Polymorphism rs7079 in miR-31/-584 Binding Site in Angiotensinogen Gene Associates with Earlier Onset of Coronary Artery Disease in Central European Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Remote Follow up after 15 Years
2.3. Genotyping
2.4. Statistical Analysis
3. Results
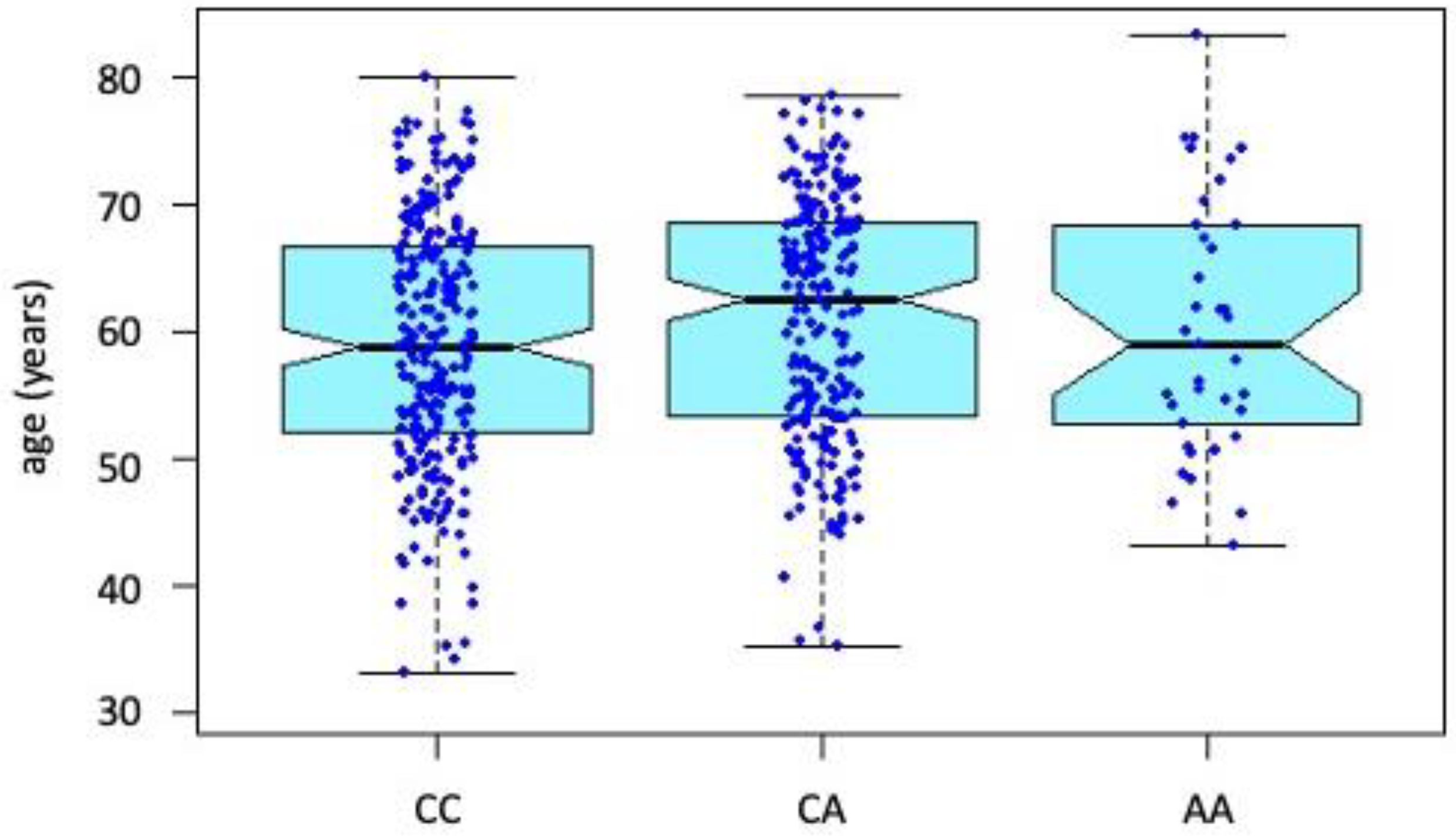
3.1. Basic Description of the Study Group
3.2. Comparison of Individual Genotypes
3.3. Correlation of AGT Genotypes with Categorical Variables
3.4. Logistic Regression Modeling Focused on Restenosis Occurrence
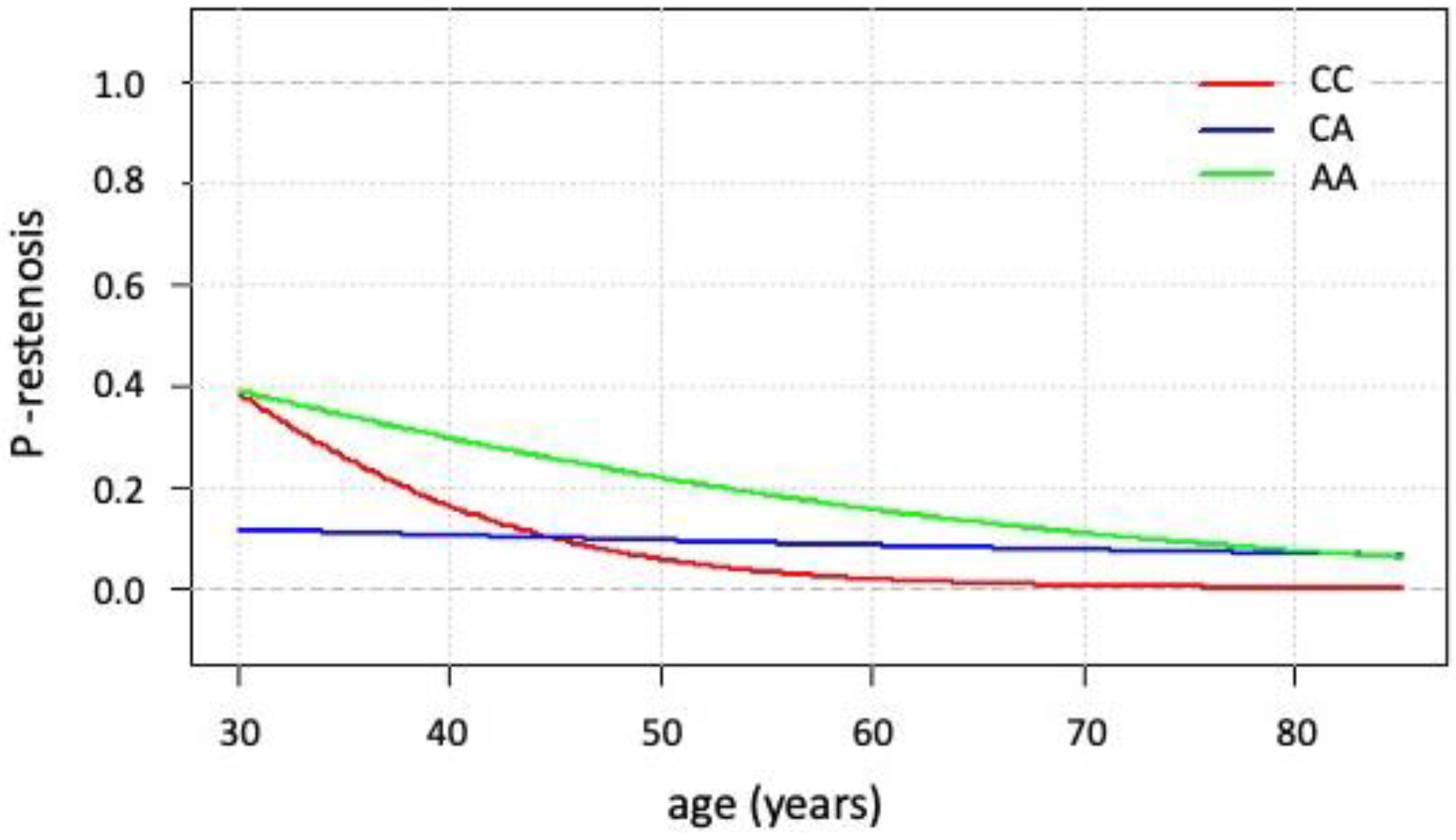
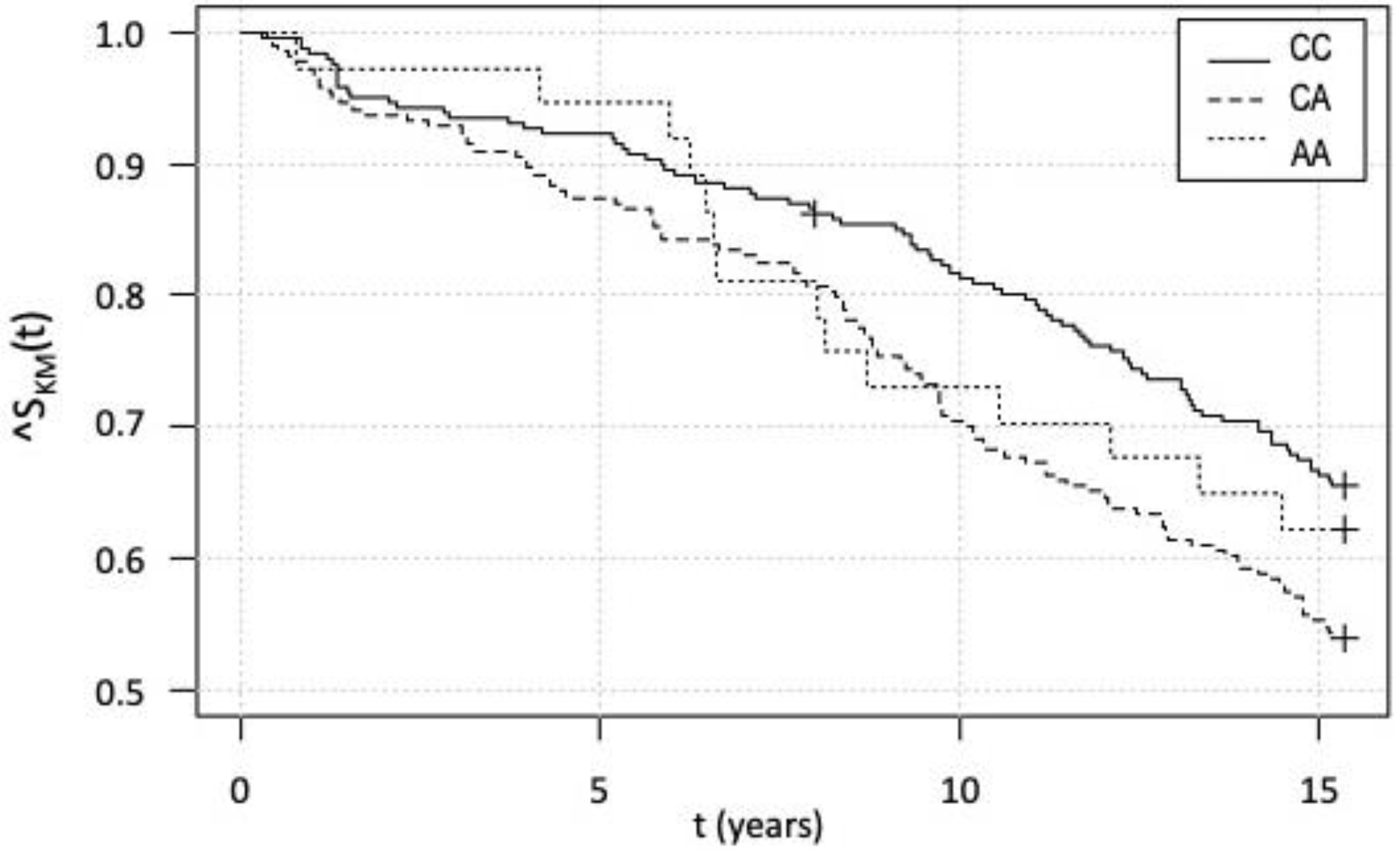
3.5. Kaplan–Meier Survival Analysis and Cox Analysis
4. Discussion
5. Conclusions
6. Strengths and Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benigni, A.; Cassis, P.; Remuzzi, G. Angiotensin II revisited: New roles in inflammation, immunology and aging. EMBO Mol. Med. 2010, 2, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.M.; França-Falcão, M.S.; Calzerra, N.T.M.; Luz, M.S.; Gadelha, D.D.A.; Balarini, C.M.; Queiroz, T.M. Role of Renin-Angiotensin System Components in Atherosclerosis: Focus on Ang-II, ACE2, and Ang-1-7. Front. Physiol. 2020, 11, 1067. [Google Scholar] [CrossRef] [PubMed]
- Furuya, K.; Yamaguchi, E.; Itoh, A.; Hizawa, N.; Ohnuma, N.; Kojima, J.; Kodama, N.; Kawakami, Y. Deletion polymorphism in the angiotensin I converting enzyme (ACE) gene as a genetic risk factor for sarcoidosis. Thorax 1996, 51, 777–780. [Google Scholar] [CrossRef] [PubMed]
