Abstract
Faba bean (Vicia faba L.), a drought-sensitive crop, is drastically affected by drought stresses compromising its growth and yield. However, wild relatives of faba bean are considered a reservoir of potential genetic resources for tolerance against abiotic stresses. This study was conducted to characterize wild relatives of faba bean for identification of a specific tolerance system required for its improvement against drought stress. The study focused on physiological, biochemical, and anatomical responses of wild Vicia species under drought stress conditions. The experiment was carried out under various levels of drought stress imposed through different field capacities (FC) which included 80% FC ie (well-watered condition), 55% FC (moderate stress), and 30% FC (severe stress). When compared to plants grown in a control environment, drought stress significantly reduced the studied physiological attributes including soluble sugars (21.3% and 15.8%), protein contents (14.7 and 14.6%), and chlorophyll (8.4 and 28.6%) under moderate (55% FC) and severe drought stress (30% FC), respectively. However, proline content increased by 20.5% and 27.6%, peroxidase activity by 48.5% and 57.1%, and superoxide dismutase activity by 72.6% and 64.8% under moderate and severe stress, respectively. The studied anatomical attributes were also affected under drought stress treatments, including diameter of stem xylem vessels (9.1% and 13.7%), leaf lower epidermal thickness (8.05% and 13.34%), and leaf phloem width (5.3% and 10.1%) under moderate and severe stress, respectively. Wild Vicia spp. showed better tolerance to water-deficit conditions as compared to cultivated Vicia L. The observed potential diversity for drought tolerance in wild Vicia spp. may assist in improvement of faba bean and may also help in understanding the mechanisms of adaptations in drought-prone environments.
1. Introduction
Among legumes, the genus Vicia has remarkable economic importance for humans and animals on account of its food, fodder, and medicinal value [1]. Faba bean (Vicia L. Fabaceae) is a cool-season annual legume that is consumed by humans for nutritional purposes as it contains about 35% proteins, in addition to complex carbohydrates, choline, lecithin, and dietary fibers, and because it is a rich source of nutrients such as calcium, magnesium, potassium, and zinc [2,3]. Moreover, the seeds of faba bean have lower amounts of cholesterol, fats, and sodium [4]. Its seeds are routinely used as a substitute of fish and meat proteins in the developing countries of Asia and Africa [5]. It also plays an important role in increasing soil fertility by fixing atmospheric nitrogen and solubilizing phosphorus (P) in the soil, enhancing plant–microbe interactions [6,7]. Medicinally, faba bean is valuable in accumulating a substantial concentration of L-Dopa, a precursor of dopamine used for treating hormonal imbalance [3,8]. Various species of Vicia possess antioxidant, antimicrobial, anti-Parkinson, and antidiabetic potentials [9]. Its annual total production has been estimated as 14.2 Mt on cultivated land of 13.7 Mha; however, there are reports of a decreased global acreage and yield of faba bean since 1980 [10,11,12,13]. The yield of Vicia L. is reduced by deficiency of water, subjected at any stage of the life cycle; however, flowering, podding, and grain-filling phases are the most sensitive [14,15,16]. Further, different varieties of faba bean may have differential responses toward abiotic stresses [17,18]. To adopt plants with deficient water conditions, plant breeders and physiologists are trying to improve the phenology and physiology of crops by developing drought-tolerant cultivars [19,20,21]. However, improvement in the development of cultivars for drought tolerance is slow because of a lack of efficient screening techniques and large seasonal variation [22,23,24,25].
Crop wild relatives (CWRs) can play important roles in the improvement of cultivated crops because of exhibiting resistance to various abiotic and biotic stresses [26,27,28]. Different genes which are present in CWRs can be utilized to develop new varieties which produce better yield and tolerance [29]. For instance, a few genes reported in Oryza nivara, a wild relative of rice, have shown resistance to various plant diseases [30]. Hence, different genes can be isolated from CWRs and transferred to cultivated crops to achieve high yields, because they possess resistance to abiotic and biotic stresses [31].
Vetches are mostly grown in the wild and are found naturally in rain-fed conditions [29]. Vicia is a taxonomically large and complex genus represented by about 210 to 240 species, distributed primarily in temperate regions of the northern hemisphere in Asia, Europe, and North America, and extending into extratropical South America as well [32]. Mediterranean and Irano-Turanian regions are considered the center of origin and diversity for the genus Vicia [33]. Vicia sativa subsp. sativa L. can grow in humid as well as in semi-dry areas and is used as livestock feed. Vicia villosa subsp. dasycarpa Roth. is a cool-season legume, grown for pasture, hay, silage, and grains for livestock. Vicia narbonensis L. is also a cool-season annual legume, having the potential to produce more grains in non-tropical areas in comparison to Vicia sativa, Vicia ervilia, and Vicia villosa subsp. dasycarpa [34]. Members of the Vicia genus have medicinal importance and also fix atmospheric nitrogen to a usable form and hence increase the fertility of soil [35,36].
The production of the legumes crop is mostly affected by various abiotic stresses such as cold, salinity. and drought, which cause a reduction in yield in different parts of the world [37]. Drought stress is a major abiotic stress which affects the yield of cereals, faba bean, and other legumes. It affects various physiological processes of plants such as cell wall turgidity, reduced carbon assimilation rate, and increased oxidative changes, the result being decreased yield [38,39]. Further, it also affects flowering times in plants [40,41]. Due to drought stress, metabolic activities of plants may lead to the formation of reactive oxygen species (ROS) which cause the oxidation of organic molecules such as DNA, RNA, lipids, and proteins [42,43]. After thorough review, it was revealed that there is no work reported to date on the biochemical and anatomical characterization of wild Vicia spp. of the study area. Therefore, the present work aimed to evaluate selected wild relatives of faba bean under progressive drought stress. The main focus was to investigate their physiological, biochemical, and anatomical responses under water-deficit conditions and to explore potential Vicia spp. with future prospects in breeding for drought tolerance.
2. Materials and Methods
2.1. Plant Specimen and Seed Collection
The genus Vicia is represented in Pakistan by 14 species, mostly confined to the northern temperate regions of Gilgit-Baltistan, Khyber Pakhtunkhwa, Upper Punjab, and Northern Baluchistan. Of these, V. faba L. is known only in cultivation, while the rest of the species are wild, or have become naturalized (V. narbonensis, V. villosa). During the fieldwork, about 450 specimens belonging to Vicia sativa subsp. sativa, V. sativa subsp. nigra, V. narbonensis, V. bithynica, and V. monantha were collected from different localities in twelve districts of Northern Pakistan including Swat, Dir, Bajour, Chitral, Mansehra, Battagram, Abbotabad, Islamabad, Skardu, Gilgit, Muzaffarabad and Neelum valley Azad Jammu, and Kashmir. Collections were done during flowering and fruiting periods of the species from March to September 2019 in different ecological habitats. Sampling was done randomly, and at least five specimens per species were sampled in each population in different areas, and, in total, more than sixty populations were sampled. The majority of the sampling was done in Swat, followed by Chitral and Mansehra. During fieldwork information on locality, phenology, population size, habitat features, number of flowers, number of pods, and seeds per pod were noted in a field notebook. A specific collection number was assigned to each specimen and field photographs of the whole plant and plant parts were taken. The specimens were dried, preserved, and mounted on herbarium sheets and submitted as vouchers to the Herbarium University of Swat (SWAT). Specimens were identified to species and subspecific levels by the second author using the flora of Pakistan and flora of Iran [44,45]. The nomenclature of scientific names is in accordance with Kew database Plants of the World Online “https://powo.science.kew.org/ (accessed on 23 June 2021)” and World Flora Online database “http://www.worldfloraonline.org/ (accessed on 23 June 2021)”. Seeds were collected from mature pods during May and September in cotton bags, dried, and stored in dark sealed bottles. Seeds of cultivated faba bean were provided by NARC (National Agriculture Research Centre) Islamabad. Species used during the current study included Vicia sativa ssp. Sativa L., V. sativa ssp. Nigra Ehrh., V. narbonensis L., V. bithynica (L.) L., V. faba L., and V. monantha Retz.
2.2. Pot Experiment
An experiment was carried out in green house at the Center for Plant Sciences and Biodiversity, University of Swat, for comparing the physiological, biochemical, and anatomical responses of the experimental plant materials comprising of wild Vicia species under drought stress conditions. Seeds were germinated in petri plates, and healthy seedlings were transplanted into plastic pots filled with soil and exposed to sunlight for better growth. The field capacity (FC) was determined gravimetrically following the method of Liu and Li [46] and Graber et al. [47]. Treatments in the pot experiment included 80% FC ie (well-watered condition), 55% FC (moderate stress), and 30% FC (severe stress). The experimental design employed during the study was a randomized complete block design (RCBD) replicated three times. To monitor drought stress, each pot was weighted daily, and water lost through evapotranspiration was added to pots. Thirty days after the imposition of stress, leaf samples were collected, weighted, wrapped in aluminum foil, and kept in a refrigerator at 40 °C for the determination of various anatomical, physiological, and biochemical attributes as described in Ali et al. [23].
2.3. Leaf Epidermal Anatomy
For leaf epidermal anatomy, fresh leaves were collected and boiled in 88% lactic acid for 30–40 min at 100 °C in a water bath according to the method used by Clark [48] and Ullah et al. [49]. To attain the peel of the abaxial surface of leaf, the adaxial surface was removed, along with mesophyll tissues by using a sharp blade, leaving the peel of the abaxial surface. To attain the peel of the adaxial epidermis, the same procedure was repeated. The leaves were softened with lactic acid, and the abaxial and adaxial peels were removed using a sharp razor blade. Epidermal peels were stained with safranin and mounted on glass slides in lactic acid. The studied qualitative traits were the epidermal cell shape, wall morphology, and stomata types, while quantitative traits included the subsidiary cell number and shape, length, width, and abundance of stomata, as well as the trichome type and size examined by using a light microscope equipped with a digital camera (Meiji infinity DK-5000, Tokyo, Japan).
2.4. Anatomical Features of Leaf and Stem
The anatomical study of leaves and stems was carried out to study variations in different traits in response to drought stress according to Ruzin [50]. Small pieces of plant tissue were fixed for 24 h in formalin acetic alcohol (FAA) solution containing formalin (10 mL), 70% ethyl alcohol (85 mL), and glacial acidic acid (85 mL). Slides were prepared by free-hand sectioning using sharp blades. Sections were then dehydrated by ethanol series (30%, 50%, 70%, and 90%). For clear visibility, sections were stained with light green and safranin, which were studied under light microscopes. Different anatomical features of stems and leaves such as epidermal cell length and width, stomatal length and width, stomatal complex length and width, trichome length and width, phloem length and width, and metaxylem diameter were recorded accordingly.
