Evolutionary Divergence between Toona ciliata and Toona sinensis Assayed with Their Whole Genome Sequences
Abstract
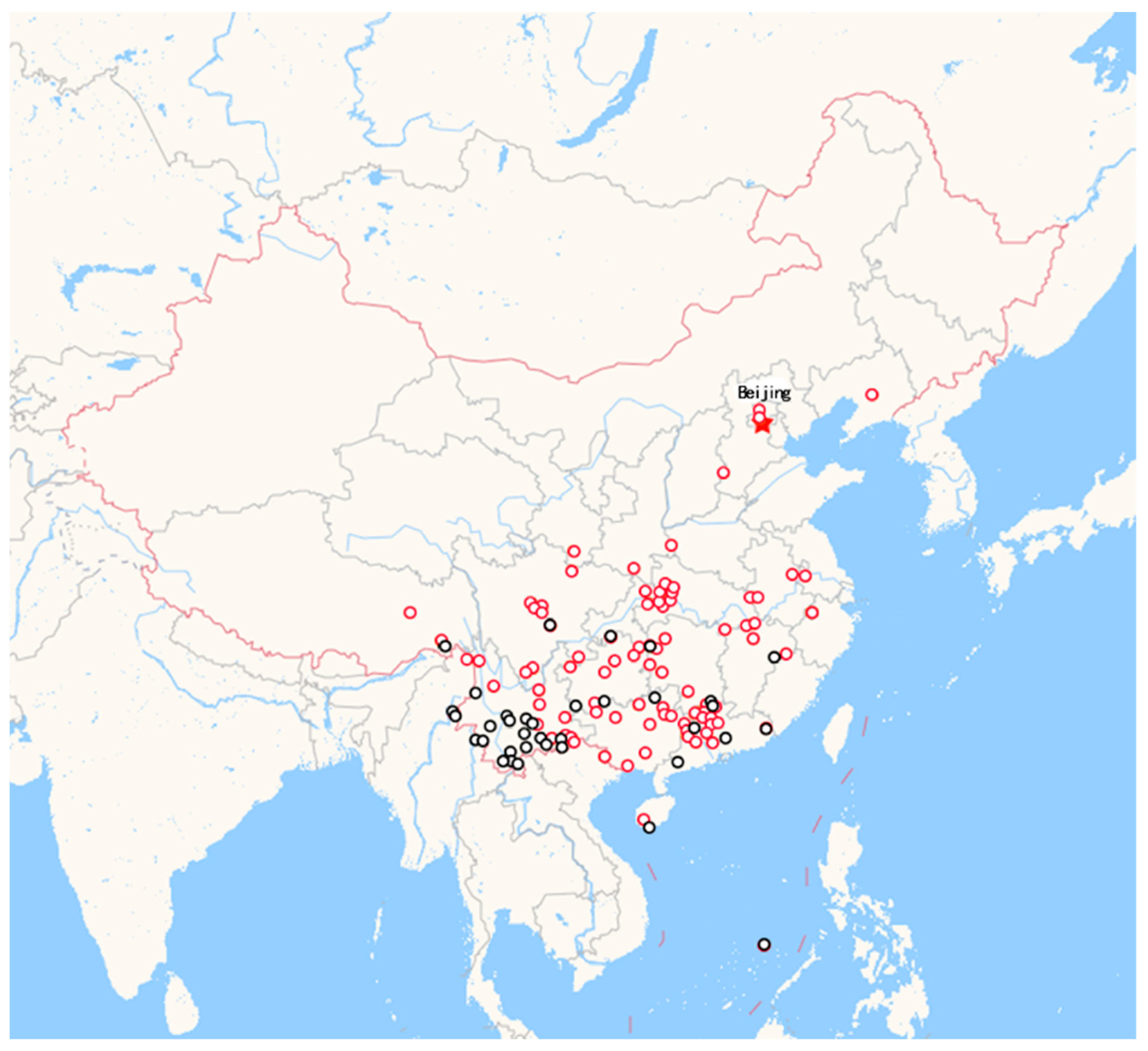
1. Introduction
2. Materials and Methods
3. Results
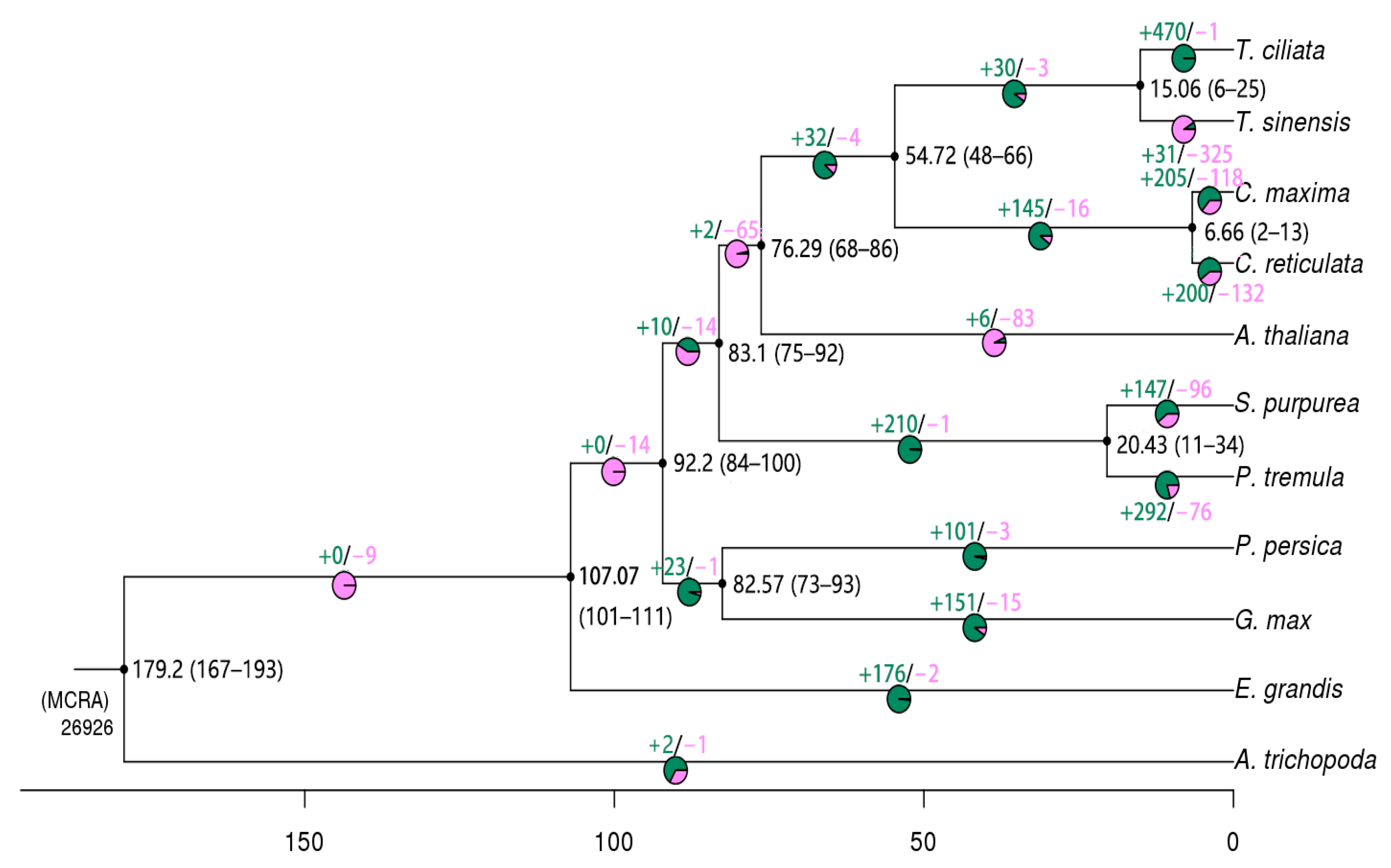
3.1. Expansion and Contraction of Gene Families
3.2. Detection of Positive Selection
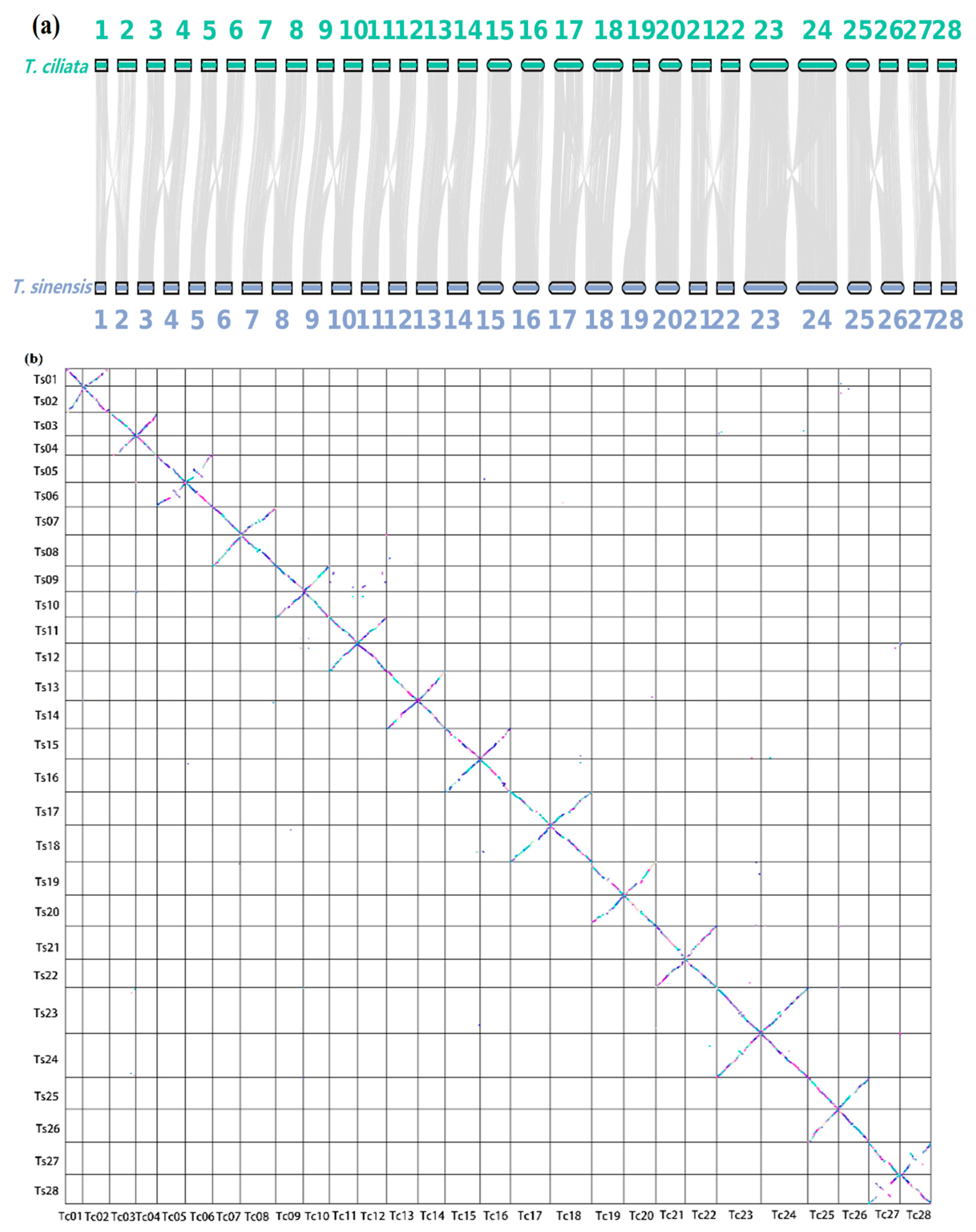
3.3. Genome Collinearity Analysis
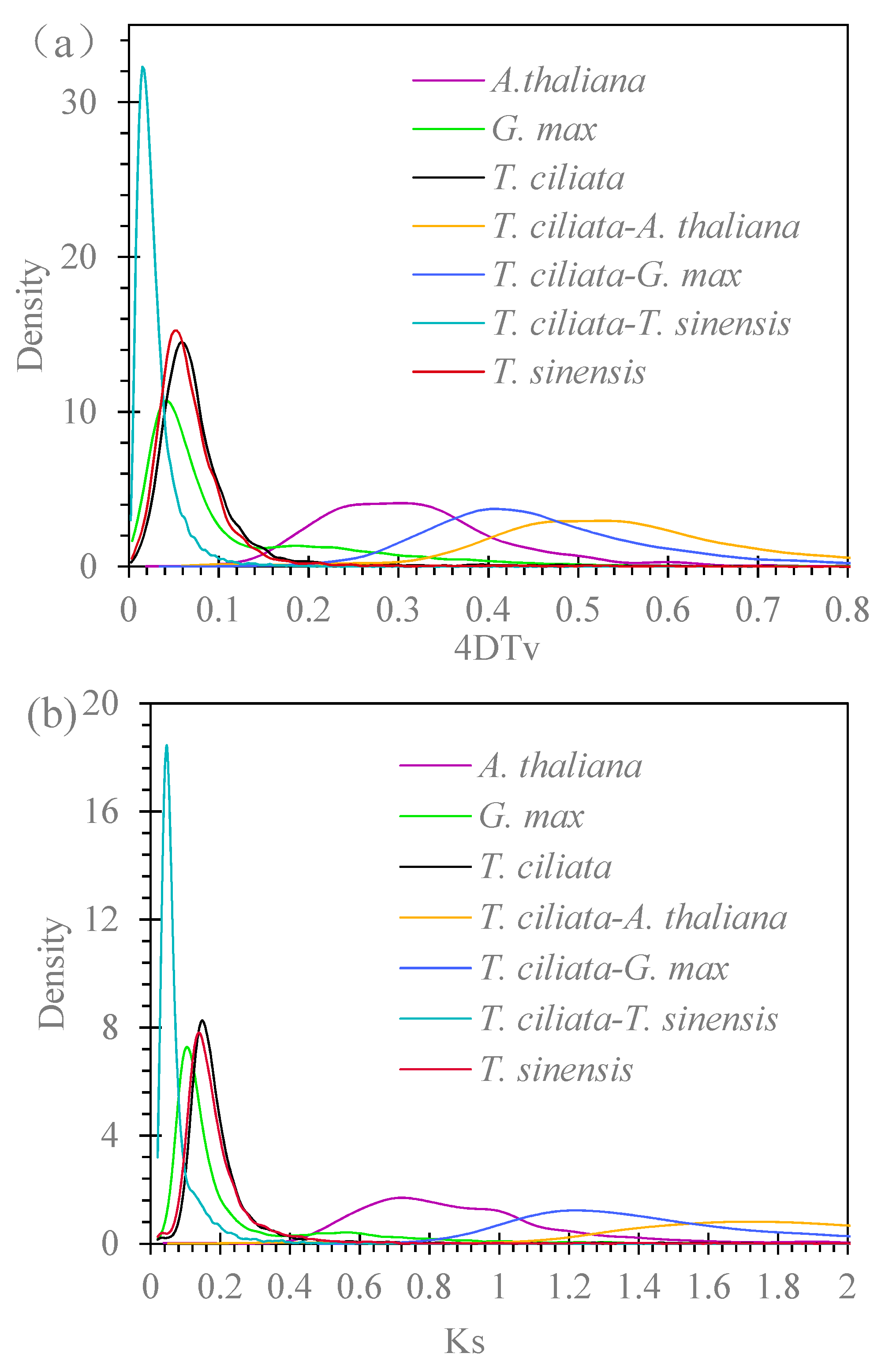
3.4. Genome Duplications
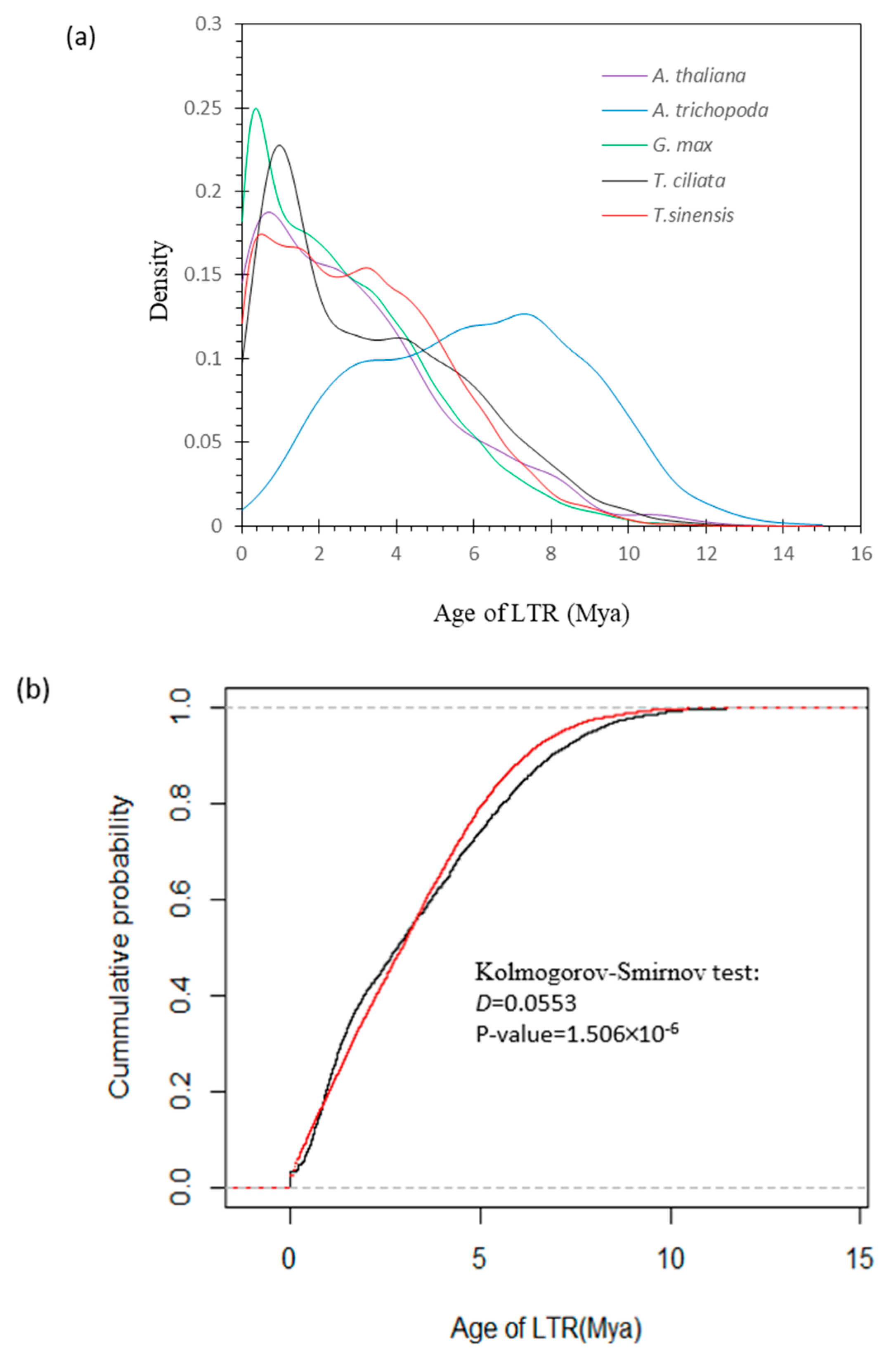
3.5. Divergence in LTR Insertion Time
4. Discussion
4.1. Genome Comparison
4.2. Evolutionary Divergence and Its Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.-K.; Li, H.; Chen, B.-Y. Meliaceae. In Flora of China 43; Science Press: Beijing China, 1997. [Google Scholar]
- Cheng, D.S.; Cui, T.L. Utilization value and cultivation techniques of Toona sureni. For. By-Prod. Spec. China 2010, 4, 39–40. [Google Scholar] [CrossRef]
- Fu, L.G. China Plant Red Data Book: Rare and Endangered Plants; Science Press: Beijing, China, 1992; Volume 1. [Google Scholar]
- Liang, R.L.; Liao, R.Y.; Dai, J. Endangered causes and protection strategy of Toona ciliata. Guangxi For. Sci. 2011, 40, 201–203. [Google Scholar] [CrossRef]
- Jiao, Z.Y.; Zheng, J.W.; Yan, R.C.; Zhang, Y.; Tang, L.L. Overview of the main research on the multifunctional precious tree species Toona sinensis. J. Jiangsu For. Sci. Technol. 2019, 46, 51–56. [Google Scholar] [CrossRef]
- Edmonds, J.M. The potential value of Toona species (Meliaceae) as multipurpose and plantation trees in Southeast Asia. Commonw. For. Rev. 1993, 72, 181–186. [Google Scholar]
