 78, 3522–3531. [Google Scholar] [CrossRef]
- Han, H.Y.; Fan, G.; Song, S.; Jiang, Y.X.; Qian, C.A.; Zhang, W.M.; Su, Q.; Xue, X.F.; Zhuang, W.Z.; Li, B.Z. piRNA-30473 contributes to tumorigenesis and poor prognosis by regulating m6A RNA methylation in DLBCL. Blood 2021, 137, 1603–1614. [Google Scholar] [CrossRef]
- Chen, S.; Xu, M.; Zhao, J.; Shen, J.Q.; Li, J.H.; Liu, Y.; Cao, G.; Ma, J.C.; He, W.Z.; Chen, X.; et al. MicroRNA-4516 suppresses pancreatic cancer development via negatively regulating orthodenticle homeobox 1. Int. J. Biol. Sci. 2020, 16, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Xing, S.; Shen, B.Y.; Chen, H.T.; Sun, B.; Wang, Z.T.; Wang, J.W.; Lu, X.X. PIWIL1 interacting RNA piR-017061 inhibits pancreatic cancer growth via regulating EFNA5. Hum. Cell 2021, 34, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Yang, L.; Wang, W.; Yuan, N.; Zhan, S.; Yang, P.; Chen, X.; Ma, T.; Wang, Y. A novel class of tsRNA signatures as biomarkers for diagnosis and prognosis of pancreatic cancer. Mol. Cancer 2021, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Norgard, R.J.; Stanger, B.Z. Cellular Plasticity in Cancer. Cancer Discov. 2019, 9, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, C.; Campbell, J.D.; Gerrein, J.; Tellez, C.S.; Garrison, C.B.; Walser, T.C.; Drizik, E.; Si, H.; Gower, A.C.; Vick, J.; et al. MicroRNA 4423 is a primate-specific regulator of airway epithelial cell differentiation and lung carcinogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 18946–18951. [Google Scholar] [CrossRef]
- Fuziwara, C.S.; Saito, K.C.; Kimura, E.T. Thyroid Follicular Cell Loss of Differentiation Induced by MicroRNA miR-17-92 Cluster Is Attenuated by CRISPR/Cas9n Gene Silencing in Anaplastic Thyroid Cancer. Thyroid 2020, 30, 81–94. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Y.; Xie, C.; Zhang, Y. miR-34a exerts as a key regulator in the dedifferentiation of osteosarcoma via PAI-1-Sox2 axis. Cell Death Dis. 2018, 9, 777. [Google Scholar] [CrossRef]
- Jones, M.F.; Hara, T.; Francis, P.; Li, X.L.; Bilke, S.; Zhu, Y.; Pineda, M.; Subramanian, M.; Bodmer, W.F.; Lal, A. The CDX1-microRNA-215 axis regulates colorectal cancer stem cell differentiation. Proc. Natl. Acad. Sci. USA 2015, 112, E1550–E1558. [Google Scholar] [CrossRef]
- Ma, Y.S.; Lv, Z.W.; Yu, F.; Chang, Z.Y.; Cong, X.L.; Zhong, X.M.; Lu, G.X.; Zhu, J.; Fu, D. MicroRNA-302a/d inhibits the self-renewal capability and cell cycle entry of liver cancer stem cells by targeting the E2F7/AKT axis. J. Exp. Clin. Cancer Res. 2018, 37, 252. [Google Scholar] [CrossRef]
- Huang, S. Tumor progression: Chance and necessity in Darwinian and Lamarckian somatic (mutationless) evolution. Prog. Biophys. Mol. Biol. 2012, 110, 69–86. [Google Scholar] [CrossRef]
- Darwiche, N. Epigenetic mechanisms and the hallmarks of cancer: An intimate affair. Am. J. Cancer Res. 2020, 10, 1954–1978. [Google Scholar] [PubMed]
- Feng, Y.; Liu, X.; Pauklin, S. 3D chromatin architecture and epigenetic regulation in cancer stem cells. Protein Cell 2021, 12, 440–454. [Google Scholar] [CrossRef] [PubMed]
