Cloning of Toll-like Receptor 3 Gene from Schizothorax prenanti (SpTLR3), and Expressions of Seven SpTLRs and SpMyD88 after Lipopolysaccharide Induction
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Treatment
2.2. RNA Extraction and cDNA Synthesis
2.3. Full Length Cloning of the SpTLR3
2.4. Sequence Analysis
2.5. Tissue Distribution of TLR3 mRNA in Unstressed Conditions
2.6. Detection of the Expression Patterns Induced by LPS
2.7. Statistical Analysis
3. Results
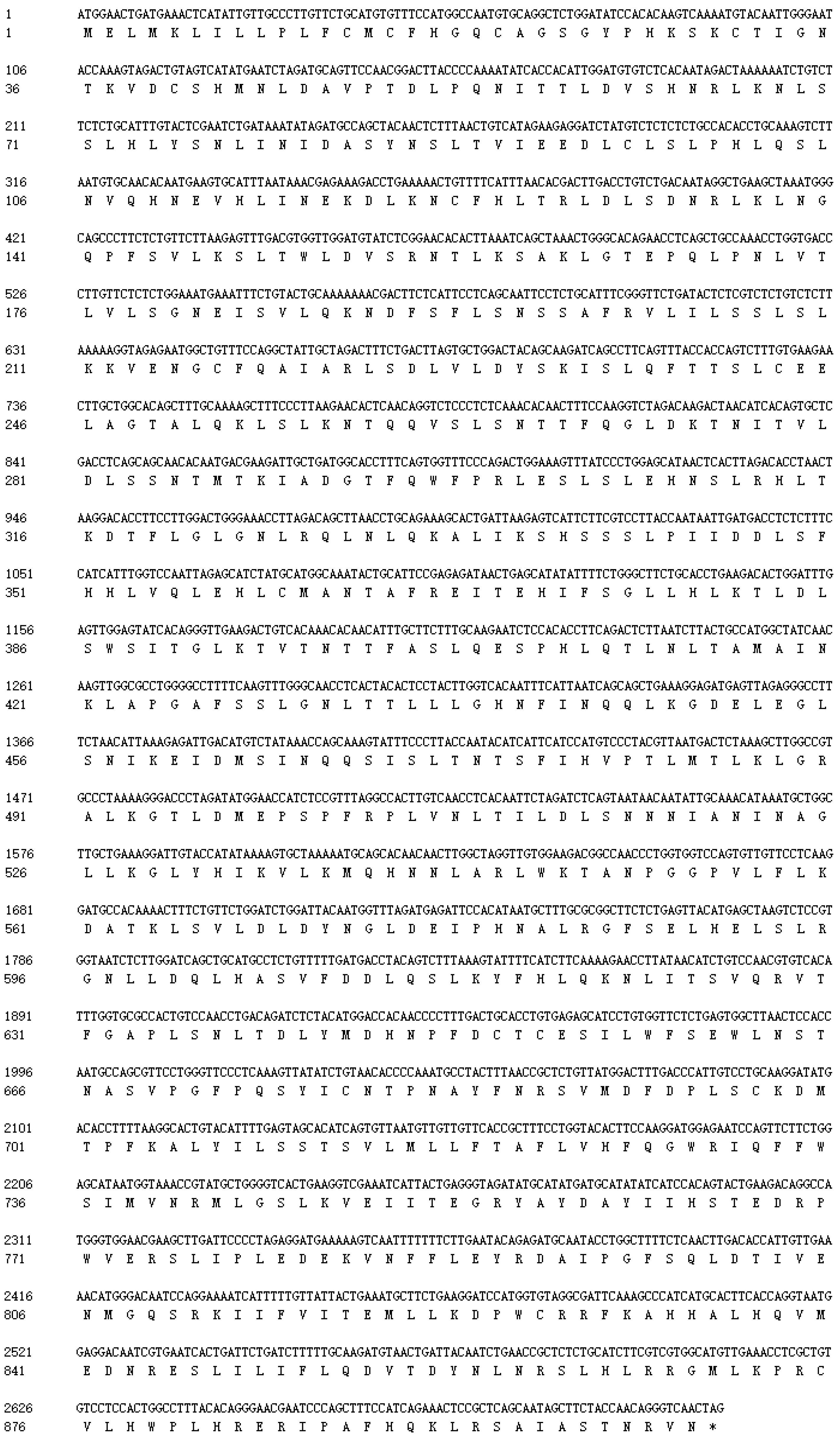
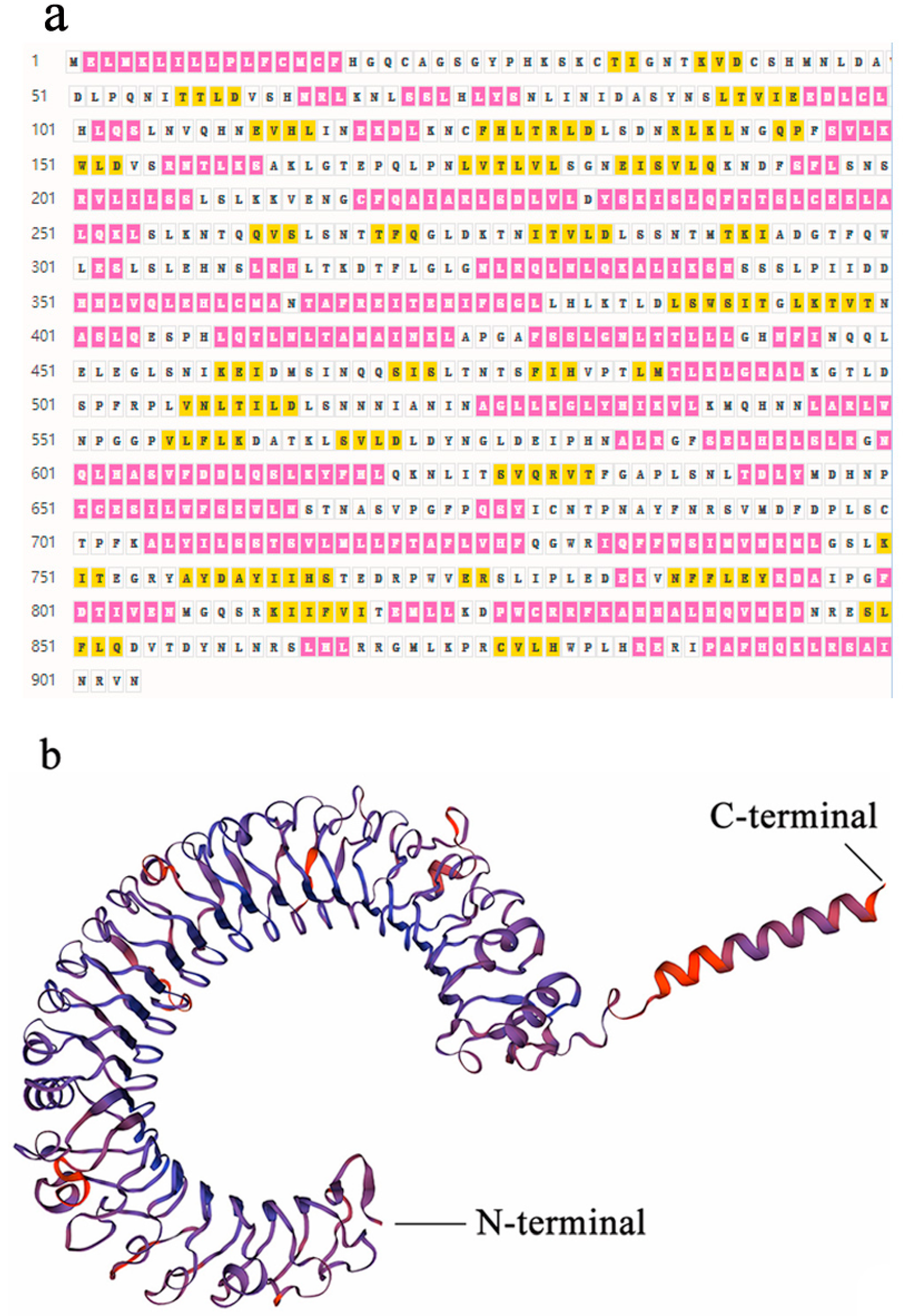
3.1. Identification, Structural, and Phylogenetic Analysis of SpTLR3
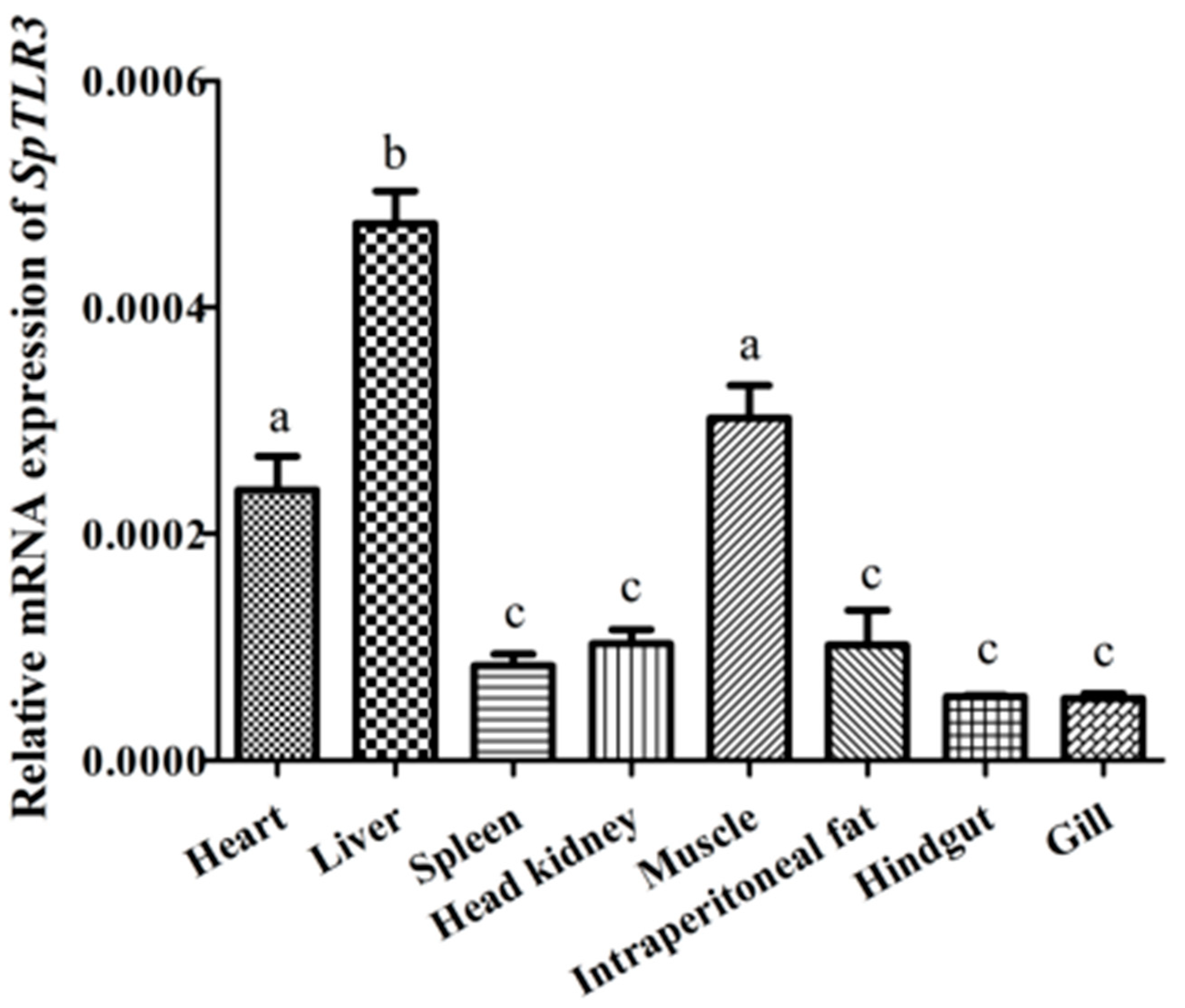
3.2. Tissue Distribution of SpTLR3 Expression in S. prenanti
3.3. Expression of SpMyD88 and SpTLRs after Injection of LPS
3.3.1. Expression of SpMyD88 after Injection of LPS
3.3.2. Expression of SpTLR2 after Injection with LPS
3.3.3. Expression Levels of SpTLR3 after Injection with LPS
3.3.4. Expression of SpTLR4 after Injection with LPS
3.3.5. Expression of SpTLR18 after Injection with LPS
3.3.6. Expression of SpTLR22s after Injection with LPS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alcorn, S.W.; Murra, A.L.; Pascho, R.J. Effects of rearing temperature on immune functions in sockeye salmon (Oncorhynchus nerka). Fish Shellfish Immunol. 2002, 12, 303–334. [Google Scholar] [CrossRef] [PubMed]
- Pietretti, D.; Wiegertjes, G.F. Ligand specificities of Toll-like receptors in fish: Indications from infection studies. Dev. Comp. Immunol. 2014, 43, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.D.; Vojtech, L.N.; Laing, K.J. Sensing disease and danger: A survey of vertebrate PRRs and their origins. Dev. Comp. Immunol. 2011, 35, 886–897. [Google Scholar] [CrossRef]
- Anderson, K.V.; Jurgens, G.; Nusslein-Volhard, C. Establishment of dorsal-ventral polarity in the Drosophila embryo: Genetic studies on the role of the Toll gene product. Cell 1985, 42, 779–789. [Google Scholar] [CrossRef]
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997, 388, 394–397. [Google Scholar] [CrossRef]