- Liao, J.W.; Yeh, J.Y.; Lin, Y.C.; Wei, M.M.; Chung, Y.C. Mutagenicity and safety evaluation of water extract of fermented Toona sinensis Roem or leaves. J. Food Sci 2009, 74, T7–T13. [Google Scholar] [CrossRef]
- Hao, J.W. On the resource value and utilization of Toona sinensis. Jiangsu Agric. Sci. 2003, 5, 102–103. [Google Scholar] [CrossRef]
- Zhang, J.F.; Zhang, Q.; Lu, G. Effects of Toona sinensis leaves extract on lipid metabolism and antioxidant activity of mice with hyperlipidemia. J. Chin. Inst. Food Sci. Technol. 2007, 7, 3–7. [Google Scholar] [CrossRef]
- Edmonds, J.M.; Staniforth, M. Toona sinensis (Meliaceae). Curtis’s Bot. Mag. 1998, 15, 186–193. [Google Scholar] [CrossRef]
- Park, J.C.; Yu, Y.B.; Lee, J.H.; Choi, J.S.; Ok, K.D. Phenolic compounds from the rachis of Cedrela sinensis. Korean J. Pharmacogn. 1996, 27, 219–223. [Google Scholar]
- Zhou, W.; Zhang, X.-X.; Ren, Y.; Li, P.; Chen, X.-Y.; Hu, X.-S. Mating system and population structure in the natural distribution of Toona ciliata (Meliaceae) in South China. Sci. Rep. 2020, 10, 16998. [Google Scholar] [CrossRef]
- Mullner, A.N.; Pennington, T.D.; Koecke, A.V.; Renner, S.S. Biogeography of Cedrela (Mellaceae, Sapindales) in central and south America. Am. J. Bot. 2010, 97, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhan, X.; Que, Q.-M.; Qu, W.-T.; Liu, M.-Q.; OuYang, K.-X.; Li, J.-C.; Deng, X.-M.; Zhang, J.-J.; Liao, B.-Y.; et al. Genetic diversity and population structure of Toona ciliata Roem. based on sequence-related amplified polymorphism (SRAP) markers. Forests 2015, 6, 1094–1106. [Google Scholar] [CrossRef]
- Zhan, X.; Li, P.; Hui, W.-K.; Deng, Y.-W.; Gan, S.-M.; Sun, Y.; Zhao, X.-H.; Chen, X.-Y.; Deng, X.-M. Genetic diversity and population structure of Toona ciliata revealed by simple sequence repeat markers. Biotechnol. Biotechnol. Equip. 2019, 33, 214–222. [Google Scholar] [CrossRef]