- Nam, A.S.; Chaligne, R.; Landau, D.A. Integrating genetic and non-genetic determinants of cancer evolution by single-cell multi-omics. Nat. Rev. Genet 2021, 22, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.D.; Jiang, H.; Zhang, Y.F.; Zhang, Y.; Qian, L.L.; Zhang, Y.F. The regulatory function of piRNA/PIWI complex in cancer and other human diseases: The role of DNA methylation. Int. J. Biol. Sci. 2022, 18, 3358–3373. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, M.; Cao, Z.; Li, X.; Zhang, Y.; Shi, J.; Feng, G.H.; Peng, H.; Zhang, X.; Zhang, Y.; et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 2016, 351, 397–400. [Google Scholar] [CrossRef]
- Sharma, U.; Conine, C.C.; Shea, J.M.; Boskovic, A.; Derr, A.G.; Bing, X.Y.; Belleannee, C.; Kucukural, A.; Serra, R.W.; Sun, F.; et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 2016, 351, 391–396. [Google Scholar] [CrossRef]
- Dzutsev, A.; Badger, J.H.; Perez-Chanona, E.; Roy, S.; Salcedo, R.; Smith, C.K.; Trinchieri, G. Microbes and Cancer. Annu. Rev. Immunol. 2017, 35, 199–228. [Google Scholar] [CrossRef]
- Helmink, B.A.; Khan, M.A.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The microbiome, cancer, and cancer therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef]
- Malmuthuge, N.; Guan, L.L. Noncoding RNAs: Regulatory Molecules of Host-Microbiome Crosstalk. Trends. Microbiol. 2021, 29, 713–724. [Google Scholar] [CrossRef]
- Dong, J.; Tai, J.W.; Lu, L.F. miRNA-Microbiota Interaction in Gut Homeostasis and Colorectal Cancer. Trends. Cancer 2019, 5, 666–669. [Google Scholar] [CrossRef]
- Burns, M.B.; Montassier, E.; Abrahante, J.; Priya, S.; Niccum, D.E.; Khoruts, A.; Starr, T.K.; Knights, D.; Blekhman, R. Colorectal cancer mutational profiles correlate with defined microbial communities in the tumor microenvironment. PLoS Genet 2018, 14, e1007376. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Liao, Y.; Zhang, H.; Zhang, W.; Zhang, Z.; Zhang, J.; Wang, D.; Tang, D. Impacts of MicroRNAs Induced by the Gut Microbiome on Regulating the Development of Colorectal Cancer. Front. Cell Infect. Microbiol. 2022, 12, 804689. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Weng, W.; Peng, J.; Hong, L.; Yang, L.; Toiyama, Y.; Gao, R.; Liu, M.; Yin, M.; Pan, C.; et al. Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-kappaB, and Up-regulating Expression of MicroRNA-21. Gastroenterology 2017, 152, 851–866.e824. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Kim, N.; Park, J.H.; Nam, R.H.; Choi, Y.J.; Lee, H.S.; Yoon, H.; Shin, C.M.; Park, Y.S.; Kim, J.M.; et al. Different MicroRNA Expression Levels in Gastric Cancer Depending on Helicobacter pylori Infection. Gut Liver 2015, 9, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Rahbar Saadat, Y.; Pourseif, M.M.; Zununi Vahed, S.; Barzegari, A.; Omidi, Y.; Barar, J. Modulatory Role of Vaginal-Isolated Lactococcus lactis on the Expression of miR-21, miR-200b, and TLR-4 in CAOV-4 Cells and In Silico Revalidation. Probiot. Antimicrob. Proteins 2020, 12, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.H.; Yang, X.S.; Wang, F.L.; Cui, Z.M.; Huang, Y. MicroRNA-21 promotes the cell proliferation, invasion and migration abilities in ovarian epithelial carcinomas through inhibiting the expression of PTEN protein. Int. J. Mol. Med. 2010, 26, 819–827. [Google Scholar] [CrossRef]
