VDR Polymorphic Variants Are Related to Improvements in CRP and Disease Activity in Patients with Axial Spondyloarthritis That Undergo Anti-TNF Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. SNP Selection
2.3. Sample Collection and Genotyping
2.4. Statistical Analysis
3. Results
3.1. Linkage Disequilibrium and Hardy-Weinberg Equilibrium (HWE)
3.2. Patients vs. Healthy Controls
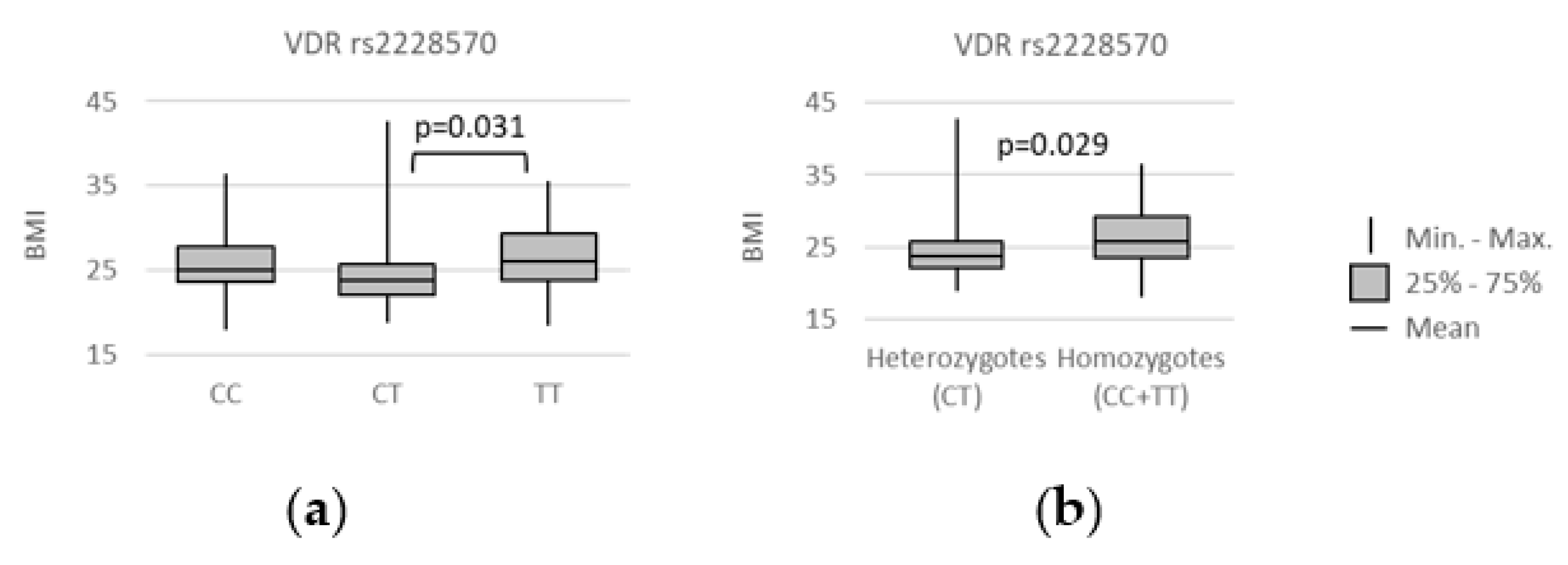
3.3. VDR Polymorphisms and Patients’ Characteristics
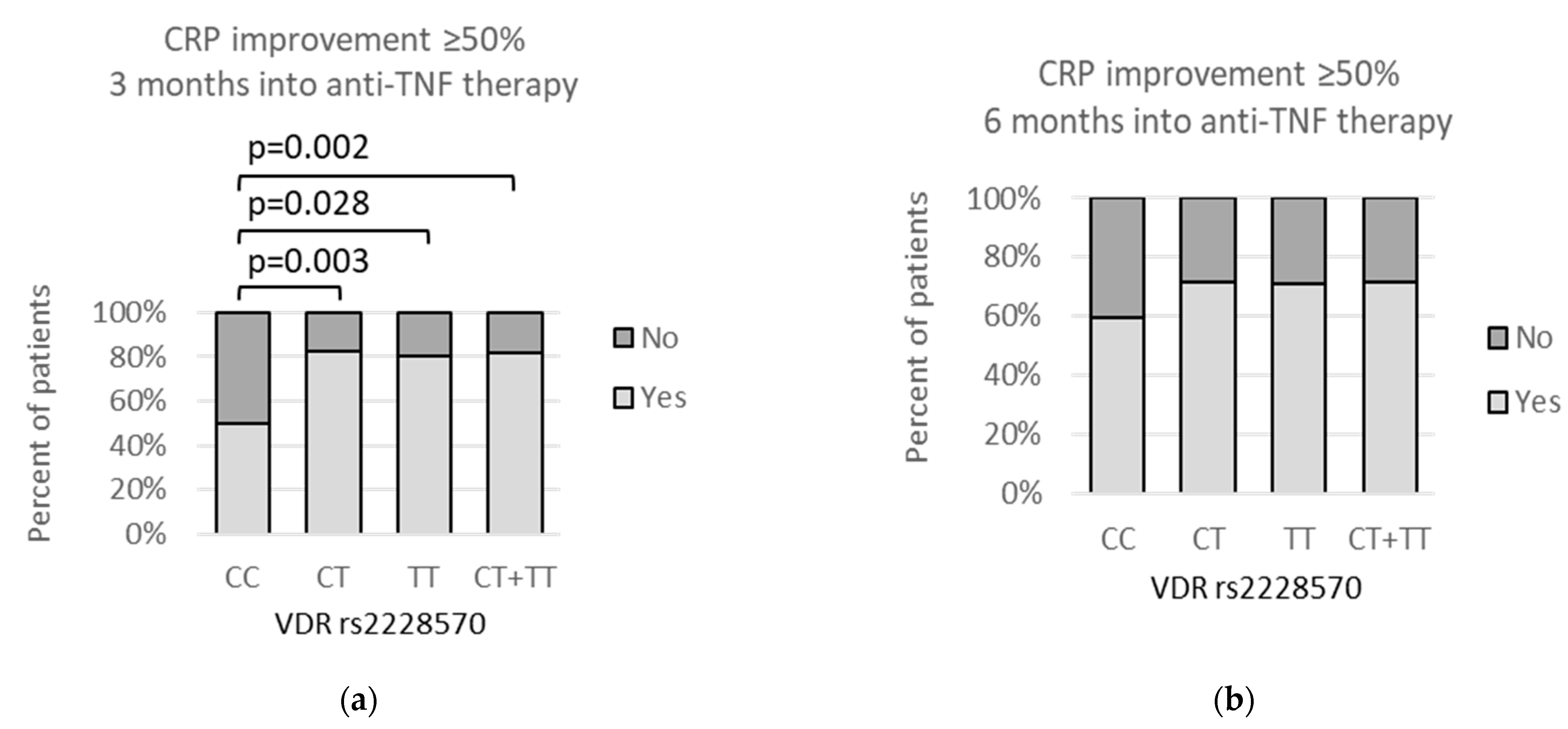
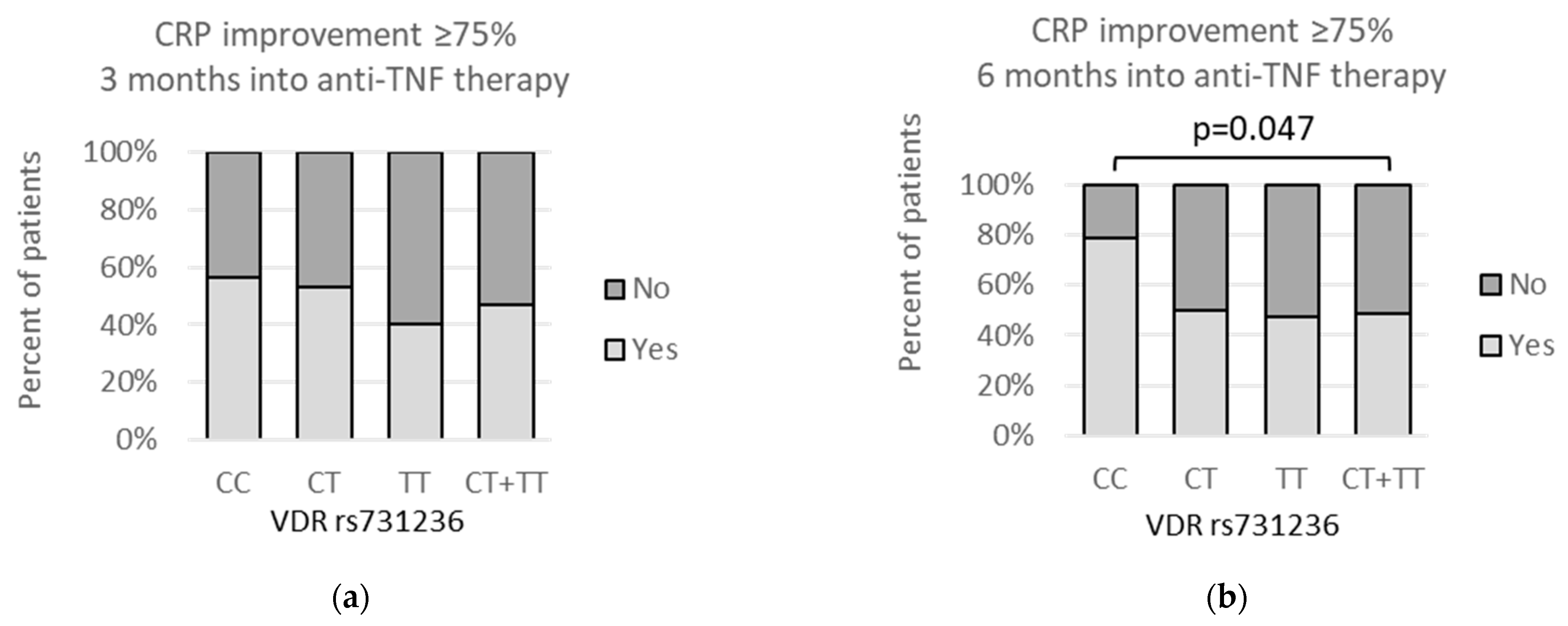
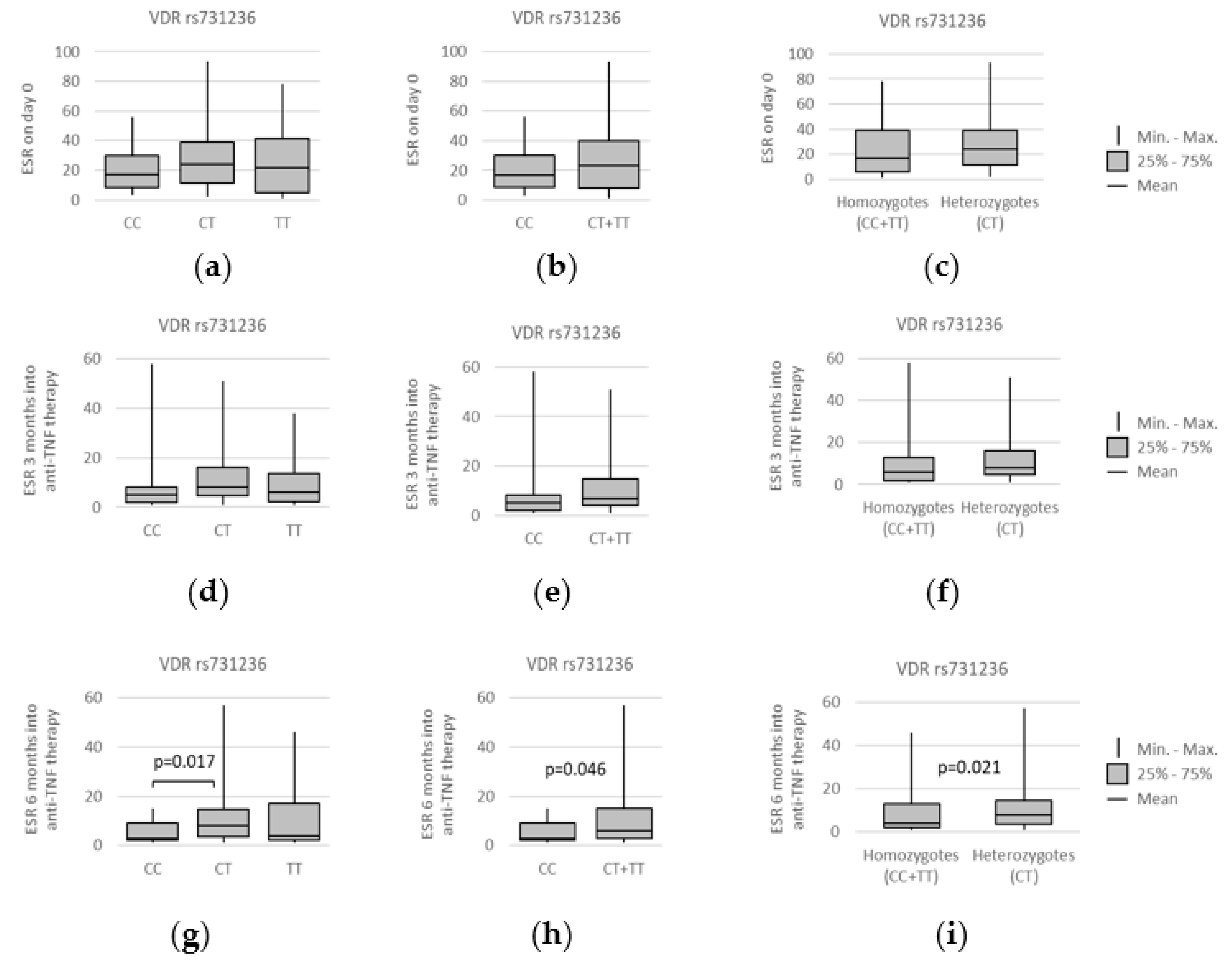
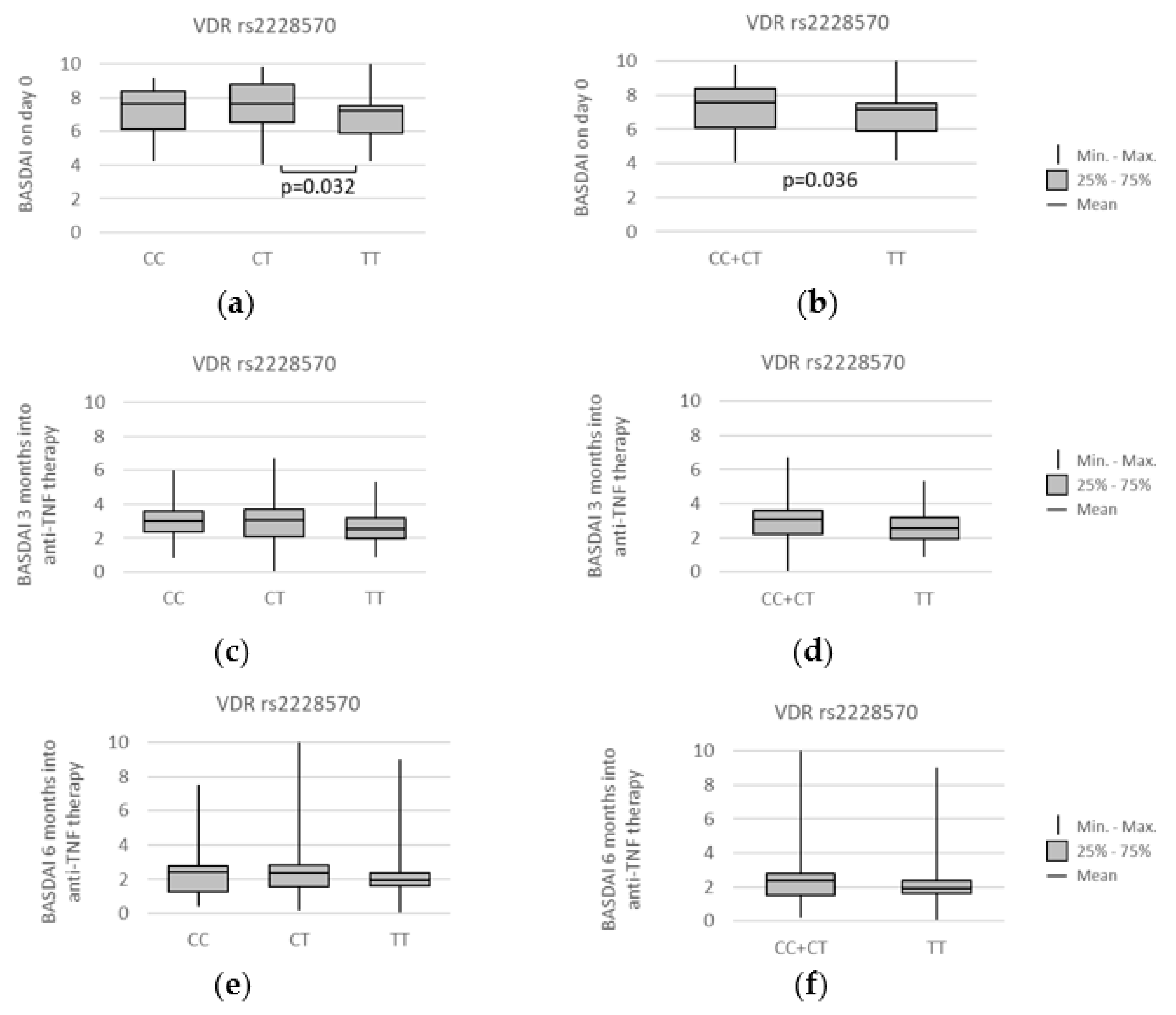
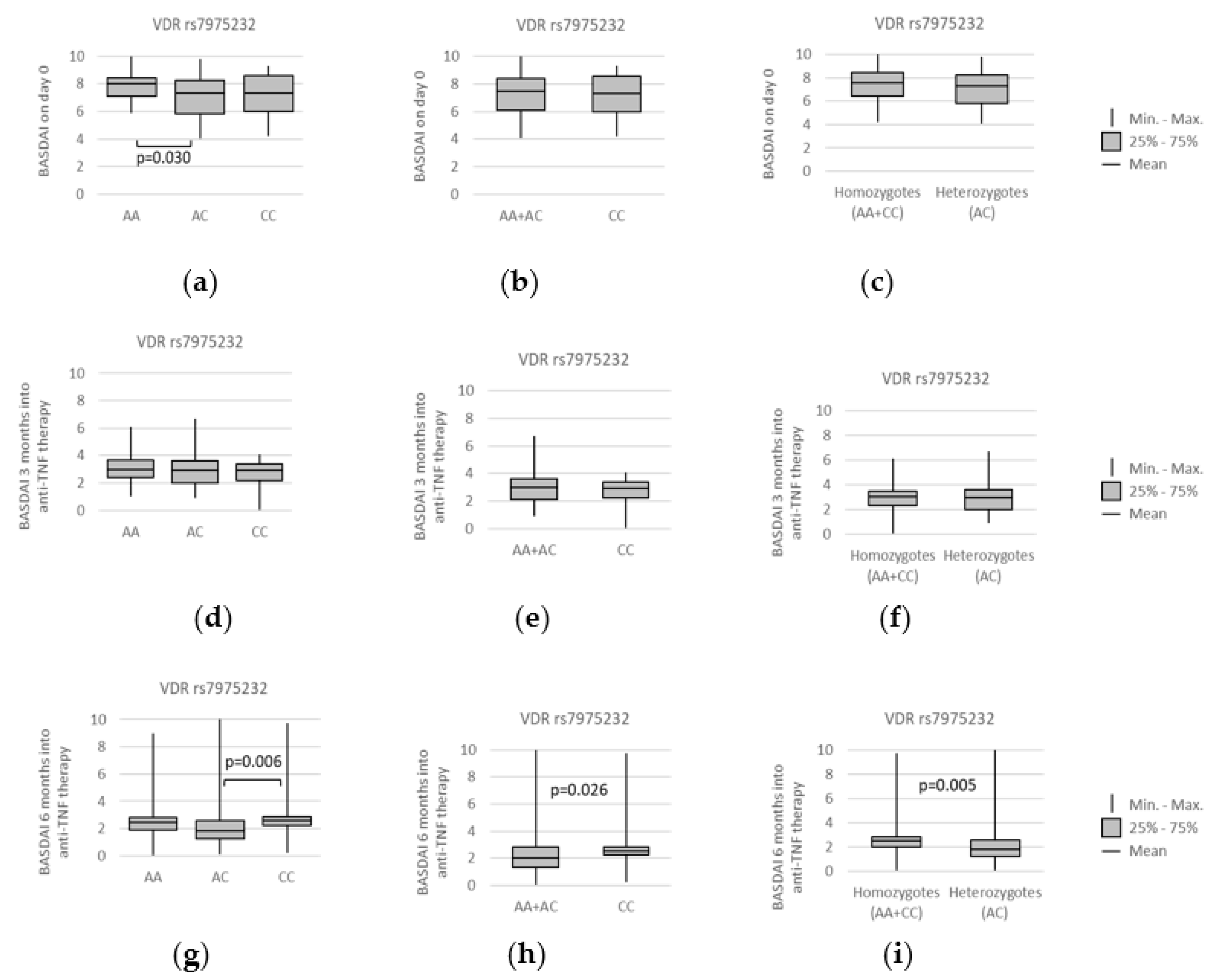
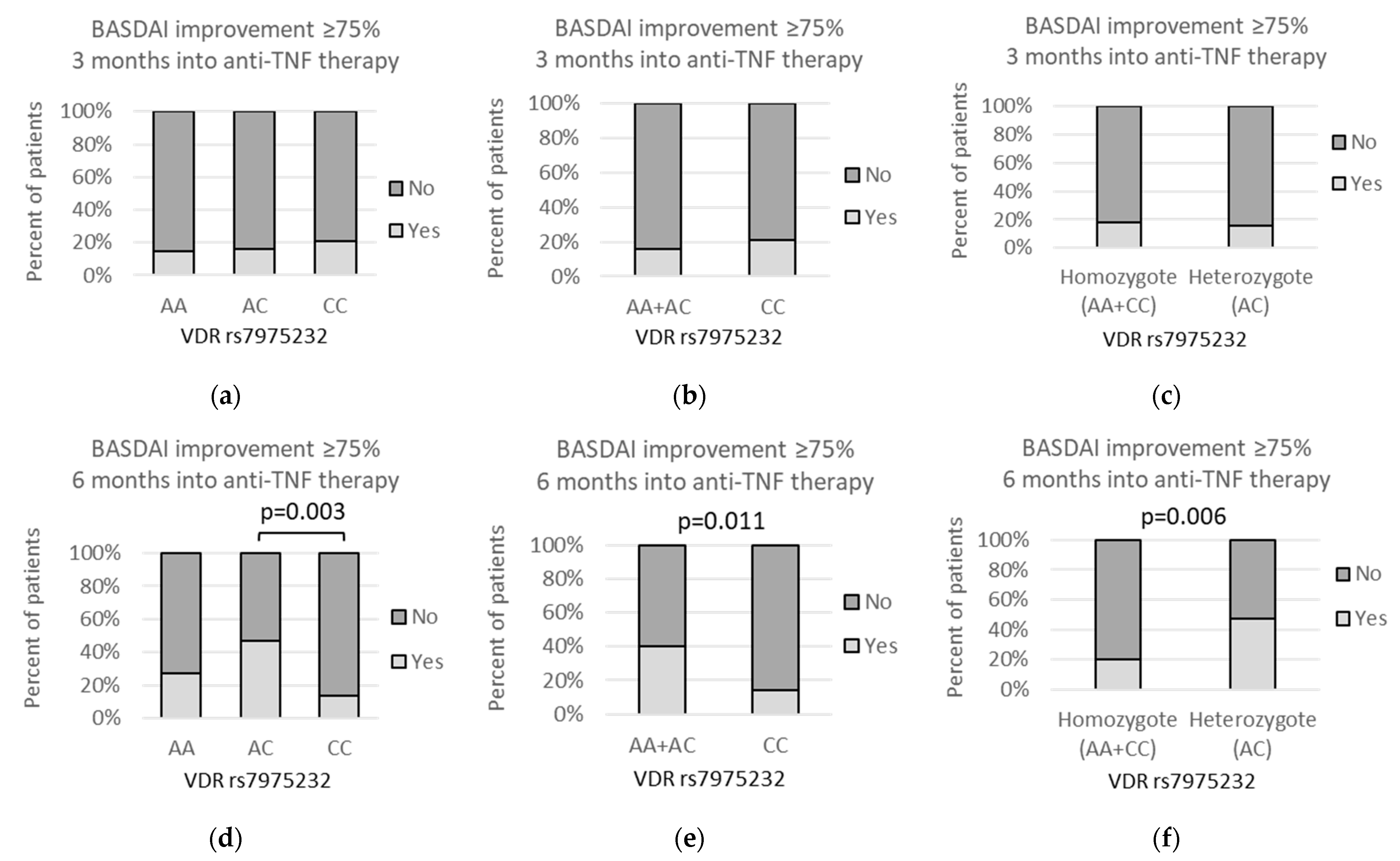
3.4. VDR polymorphisms and clinical parameters
3.5. VDR Polymorphisms and Other Symptoms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bizzaro, G.; Antico, A.; Fortunato, A.; Bizzaro, N. Vitamin D and Autoimmune Diseases: Is Vitamin D Receptor (VDR) Polymorphism the Culprit? Isr. Med. Assoc. J. 2017, 19, 438–443. [Google Scholar] [PubMed]
- Pike, J.W.; Christakos, S. Biology and Mechanisms of Action of the Vitamin D Hormone. Endocrinol. Metab. Clin. North Am. 2017, 46, 815–843. [Google Scholar] [CrossRef] [PubMed]
- Goltzman, D. Functions of Vitamin D in Bone. Histochem. Cell Biol. 2018, 149, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Bikle, D.D. Extraskeletal Actions of Vitamin D. Ann. N. Y. Acad. Sci. 2016, 1376, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef]
- Pokhai, G.G.; Bandagi, S.; Abrudescu, A. Vitamin D Levels in Ankylosing Spondylitis: Does Deficiency Correspond to Disease Activity? Rev. Bras. Reumatol. 2014, 54, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Illescas-Montes, R.; Melguizo-Rodríguez, L.; Ruiz, C.; Costela-Ruiz, V.J. Vitamin D and Autoimmune Diseases. Life Sci. 2019, 233, 116744. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Lee, Y.H.; Bae, S.C. Vitamin D Level in Rheumatoid Arthritis and Its Correlation with the Disease Activity: A Meta-Analysis. Clin. Exp. Rheumatol. 2016, 34, 827–833. [Google Scholar] [PubMed]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Tonacci, A.; Negrini, S.; Greco, M.; Borro, M.; Puppo, F.; Gangemi, S. Emerging Role of Vitamin D in Autoimmune Diseases: An Update on Evidence and Therapeutic Implications. Autoimmun. Rev. 2019, 18, 102350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.T.; Jin, T.F.; Chen, L. Associations of Four Common VDR Polymorphisms with Rheumatoid Arthritis and Systemic Lupus Erythematosus: Evidence from a Meta-Analysis. Lupus 2020, 29, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Vaquero, C.; Fiter, J.; Enjuanes, A.; Nogués, X.; Díez-Pérez, A.; Nolla, J.M. Influence of the BsmI Polymorphism of the Vitamin D Receptor Gene on Rheumatoid Arthritis Clinical Activity—PubMed. J. Rheumatol. 2007, 64, 51–58. [Google Scholar]
- Khoja, S.O.; El-Miedany, Y.; Iyer, A.P.; Bahlas, S.M.; Balamash, K.S.; Elshal, M.F. Associations of Vitamin D Levels and Vitamin D Receptor Genotypes with Patient-Reported Outcome/Disease Activity in Patients with Rheumatoid Arthritis. Clin. Lab. 2018, 64, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Punceviciene, E.; Gaizevska, J.; Sabaliauskaite, R.; Venceviciene, L.; Puriene, A.; Vitkus, D.; Jarmalaite, S.; Butrimiene, I. Vitamin D and VDR Gene Polymorphisms’ Association with Rheumatoid Arthritis in Lithuanian Population. Med. Kaunas. 2021, 57, 346. [Google Scholar] [CrossRef] [PubMed]