- Xing, P.-Y.; Liu, T.; Song, Z.-Q.; Li, X.-F. Genetic diversity of Toona sinensis Roem in China revealed by ISSR and SRAP markers. Genet. Mol. Res. 2016, 15, 1–12. [Google Scholar] [CrossRef]
- Chen, Q.; Rong, L.; Shao, Z.; Liu, T.; Wei, L.; Song, Z. Genetic diversity analysis of Toona sinensis germplasms based on SRAP and EST-SSR markers. Acta Hortic. Sin. 2018, 45, 967–976. [Google Scholar] [CrossRef]
- Zhou, W. Studies on Geographic Variation of Seeds and Seedling Traits and Genetic Diversity of Toona sinensis (Meliaceae). Ph.D. Thesis, South China Agricultural University, Guangzhou, China, 2020. [Google Scholar]
- Nobrega, M.A.; Pennacchio, L.A. Comparative genomic analysis as a tool for biological discovery. J. Physiol. 2004, 554, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Baloch, A.A.; Kakar, K.U.; Nawaz, Z.; Mushtaq, M.; Abro, A.; Khan, S.; Latif, A. Comparative genomics and evolutionary analysis of plant CNGCs. Biol. Methods Protoc. 2022, 7, bpac018. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.-T.; Xiu, Z.-H.; Chen, C.-H.; Wang, Y.; Yang, J.-X.; Sui, J.-J.; Jiang, S.-J.; Wang, P.; Yue, S.-Y.; Zhang, Q.-Q.; et al. Long read sequencing of Toona sinensis (A. Juss) Roem: A chromosome-level reference genome for the family Meliaceae. Mol. Ecol. Resour. 2021, 21, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiao, Y.; He, Z.H.; Li, L.L.; Song, H.Y.; Zhang, J.J.; Cheng, X.; Chen, X.Y.; Li, P.; Hu, X.S. A chromosome-level genome assembly of Toona ciliata (Meliaceae). Genome Biol. Evol. 2022, 14, evac121. [Google Scholar] [CrossRef]