2.5. Physiological and Biochemical Analysis
Chlorophyll contents were determined according to the method used by Hiscox and Israelstam [51] using dimethyl sulfoxide (DMSO). Proline contents were determined in leaf samples using 3% sulfosalicylic acid by following the method of Bates et al. [52]. Protein contents in leaves were determined by following the method of Lowery et al. [53], using bovine serum albumin (BSA) as a standard. Soluble sugar contents were determined according to the method used by Dubois et al. [54], using the phenol sulfuric acid method, with a little bit of modification. For the determination of superoxide dismutase (SOD) activity, the method of Beauchamp and Fridovich [55] was followed, and peroxidase (POD) activity was accomplished according to Gorin and Heidema [56].
2.6. Statistical Analysis
For statistical analysis, the data of samples were recorded in Microsoft excel. The experimental design employed was a two-factor factorial RCBD (randomized complete block design) with three replications. The two treatment factors were six Vicia species and three drought stresses. Quantitative data were shown by drawing graphs and were also subjected to an analysis of variance (ANOVA) and least significant differences using Statistix 8.1., Tallahassee, FL, USA. Comparison among treatment means was made as described in Ali et al. [42]. For graphical representation of the association among different studied traits, the data were subjected to a Pearson correlation coefficient and principle component analysis by employing the Jamovi software.
3. Results
3.1. Leaf Micromorphological Attributes
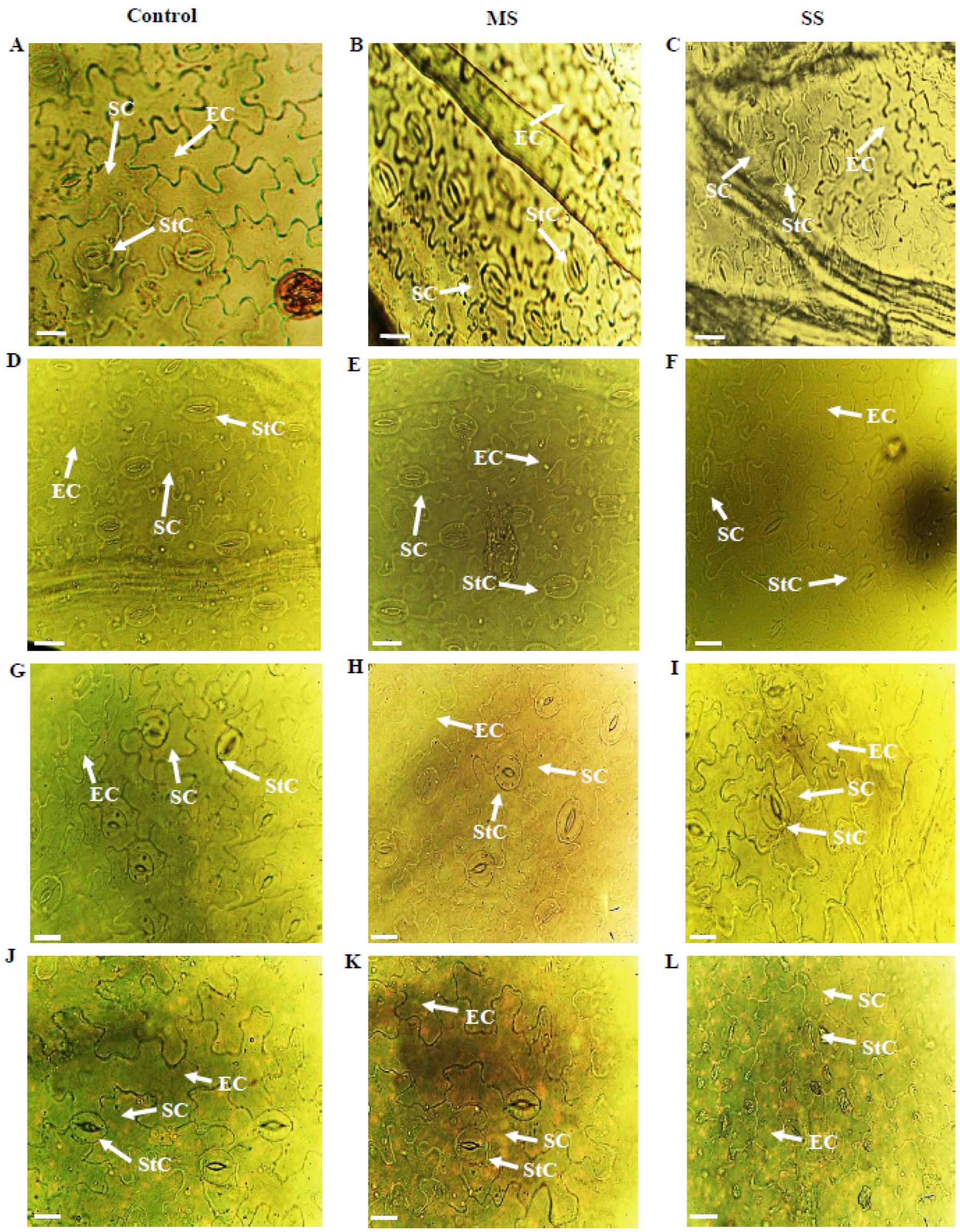
Results showed that both moderate and severe drought stress significantly affected various biochemical and physiological characteristics of Vicia spp. Mean square results showed that Vicia spp. and treatments highly differed for all the studied attributes in abaxial and adaxial leaf surfaces. However, their interaction was different only for stomatal width, while no significant differences were found for the rest of the studied traits (Table 1, Table 2, Table 3, Table 4 and Table 5; Figure 1). Drought stress significantly reduced all the studied attributes of both epidermal surfaces of leaves. Epidermal cell length decreased by 6.1% and 9.4% under moderate and severe drought stress, respectively. Similarly, epidermal cell width declined by 9.9% and 11.2% in moderate and severe stress, respectively. The stomatal length increased by 4.1% under moderate stress and 9.7% under severe stress. Under moderate stress, the stomatal width decreased by 5.8% while the decrease was 11.8% in severe drought stress. Stomatal complex length, trichome length, trichome width, and stomatal complex width also declined in response to progressive drought stress.

Table 1.
Analysis of variance, mean values, and percentage changes in leaf abaxial surfaces of Vicia species.

Table 2.
Analysis of variance, mean values, and percentage changes in leaf adaxial surfaces of Vicia species.

Table 3.
Analysis of variance, mean values, and percentage changes in stem and leaf anatomical traits of Vicia species.

Table 4.
Percentage changes in studied traits of leaf abaxial surfaces in studied Vicia species under drought stress.

Table 5.
Percentage changes in various traits of leaf adaxial surfaces studied Vicia species under drought stress.
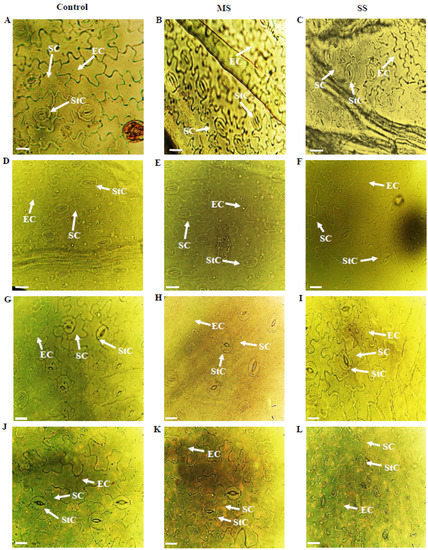
Figure 1.
Epidermal anatomy of abaxial (AB) surface of Vicia species showing variations in epidermal cells (EC), stomatal cells (SC) and stomatal complexes (StC). (A) V. sativa subsp. sativa control, (B) V. sativa subsp. sativa 55% FC, (C) V. sativa subsp. sativa 30% FC, (D) V. sativa subsp. nigra control, (E) V. sativa subsp. nigra 55% FC, (F) V. sativa subsp. nigra 30% FC, (G) V. narbonensis control, (H) V. narbonensis 55% FC, (I) V. narbonensis 30% FC, (J) V. bithynica control, (K) V. bithynica 55% FC, (L) V. bithynica 30% FC. Bar scale 20 μm.