- Van Der Linden, S.; Valkenburg, H.A.; Cats, A. Evaluation of Diagnostic Criteria for Ankylosing Spondylitis. Arthritis Rheum. 1984, 27, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; Van Der Heijde, D.; Landewé, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The Development of Assessment of SpondyloArthritis International Society Classification Criteria for Axial Spondyloarthritis (Part II): Validation and Final Selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, L.; Chai, K. Systemic Immune-Inflammation Index Is Associated with Disease Activity in Patients with Ankylosing Spondylitis. J. Clin. Lab. Anal. 2021, 35, e23964. [Google Scholar] [CrossRef] [PubMed]
- Litao, M.K.S.; Kamat, D. Erythrocyte Sedimentation Rate and C-Reactive Protein: How Best to Use Them in Clinical Practice. Pediatr. Ann. 2014, 43, 417–420. [Google Scholar] [CrossRef]
- van den Berg, R.; van der Heijde, D.M.F.M. How Should We Diagnose Spondyloarthritis According to the ASAS Classification Criteria: A Guide for Practicing Physicians. Pol. Arch. Med. Wewn. 2010, 120, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Garrett, S.; Jenkinson, T.; Kennedy, L.G.; Whitelock, H.; Gaisford, P.; Calin, A. A New Approach to Defining Disease Status in Ankylosing Spondylitis: The Bath Ankylosing Spondylitis Disease Activity Index. J. Rheumatol. 1994, 21, 2286–2291. [Google Scholar] [PubMed]
- Gleba, J.J.; Kłopotowska, D.; Banach, J.; Turlej, E.; Mielko, K.A.; Gębura, K.; Bogunia-Kubik, K.; Kutner, A.; Wietrzyk, J. Polymorphism of VDR Gene and the Sensitivity of Human Leukemia and Lymphoma Cells to Active Forms of Vitamin D. Cancers 2022, 14, 387. [Google Scholar] [CrossRef]
- Arai, H.; Miyamoto, K.I.; Taketani, Y.; Yamamoto, H.; Iemori, Y.; Morita, K.; Tonai, T.; Nishisho, T.; Mori, S.; Takeda, E. A Vitamin D Receptor Gene Polymorphism in the Translation Initiation Codon: Effect on Protein Activity and Relation to Bone Mineral Density in Japanese Women. J. Bone Miner. Res. 1997, 12, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Uitterlinden, A.G.; Fang, Y.; Van Meurs, J.B.J.; Pols, H.A.P.; Van Leeuwen, J.P.T.M. Genetics and Biology of Vitamin D Receptor Polymorphisms. Gene 2004, 338, 143–156. [Google Scholar] [CrossRef]