- Koutsaki, M.; Spandidos, D.A.; Zaravinos, A. Epithelial-mesenchymal transition-associated miRNAs in ovarian carcinoma, with highlight on the miR-200 family: Prognostic value and prospective role in ovarian cancer therapeutics. Cancer Lett. 2014, 351, 173–181. [Google Scholar] [CrossRef]
- Lee, S.; Schmitt, C.A. The dynamic nature of senescence in cancer. Nat. Cell Biol. 2019, 21, 94–101. [Google Scholar] [CrossRef]
- Ramalho-Carvalho, J.; Graca, I.; Gomez, A.; Oliveira, J.; Henrique, R.; Esteller, M.; Jeronimo, C. Downregulation of miR-130b similar to 301b cluster is mediated by aberrant promoter methylation and impairs cellular senescence in prostate cancer. J. Hematol. Oncol. 2017, 10, 281–297. [Google Scholar] [CrossRef]
- Su, W.; Hong, L.; Xu, X.; Huang, S.; Herpai, D.; Li, L.; Xu, Y.; Truong, L.; Hu, W.Y.; Wu, X.; et al. miR-30 disrupts senescence and promotes cancer by targeting both p16(INK4A) and DNA damage pathways. Oncogene 2018, 37, 5618–5632. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, X.; Qin, Z.L.; Wei, L.Y.; Lu, Y.J.; Peng, Q.; Gao, Y.X.; Zhang, X.M.; Zhang, X.Y.; Li, Z.S.; et al. Epstein-Barr virus miR-BART3-3p promotes tumorigenesis by regulating the senescence pathway in gastric cancer. J. Biol. Chem. 2019, 294, 4854–4866. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.Q.; Chen, H.B.; Zhang, T.Y.; Chen, Z.; Tian, L.; Gu, D.N. MicroRNA-7 modulates cellular senescence to relieve gemcitabine resistance by targeting PARP1/NF-kappaB signaling in pancreatic cancer cells. Oncol. Lett. 2021, 21, 139. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kohli, J.; Demaria, M. Senescent Cells in Cancer Therapy: Friends or Foes? Trends. Cancer 2020, 6, 838–857. [Google Scholar] [CrossRef]
- Kowald, A.; Passos, J.F.; Kirkwood, T.B.L. On the evolution of cellular senescence. Aging Cell 2020, 19, e13270. [Google Scholar] [CrossRef] [PubMed]
- Birch, J.; Gil, J. Senescence and the SASP: Many therapeutic avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef]
- Faget, D.V.; Ren, Q.; Stewart, S.A. Unmasking senescence: Context-dependent effects of SASP in cancer. Nat. Rev. Cancer 2019, 19, 439–453. [Google Scholar] [CrossRef]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef]
- Kabir, T.D.; Leigh, R.J.; Tasena, H.; Mellone, M.; Coletta, R.D.; Parkinson, E.K.; Prime, S.S.; Thomas, G.J.; Paterson, I.C.; Zhou, D.; et al. A miR-335/COX-2/PTEN axis regulates the secretory phenotype of senescent cancer-associated fibroblasts. Aging 2016, 8, 1608–1635. [Google Scholar] [CrossRef]