- Rock, F.L.; Hardiman, G.; Timans, J.C.; Kastelein, R.A.; Bazan, J.F. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA 1998, 95, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Sangrador-Vegas, A.; Martin, S.A.; O’Dea, P.G.; Smith, T.J. Cloning and characterization of the rainbow trout (Oncorhynchus mykiss) type II interleukin-1 receptor cDNA. Eur. J. Biochem. 2000, 267, 7031–7037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kong, X.H.; Zhou, C.J.; Li, L.; Nie, G.X.; Li, X.J. Toll-like receptor recognition of bacteria in fish: Ligand specificity and signal pathways. Fish Shellfish Immunol. 2014, 41, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Zhang, Z.; Liu, J.; Li, F.; Chang, F.; Fu, H.; Zhao, J.; Yin, D.L. Structural characterization and evolutionary analysis of fish-specific TLR27. Fish Shellfish Immunol. 2015, 45, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Li, J.R.; Han, J.J.; Shu, C.; Xu, T.J. Identification and characteristic analysis of TLR28: A novel member of the TLR1 family in teleost. Dev. Comp. Immunol. 2016, 62, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Hikima, J.; Hwang, S.D.; Jung, T.S. Innate immunity of finfish: Primordial conservation and function of viral RNA sensors in teleosts. Fish Shellfish Immunol. 2013, 35, 1689–1702. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Don, H.S.; Kondo, H.; Hirono, I.; Aoki, T.; Sano, M. Evidence of molecular toll-like receptor mechanisms in teleosts. Fish Pathol. 2010, 45, 1–16. [Google Scholar] [CrossRef]
- Venkatesh, B. Evolution and diversity of fish genomes. Curr. Opin. Genet. Dev. 2003, 13, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Lo, Y.C.; Wu, H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 2010, 465, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Deng, L.; Hong, M.; Akkaraju, G.R.; Inoue, J.; Chen, Z.J. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 2001, 412, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.M.; Chen, Z.J. The role of ubiquitylation in immune defence and pathogen evasion. Nat. Rev. Immunol. 2011, 12, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhao, C.; Wang, C.; Fan, S.; Yan, L.; Qiu, L. TLR3 gene in Japanese sea perch (Lateolabrax japonicus): Molecular cloning, characterization and expression analysis after bacterial infection. Fish Shellfish Immunol. 2018, 76, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.F.; Wiens, G.D.; Purcell, M.K.; Palti, Y. Characterization of Toll-like receptor 3 gene in rainbow trout (Oncorhynchus mykiss). Immunogenetics 2005, 57, 510–519. [Google Scholar] [CrossRef]