3.2. Anatomical Attributes
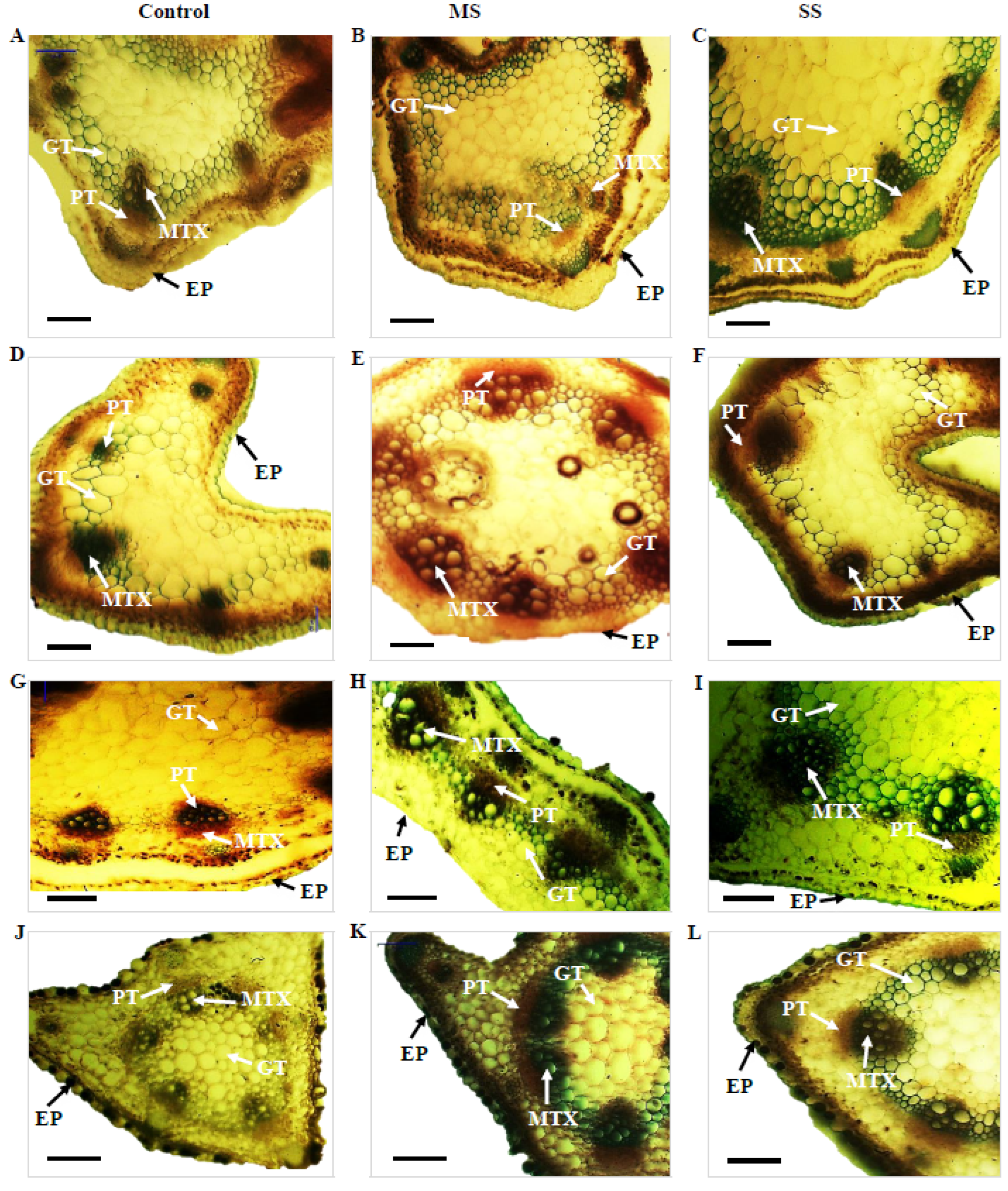
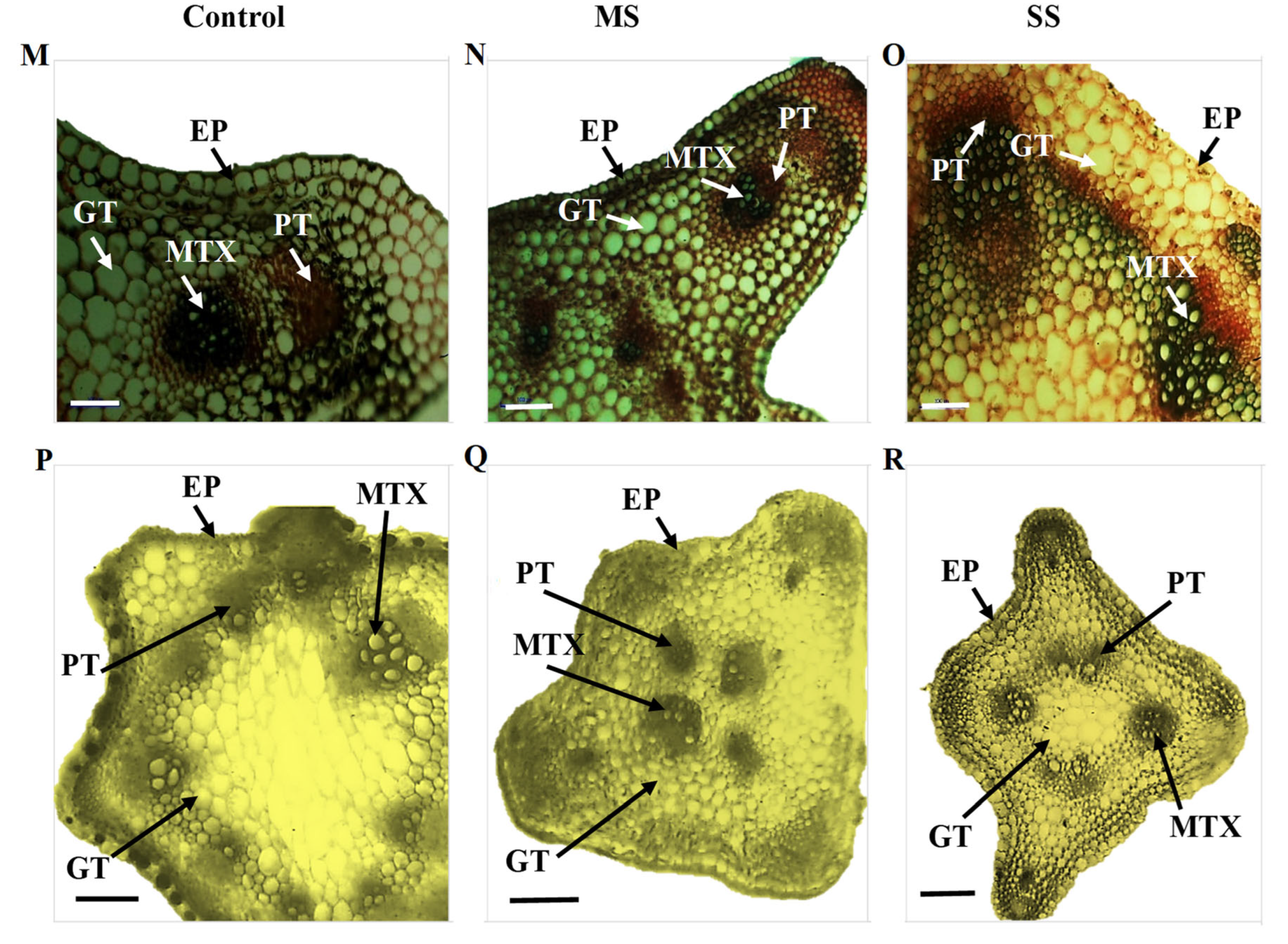
Mean square results concerning anatomical attributes revealed that epidermis thickness, phloem length, phloem width, and metaxylem diameter were highly different among the studied Vicia spp. Similarly, the interaction between Vicia spp. and drought stress treatment resulted in high differences in epidermis thickness, phloem length, phloem width, and metaxylem diameter (Table 6). Drought stress significantly reduced all the studied attributes of stem vascular anatomy. For instance, epidermis thickness decreased by 8.8% under moderate and 15.2% under severe drought stress. The decrease in phloem length was also recorded under moderate (3.0%) and severe (6.4%) drought stress. Similarly, phloem width declined by 3.9% under moderate and 8.4% under severe drought stress. Likewise, metaxylem diameter decreased by 9.1% under moderate drought stress and 13.7% under severe drought stress (Table 6; Figure 2). The maximum change (18.9%) in epidermal cells thickness under moderate drought stress was recorded in Vicia sativa subsp. sativa while minimum change (3.6%) was recorded in Vicia bithynica. Similarly, the maximum change (5.3%) in phloem width was recorded in Vicia under moderate stress while the minimum change (4.9%) was recorded in Vicia sativa subsp. sativa. Similarly, the maximum change in metaxylem diameter was recorded in Vicia bithynica, while the minimum change was in Vicia narbonensis under severe drought stress (Table 6; Figure 2).

Table 6.
Percentage changes in various stem and leaf anatomical traits of Vicia species under drought stress.
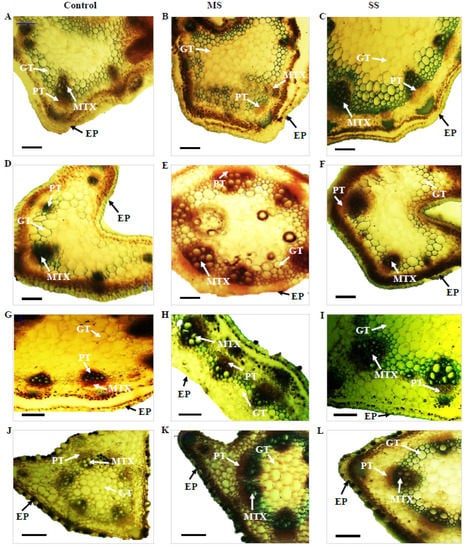
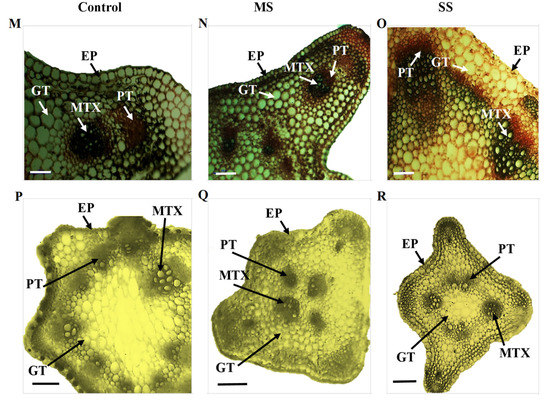
Figure 2.
Transverse section of the stem of Vicia species showing variation in the studied anatomical traits, i.e., epidermis (EP), metaxylem (MTX), phloem (PT), and ground tissues (GT); (A) V. sativa subsp. sativa control, (B) V. sativa subsp. sativa 55% FC, (C) V. sativa subsp. sativa 30% FC, (D) V. sativa subsp. nigra control (E) V. sativa subsp. nigra 55% FC, (F) V. sativa subsp. nigra 30% FC, (G) V. narbonensis control, (H) V. narbonensis 55% FC, (I) V. narbonensis 30% FC, (J) V. bithynica control, (K) V. bithynica 55% FC, (L) V. bithynica 30% FC, (M) V. faba control, (N) V. faba 55% FC, (O) V. faba 30% FC, (P) V. monantha control, (Q) V. monantha 55% FC, (R) V. monantha 30% FC. Bar scale 100 μm.