- Imaoka, H.; Toiyama, Y.; Fujikawa, H.; Hiro, J.; Saigusa, S.; Tanaka, K.; Inoue, Y.; Mohri, Y.; Mori, T.; Kato, T.; et al. Circulating microRNA-1290 as a novel diagnostic and prognostic biomarker in human colorectal cancer. Ann. Oncol. 2016, 27, 1879–1886. [Google Scholar] [CrossRef]
- Nakamura, K.; Sawada, K.; Yoshimura, A.; Kinose, Y.; Nakatsuka, E.; Kimura, T. Clinical relevance of circulating cell-free microRNAs in ovarian cancer. Mol. Cancer 2016, 15, 48. [Google Scholar] [CrossRef]
- Nassar, F.J.; Nasr, R.; Talhouk, R. MicroRNAs as biomarkers for early breast cancer diagnosis, prognosis and therapy prediction. Pharmacol. Ther. 2017, 172, 34–49. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.C.; Vira, M.; Shen, J.; Sanda, M.; Raman, J.D.; Liao, J.; Patil, D.; Taioli, E. Circulating microRNAs in plasma as potential biomarkers for the early detection of prostate cancer. Prostate 2018, 78, 411–418. [Google Scholar] [CrossRef]
- Zhu, L.; Li, J.; Gong, Y.; Wu, Q.; Tan, S.; Sun, D.; Xu, X.; Zuo, Y.; Zhao, Y.; Wei, Y.Q.; et al. Exosomal tRNA-derived small RNA as a promising biomarker for cancer diagnosis. Mol. Cancer 2019, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Preethi, K.A.; Selvakumar, S.C.; Ross, K.; Jayaraman, S.; Tusubira, D.; Sekar, D. Liquid biopsy: Exosomal microRNAs as novel diagnostic and prognostic biomarkers in cancer. Mol. Cancer 2022, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lin, Y.; Luo, Y.; Xiong, X.; Wang, L.; Durante, K.; Li, J.; Zhou, F.; Guo, Y.; Chen, S.; et al. A signature of saliva-derived exosomal small RNAs as predicting biomarker for esophageal carcinoma: A multicenter prospective study. Mol. Cancer 2022, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, W.; Wang, F.; Yang, S.; Hu, J.; Lu, B.; Pan, Z.; Ma, Y.; Zheng, M.; Zhou, L.; et al. Plasma-derived exosomal miR-15a-5p as a promising diagnostic biomarker for early detection of endometrial carcinoma. Mol. Cancer 2021, 20, 57. [Google Scholar] [CrossRef]
- Zhong, S.; Golpon, H.; Zardo, P.; Borlak, J. miRNAs in lung cancer. A systematic review identifies predictive and prognostic miRNA candidates for precision medicine in lung cancer. Transl. Res. 2021, 230, 164–196. [Google Scholar] [CrossRef]
- Gao, Z.; Jijiwa, M.; Nasu, M.; Borgard, H.; Gong, T.; Xu, J.; Chen, S.; Fu, Y.; Chen, Y.; Hu, X.; et al. Comprehensive landscape of tRNA-derived fragments in lung cancer. Mol. Ther. Oncolytics 2022, 26, 207–225. [Google Scholar] [CrossRef]
- Li, D.; Luo, Y.; Gao, Y.; Yang, Y.; Wang, Y.; Xu, Y.; Tan, S.; Zhang, Y.; Duan, J.; Yang, Y. piR-651 promotes tumor formation in non-small cell lung carcinoma through the upregulation of cyclin D1 and CDK4. Int. J. Mol. Med. 2016, 38, 927–936. [Google Scholar] [CrossRef]
- Tang, S.; Li, S.; Liu, T.; He, Y.; Hu, H.; Zhu, Y.; Tang, S.; Zhou, H. MicroRNAs: Emerging oncogenic and tumor-suppressive regulators, biomarkers and therapeutic targets in lung cancer. Cancer Lett. 2021, 502, 71–83. [Google Scholar] [CrossRef]