- Wu, M.; Zhu, K.C.; Guo, H.Y.; Guo, L.; Liu, B.; Jiang, S.G.; Zhang, D.C. Characterization, expression and function analysis of the TLR3 gene in golden pompano (Trachinotus ovatus). Dev. Comp. Immunol. 2021, 117, 103977. [Google Scholar] [CrossRef]
- Su, J.G.; Jang, S.H.; Yang, C.R.; Wang, Y.P.; Zhu, Z.Y. Genomic organization and expression analysis of Toll-like receptor 3 in grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2009, 27, 433–439. [Google Scholar] [CrossRef]
- Bilodeau, A.L.; Waldbieser, G.C. Activation of TLR3 and TLR5 in channel catfish exposed to virulent Edwardsiella ictaluri. Dev. Comp. Immunol. 2005, 29, 713–721. [Google Scholar] [CrossRef]
- Moriwaki, Y.; Begum, N.A.; Kobayashi, M.; Matsumoto, M.; Toyoshima, K.; Seya, T. Mycobacterium bovis Bacillus Calmette-Guerin and its cell wall complex induce a novel lysosomal membrane protein, SIMPLE, that bridges the missing link between lipopolysaccharide and p53-inducible gene, LITAF (PIG7), and estrogen-inducible gene, EET-1. J. Biol. Chem. 2001, 276, 23065. [Google Scholar] [CrossRef]
- Wei, R.B.; Yuan, D.Y.; Zhou, C.W.; Wang, T.; Lin, F.J.; Chen, H.; Wu, H.W.; Xin, Z.M.; Yang, S.Y.; Chen, D.F.; et al. Cloning, distribution and effects of fasting status of melanocortin 4 receptor (MC4R) in Schizothorax prenanti. Gene 2013, 532, 100–107. [Google Scholar] [CrossRef]
- Song, J.; Song, Z.; Yue, B.; Zheng, W. Assessing genetic diversity of wild populations of Prenant’s schizothoracin, Schizothorax prenanti, using AFLP markers. Environ. Biol. Fish. 2006, 77, 79–86. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Zhang, X.; Yang, S.; Yan, T.; Song, Z. Molecular cloning and expression of two heat-shock protein genes (HSC70/HSP70) from Prenant’s schizothoracin (Schizothorax prenanti). Fish Physiol. Biochem. 2015, 41, 573–585. [Google Scholar] [CrossRef]
- Zheng, Q.R.; Wu, Y.L.; Xu, H.L.; Wang, H.J.; Tang, H.L.; Xia, X.J.; Feng, J. Immune responses to Aeromonas hydrophila infection in Schizothorax prenanti fed with oxidized konjac glucomannan and its acidolysis products. Fish Shellfish Immunol. 2016, 49, 260–267. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, K.Y.; Huang, X.L.; Chen, D.F.; Li, C.W.; Ren, S.Y.; Liao, Y.T.; Zhou, Z.Y.; Liu, Q.F.; Du, Z.; et al. Streptococcus agalactiae, an emerging pathogen for cultured Ya-fish, Schizothorax prenanti, in China. Transbound. Emerg. Dis. 2012, 59, 369–375. [Google Scholar] [CrossRef]