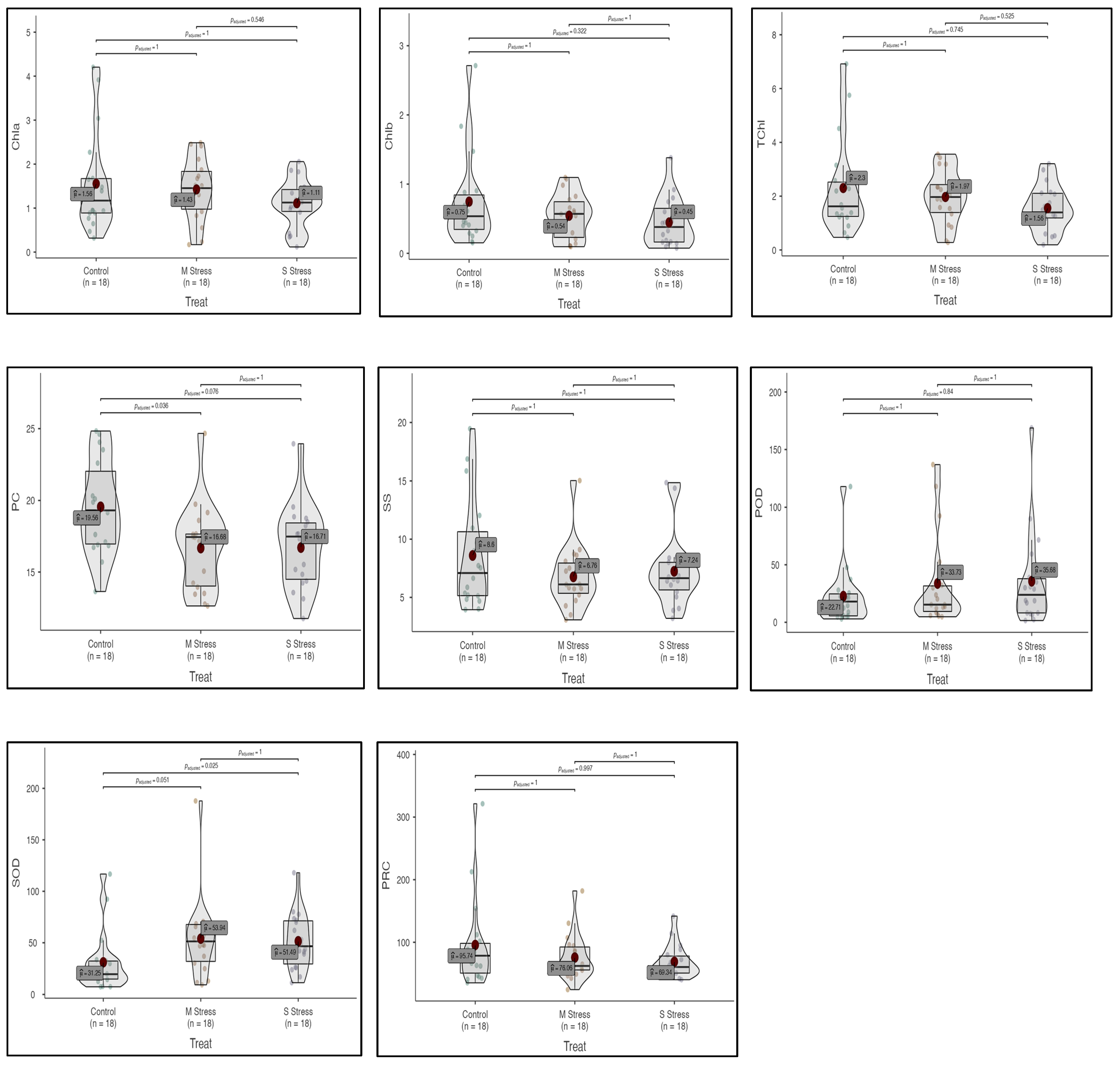
3.3. Chlorophyll Contents
Photosynthetic pigments were also affected by both moderate and severe drought stress as compared to the control. Chlorophyll a (Chla) content decreased both in moderate and severe stress by (8.4% and 28.6%), respectively. Chlorophyll b (Chlb) contents were decreased by 27.3% and 40.4% in moderate and severe stress, respectively. Similarly, total chlorophyll contents also decreased by 14.4% and 32.4% in both stresses, respectively (ESM 1; Figure 3). Among Vicia species, the maximum change in Chla contents was recorded in V. narbonensis, while the minimum change was in Vicia sativa subsp. sativa under moderate and severe drought stress, respectively. Similarly, the maximum change in TChl content was observed in V. narbonensis, while the minimum change was in V. sativa subsp. sativa under moderate and severe stress treatments, respectively (ESM 1, 2; Figure 3).

Figure 3.
Box–violin plots presenting physiological and biochemical traits, i.e., chlorophyll a (Chla), chlorophyll b (Chlb), total chlorophyll (TChl), protein content (PC), soluble sugars (SS), peroxidase activity (POD), superoxide dismutase activity (SOD), and proline content (PRC) under control (C), moderate (MS) and severe (SS) drought stress conditions.
3.4. Biochemical Attributes
Soluble sugar (SS) decreased by 21.3% and 15.8% in moderate and severe stress, respectively. A reduction in protein contents was observed, which was 14.7% and 14.6% in moderate and severe stress, respectively. Proline contents also decreased by 20.5% under moderate drought stress, but increased by 27.58% under severe drought stress. Peroxidase activity (POD) increased by 48.5% and 57.1% in moderate and severe stress, respectively. An increase in superoxide dismutase (SOD) activity was also observed at 72.6% and 64.8% in moderate and severe stress, respectively (ESM 1,3,4; Figure 2 and Figure 3). A maximum change in peroxidase (POD) activity was recorded in Vicia sativa subsp. nigra under moderate stress, while a minimum change of 12.5% was recorded in Vicia narbonensis under severe drought stress (ESM 4; Figure 2).
A maximum change in soluble sugar (SS) was recorded at 55.23% in Vicia sativa subsp. nigra while a minimum change in SS was recorded at 4.25% in Vicia bithynica under severe drought stress. Similarly, a maximum change in protein contents was recorded at 30.94% in Vicia narbonensis under moderate stress, while a minimum change was recorded at 3.54% in Vicia bithynica in severe drought stress. A maximum change in proline contents under moderate drought stress was recorded at 53.43% in Vicia sativa ssp. Nigra, while a minimum change in proline contents was recorded 4.57% in Vicia bithynica in severe drought stress (ESM 5; Figure 2).
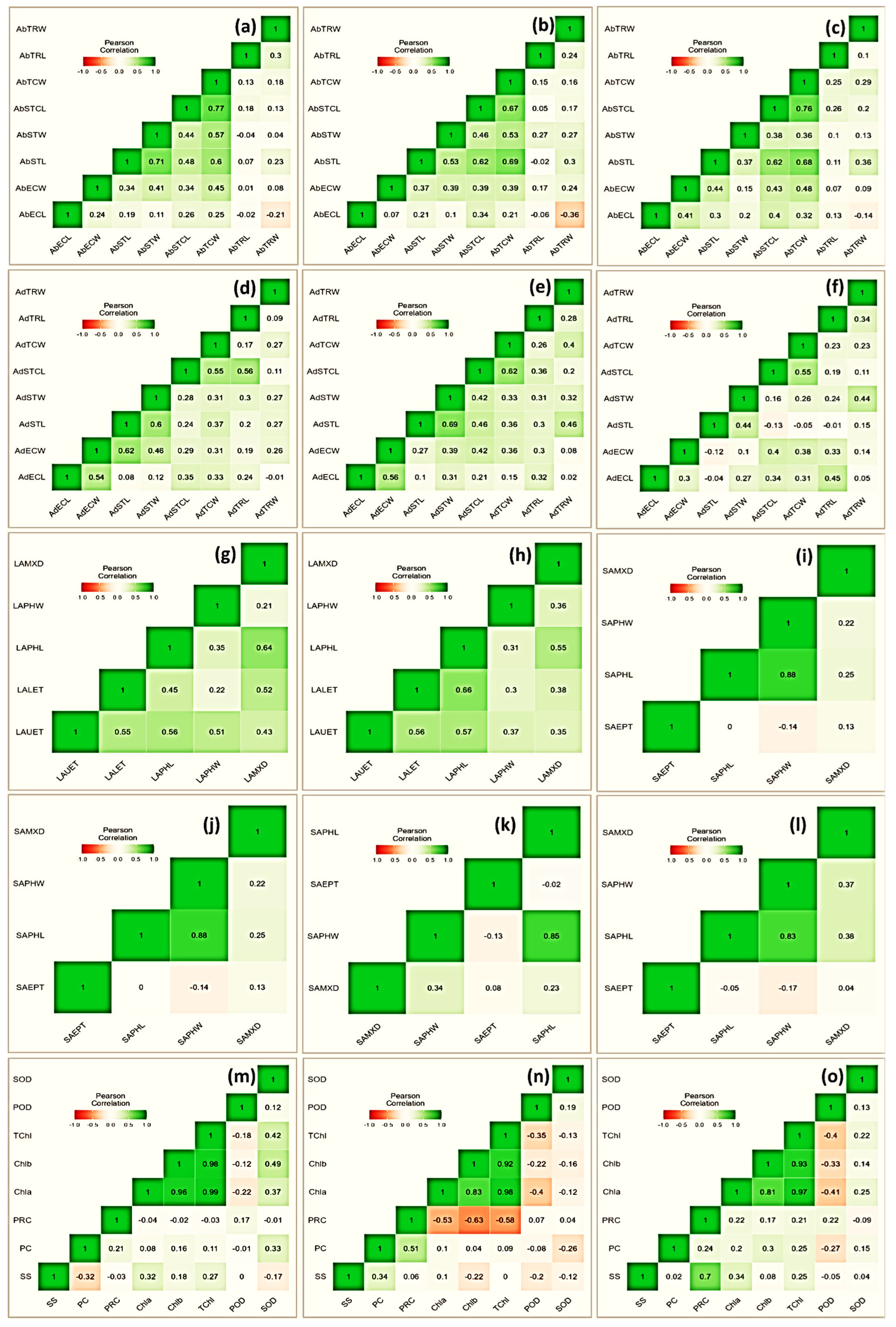
3.5. Heatmap of Pearson’s Coefficient
A heatmap of Pearson’s coefficient of correlation (r) analysis of the investigated attributes depicted variable associations (Figure 4). Among physiological traits, superoxide dismutase exhibited a significantly positive correlation. Specifically, its association was more prominent with Chla (r = 0.37), Chlb (r = 49), TChl (r = 0.42), and PRC (r = 0.33). The association of proline content was noticeable with chlorophyll with r = −0.53, −0.63 and −0.58 for Chla, Chlb, and TChl, respectively, under a moderate stress environment. The protein content correlated inconsistently with most of the studied traits, especially under stress treatment; however, its association was positive with SS (r = 0.34) and PRC (r = 0.51). Similarly, among leaf micromorphological traits, stomatal length in abaxial surface correlated strongly with stomatal width (r = 0.53 and 0.37), trichome cell length (r = 0.62 and 0.63), trichome cell width (r = 0.69 and 0.68), and epidermal cell width (r = 0.0.37 and 0.41), under moderate and severe drought stress, respectively. Among leaf anatomical traits, phloem length was in positive association with metaxylem diameter (r = 0.55), and upper and lower epidermal thicknesses (r = 0.57 and 0.66, respectively) under moderate drought stress. Further, with respect to the studied stem anatomical traits, the highest positive association was recorded between phloem length and width (r = 0.85 and 0.83) under moderate and severe stress, respectively.
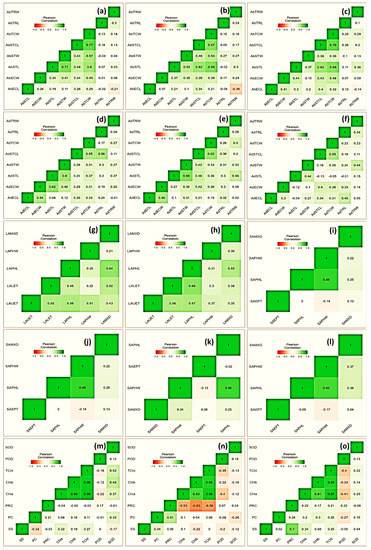
Figure 4.