- White, P.C.; Slutsker, L. Haplotype analysis of CYP11B2. Endocr. Res. 1995, 21, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Slaby, O.; Bienertova-Vasku, J.; Svoboda, M.; Vyzula, R. Genetic polymorphisms, and microRNAs: New direction in molecular epidemiology of solid cancer. J. Cell. Mol. Med. 2012, 16, 8–21. [Google Scholar] [CrossRef]
- Mopidevi, B.; Ponnala, M.; Kumar, A. Human angiotensinogen +11525 C/A polymorphism modulates its gene expression through microRNA binding. Physiol. Genom. 2013, 45, 901–906. [Google Scholar] [CrossRef][Green Version]
- Machal, J.; Novak, J.; Hezova, R.; Zlamal, F.; Vasku, A.; Slaby, O.; Bienertova-Vasku, J. Polymorphism in miR-31 and miR-584 binding site in the angiotensinogen gene differentially influences body fat distribution in both sexes. Genes Nutr. 2015, 10, 488. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, M.; Zhang, J.; Sun, N.; Li, C. A new model of the mechanism underlying lead poisoning: SNP in miRNA target region influence the AGT expression level. Hereditas 2019, 156, 6. [Google Scholar] [CrossRef]
- Máchal, J.; Vašků, A.; Kincl, V.; Hlavna, M.; Bartáková, V.; Jurajda, M.; Meluzín, J. Association between three single nucleotide polymorphisms in eotaxin (CCL 11) gene, hexanucleotide repetition upstream, severity and course of coronary atherosclerosis. J. Appl. Genet. 2012, 53, 271–278. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Al-Najai, M.; Muiya, P.; Tahir, A.I.; Elhawari, S.; Gueco, D.; Andres, E.; Mazhar, N.; Altassan, N.; Alshahid, M.; Dzimiri, N. Association of the angiotensinogen gene polymorphism with atherosclerosis and its risk traits in the Saudi population. BMC Cardiovasc. Disord. 