- Du, X.; Li, D.; Li, Y.; Wu, J.; Huang, A.; Bu, G.; Meng, F.; Kong, F.; Cao, X.; Han, X.; et al. Clone, identification and functional character of two toll-like receptor 5 molecules in Schizothorax prenanti. Fish Shellfish Immunol. 2019, 95, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, J.; Li, D.; Huang, A.; Bu, G.; Meng, F.; Kong, F.; Cao, X.; Han, X.; Pan, X.; et al. Teleost-specific TLR25 identified from Schizothorax prenanti may recognize bacterial/viral components and activate NF-κB and type I IFNs signaling pathways. Fish Shellfish Immunol. 2018, 82, 361–370. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The Clustal_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nuclc. Acids. Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Koichiro, T.; Joel, D.; Masatoshi, N.; Sudhir, K. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Pellitero, P. Fish immunity and parasite infections: From innate immunity to immunoprophylactic prospects. Vet. Immunol. Immunopathol. 2008, 126, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, J.; Li, D.; Huang, A.; Bu, G.; Meng, F.; Kong, F.; Cao, X.; Han, X.; Pan, X.; et al. Transcriptome analysis of spleen reveals the signal transduction of toll-like receptors after Aeromonas hydrophila infection in Schizothorax prenanti. Fish Shellfish Immunol. 2019, 84, 816–824. [Google Scholar] [CrossRef]
- Hwang, S.D.; Asahi, T.; Kondo, H.; Hirono, I.; Aoki, T. Molecular cloning and expression study on Toll-like receptor 5 paralogs in Japanese flounder, Paralichthys olivaceus. Fish Shellfish Immunol. 2010, 29, 630–638. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Fitzgerald, K.A.; Bowie, A.G. The Toll-IL-1 receptor adaptor family grows to five members. Trends Immunol. 2003, 24, 286–290. [Google Scholar] [CrossRef]
- Slack, J.L.; Schooley, K.; Bonnert, T.P.; Mitcham, J.L.; Qwarnstrom, E.E.; Sims, J.E.; Dower, S.K. Identification of two major sites in the type I interleukin-1 receptor cytoplasmic region responsible for coupling to pro-inflammatory signaling pathways. J. Biol. Chem. 2000, 275, 4670–4678. [Google Scholar] [CrossRef]