Heatmap showing Pearson correlation coefficient and associated probabilities (p ≤ 0.05, ≤ 0.01, ≤ 0.001, respectively) among Vicia species for the studied traits (n = 10) evaluated under drought stress (pot experiment with three treatments, i.e., control, FC = 80%; MS, FC = 50%; SS, FC = 30%). Micromorphological traits represent both abaxial and adaxial surfaces, i.e., trichome width (AbTRW and AdTRW), trichome length (AbTRL and AdTRL), stomatal complex length (AbSTCL and AdSTCL), stomatal complex width (AbSTCW and AdSTCW), stomatal length (AbSTW and AdSTW), stomatal width (AbSTL and AbSTL), epidermal cell length (AbECL and AdECL), and epidermal cell width (AbECW and AdECW). Leaf anatomical traits included metaxylem diameter (LAMXD), phloem width (LAPHW), phloem length (LAPHL), lower epidermis thickness (LALET), and upper epidermis thickness (LAUET). Stem anatomical traits included metaxylem diameter (SAMXD), phloem width (SAPHW), phloem length (SAPHL), and epidermis thickness (SAEPT). (a) Control, (b) MS, and (c); SS (d) control, (e) MS, and (f) SS showing relationship for leaf micromorphological traits; (g) control, (h) MS, and (i) SS showing relationship for leaf anatomical traits; (j) control, (k) MS, and (l) SS showing relationship for stem anatomical traits; and (m) control, (n) MS, and (o) SS showing relationship for physiological traits.
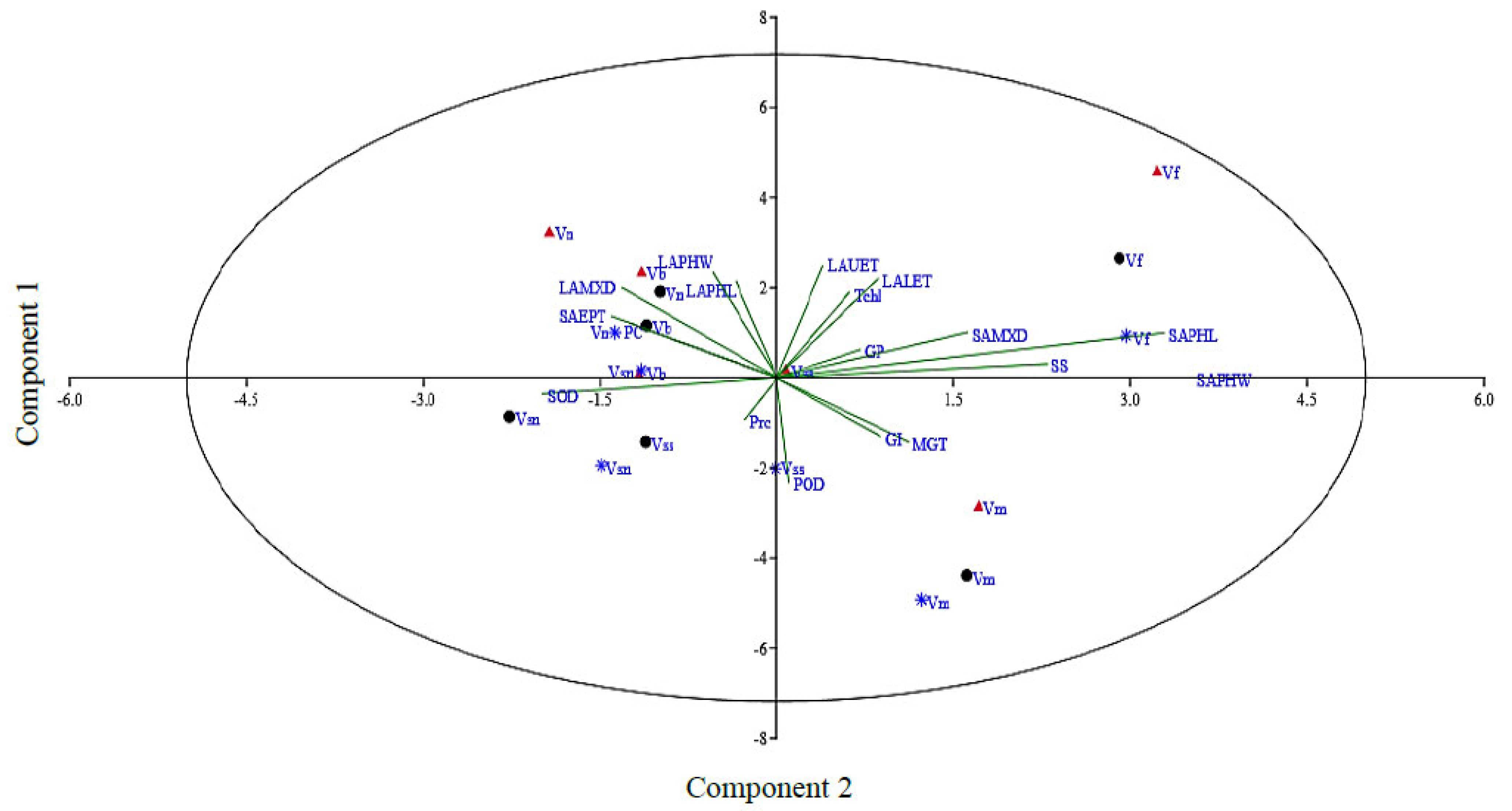
3.6. Principle Component Analysis (PCA)
Principle component analysis can be utilized to select traits that can be categorized into main groups and subgroups based on homogeneity and dissimilarity. In order to find out the most appropriate combination of the studied attributes, PCA and biplot analysis were conducted using mean values (Figure 5 and ESM 5). The vector length shows the extent of variation explained by respective traits in the PCA. The first two axes, i.e., PC1 (eigen value = 6.68) and PC2 (eigen value = 3.24), explained up to 55% of the total variability. Considering PC1 and PC2, it is very clear that mostly morpho-physiological, biochemical, and leaf epidermal attributes contributed to PC1, while the attributes with major contributions to PC2 were mostly leaf epidermal and biochemical traits. The attributes in order of their positive contribution to PC1 included leaf upper epidermis thickness (0.37) and phloem width (0.35). The contribution from the remaining studied attributes to this principal component was either positive or negative but non-significant. Similarly, for PC2, the major contributing attributes were stem phloem width (0.52), phloem length (0.49), and SS (0.34). The contribution of all other studied traits to this principal component was minor. For PC3, stem metaxylem diameter (0.27) was noted for its prominent contribution. For PC4, leaf phloem length (0.38) was noted for its prominent contribution. Further, in our data set, four groups of traits were identified in the PCA biplot considering both PC1 and PC2 simultaneously (Figure 5). The most prominent among those in group 1 were leaf lower epidermis thickness, total chlorophyll, stem metaxylem diameter, soluble sugars, and stem phloem length, while stem epidermis thickness, protein content, leaf metaxylem diameter, leaf phloem length, and width were the most prominent in group II.
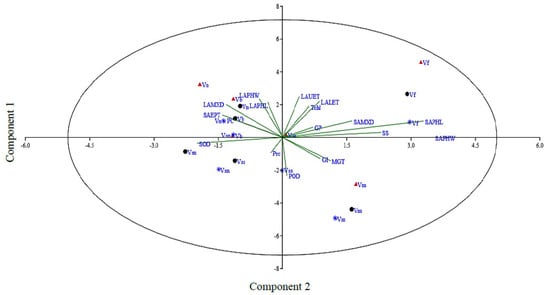
Figure 5.
Principal component analysis of the studied attributes in Vicia species evaluated under pot induced drought stress (control, FC = 80%; moderate stress, FC = 50%; severe stress, FC = 30%, represented by black, blue, and crimson colors, respectively). Vss, V. sativa subsp. sativa; Vsn, V. sativa subsp. nigra; Vn, V. narbonensis; Vb, V. bithynica; Vf, V. faba; Vm, V. monantha.
4. Discussion
The faba bean cultivars can produce high yields, but they are highly sensitive to environmental stresses, especially drought stress. Therefore, determining suitable attributes in the wild relative to legumes for boosting legume growth and production under water-deficient conditions is crucial. In the current study, Vicia wild relatives were evaluated for physiological, biochemical, and anatomical attributes. Photosynthetic pigments are vital for plant normal growth and are important predictors of describing the health condition of plants, especially under stress [57]. A reduction in photosynthetic pigments is the early response of plants toward water-deficit conditions, which leads to a reduction in the final yield and accumulation of metabolites [37,58]. Concerning photosynthetic pigments, current results revealed a decrease with an increase in the severity of drought stress. Our current results support the findings of Abbasi et al. [59] who also reported a reduction in chlorophyll contents under drought stress conditions in Vicia sativa. A similar reduction in photosynthetic pigments under drought stress conditions was also reported by [57,60].
Soluble sugars may have a prominent role in osmotic protection, the stability of cell membranes, and turgor pressure. Therefore, variances in the accumulation of SS may be considered genetic factors which can affect plant morpho-physiological responses under water-deficit conditions [61]. The current results revealed a reduction in SS under drought stress conditions. Our results support the findings of Hammad and Ali [62], who reported decrease in soluble sugar contents with an increase in drought stress.
The accumulation of proteins such as dehydrins late embryogenesis, abundant under water-deficit conditions, indicates drought-tolerant ability of plants. However, the reduction in certain other proteins under stress conditions and the extent of decline or accumulation largely depends on a genetic variation of different plant species [63,64]. Concerning protein contents, the current study revealed a decline under water-deficit conditions. Our findings supported the results of Parida et al. [65] who also reported a reduction in protein contents under drought stress conditions. Similarly, Mansour et al. [66] also reported that protein contents were significantly reduced in the studied faba bean under drought stress conditions.
Osmotic adjustment is a vital process of plants for maintaining water uptakes and cell wall pressure under drought stress conditions [67,68]. An increase in the accumulation of proline contents under water-deficit conditions is considered to be beneficial for the osmoprotection of plants and, generally, it is recommended as the criteria for selection tolerance against drought stress [69,70]. Our findings suggested that an increase in proline contents may have improved the plant’s ability to tolerate osmotic stress and hence, is the best indicator of stress tolerance. The present study also revealed enhanced proline contents in moderate and severe drought stresses, respectively. Our finding supports the findings of Kabbadj et al. [60] who reported an increase in proline contents with an increase in drought stress conditions. Similarly, our finding is also supported by the findings of Selim et al. [71] who work on tomato and reported the same findings. Our findings were also supported by Yadav et al. [72] who reported an increase in proline contents with an increase in drought stress.