- Ruiz-Ballesteros, A.I.; Meza-Meza, M.R.; Vizmanos-Lamotte, B.; Parra-Rojas, I.; de la Cruz-Mosso, U. Association of Vitamin D Metabolism Gene Polymorphisms with Autoimmunity: Evidence in Population Genetic Studies. Int. J. Mol. Sci. 2020, 21, 9626. [Google Scholar] [CrossRef]
- Maj, E.; Trynda, J.; Maj, B.; Gębura, K.; Bogunia-Kubik, K.; Chodyński, M.; Kutner, A.; Wietrzyk, J. Differential Response of Lung Cancer Cell Lines to Vitamin D Derivatives Depending on EGFR, KRAS, P53 Mutation Status and VDR Polymorphism. J. Steroid Biochem. Mol. Biol. 2019, 193, 105431. [Google Scholar] [CrossRef]
- Mahto, H.; Tripathy, R.; Das, B.K.; Panda, A.K. Association between Vitamin D Receptor Polymorphisms and Systemic Lupus Erythematosus in an Indian Cohort. Int. J. Rheum. Dis. 2018, 21, 468–476. [Google Scholar] [CrossRef]
- Goswami, R. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Indian J. Med. Res. 2016, 144, 489–490. [Google Scholar] [CrossRef]
- Latini, A.; De Benedittis, G.; Perricone, C.; Colafrancesco, S.; Conigliaro, P.; Ceccarelli, F.; Chimenti, M.S.; Novelli, L.; Priori, R.; Conti, F.; et al. VDR Polymorphisms in Autoimmune Connective Tissue Diseases: Focus on Italian Population. J. Immun. Res. 2021, 2021, 5812136. [Google Scholar] [CrossRef]
- Sieper, J.; Poddubnyy, D. Axial Spondyloarthritis. Lancet 2017, 390, 73–84. [Google Scholar] [CrossRef]
- Dube, C.E.; Lapane, K.L.; Ferrucci, K.A.; Beccia, A.L.; Khan, S.K.; Yi, E.; Kay, J.; Kuhn, K.A.; Ogdie, A.; Liu, S.H. Personal Experiences with Diagnostic Delay Among Axial Spondyloarthritis Patients: A Qualitative Study. Rheumatol. Ther. 2021, 8, 1015–1030. [Google Scholar] [CrossRef] [PubMed]
- Juanola, X.; Ramos, M.J.M.; Belzunegui, J.M.; Fernández-Carballido, C.; Gratacós, J. Treatment Failure in Axial Spondyloarthritis: Insights for a Standardized Definition. Adv. Ther. 2022, 39, 1490–1501. [Google Scholar] [CrossRef]