- Du, X.; Wu, J.; Li, Y.; Xia, P.; Li, D.; Yang, X.; Yu, G.; Bu, G.; Huang, A.; Meng, F.; et al. Multiple subtypes of TLR22 molecule from Schizothorax prenanti present the functional diversity in ligand recognition and signal activation. Fish Shellfish Immunol. 2019, 93, 986–996. [Google Scholar] [CrossRef]
- Lin, F.; Wu, H.; Chen, H.; Xin, Z.; Yuan, D.; Wang, T.; Liu, J.; Gao, Y.; Zhang, X.; Zhou, C.; et al. Molecular and physiological evidences for the role in appetite regulation of apelin and its receptor APJ in Ya-fish (Schizothorax prenanti). Mol. Cell Endocrinol. 2014, 396, 46–57. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Lin, F.; Zhou, C.; Chen, H.; Wu, H.; Xin, Z.; Liu, J.; Gao, Y.; Yuan, D.; Wang, T.; Wei, R.; et al. Molecular characterization, tissue distribution and feeding related changes of NUCB2A/nesfatin-1 in Ya-fish (Schizothorax prenanti). Gene 2014, 536, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Kondo, H.; Hirono, I.; Saito-Taki, T.; Endo, M.; Aoki, T. Identification and characterization of a myeloid differentiation factor 88 (MyD88) cDNA and gene in Japanese flounder, Paralichthys olivaceus. Dev. Comp. Immunol. 2006, 30, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yang, M.; Liu, J.; Zheng, L.; Xu, D.; Chi, C.; Lv, Z.; Liu, H. Identification, functional characterization and expression pattern of myeloid differentiation factor 88 (MyD88) in Nibea albiflora. Fish Shellfish Immunol. 2022, 124, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.L.; Kong, P.; Wang, Z.Y.; Ji, P.F.; Liu, X.D.; Cai, M.Y.; Han, X.Z. Molecular cloning and expression of MyD88 in large yellow croaker, Pseudosciaena crocea. Fish Shellfish Immunol. 2009, 26, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 2005, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Palti, Y. Toll-like receptors in bony fish: From genomics to function. Dev. Comp. Immunol. 2011, 35, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Quiniou, S.M.; Boudinot, P.; Bengtén, E. Comprehensive survey and genomic characterization of Toll-like receptors (TLRs) in channel catfish, Ictalurus punctatus: Identification of novel fish TLRs. Immunogenetics 2013, 65, 511–530. [Google Scholar] [CrossRef]
- Wei, Y.C.; Pan, T.S.; Chang, M.X.; Huang, B.; Xu, Z.; Luo, T.R.; Nie, P. Cloning and expression of Toll-like receptors 1 and 2 from a teleost fish, the orange-spotted grouper Epinephelus coioides. Vet. Immunol. Immunop. 2011, 141, 173–182. [Google Scholar] [CrossRef]