- Doyle, J.J. A rapid isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Sloan, D.B.; Wu, Z.; Sharbrough, J. Correction of persistent errors in Arabidopsis reference mitochondrial genomes. Plant Cell 2018, 30, 525–527. [Google Scholar] [CrossRef]
- Amborella Genome Project. The Amborella genome and the evolution of flowering plants. Science 2013, 342, 1241089. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Zhang, S.; Cao, L.; Huang, Y.; Cheng, J.; Wu, G.; Tian, S.; Chen, C.; Liu, Y.; et al. Genomic analyses of primitive, wild and cultivated citrus provide insights into asexual reproduction. Nat. Genet. 2017, 49, 765–772. [Google Scholar] [CrossRef]
- Wang, L.; He, F.; Huang, Y.; He, J.; Yang, S.; Zeng, J.; Deng, C.; Jiang, X.; Fang, Y.; Wen, S.; et al. Genome of wild mandarin and domestication history of mandarin. Mol. Plant 2018, 11, 1024–1037. [Google Scholar] [CrossRef]
- Bartholomé, J.; Mandrou, E.; Mabiala, A.; Jenkins, J.; Nabihoudine, I.; Klopp, C.; Schmutz, J.; Plomion, C.; Gion, J.-M. High-resolution genetic maps of Eucalyptus improve Eucalyptus grandis genome assembly. New Phytol. 2015, 206, 1283–1296. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.; Thelen, J.J.; Cheng, J.; et al. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef]
- International Peach Genome Initiative; Verde, I.; Abbott, A.G.; Scalabrin, S.; Jung, S.; Shu, S.; Marroni, F.; Zhebentyayeva, T.; Dettori, M.T.; Grimwood, J.; et al. The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nat. Genet. 2013, 45, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Wang, J.; Delhomme, N.; Schiffthaler, B.; Sundström, G.; Zuccolo, A.; Nystedt, B.; Hvidsten, T.R.; de la Torre, A.; Cossu, R.M.; et al. Functional and evolutionary genomic inferences in Populus through genome and population sequencing of American and European aspen. Proc. Natl. Acad. Sci. USA 2018, 115, E10970–E10978. [Google Scholar] [CrossRef]