- García-Carrasco, M.; Romero, J.L.G. Vitamin D and Autoimmune Rheumatic Disease. Reumatol. Clin. 2015, 11, 333–334. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F.; Tamone, C.; D’amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef]
- El-Fakhri, N.; McDevitt, H.; Shaikh, M.G.; Halsey, C.; Ahmed, S.F. Vitamin D and Its Effects on Glucose Homeostasis, Cardiovascular Function and Immune Function. Horm. Res. Paediatr. 2014, 81, 363–378. [Google Scholar] [CrossRef]
- Lemke, D.; Klement, R.J.; Schweiger, F.; Schweiger, B.; Spitz, J. Vitamin D Resistance as a Possible Cause of Autoimmune Diseases: A Hypothesis Confirmed by a Therapeutic High-Dose Vitamin D Protocol. Front. Immunol. 2021, 12, 655739. [Google Scholar] [CrossRef] [PubMed]
- Dankers, W.; Colin, E.M.; van Hamburg, J.P.; Lubberts, E. Vitamin D in Autoimmunity: Molecular Mechanisms and Therapeutic Potential. Front. Immunol. 2017, 7, 697. [Google Scholar] [CrossRef]
- Wright, G.; Kaine, J.; Deodhar, A. Understanding Differences between Men and Women with Axial Spondyloarthritis. Semin. Arthritis Rheum. 2020, 50, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Vasile, M.; Corinaldesi, C.; Antinozzi, C.; Crescioli, C. Vitamin D in Autoimmune Rheumatic Diseases: A View inside Gender Differences. Pharmacol. Res. 2017, 117, 228–241. [Google Scholar] [CrossRef]
- Dupuis, M.L.; Pagano, M.T.; Pierdominici, M.; Ortona, E. The Role of Vitamin D in Autoimmune Diseases: Could Sex Make the Difference? Biol. Sex Differ. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, I.; Mateus, H.; Rodrigues-Manica, S.; Pinheiro-Torres, R.; Neto, A.; Domingues, L.; Lage Crespo, C.; Sardoo, A.; Machado, P.; Branco, J.C.; et al. The Effect of ACTN3 and VDR Polymorphisms on Skeletal Muscle Performance in Axial Spondyloarthropathies. Front. Genet. 