- Meijer, A.H.; Gabby, K.S.F.; Medina, R.I.A.; He, S.; Bitter, W.; Ewa, S.J.B.; Spaink, H.P. Expression analysis of the Toll-like receptor and TIR domain adaptor families of zebrafish. Mol. Immunol. 2004, 40, 773–783. [Google Scholar] [CrossRef]
- Lee, P.T.; Zou, J.; Holland, J.W.; Martin, S.A.M.; Collet, B.; Kanellos, T.; Secombes, C.J. Identification and characterisation of TLR18-21 genes in Atlantic salmon (Salmo salar). Fish Shellfish Immunol. 2014, 41, 549–559. [Google Scholar] [CrossRef]
- Huang, W.J.; Shen, Y.b.; Xu, X.Y.; Hu, M.Y.; Li, J.L. Identification and characterization of the TLR18 gene in grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2015, 47, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Ji, W.; Zhang, G.R.; Wei, K.J.; Shi, Z.C.; Zhang, X.T.; Zheng, H.; Fan, Q.X. Molecular characterization and expression analysis of three TLR genes in yellow catfish (Pelteobagrus fulvidraco): Responses to stimulation of Aeromonas hydrophila and TLR ligands. Fish and Shellfish Immunol. 2017, 66, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Liu, D.; Liu, R.; Zhu, Y.; Li, T.; Zhang, F.; An, L.; Yang, G.; Li, H. Non-mammalian Toll-like receptor 18 (Tlr18) recognizes bacterial pathogens in common carp (Cyprinus carpio L.): Indications for a role of participation in the NF-κB signaling pathway. Fish Shellfish Immunol. 2018, 72, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Osamu, T.; Shizuo, A. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Sullivan, C.; Charette, J.; Catchen, J.; Lage, C.R.; Giasson, G.; Postlethwait, J.H.; Millard, P.J.; Kim, C.H. The gene history of zebrafish tlr4a and tlr4b is predictive of their divergent functions. J. Immunol. 2009, 183, 5896–5908. [Google Scholar] [CrossRef]
- Sepulcre, M.P.; Alcaraz-Perez, F.; Lopez-Munoz, A.; Roca, F.J.; Meseguer, J.; Cayuela, M.L.; Mulero, V. Evolution of lipopolysaccharide (LPS) recognition and signaling: Fish TLR4 does not recognize LPS and negatively regulates NF-κB activation. J. Immunol. 2009, 182, 1836–1845. [Google Scholar] [CrossRef]