- Yang, W.; Gao, K.; Qian, Y.; Huang, Y.; Xiang, Q.; Chen, C.; Chen, Q.; Wang, Y.; Fang, F.; He, Q.; et al. A novel tRNA-derived fragment AS-tDR-007333 promotes the malignancy of NSCLC via the HSPB1/MED29 and ELK4/MED29 axes. J. Hematol. Oncol. 2022, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Giglio, S.; De Nunzio, C.; Cirombella, R.; Stoppacciaro, A.; Faruq, O.; Volinia, S.; Baldassarre, G.; Tubaro, A.; Ishii, H.; Croce, C.M.; et al. A preliminary study of micro-RNAs as minimally invasive biomarkers for the diagnosis of prostate cancer patients. J. Exp. Clin. Cancer Res. 2021, 40, 79. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.P.; Lai, H.M.; Guo, Z. Prostate cancer early diagnosis: Circulating microRNA pairs potentially beyond single microRNAs upon 1231 serum samples. Brief. Bioinform. 2021, 22, bbaa111. [Google Scholar] [CrossRef] [PubMed]
- Baumann, B.; Acosta, A.M.; Richards, Z.; Deaton, R.; Sapatynska, A.; Murphy, A.; Kajdacsy-Balla, A.; Gann, P.H.; Nonn, L. Association of High miR-182 Levels with Low-Risk Prostate Cancer. Am. J. Pathol. 2019, 189, 911–923. [Google Scholar] [CrossRef]
- Wang, J.; Ni, J.; Beretov, J.; Thompson, J.; Graham, P.; Li, Y. Exosomal microRNAs as liquid biopsy biomarkers in prostate cancer. Crit. Rev. Oncol. Hematol. 2020, 145, 102860. [Google Scholar] [CrossRef]
- So, J.B.Y.; Kapoor, R.; Zhu, F.; Koh, C.; Zhou, L.; Zou, R.; Tang, Y.C.; Goo, P.C.K.; Rha, S.Y.; Chung, H.C.; et al. Development and validation of a serum microRNA biomarker panel for detecting gastric cancer in a high-risk population. Gut 2021, 70, 829–837. [Google Scholar] [CrossRef]
- Ouyang, J.; Xie, Z.; Lei, X.; Tang, G.; Gan, R.; Yang, X. Clinical crosstalk between microRNAs and gastric cancer (Review). Int. J. Oncol. 2021, 58, 7. [Google Scholar] [CrossRef]
- Abe, S.; Matsuzaki, J.; Sudo, K.; Oda, I.; Katai, H.; Kato, K.; Takizawa, S.; Sakamoto, H.; Takeshita, F.; Niida, S.; et al. A novel combination of serum microRNAs for the detection of early gastric cancer. Gastric. Cancer 2021, 24, 835–843. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, X.; Qin, X.; Huang, Y.; Ju, S. Evaluation of serum tRF-23-Q99P9P9NDD as a potential biomarker for the clinical diagnosis of gastric cancer. Mol. Med. 2022, 28, 63. [Google Scholar] [CrossRef]
- Chen, B.; Xia, Z.; Deng, Y.N.; Yang, Y.; Zhang, P.; Zhu, H.; Xu, N.; Liang, S. Emerging microRNA biomarkers for colorectal cancer diagnosis and prognosis. Open Biol. 2019, 9, 180212. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Z.; Cai, H.; Peng, Y.; Yang, L.; Wang, Z. Identifying Differentially Expressed tRNA-Derived Small Fragments as a Biomarker for the Progression and Metastasis of Colorectal Cancer. Dis. Markers 2022, 2022, 2646173. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, P.; Kolhe, R.; Gahlay, G.K. The clinical relevance of gene expression based prognostic signatures in colorectal cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188513. [Google Scholar] [CrossRef] [PubMed]