Under drought stress conditions, plants not only face turgidity loss but also suffer from oxidative stress due to the accumulation of ROS. For the removal of these ROS, plants possess antioxidant mechanisms [73]. Peroxidase (POD) and superoxide dismutase (SOD) are major enzymes that play a key role in the defense mechanism of plants against various stress conditions by scavenging H2O2 in chloroplasts [74]. In the current study, drought stress resulted in an increased antioxidative defense system, and hence we observed variably enhanced SOD and POD activities in the studied Vicia species. Similar findings regarding peroxidase and superoxide dismutase activity are supported by those reported by Acar et al. [75]. The current results are also in general agreement with those previously reported [60,76].
Principle component analysis is a powerful statistical procedure to reduce the dimensions of variables and to divulge constructive evidence-driven feedback from a highly correlated dataset. PCA biplot analysis has been used widely and effectively by other researchers for screening drought-tolerant cultivars of Vicia and lentil species [27,77,78]. Current results clearly demonstrated that correlations of a trait pair were well coordinated with the approximation of the vector angles. PCA was a better approach in the identification of species tolerant and sensitive to progressive drought stresses.
Changes in anatomical attributes of root, stem, and leaves are diverse mechanisms through which plants can tolerate the harmful effects of drought stress [79,80,81]. Drought significantly affected the various studied leaf micromorphological attributes. Concerning epidermal cell length and width, the current study revealed a decreasing trend in both moderate and severe drought stress which are in general agreement with those reported by Boughalleb et al. [79] and Haffani et al. [82]. However, comparatively, less reduction in epidermal cell length and width was observed in V. monantha and V. sativa subsp. sativa in both drought stress treatments. This may have contributed toward its better water retention properties and prevention of excessive loss through transpiration. Further, a relatively lower decrease in leaf upper epidermal cell thickness under drought stress conditions may be regarded as a growth promoting/sustaining factor which may further assist its adaptation in arid environments as discussed by Zhang et al. [83]. The maximum CO2 assimilation in drought stress environment is considered as an adaptation strategy which can be achieved if stomatal changes in plants synchronize the interrelationship between water, transpiration, and photosynthesis, with the ultimate result being reduced levels of tissue damages [84]. We noted the enhanced stomatal length and decreased width with an increase in drought stress conditions as reported by Li et al. [85]. Our findings are similar to findings of Makbul et al. [86], who also reported a decrease in stomatal width with an increase in drought stress in soybean plants, concerning trichome length and width showed a decreasing trend with an increase in drought stress. Similar findings were also reported by Yadav et al. [72] who reported an increase in trichome length with an increase in the concentration of drought stress. Chen et al. [87] also discussed the densities of leaf trichomes in relation to the availability of water and its effects on plant growth and development in terms of increased cell size and expansion in the leaf area.
There was a decreasing trend in the studied stem anatomical attributes with increasing water deficit. For instance, epidermal and ground tissue, phloem length and width, and xylem elements exhibited progressive reduction both under MS and SS. The adaptation of wild Vicia spp. in these traits may be used for the identification of drought-tolerant species to overcome current food security crises [88,89]. Concerning epidermal tissue thickness, the current results revealed a declining trend under drought stress environments in comparison to the control-grown plants. Boughalleb et al. [79] also reported a reduction in epidermal thickness in plants under drought stress conditions. Similarly, a significant decrease in metaxylem thickness with increase in drought stress was recorded which was in agreement with those reported by Haffani et al. [82]. Our result revealed that the decreased xylem vessel diameter, and the phloem length and width increased in drought stress. This reduction may be due to a decrease in the radius of the sieve tube and an increase in viscosity of phloem sap, which in turn may affect the capacity of phloem transport due to lesser phloem conductance in the stem as previously reported [28,90,91]. The dissection of the complex architecture of quantitative traits in drought stress environments may lead to identification of favorable SNPs and haplotypes, underpinning traits of breeding interest through crop-wild introgressions [92,93,94,95]. The findings of current research work suggest its implications for detailed molecular characterization of wild Vicia species which may further help researchers for its rational use in their breeding efforts. The study provided the basis for further genetic diversity studies of wild Vicia species through high-throughput genotyping platforms which ultimately will lead to marker-assisted selection (MAS) in breeding programs in climate change perspectives. De la Rosa et al. [96] recently conducted similar genetic diversity studies of a large collection of Vicia sativa accessions and reported some potential drought-tolerant accessions to be utilized as promising sources in breeding programs. Proline metabolism has been reported as among the important pathways enriched in the leaves and roots of Vicia sativa. In a study by Min et al. [97] related to differentially expressed genes (DEG) of sucrose non-fermenting 1-related protein kinase (SnRK) in Vicia sativa, two SnRK DEGs were observed to be downregulated and two upregulated in both leaves and roots, suggesting its role as signal transducers and controlling phosphorylation of stress-responsive genes in a drought stress environment.
5. Conclusions
Variable responses were observed for the investigated physiological, biochemical, and anatomical traits among studied Vicia species which may/could be considered effective indices for selection against drought tolerance in breeding. In comparison with other species, V. sativa subsp. sativa, V. narbonensis, and V. monantha were found most tolerant to drought stress because of a lower decrease in chlorophyll contents and soluble sugar under drought stress. These species also showed better tolerance to drought stress by exhibiting increased proline contents, and POD and SOD activities. Concerning anatomical traits, the most tolerant species were V. sativa ssp. sativa, V. narbonensis, and V. bithynica, which showed comparatively less reduction in epidermal thickness, stomatal length and width, stomatal complex length and width, trichome length and width, phloem length and width, and metaxylem diameter. The research work depicted the importance of Vicia wild species and its further utilization in breeding faba bean and other legume crops for drought tolerance. The study provided the basis for further genetic diversity studies of wild Vicia species through high-throughput genotyping platforms which ultimately will lead to marker-assisted selection (MAS) in breeding programs in climate change perspectives. Moreover, further research is recommended in order to investigate the underlying mechanism of drought stress tolerance at the molecular and cellular level.
Author Contributions
Z.U. and A.A. designed the research; I.H. and Z.U. conducted the research; I.A., A.A. and D.N.B. analyzed the data; A.A., I.A. and H.S. checked the final content of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to acknowledge Princess Nourah bint Abdulrahman University researchers supporting project number (PNURSP2022R155) for its support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the generated data are represented in the manuscript.
Conflicts of Interest
The authors report there are no competing interests to declare.
References
- Kopke, U.; Nemecek, T. Ecological services of faba bean. Field Crops Res. 2010, 115, 217–233. [Google Scholar] [CrossRef]
- Bilalis, D.; Sidiras, N.; Economou, G.; Vakali, C. Effect of different levels of wheat straw soil surface coverage on weed flora in Vicia faba crops. J. Agron. Crop Sci. 2003, 189, 233–241. [Google Scholar] [CrossRef]
- Etemadi, F.; Hashemi, M.; Randhir, R.; ZandVakili, O.; Ebadi, A. Accumulation of L-DOPA in various organs of faba bean and influence of drought, nitrogen stress, and processing methods on L-DOPA yield. Crop, J. 2018, 6, 426–434. [Google Scholar] [CrossRef]
- Adamu, G.O.L.; Ezeokoli, O.T.; Dawodu, A.O.; Adebayo-Oyetoro, A.O.; Ofodile, L.N. Macronutrients and micronutrients profile of some underutilized beans in south western Nigeria. Int. J. Biochem. Res. Rev. 2015, 7, 80–89. [Google Scholar] [CrossRef]
- Lizarazo, C.I.; Lampi, A.M.; Liu, J.; Sontag-Strohm, T.; Piironen, V.; Stoddard, F.L. Nutritive quality and protein production from grain legumes in a boreal climate. J. Sci. Food Agric. 2015, 95, 2053–2064. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef]
- Siddique, K.H.M. Drought stress in grain legumes during reproduction and grain filling. J. Agron. Crop Sci. 2016, 203, 81–102. [Google Scholar]
- Etemadi, F.; Hashemi, M.; Barker, A.V.; Zandvakili, O.R.; Liu, X. Agronomy, nutritional value, and medicinal application of faba bean (Vicia faba L.). Hortic. Plant J. 2019, 5, 170–182. [Google Scholar] [CrossRef]