- Samanta, M.; Basu, M.; Swain, B.; Paichha, M.; Lenka, S.S.; Das, S.; Jayasankar, P.; Maiti, N.K. Molecular cloning and characterization of LrTLR4, analysis of its inductive expression and associated down-stream signaling molecules following lipopolysaccharide stimulation and Gram-negative bacterial infection. Fish Shellfish Immunol. 2017, 60, 164–176. [Google Scholar] [CrossRef]
- Stafford, J.L.; Ellestad, K.K.; Magor, K.E.; Belosevic, M.; Magor, B.G. A toll-like receptor (tlr) gene is up-regulated in activated goldfish macrophages. Dev. Comp. Immunol. 2003, 27, 685–698. [Google Scholar] [CrossRef]
- Hirono, I.; Takami, M.; Miyata, M.; Miyazaki, T.; Han, H.J.; Takano, T.; Endo, M.; Aoki, T. Characterization of gene structure and expression of two toll-like receptors from Japanese flounder, Paralichthys olivaceus. Immunogenetics 2004, 56, 38–46. [Google Scholar] [CrossRef]
- Salazar, C.; Haussmann, D.; Kausel, G.; Figueroa, J. Molecular cloning of Salmo salar Toll-like receptors (TLR1, TLR22, TLR5M and TLR5S) and expression analysis in SHK-1 cells during Piscirickettsia salmonis infection. J. Fish Dis. 2016, 39, 239–248. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Ascencio-Valle, F.; Alamillo, E.; Hirono, I.; Kondo, H.; Jirapongpairoj, W.; Angulo, C. Molecular cloning and comparative responses of Toll-like receptor 22 following ligands stimulation and parasitic infection in yellowtail (Seriola lalandi). Fish Shellfish Immunol. 2015, 46, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, G.; Ma, F.; Li, T.; Yang, H.; Rombout, J.H.; An, L. Molecular characterization of a fish-specific toll-like receptor 22 (TLR22) gene from common carp (Cyprinus carpio L.): Evolutionary relationship and induced expression upon immune stimulants. Fish Shellfish Immunol. 2017, 63, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Rebl, A.; Siegl, E.; Köllner, B.; Fischer, U.; Seyfert, H.M. Characterization of twin toll-like receptors from rainbow trout (Oncorhynchus mykiss): Evolutionary relationship and induced expression by Aeromonas salmonicida salmonicida. Dev. Comp. Immunol. 2007, 31, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, A.Y.M.; Kiron, V.; Dopazo, J.; Fernandes, J.M.O. Diversification of the expanded teleost-specific toll-like receptor family in Atlantic cod, Gadus morhua. BMC Evol. Biol. 2012, 12, 256. [Google Scholar] [CrossRef] [PubMed]



| Primer | Sequence (5′-3′) | Annealing Temperature (°C) | Size (bp) |