- Tsiakanikas, P.; Adamopoulos, P.G.; Tsirba, D.; Artemaki, P.I.; Papadopoulos, I.N.; Kontos, C.K.; Scorilas, A. High Expression of a tRNA(Pro) Derivative Associates with Poor Survival and Independently Predicts Colorectal Cancer Recurrence. Biomedicines 2022, 10, 1120. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Bhowmik, S.; Majumdar, S.; Goswami, A.; Chakraborty, J.; Gupta, S.; Aggarwal, S.; Ray, S.; Chatterjee, R.; Bhattacharyya, S.; et al. The exosome encapsulated microRNAs as circulating diagnostic marker for hepatocellular carcinoma with low α-fetoprotein. Int. J. Cancer 2020, 147, 2934–2947. [Google Scholar] [CrossRef]
- Zhan, S.; Yang, P.; Zhou, S.; Xu, Y.; Xu, R.; Liang, G.; Zhang, C.; Chen, X.; Yang, L.; Jin, F.; et al. Serum mitochondrial tsRNA serves as a novel biomarker for hepatocarcinoma diagnosis. Front. Med. 2022, 16, 216–226. [Google Scholar] [CrossRef]
- Yang, B.; Feng, X.; Liu, H.; Tong, R.; Wu, J.; Li, C.; Yu, H.; Chen, Y.; Cheng, Q.; Chen, J.; et al. High-metastatic cancer cells derived exosomal miR92a-3p promotes epithelial-mesenchymal transition and metastasis of low-metastatic cancer cells by regulating PTEN/Akt pathway in hepatocellular carcinoma. Oncogene 2020, 39, 6529–6543. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, L.; Ren, R.; Ji, F.; Xue, S.; Zhang, J.; Liu, Z.; Ma, Z.; Wang, X.W.; Wong, L.; et al. MiR-125b Loss Activated HIF1alpha/pAKT Loop, Leading to Transarterial Chemoembolization Resistance in Hepatocellular Carcinoma. Hepatology 2021, 73, 1381–1398. [Google Scholar] [CrossRef]
- Zhao, J.; Li, H.; Zhao, S.; Wang, E.; Zhu, J.; Feng, D.; Zhu, Y.; Dou, W.; Fan, Q.; Hu, J.; et al. Epigenetic silencing of miR-144/451a cluster contributes to HCC progression via paracrine HGF/MIF-mediated TAM remodeling. Mol. Cancer 2021, 20, 46. [Google Scholar] [CrossRef]
- Yokota, Y.; Noda, T.; Okumura, Y.; Kobayashi, S.; Iwagami, Y.; Yamada, D.; Tomimaru, Y.; Akita, H.; Gotoh, K.; Takeda, Y.; et al. Serum exosomal miR-638 is a prognostic marker of HCC via downregulation of VE-cadherin and ZO-1 of endothelial cells. Cancer Sci. 2021, 112, 1275–1288. [Google Scholar] [CrossRef]
- Sharma, G.G.; Okada, Y.; Von Hoff, D.; Goel, A. Non-coding RNA biomarkers in pancreatic ductal adenocarcinoma. Semin Cancer Biol. 2021, 75, 153–168. [Google Scholar] [CrossRef]
- Yang, J.; Xu, R.; Wang, C.; Qiu, J.; Ren, B.; You, L. Early screening and diagnosis strategies of pancreatic cancer: A comprehensive review. Cancer Commun. 2021, 41, 1257–1274. [Google Scholar] [CrossRef] [PubMed]
- Daoud, A.Z.; Mulholland, E.J.; Cole, G.; McCarthy, H.O. MicroRNAs in Pancreatic Cancer: Biomarkers, prognostic, and therapeutic modulators. BMC Cancer 2019, 19, 1130. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yoo, J.; Ho, J.Y.; Jung, Y.; Lee, S.; Hur, S.Y.; Choi, Y.J. Plasma-derived exosomal miR-4732-5p is a promising noninvasive diagnostic biomarker for epithelial ovarian cancer. J. Ovarian Res. 2021, 14, 59. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Chen, Z.; Wang, M.; Zhang, M.; Chen, Y.; Yang, X.; Zhou, C.; Liu, Y.; Hong, L.; Zhang, L. Detection of plasma exosomal miRNA-205 as a biomarker for early diagnosis and an adjuvant indicator of ovarian cancer staging. J. Ovarian Res. 2022, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Hulstaert, E.; Morlion, A.; Levanon, K.; Vandesompele, J.; Mestdagh, P. Candidate RNA biomarkers in biofluids for early diagnosis of ovarian cancer: A systematic review. Gynecol. Oncol. 2021, 160, 633–642. [Google Scholar] [CrossRef]