- Zhou, R.; Macaya-Sanz, D.; Carlson, C.H.; Schmutz, J.; Jenkins, J.W.; Kudrna, D.; Sharma, A.; Sandor, L.; Shu, S.; Barry, K.; et al. A willow sex chromosome reveals convergent evolution of complex palindromic repeats. Genome Biol. 2020, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.M.; Pattnaik, S.; Jain, P.; Gaur, P.; Choudhary, R.; Vaiyanathan, S.; Deepak, S.; Hariharan, A.K.; Krishan, P.B.; Nair, J.; et al. A draft of the genome and four transcriptomes of a medicinal and pesticidal angiosperm Azadirachta indica. BMC Genom. 2012, 13, 464. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. ClusterProfiler: An R package for comparing biological themes among gene clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic orthology inference for comparative genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Ebert, D.; Huang, X.; Thomas, P.D. PANTHER version 14: More genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019, 47, D419–D426. [Google Scholar] [CrossRef]
- Puttick, M.N. MCMCtreeR: Functions to prepare MCMCtree analyses and visualize posterior ages on trees. Bioinformatics 2019, 35, 5321–5322. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. PAML: A program package for phylogenetic analysis by maximum likelihood. Bioinformatics 1997, 13, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Asimenos, G.; Toh, H. Multiple alignment of DNA sequences with MAFFT. Methods Mol. Biol. 2009, 537, 39–64. [Google Scholar] [CrossRef] [PubMed]