- Salehi, B.; Abu-Reidah, I.M.; Sharopov, F.; Karazhan, N.; Sharifi-Rad, J.; Akram, M.; Pezzani, R. Vicia plants—A comprehensive review on chemical composition and phytopharmacology. Phytother. Res. 2021, 35, 790–809. [Google Scholar] [CrossRef]
- McDonald, G.K.; Paulsen, G.M. High temperature effects on photosynthesis and water relations of grain legumes. Plant Soil 1997, 196, 47–58. [Google Scholar] [CrossRef]
- Micheletto, S.; Rodriguez-Uribe, L.; Hernandez, R.; Richins, R.D.; Curry, J.; O’Connell, M.A. Comparative transcript profiling in roots of Phaseolus acutifolius and P. vulgaris under water deficit stress. Plant Sci. 2007, 173, 510–520. [Google Scholar] [CrossRef]
- Tiwari, N.; Kumar, T.; Saxena, D.R.; Swain, N.; Maalouf, F.; Ahmed, S.; Sarker, A. Evaluation of disease resistant and high yielding faba bean germplasm in India. J. Gen. 2021, 100, 1–8. [Google Scholar] [CrossRef]
- Eker, T.; Sari, D.; Sari, H.; Tosun, H.S.; Toker, C. A kabuli chickpea ideotype. Sci. Rep. 2022, 12, 1–17. [Google Scholar]
- Khan, H.R.; Paull, J.G.; Siddique, K.H.M.; Stoddard, F.L. Faba bean breeding for drought-affected environments: A physiological and agronomic perspective. Field Crops Res. 2010, 115, 279–286. [Google Scholar] [CrossRef]
- Katerji, N.; Mastrorilli, M.; Lahmer, F.Z.; Maalouf, F.; Oweis, T. Faba bean productivity in saline–drought conditions. Eur. J. Agron. 2011, 35, 2–12. [Google Scholar] [CrossRef]
- Nguyen, V.; Riley, S.; Nagel, S.; Fisk, I.; Searle, I.R. Common vetch: A drought tolerant, high protein neglected leguminous crop with potential as a sustainable food source. Front. Plant Sci. 2020, 11, 818. [Google Scholar] [CrossRef]
- Link, W.; Abdelmula, A.A.; Kittlitz, E.V.; Bruns, S.; Riemer, H.; Stelling, D. Genotypic variation for drought tolerance. Vicia Faba. Plant Breed. 1999, 118, 477–484. [Google Scholar] [CrossRef]
- Girma, F.; Haile, D. Effects of supplemental irrigation on physiological parameters and yield of faba bean (Vicia faba L.) varieties in the highlands of Bale, Ethiopia. J. Agron. 2014, 13, 29–34. [Google Scholar] [CrossRef]
- Grzesiak, S.; Iijima, M.; Kono, Y.; Yamauchi, A. Differences in drought tolerance between cultivars of field bean and field pea. Morphological characteristics, germination and seedling growth. Acta Physiol. Plant. 1997, 19, 339–348. [Google Scholar] [CrossRef]
- Ali, Z.; Ali, B.; Mohammad, A.; Ahmad, M.; Ahmad, I.; Napar, A.A.; Kazi, A.G.; Ali, A.; Shah, S.S.; Mujeeb-Kazi, A. Combating water scarcity for global food security. In Agricultural Systems in the 21st Century; NOVA New York Press: Hauppauge, NY, USA, 2013; pp. 1–30. [Google Scholar]
- Salam, A.; Ali, A.; Afridi, M.S.; Ali, S.; Ullah, Z. Agrobiodiversity: Effect of Drought stress on the eco-physiology and morphology of wheat. In Biodiversity, Conservation and Sustainability in Asia; Springer: Cham, Switzerland, 2022; pp. 597–618. [Google Scholar]
- Stoddard, F.L.; Balko, C.; Erskine, W.; Khan, H.R.; Link, W.; Sarker, A. Screening techniques and sources of resistance to abiotic stresses in cool-season food legumes. Euphytica 2006, 147, 167–186. [Google Scholar] [CrossRef]
- Ali, A.; Arshad, M.; Naqvi, S.M.S.; Rasheed, A.; Sher, H.; Kazi, A.G.; Mujeeb-Kazi, A. Comparative assessment of synthetic-derived and conventional bread wheat advanced lines under osmotic stress and implications for molecular analysis. Plant Mol. Biol. Rep. 2015, 33, 1907–1917. [Google Scholar] [CrossRef]
- Gul, A.; Rasheed, A.; Afzal, F.; Napar, A.A.; Ali, A.; Jamil, M.; Khalid, M.; Bux, H.; Mujeeb-Kazi, A. Characterization of synthetic hexaploids derived from same Aegilops tauschii accessions and different durum cultivars. Cytologia 2015, 80, 427–440. [Google Scholar] [CrossRef]
- Kazi, A.G.; Rasheed, A.; Bux, H.; Napar, A.A.; Ali, A.; Mujeeb-Kazi, A. Cytological, phenological and molecular characterization of B (S)-genome synthetic hexaploids (2n = 6x = 42; AABBSS). Cereal Res. Commun. 2015, 43, 179–188. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.; Mittal, N.; Leamy, L.J.; Barazani, O.; Song, B.H. Back into the wild—Apply untapped genetic diversity of wild relatives for crop improvement. Evol. Appl. 2017, 10, 5–24. [Google Scholar] [CrossRef]
- Afzal, M.; Alghamdi, S.S.; Migdadi, H.H.; El-Harty, E.; Al-Faifi, S.A. Agronomical and physiological responses of faba bean genotypes to salt stress. Agricultural 2022, 12, 235. [Google Scholar] [CrossRef]
- Khan, A.; Ali, A.; Ullah, Z.; Ali, I.; Kaushik, P.; Alyemeni, M.N.; Rasheed, A.; Sher, H. Exploiting the drought tolerance of wild Elymus species for bread wheat improvement. Front. Plant Sci. 2022, 13, 982844. [Google Scholar] [CrossRef]
- Adhikari, K.N.; Khazaei, H.; Ghaouti, L.; Maalouf, F.; Vandenberg, A.; Link, W.; O’Sullivan, D.M. Conventional and molecular breeding tools for accelerating genetic gain in faba bean (Vicia faba L.). Front. Plant Sci. 2021, 12, 744259. [Google Scholar] [CrossRef]
- Brar, D.S.; Khush, G.S. Alien introgression in rice. In Oryza: From Molecule to Plant; Springer: Dordrecht, The Netherland, 1997; pp. 35–47. [Google Scholar]
- Zhou, Z.; Jiang, Y.; Wang, Z.; Gou, Z.; Lyu, J.; Li, W.; Fang, C. Resequencing 302 wild and cultivated accessions identifies genes related to domestication and improvement in soybean. Nat. Biotechnol. 2015, 33, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Leht, M. Phylogenetics of Vicia (Fabaceae) based on morphological data. Feddes Repert. 2009, 120, 379–393. [Google Scholar] [CrossRef]
- Maxted, N. A phenetic investigation of Vicia L. subgenus Vicia (Leguminosae, Vicieae). Bot. J. Linn. Soc. 1993, 111, 155–182. [Google Scholar] [CrossRef]
- Larbi, A.; Hassan, S.; Kattash, G.; El-Moneim, A.M.A.; Jammal, B.; Nabil, H.; Nakkul, H. Annual feed legume yield and quality in dryland environments in north-west Syria: 1. Herbage yield and quality. Anim. Feed. Sci. Technol. 2010, 160, 81–89. [Google Scholar] [CrossRef]
- Bourion, V.; Heulin-Gotty, K.; Aubert, V.; Tisseyre, P.; Chabert-Martinello, M.; Pervent, M.; Brunel, B. Co-inoculation of a pea core-collection with diverse rhizobial strains shows competitiveness for nodulation and efficiency of nitrogen fixation are distinct traits in the interaction. Front. Plant Sci. 2018, 8, 2249. [Google Scholar] [CrossRef]
- Rahman, S.U.; Ullah, Z.; Ali, A.; Aziz, M.A.; Alam, N.; Sher, H.; Ali, I. Traditional knowledge of medicinal flora among tribal communities of Buner Pakistan. Phytomed. Plus 2022, 2, 100277. [Google Scholar] [CrossRef]
- Desoky, E.S.M.; Mansour, E.; El-Sobky, E.S.E.; Abdul-Hamid, M.I.; Taha, T.F.; Elakkad, H.A.; Arnaout, S.M.A.I.; Eid, R.S.M.; El-Tarabily, K.A.; Yasin, M.A. Physio-biochemical and agronomic responses of faba beans to exogenously applied nano-silicon under drought stress conditions. Front. Plant Sci. 2021, 12, 637783. [Google Scholar] [CrossRef]
- Ali, A.; Ali, Z.; Quraishi, U.M.; Kazi, A.G.; Malik, R.N.; Sher, H.; Mujeeb-Kazi, A. Integrating physiological and genetic approaches for improving drought tolerance in crops. In Emerging Technologies and Management of Crop Stress Tolerance; Academic Press: Cambridge, MA, USA, 2014; pp. 315–345. [Google Scholar]
- Chowdhury, J.A.; Karim, M.A.; Khaliq, Q.A.; Ahmed, A.U.; Khan, M.S.A. Effect of drought stress on gas exchange characteristics of four soybean genotypes. Bangladesh J. Agric. Res. 2016, 41, 195–205. [Google Scholar] [CrossRef]
- Ali, I.; Salah, K.B.H.; Sher, H.; Ali, H.; Ullah, Z.; Ali, A.; Alam, N.; Shah, S.A.; Iqbal, J.; Ilyas, M.; et al. Drought stress enhances the efficiency of floral dip method of Agrobacterium-mediated transformation in Arabidopsis Thal. Braz. J. Biol. 2022, 84, 1–5. [Google Scholar]
- Ali, I.; Sher, H.; Ali, A.; Hussain, S.; Ullah, Z. Simplified floral dip transformation method of Arabidopsis thaliana. J. Microbiol. Methods 2022, 197, 106492. [Google Scholar] [CrossRef]
- Ali, A.; Arshad, M.; Naqvi, S.M.; Ahmad, M.; Sher, H.; Fatima, S.; Kazi, A.G.; Rasheed, A.; Mujeeb-Kazi, A. Exploitation of synthetic-derived wheats through osmotic stress responses for drought tolerance improvement. Acta Physiol. Plant. 2014, 36, 2453–2465. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Arshad, M.; Shahzad, S.M. Variation in growth and ion uptake of maize due to inoculation with plant growth promoting rhizobacteria under salt stress. Soil Environ. 2018, 25, 78–84. [Google Scholar]
- Ali, S.I. Flora of Pakistan No.100. In Flora of Pakistan, Papilionaceae; Ali, S.I., Nasir, E., Eds.; Department of Botany, University of Karachi: Karachi, Pakistan, 1977. [Google Scholar]
- Jalilian, N.; Rahiminejad, M.R.; Maassoumi, A.A.; Maroofi, H. Taxonomic revision of the genus Vicia L. (Fabaceae) in Iran. Iran J. Bot. 2014, 20, 155–164. [Google Scholar]
- Liu, H.S.; Li, F.M. Root respiration, photosynthesis and grain yield of two spring wheat in response to soil drying. Plant Growth Regul. 2005, 46, 233–240. [Google Scholar] [CrossRef]
- Graber, E.R.; Harel, Y.M.; Kolton, M.; Cytryn, E.; Silber, A.; David, D.R.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Clark, J. Preparation of leaf epidermis for topographic study. Stain. Technol. 2016, 35, 35–39. [Google Scholar] [CrossRef]
- Ullah, Z.; Khan, M.A.; Ahmad, M.; Zafar, M.; Ullah, K. Systematic implications of foliar epidermis in andropogoneae (poaceae) from Hindukush-himalayas Pakistan. J. Med. Plants Res. 2011, 5, 949–957. [Google Scholar]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: New York, NY, USA, 1999; Volume 198. [Google Scholar]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Lowery, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Gorin, N.; Heidema, F.T. Peroxidase activity in Golden Delicious apples as a possible parameter of ripening and senescence. J. Agric. Food Chem. 1976, 24, 200–201. [Google Scholar] [CrossRef]