- Tung, C.H.; Kuo, L.W.; Huang, M.F.; Wu, Y.Y.; Tsai, Y.T.; Wu, J.E.; Hsu, K.F.; Chen, Y.L.; Hong, T.M. MicroRNA-150-5p promotes cell motility by inhibiting c-Myb-mediated Slug suppression and is a prognostic biomarker for recurrent ovarian cancer. Oncogene 2020, 39, 862–876. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, W.; Xu, L.; Yi, T.; Zhao, X.; Wei, Y.; Vermeulen, L.; Goel, A.; Zhou, S.; Wang, X. Integrative network biology analysis identifies miR-508-3p as the determinant for the mesenchymal identity and a strong prognostic biomarker of ovarian cancer. Oncogene 2019, 38, 2305–2319. [Google Scholar] [CrossRef]
- Todeschini, P.; Salviato, E.; Romani, C.; Raimondi, V.; Ciccarese, F.; Ferrari, F.; Tognon, G.; Marchini, S.; D’Incalci, M.; Zanotti, L.; et al. Comprehensive Profiling of Hypoxia-Related miRNAs Identifies miR-23a-3p Overexpression as a Marker of Platinum Resistance and Poor Prognosis in High-Grade Serous Ovarian Cancer. Cancers 2021, 13, 3358. [Google Scholar] [CrossRef]
- Panoutsopoulou, K.; Avgeris, M.; Mavridis, K.; Dreyer, T.; Dorn, J.; Obermayr, E.; Reinthaller, A.; Michaelidou, K.; Mahner, S.; Vergote, I.; et al. miR-203 is an independent molecular predictor of prognosis and treatment outcome in ovarian cancer: A multi-institutional study. Carcinogenesis 2020, 41, 442–451. [Google Scholar] [CrossRef]
- Panoutsopoulou, K.; Dreyer, T.; Dorn, J.; Obermayr, E.; Mahner, S.; Gorp, T.V.; Braicu, I.; Zeillinger, R.; Magdolen, V.; Avgeris, M.; et al. tRNA(GlyGCC)-Derived Internal Fragment (i-tRF-GlyGCC) in Ovarian Cancer Treatment Outcome and Progression. Cancers 2021, 14, 24. [Google Scholar] [CrossRef]
- Bao, Z.; Zhang, N.; Niu, W.; Mu, M.; Zhang, X.; Hu, S.; Niu, C. Exosomal miR-155-5p derived from glioma stem-like cells promotes mesenchymal transition via targeting ACOT12. Cell Death Dis. 2022, 13, 725. [Google Scholar] [CrossRef] [PubMed]
- Shao, N.; Xue, L.; Wang, R.; Luo, K.; Zhi, F.; Lan, Q. miR-454-3p Is an Exosomal Biomarker and Functions as a Tumor Suppressor in Glioma. Mol. Cancer Ther. 2019, 18, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xiao, Z.; Li, B.; Li, H.; Yang, B.; Li, T.; Mei, Z. miRNA-21 may serve as a promising noninvasive marker of glioma with a high diagnostic performance: A pooled analysis of 997 patients. Ther. Adv. Med. Oncol. 2021, 13, 1758835920987650. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhao, X.; Ming, J.; Liu, X.; Liu, D.; Jiang, C. Stepwise detection and evaluation reveal miR-10b and miR-222 as a remarkable prognostic pair for glioblastoma. Oncogene 2019, 38, 6142–6157. [Google Scholar] [CrossRef]