- Suyama, M.; Torrents, D.; Bork, P. PAL2NAL: Robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 2006, 34 (Suppl. S2), W609–W612. [Google Scholar] [CrossRef]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; Haeseler, V.A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587. [Google Scholar] [CrossRef] [PubMed]
- Han, M.V.; Thomas, G.W.; Lugo-Martinez, J.; Hahn, M.W. Estimating gene gain and loss rates in the presence of error in genome assembly and annotation using CAFE 3. Mol. Biol. Evol. 2013, 30, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Deely, J.J.; Lindley, D.V. Bayes empirical Bayes. J. Am. Stat. Assoc. 1981, 76, 833–841. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Krishnakumar, V.; Li, J. jcvi: JCVI utility libraries. Zenodo 2015. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Comeron, J.M. A method for estimating the numbers of synonymous and nonsynonymous substitutions per site. J. Mol. Evol. 1995, 41, 1152–1159. [Google Scholar] [CrossRef]
- Zwaenepoel, A.; Van-de-Peer, Y. Wgd-simple command line tools for the analysis of ancient whole-genome duplications. Bioinformatics 2019, 35, 2153–2155. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, H. LTR_FINDER: An efficient tool for the prediction of full-length LTR retrotransposons. Nucleic Acids Res. 2007, 35, W265–W268. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. Estimation of evolutionary distances between homologous nucleotide sequences. Proc. Natl. Acad. Sci. USA 1981, 78, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.; Longden, I.; Bleasby, A. EMBOSS: The European molecular biology open software suite. Trends Genet. 2000, 16, 276–277. [Google Scholar] [CrossRef]