2021, 12, 1408. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.S.F.; Visentainer, J.E.L.; da Silva Reis, D.M.; Loures, M.A.R.; Alves, H.V.; Lara-Armi, F.F.; De Alencar, J.B.; Valentin Zacarias, J.M.; Sell, A.M. The Influence of Vitamin D Receptor Gene Polymorphisms in Spondyloarthritis. Int. J. Inflam. 2020, 2020, 8880879. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Avila, R.G.; González-Castro, T.B.; Tovilla-Zárate, C.A.; Juárez-Rojop, I.E.; López-Narváez, M.L.; Rodríguez-Pérez, J.M.; Pérez-Hernández, N. The Role of TaqI, ApaI and BsmI Polymorphisms of VDR Gene in Lumbar Spine Pathologies: Systematic Review and Meta-Analysis. Eur. Spine J. 2021, 30, 2049–2059. [Google Scholar] [CrossRef]
- Cai, G.; Zhang, X.; Xin, L.; Wang, L.; Wang, M.; Yang, X.; Li, X.; Xia, Q.; Xu, S.; Ding, C.; et al. Associations between Vitamin D Receptor Gene Polymorphisms and Ankylosing Spondylitis in Chinese Han Population: A Case–Control Study. Osteoporos. Int. 2016, 27, 2327–2333. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Q.; Qi, J.; Lv, Q.; Zheng, X.; Wu, X.; Gu, J. Association between Vitamin D Receptor Gene Polymorphism and Ankylosing Spondylitis in Han Chinese. Int. J. Rheum. Dis. 2016, 20, 1510–1516. [Google Scholar] [CrossRef]
- Obermayer-Pietsch, B.M.; Lange, U.; Tauber, G.; Frühauf, G.; Fahrleitner, A.; Dobnig, H.; Hermann, J.; Aglas, F.; Teichmann, J.; Neeck, G.; et al. Vitamin D Receptor Initiation Codon Polymorphism, Bone Density and Inflammatory Activity of Patients with Ankylosing Spondylitis. Osteoporos. Int. 2003, 14, 995–1000. [Google Scholar] [CrossRef]
- Idriss, N.K.; Selim, Z.I.; El-Hakeim, E.H.; El Nouby, F.H.; Ibrahim, A.K.; Sayyed, H.G.; Elgamal, D.A.; Ibrahim, M.A.; Kamal, D.; Goma, S.H. Is There a Feasible Link between Vitamin D Receptor Genotypic and Allelic Frequencies with Analytical Biomarkers of Rheumatoid Arthritis Disease? J. Nutr. Sci. Vitaminol. Tokyo 2020, 66, 526–535. [Google Scholar] [CrossRef]