- Mansour, E.; Moustafa, E.S.; Desoky, E.S.M.; Ali, M.; Yasin, M.A.; Attia, A.; Alsuhaibani, N.; Tahir, M.U.; El-Hendawy, S. Multidimensional evaluation for detecting salt tolerance of bread wheat genotypes under actual saline field growing conditions. Plants 2020, 9, 1324. [Google Scholar] [CrossRef] [PubMed]
- Janusauskaite, D.; Razbadauskiene, K. Comparison of productivity and physiological traits of faba bean (Vicia faba L.) varieties under conditions of boreal climatic zone. Agronomy 2021, 11, 707. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Sarvestani, R.; Mohammadi, B.; Baghery, A. Drought stress-induced changes at physiological and biochemical levels in some common vetch (Vicia sativa L.) genotypes. J. Agric. Sci. Technol. 2014, 16, 505–516. [Google Scholar]
- Kabbadj, A.; Makoudi, B.; Mouradi, M.; Pauly, N.; Frendo, P.; Ghoulam, C. Physiological and biochemical responses involved in water deficit tolerance of nitrogen-fixing Vicia faba. PLoS ONE 2017, 12, e0190284. [Google Scholar] [CrossRef]
- Banik, P.; Zeng, W.; Tai, H.; Bizimungu, B.; Tanino, K. Effects of drought acclimation on drought stress resistance in potato (Solanum tuberosum L.) genotypes. Environ. Exp. Bot. 2016, 126, 76–89. [Google Scholar] [CrossRef]
- Hammad, S.A.; Ali, O.A. Physiological and biochemical studies on drought tolerance of wheat plants by application of amino acids and yeast extract. Ann. Agric. Sci. 2014, 59, 133–145. [Google Scholar] [CrossRef]
- Sadiq, M.; Asghar, R.; Qureshi, R.; Ali, A. Study of polypeptides induced by drought stress in some local varieties of barley from Pakistan. Pak. J. Bot. 2013, 45, 1251–1254. [Google Scholar]
- Ahmadi, J.; Pour-Aboughadareh, A.; Ourang, S.F.; Mehrabi, A.A.; Siddique, K.H. Wild relatives of wheat: Aegilops–Triticum accessions disclose differential antioxidative and physiological responses to water stress. Acta Physiol. Plant. 2018, 40, 90. [Google Scholar] [CrossRef]
- Parida, A.K.; Dagaonkar, V.S.; Phalak, M.S.; Umalkar, G.V.; Aurangabadkar, L.P. Alterations in photosynthetic pigments, protein and osmotic components in cotton genotypes subjected to short-term drought stress followed by recovery. Plant Biotech. Rep. 2007, 1, 37–48. [Google Scholar] [CrossRef]
- Mansour, E.; Mahgoub, H.A.; Mahgoub, S.A.; El-Sobky, E.S.E.; Abdul-Hamid, M.I.; Kamara, M.M.; AbuQamar, S.F.; El-Tarabily, K.A.; Desoky, E.S.M. Enhancement of drought tolerance in diverse Vicia faba cultivars by inoculation with plant growth-promoting rhizobacteria under newly reclaimed soil conditions. Sci. Rep. 2021, 11, 1–20. [Google Scholar]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Cyriac, D.; Hofmann, R.W.; Stewart, A.; Sathish, P.; Winefield, C.S.; Moot, D.J. Intraspecific differences in long-term drought tolerance in perennial ryegrass. PLoS ONE 2018, 13, e0194977. [Google Scholar] [CrossRef]
- Hessini, K.; Martínez, J.P.; Gandour, M.; Albouchi, A.; Soltani, A.; Abdelly, C. Effect of water stress on growth, osmotic adjustment, cell wall elasticity and water-use efficiency in Spartina Alterniflora Environ. Exp. Bot. 2009, 67, 312–319. [Google Scholar] [CrossRef]
- Zada, A.; Ali, A.; Ullah, Z.; Sher, H.; Shah, A.H.; Khan, I.; Shah, A.Z. Improvement of osmotic stress tolerance in wheat by seed priming. Int. J. Biosci. 2020, 17, 133–142. [Google Scholar]
- Selim, A.F.H.; El-Nady, M.F. Physio-anatomical responses of drought stressed tomato plants to magnetic field. Acta Astronaut. 2011, 69, 387–396. [Google Scholar] [CrossRef]
- Yadav, R.K.; Sangwan, R.S.; Sabir, F.; Srivastava, A.K.; Sangwan, N.S. Effect of prolonged water stress on specialized secondary metabolites, peltate glandular trichomes, and pathway gene expression in Artemisia annua L. Plant Physiol. Biochem. 2014, 74, 70–83. [Google Scholar] [CrossRef]
- Bouchemal, K.; Bouldjadj, R.; Belbekri, M.N.; Ykhlef, N.; Djekoun, A. Differences in antioxidant enzyme activities and oxidative markers in ten wheat (Triticum durum Desf.) genotypes in response to drought, heat and paraquat stress. Arch. Agron. Soil Sci. 2017, 63, 710–722. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Acar, O.; Türkan, I.; Ozdemir, F. Superoxide dismutase and peroxidase activities in drought sensitive and resistant barley (Hordeum vulgare L.) varieties. Acta Physiol. Plant. 2010, 23, 351–356. [Google Scholar] [CrossRef]
- Mohammadi, A.; Habibi, D.; Rohami, M.; Mafakheri, S. Effect of drought stress on antioxidant enzymes activity of some chickpea cultivars. Am. Euras. J. Agric. Environ. Sci. 2007, 11, 782–785. [Google Scholar]
- Karadavut, U. Path analysis for yield and yield components in lentil (Lens culinaris Medik.). Turkish J. Field Crop 2009, 14, 97–104. [Google Scholar]
- Belachew, K.Y.; Nagel, K.A.; Poorter, H.; Stoddard, F.L. Association of shoot and root responses to water deficit in young faba bean (Vicia faba L.) plants. Front. Plant Sci. 2019, 10, 1063. [Google Scholar] [CrossRef]
- Boughalleb, F.; Hajlaoui, H. Physiological and anatomical changes induced by drought in two olive cultivars (cv Zalmati and Chemlali). Acta Physiol. Plant. 2011, 33, 53–65. [Google Scholar] [CrossRef]
- Carrera, C.S.; Solis, S.M.; Ferrucci, M.S.; Vega, C.C.; Galati, B.G.; Ergo, V.; Lascano, R.H. Leaf structure and ultrastructure changes induced by heat stress and drought during seed filling in field-grown soybean and their relationship with grain yield. An. Acad. Bras. Cienc. 2021, 93, e20191388. [Google Scholar] [CrossRef]
- Shafqat, W.; Mazrou, Y.S.; Nehela, Y.; Ikram, S.; Bibi, S.; Naqvi, S.A.; Hameed, M.; Jaskani, M.J. Effect of three water regimes on the physiological and anatomical structure of stem and leaves of different citrus rootstocks with distinct degrees of tolerance to drought stress. Horticulturae 2021, 7, 554. [Google Scholar] [CrossRef]
- Haffani, S.; Mezni, M.; Nasri, M.B.; Chaibi, W. Comparative leaf water relations and anatomical responses of three vetch species (Vicia narbonensis L., V. sativa L. and V. villosa Roth.) to cope with water stress. Crop Pasture Sci. 2006, 68, 691–702. [Google Scholar] [CrossRef]
- Zhang, C.; Li, W.; Gao, Y.; Xu, Z.; Tian, X. Artificial Regulation Effect of Plant Retardants on Leaf Anatomical Characteristics of Elaeagnus Angustifolia Front. Environ. Sci. 2022, 10, 900960. [Google Scholar]
- Yongping, Z.; Zhimin, W. Stomatal characteristics of different green organs in wheat under different irrigation regimes. Acta Agron. Sin. 2006, 31, 70–75. [Google Scholar]
- Li, Y.; Li, H.; Li, Y.; Zhang, S. Improving water-use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought-resistant wheat. Crop J. 2017, 5, 231–239. [Google Scholar] [CrossRef]
- Makbul, S.; Guler, N.S.; Durmuş, N.; Güven, S. Changes in anatomical and physiological parameters of soybean under drought stress. Turk. J. Bot. 2011, 35, 369–377. [Google Scholar] [CrossRef]
- Chen, J.J.; Sun, Y.; Kopp, K.; Oki, L.; Jones, S.B.; Hipps, L. Effects of Water availability on leaf trichome density and plant growth and development of Shepherdia × utahensis. Front. Plant Sci. 2022, 13, 855858. [Google Scholar] [CrossRef] [PubMed]
- Nassar, R.M.; Kamel, H.A.; Ghoniem, A.E.; Alarcon, J.J.; Sekara, A.; Ulrichs, C.; Abdelhamid, M.T. Physiological and anatomical mechanisms in wheat to cope with salt stress induced by seawater. Plants 2020, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Papu, S.; Berli, F.; Piccoli, P.; Paton, D.; Rodriguez, D.O.; Roig, F.A. Physiological, biochemical, and anatomical responses of Araucaria araucana seedlings to controlled water restriction. Plant Physiol. Biochem. 2021, 165, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Dannoura, M.; Epron, D.; Desalme, D.; Massonnet, C.; Tsuji, S.; Plain, C.; Gérant, D. The impact of prolonged drought on phloem anatomy and phloem transport in young beech trees. Tree Physiol. 2019, 39, 201–210. [Google Scholar] [CrossRef]
- Liu, J.; Kutschke, S.; Keimer, K.; Kosmalla, V.; Schürenkamp, D.; Goseberg, N.; Bol, M. Experimental characterisation and three-dimensional modelling of Elymus for the assessment of ecosystem services. Ecol. Eng. 2021, 166, 106233. [Google Scholar] [CrossRef]
- Kim, T.-S.; Raveendar, S.; Suresh, S.; Lee, G.-A.; Lee, J.-R.; Cho, J.-H.; Lee, S.-Y.; Ma, K.-H.; Cho, G.-T.; Chung, J.-W. Transcriptome analysis of two Vicia sativa subspecies: Mining molecular markers to enhance genomic resources for vetch improvement. Genes 2015, 6, 1164–1182. [Google Scholar] [CrossRef]
- Afzal, F.; Ali, A.; Ullah, Z.; Sher, H.; Gul, A.; Mujeeb-Kazi, A.; Arshad, M. Terminal drought stress adaptability in synthetic-derived bread wheat is explained by alleles of major adaptability genes and superior phenology. Int. J. Agric. Biol. 2018, 20, 1623–1631. [Google Scholar]
- Afzal, F.; Li, H.; Gul, A.; Subhani, A.; Ali, A.; Mujeeb-Kazi, A.; Ogbonnaya, F.; Trethowan, R.; Xia, X.; He, Z.; et al. Genome-wide analyses reveal footprints of divergent selection and drought adaptive traits in synthetic-derived wheats. G3 Genes Genomes Genet. 2019, 9, 1957–1973. [Google Scholar] [CrossRef]
- Heinrich, F.; Wutke, M.; Das, P.P.; Kamp, M.; Gültas, M.; Link, W.; Schmitt, A.O. Identification of regulatory SNPs associated with vicine and convicine content of Vicia faba based on genotyping by sequencing data using deep learning. Genes 2020, 11, 614. [Google Scholar] [CrossRef]
- la Rosa, L.D.; López-Román, M.I.; González, J.M.; Zambrana, E.; Marcos-Prado, T.; Ramírez-Parra, E. Common vetch, valuable germplasm for resilient agriculture: Genetic characterization and spanish core collection development. Front. Genet. 2021, 12, 617873. [Google Scholar] [CrossRef]
- Min, X.; Lin, X.; Ndayambaza, B.; Wang, Y.; Liu, W. Coordinated mechanisms of leaves and roots in response to drought stress underlying full-length transcriptome profiling in Vicia sativa L. BMC Plant Biol. 2020, 20, 1–21. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).