- Ossowski, S.; Schneeberger, K.; Lucas-Lledó, J.I.; Warthmann, N.; Clark, R.M.; Shaw, R.G.; Weigel, D.; Lynch, M. The rate and molecular spectrum of spontaneous mutations in Arabidopsis thaliana. Science 2010, 327, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Cavers, S.; Telford, A.; Arenal Cruz, F.; Perez Castaneda, A.J.; Valencia, R.; Navarro, C.; Buonamici, A.; Lowe, A.J.; Vendramin, G.G. Cryptic species and phylogeographical structure in the tree Cedrela odorata L. throughout the Netotropics. J. Biogeogr. 2013, 40, 732–746. [Google Scholar] [CrossRef]
- Koecke, V.; Muellner-Riehl, A.N.; Pennington, T.D.; Schorr, G.; Schnitzler, J. Niche evolution through time and across continents: The story of Neotropical Cedrela (Meliaceae). Am. J. Bot. 2013, 100, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Koenen, E.J.; Clarkson, J.J.; Pennington, T.D.; Chatrou, L.W. Recently evolved diversity and convergent radiations of rainforest mahoganies (Meliaceae) shed new light on the origins of rainforest hyperdiversity. New Phytol. 2015, 207, 327–339. [Google Scholar] [CrossRef]
- Styles, B.T. The flower biology of Meliaceae and its bearing on tree breeding. Silvae Genet. 1972, 21, 175–182. [Google Scholar]
- Gouvea, C.F.; Dornelas, M.C.; Rodriguez, A.P.M. Floral development in the tribe Cedreleae (Meliaceae, sub-family Swietenioideae): Cedrela and Toona. Ann. Bot. 2008, 101, 39–48. [Google Scholar] [CrossRef]
- Li, L.-L.; Wang, X.; Xiao, Y.; Chen, X.-Y.; Hu, X.-S. On the theories of plant mating system and molecular evolution and their applications. Sci. Sin. Vitae 2021. [Google Scholar] [CrossRef]
- Wright, S.; Ncss, R.W.; Foxc, J.P.; Barrett, S.C.H. Genomic consequences of outcrossing and selfing in plants. Int. J. Plant Sci. 2008, 169, 105–118. [Google Scholar] [CrossRef]