|---|---|---|---|
| Primers for partial sequence cloning | |||
| TLR3-F | CAGACTCTTAATCTTACTG | 55 | 1558 |
| TLR3-R | GACATACCAAATAAGGAC | ||
| Primers for 5′RACE | |||
| GSP | CGACCTTCAGTGACCCCAGCATACGG | Touchdown PCR | 2543 |
| UPM (kit provided) | CTAATACGACTCACTATAGGGCAAG CAGTGGTATCAACGCAGAGT | ||
| Primers for qRT-PCR | |||
| TLR2-F | GATCAACGGCACAGTGTTTG | 62 | 170 |
| TLR2-R | CAGGTCTGAAAGGAGGTTCTG | ||
| TLR3-F | GCTGAAAGGAGATGAGTTAGAG | 62 | 110 |
| TLR3-R | ACGTAGGGACATGGATGAA | ||
| TLR4-F | CTTGGTGTCGCTTTGAGTTTG | 62 | 107 |
| TLR4-R | GTCTCTGCTCCACTTTAGGTATG | ||
| TLR18-F | ACAGACTAAATGGCCAGGGAAG | 62 | 118 |
| TLR18-R | AACCACAAGCAAGGGCAAAG | ||
| TLR22-1-F | CCTCTTCTTAGCCTTCCTTTAC | 62 | 94 |
| TLR22-1-R | CTCGTCTTTGGTGTTGTAGG | ||
| TLR22-2-F | TTCCAGGGACTGTGGTATTTG | 62 | 98 |
| TLR22-2-R | GCCCACAGATAAGGAGTGTAAG | ||
| TLR22-3-F | CCATCGGCATTCTTTGGTTT | 62 | 169 |
| TLR22-3-R | CTGTGTTCAGGAATGCCTTG | ||
| MyD88-F | GAGTTTCCCACTCCGTTAAGA | 62 | 92 |
| MyD88-R | CGCCGAGATGATGGACTTTA | ||
| β-actin-F | GACCACCTTCAACTCCATCAT | 62 | 126 |
| β-actin -R | GTGATCTCCTTCTGCATCCTATC | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Zhang, J.; Zhang, K.; Fang, C.; Li, W.; Wang, Q. Cloning of Toll-like Receptor 3 Gene from Schizothorax prenanti (SpTLR3), and Expressions of Seven SpTLRs and SpMyD88 after Lipopolysaccharide Induction. Genes 2022, 13, 1862. https://doi.org/10.3390/genes13101862
Huang J, Zhang J, Zhang K, Fang C, Li W, Wang Q. Cloning of Toll-like Receptor 3 Gene from Schizothorax prenanti (SpTLR3), and Expressions of Seven SpTLRs and SpMyD88 after Lipopolysaccharide Induction. Genes. 2022; 13(10):1862. https://doi.org/10.3390/genes13101862
Chicago/Turabian StyleHuang, Jiqin, Jianlu Zhang, Kunyang Zhang, Cheng Fang, Wanchun Li, and Qijun Wang. 2022. "Cloning of Toll-like Receptor 3 Gene from Schizothorax prenanti (SpTLR3), and Expressions of Seven SpTLRs and SpMyD88 after Lipopolysaccharide Induction" Genes 13, no. 10: 1862. https://doi.org/10.3390/genes13101862
APA StyleHuang, J., Zhang, J., Zhang, K., Fang, C., Li, W., & Wang, Q. (2022). Cloning of Toll-like Receptor 3 Gene from Schizothorax prenanti (SpTLR3), and Expressions of Seven SpTLRs and SpMyD88 after Lipopolysaccharide Induction. Genes, 13(10), 1862. https://doi.org/10.3390/genes13101862