2013, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Ochi, T.; Munekage, K.; Ogasawara, M.; Hirose, A.; Nozaki, Y.; Takahashi, M.; Okamoto, N.; Saibara, T. Angiotensinogen gene haplotype is associated with the prevalence of Japanese non-alcoholic steatohepatitis. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2011, 41, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-W.; Huang, T.-S.; Lo, H.-H.; Huang, P.-H.; Lin, C.-C.; Chang, S.-J.; Liao, K.-H.; Tsai, C.-H.; Chan, C.-H.; Tsai, C.-F.; et al. Deficiency of the microRNA-31-microRNA-720 pathway in the plasma and endothelial progenitor cells from patients with coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Baker, M.B.; Patel, R.S.; Quyyumi, A.A.; Bao, G.; Searles, C.D. MicroRNA Expression Profile in CAD Patients and the Impact of ACEI/ARB. Cardiol. Res. Pract. 2011, 2011, 532915. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Li, J.; Wu, G.; Miao, Z.; Lu, L.; Ren, G.; Wang, X. Upregulation of microRNA-335 and microRNA-584 contributes to the pathogenesis of severe preeclampsia through downregulation of endothelial nitric oxide synthase. Mol. Med. Rep. 2015, 12, 5383–5390. [Google Scholar] [CrossRef]
- Kim, S.; Lee, K.-S.; Choi, S.; Kim, J.; Lee, D.-K.; Park, M.; Park, W.; Kim, T.-H.; Hwang, J.Y.; Won, M.-H.; et al. NF-κB–responsive miRNA-31-5p elicits endothelial dysfunction associated with preeclampsia via down-regulation of endothelial nitric-oxide synthase. J. Biol. Chem. 2018, 293, 18989–19000. [Google Scholar] [CrossRef]