- Li, W.-H. Molecular Evolution; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Bucher, E.; Reinders, J.; Mirouze, M. Epigenetic control of transposon transcription and mobility in Arabidopsis. Curr. Opin. Plant Biol. 2012, 15, 503–510. [Google Scholar] [CrossRef]
- Kalendar, R.; Tanskanen, J.; Immonen, S.; Nevo, E.; Schulman, A.H. Genome evolution of wild barley (Hordeum spontaneum) by BARE-1 retrotransposon dynamics in response to sharp microclimatic divergence. Proc. Natl. Acad. Sci. USA 2000, 97, 6603–6607. [Google Scholar] [CrossRef]
- Wos, G.; Choudhury, R.R.; Kolar, F.; Parisod, C. Transcriptional activity of transposable elements along an elevational gradient in Arabidopsis arenosa. Mob. DNA 2021, 12, 7. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Xiao, Y.; He, Z.-H.; Li, L.-L.; Lv, Y.-W.; Hu, X.-S. Evolutionary Divergence between Toona ciliata and Toona sinensis Assayed with Their Whole Genome Sequences. Genes 2022, 13, 1799. https://doi.org/10.3390/genes13101799
Wang X, Xiao Y, He Z-H, Li L-L, Lv Y-W, Hu X-S. Evolutionary Divergence between Toona ciliata and Toona sinensis Assayed with Their Whole Genome Sequences. Genes. 2022; 13(10):1799. https://doi.org/10.3390/genes13101799
Chicago/Turabian StyleWang, Xi, Yu Xiao, Zi-Han He, Ling-Ling Li, Yan-Wen Lv, and Xin-Sheng Hu. 2022. "Evolutionary Divergence between Toona ciliata and Toona sinensis Assayed with Their Whole Genome Sequences" Genes 13, no. 10: 1799. https://doi.org/10.3390/genes13101799
APA StyleWang, X., Xiao, Y., He, Z.-H., Li, L.-L., Lv, Y.-W., & Hu, X.-S. (2022). Evolutionary Divergence between Toona ciliata and Toona sinensis Assayed with Their Whole Genome Sequences. Genes, 13(10), 1799. https://doi.org/10.3390/genes13101799