- Gerriets, V.; Goyal, A.; Khaddour, K. Tumor Necrosis Factor Inhibitors; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Mitoma, H.; Horiuchi, T.; Tsukamoto, H.; Ueda, N. Molecular Mechanisms of Action of Anti-TNF-α Agents—Comparison among Therapeutic TNF-α Antagonists. Cytokine 2018, 101, 56–63. [Google Scholar] [CrossRef]




| Number of Patients (N) | 106 |
|---|---|
| Age mean in years (±SD) | 42.7 (±12.9) |
| Disease duration mean in years (±SD) | 9.29 (±8.49) |
| Disease onset mean in years (±SD) | 32.7 (±10.2) |
| Sex F/M (%) | 28/78 (73.6%) |
| BMI mean (±SD) | 25.5 (±4.59) |
| HLA-B27 positive patients, % | 88% |
| Form axial/axially peripheral (%) | 58 (54.7%)/48 (45.3%) |
| nr-axSpA/AS (%) | 20 (18.9%)/86 (81.1%) |
| Extra-articular manifestations: | N (%) |
| Uveitis | 33 (31.1%) |
| Inflammatory bowel disease | 18 (17.0%) |
| Enthesitis | 17 (16.0%) |
| Psoriasis | 6 (5.7%) |
| Patients with at least one manifestation | 53 (50.0%) |
| Patients with two manifestations or more | 17 (16.0%) |
| Concomitant treatment at the start of biologic treatment: | N (%) |
| NSAIDs | 73 (69.5%) |
| MTX | 32 (30.2%) |
| Sulfasalazine/Mesalazine | 28 (26.4%) |
| Corticosteroids | 17 (16.0%) |
| Other | 2 (2.0%) |
| Anti-TNF drugs: | N (%) |
| Adalimumab | 43 (40.6%) |
| Etanercept | 28 (26.4%) |
| Certolizumab | 24 (22.6%) |
| Golimumab | 9 (8.5%) |
| Infliximab | 2 (2.0%) |
| Disease activity: | |
| BASDAI before treatment, median (range) | 7.45 (4.05–10) |
| BASDAI after 6 months of treatment, median (range) | 2.30 (0–10) |
| Low activity 1 after 6 months of treatment, N (%) | 97 (93.3%) |
| SNP/Genotype | axSpA [N (%)] | Healthy Controls [N (%)] |
|---|---|---|
| rs1544410 | ||
| AA | 17 (16.0%) | 27 (22.1%) |
| AG | 48 (45.3%) | 52 (42.6%) |
| GG | 41 (38.7%) | 43 (35.3%) |