- Navarro, E.; Mallén, A.; Cruzado, J.M.; Torras, J.; Hueso, M. Unveiling ncRNA regulatory axes in atherosclerosis progression. Clin. Transl. Med. 2020, 9, 5. [Google Scholar] [CrossRef]
- Buccheri, D.; Piraino, D.; Andolina, G.; Cortese, B. Understanding and managing in-stent restenosis: A review of clinical data, from pathogenesis to treatment. J. Thorac. Dis. 2016, 8, E1150–E1162. [Google Scholar] [CrossRef]
- Suárez, Y.; Wang, C.; Manes, T.D.; Pober, J.S. TNF-induced miRNAs Regulate TNF-induced expression of E-Selectin and ICAM-1 on Human Endothelial Cells: Feedback Control of Inflammation. J. Immunol. Baltim. Md. 1950 2010, 184, 21–25. [Google Scholar] [CrossRef]
- Huang, R.; Chen, X.; Long, Y.; Chen, R. MiR-31 promotes Th22 differentiation through targeting Bach2 in coronary heart disease. Biosci. Rep. 2019, 39, BSR20190986. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, T.; Wang, X.; Du, R.; Zhang, K.; Liu, X.; Ma, D.; Yu, S.; Su, G.; Li, Z.; et al. Elevated frequencies of circulating Th22 cell in addition to Th17 cell and Th17/Th1 cell in patients with acute coronary syndrome. PLoS ONE 2013, 8, e71466. [Google Scholar] [CrossRef]
| Parameter | |
|---|---|
| N (women) | 514 (84) |
| Age (years) | 60.10 ± 9.60 |
| BMI (kg/m2) | 27.57 ± 3.18 |
| SBP (mmHg) | 140.99 ± 18.65 |
| DBP (mmHg) | 83.92 ± 8.78 |
| Cholesterol (mmol/L) | 5.69 ± 1.10 |
| HDL (mmol/L) | 1.18 ± 0.33 |
| LDL (mmol/L) | 3.63 ± 1.05 |
| Triglycerides (mmol/L) | 2.11 ± 1.33 |
| Glucose (mmol/L) | 6.05 ± 1.70 |