- Lin, J.; Ding, S.; Xie, C.; Yi, R.; Wu, Z.; Luo, J.; Huang, T.; Zeng, Y.; Wang, X.; Xu, A.; et al. MicroRNA-4476 promotes glioma progression through a miR-4476/APC/β-catenin/c-Jun positive feedback loop. Cell Death Dis. 2020, 11, 269. [Google Scholar] [CrossRef]
- Kandettu, A.; Radhakrishnan, R.; Chakrabarty, S.; Sriharikrishnaa, S.; Kabekkodu, S.P. The emerging role of miRNA clusters in breast cancer progression. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188413. [Google Scholar] [CrossRef]
- Wang, J.; Ma, G.; Ge, H.; Han, X.; Mao, X.; Wang, X.; Veeramootoo, J.S.; Xia, T.; Liu, X.; Wang, S. Circulating tRNA-derived small RNAs (tsRNAs) signature for the diagnosis and prognosis of breast cancer. NPJ Breast Cancer 2021, 7, 4. [Google Scholar] [CrossRef]
- Takagawa, Y.; Gen, Y.; Muramatsu, T.; Tanimoto, K.; Inoue, J.; Harada, H.; Inazawa, J. miR-1293, a Candidate for miRNA-Based Cancer Therapeutics, Simultaneously Targets BRD4 and the DNA Repair Pathway. Mol. Ther. 2020, 28, 1494–1505. [Google Scholar] [CrossRef]
- Zeng, A.; Wei, Z.; Yan, W.; Yin, J.; Huang, X.; Zhou, X.; Li, R.; Shen, F.; Wu, W.; Wang, X.; et al. Exosomal transfer of miR-151a enhances chemosensitivity to temozolomide in drug-resistant glioblastoma. Cancer Lett. 2018, 436, 10–21. [Google Scholar] [CrossRef]
- Chaudhary, A.K.; Mondal, G.; Kumar, V.; Kattel, K.; Mahato, R.I. Chemosensitization and inhibition of pancreatic cancer stem cell proliferation by overexpression of microRNA-205. Cancer Lett. 2017, 402, 1–8. [Google Scholar] [CrossRef]
- Cortez, M.A.; Valdecanas, D.; Niknam, S.; Peltier, H.J.; Diao, L.; Giri, U.; Komaki, R.; Calin, G.A.; Gomez, D.R.; Chang, J.Y.; et al. In Vivo Delivery of miR-34a Sensitizes Lung Tumors to Radiation Through RAD51 Regulation. Mol. Ther. Nucleic. Acids 2015, 4, e270. [Google Scholar] [CrossRef]
- Ma, W.; Ma, C.N.; Zhou, N.N.; Li, X.D.; Zhang, Y.J. Up-regulation of miR-328-3p sensitizes non-small cell lung cancer to radiotherapy. Sci. Rep. 2016, 6, 31651. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Ohkuri, T.; Kosaka, A.; Tanahashi, K.; June, C.H.; Natsume, A.; Okada, H. Expression of miR-17-92 enhances anti-tumor activity of T-cells transduced with the anti-EGFRvIII chimeric antigen receptor in mice bearing human GBM xenografts. J. Immunother. Cancer 2013, 1, 21. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Q.; Kumar, S.; Calin, G.A.; Li, Z.; Chim, C.S. Frequent methylation of the tumour suppressor miR-1258 targeting PDL1: Implication in multiple myeloma-specific cytotoxicity and prognostification. Br. J. Haematol. 2020, 190, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Mollaei, H.; Safaralizadeh, R.; Rostami, Z. MicroRNA replacement therapy in cancer. J. Cell Physiol. 2019, 234, 12369–12384. [Google Scholar] [CrossRef]
- Di Paolo, D.; Pastorino, F.; Brignole, C.; Corrias, M.V.; Emionite, L.; Cilli, M.; Tamma, R.; Priddy, L.; Amaro, A.; Ferrari, D.; et al. Combined Replenishment of miR-34a and let-7b by Targeted Nanoparticles Inhibits Tumor Growth in Neuroblastoma Preclinical Models. Small 2020, 16, e1906426. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).