| A | 82 (38.7%) | 106 (43.4%) |
| G | 130 (61.3%) | 138 (56.6%) |
| rs2228570 | ||
| CC | 33 (31.1%) | 39 (32.0%) |
| CT | 48 (45.3%) | 63 (51.6%) |
| TT | 25 (23.6%) | 20 (16.4%) |
| C | 114 (53.8%) | 141 (57.8%) |
| T | 98 (46.2%) | 103 (42.2%) |
| rs731236 | ||
| CC | 16 (15.1%) | 15 (12.9%) |
| CT | 48 (45.3%) | 65 (56.1%) |
| TT | 42 (39.6%) | 36 (31.0%) |
| C | 80 (37.7%) | 95 (40.9%) |
| T | 132 (62.3%) | 137 (59.1%) |
| rs7975232 | ||
| AA | 27 (25.5%) | 26 (22.4%) |
| AC | 50 (47.2%) | 65 (56.0%) |
| CC | 29 (27.3%) | 25 (21.6%) |
| A | 104 (49.1%) | 117 (50.4%) |
| C | 115 (50.9%) | 115 (49.6%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bugaj, B.; Wielińska, J.; Świerkot, J.; Bogunia-Kubik, K.; Górna, K. VDR Polymorphic Variants Are Related to Improvements in CRP and Disease Activity in Patients with Axial Spondyloarthritis That Undergo Anti-TNF Treatment. Genes 2022, 13, 1873. https://doi.org/10.3390/genes13101873
Bugaj B, Wielińska J, Świerkot J, Bogunia-Kubik K, Górna K. VDR Polymorphic Variants Are Related to Improvements in CRP and Disease Activity in Patients with Axial Spondyloarthritis That Undergo Anti-TNF Treatment. Genes. 2022; 13(10):1873. https://doi.org/10.3390/genes13101873
Chicago/Turabian StyleBugaj, Bartosz, Joanna Wielińska, Jerzy Świerkot, Katarzyna Bogunia-Kubik, and Katarzyna Górna. 2022. "VDR Polymorphic Variants Are Related to Improvements in CRP and Disease Activity in Patients with Axial Spondyloarthritis That Undergo Anti-TNF Treatment" Genes 13, no. 10: 1873. https://doi.org/10.3390/genes13101873
APA StyleBugaj, B., Wielińska, J., Świerkot, J., Bogunia-Kubik, K., & Górna, K. (2022). VDR Polymorphic Variants Are Related to Improvements in CRP and Disease Activity in Patients with Axial Spondyloarthritis That Undergo Anti-TNF Treatment. Genes, 13(10), 1873. https://doi.org/10.3390/genes13101873