| LV EF (%) | 50.37 ± 10.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novák, J.; Maceková, S.; Héžová, R.; Máchal, J.; Zlámal, F.; Hlinomaz, O.; Rezek, M.; Souček, M.; Vašků, A.; Slabý, O.; et al. Polymorphism rs7079 in miR-31/-584 Binding Site in Angiotensinogen Gene Associates with Earlier Onset of Coronary Artery Disease in Central European Population. Genes 2022, 13, 1981. https://doi.org/10.3390/genes13111981
Novák J, Maceková S, Héžová R, Máchal J, Zlámal F, Hlinomaz O, Rezek M, Souček M, Vašků A, Slabý O, et al. Polymorphism rs7079 in miR-31/-584 Binding Site in Angiotensinogen Gene Associates with Earlier Onset of Coronary Artery Disease in Central European Population. Genes. 2022; 13(11):1981. https://doi.org/10.3390/genes13111981
Chicago/Turabian StyleNovák, Jan, Soňa Maceková, Renata Héžová, Jan Máchal, Filip Zlámal, Ota Hlinomaz, Michal Rezek, Miroslav Souček, Anna Vašků, Ondřej Slabý, and et al. 2022. "Polymorphism rs7079 in miR-31/-584 Binding Site in Angiotensinogen Gene Associates with Earlier Onset of Coronary Artery Disease in Central European Population" Genes 13, no. 11: 1981. https://doi.org/10.3390/genes13111981
APA StyleNovák, J., Maceková, S., Héžová, R., Máchal, J., Zlámal, F., Hlinomaz, O., Rezek, M., Souček, M., Vašků, A., Slabý, O., & Bienertová-Vašků, J. (2022). Polymorphism rs7079 in miR-31/-584 Binding Site in Angiotensinogen Gene Associates with Earlier Onset of Coronary Artery Disease in Central European Population. Genes, 13(11), 1981. https://doi.org/10.3390/genes13111981










