Integrated Bioinformatics Analysis Reveals Novel miRNA as Biomarkers Associated with Preeclampsia
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Processing
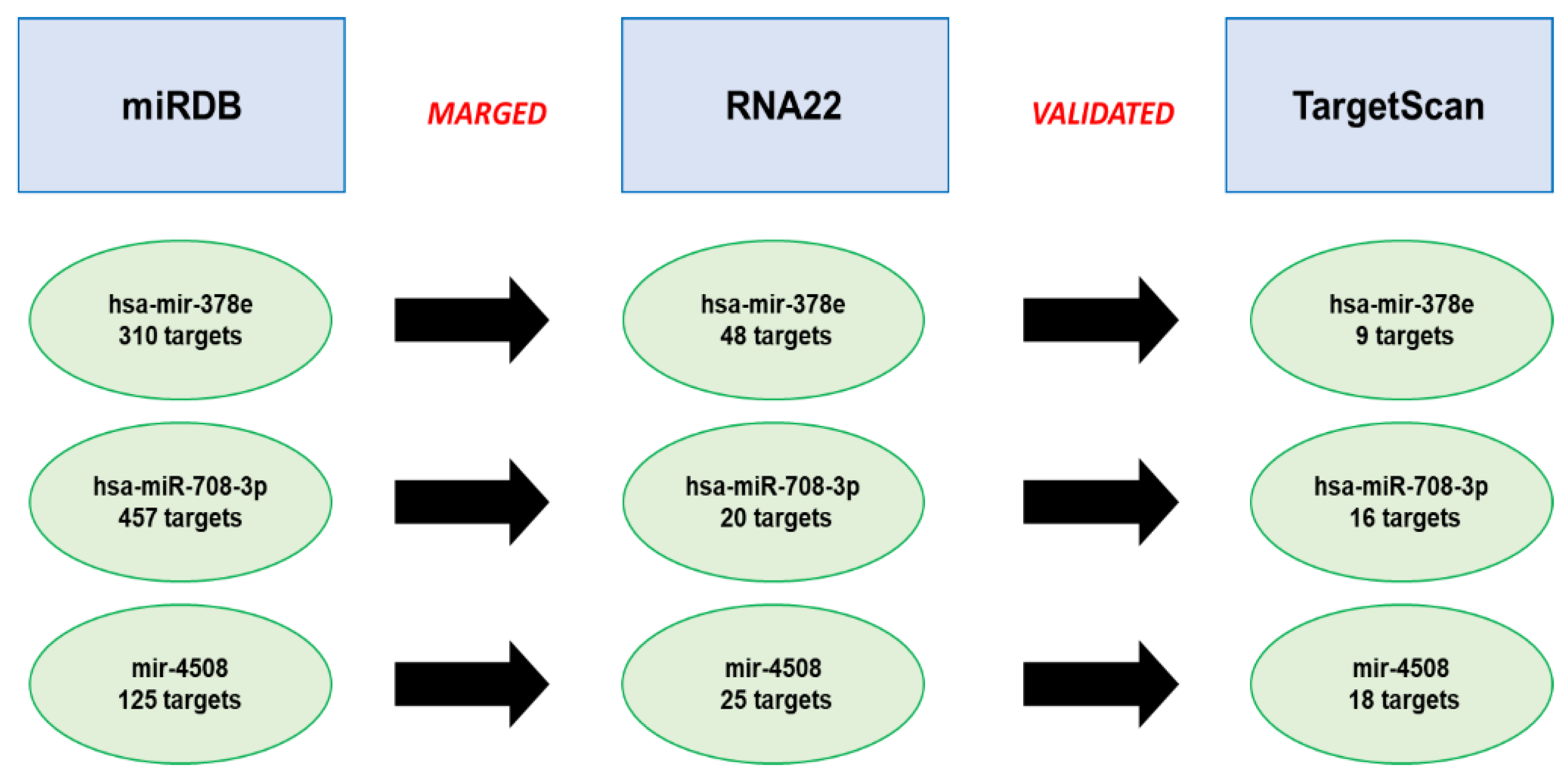
2.2. Prediction of miRNAs’ Targets
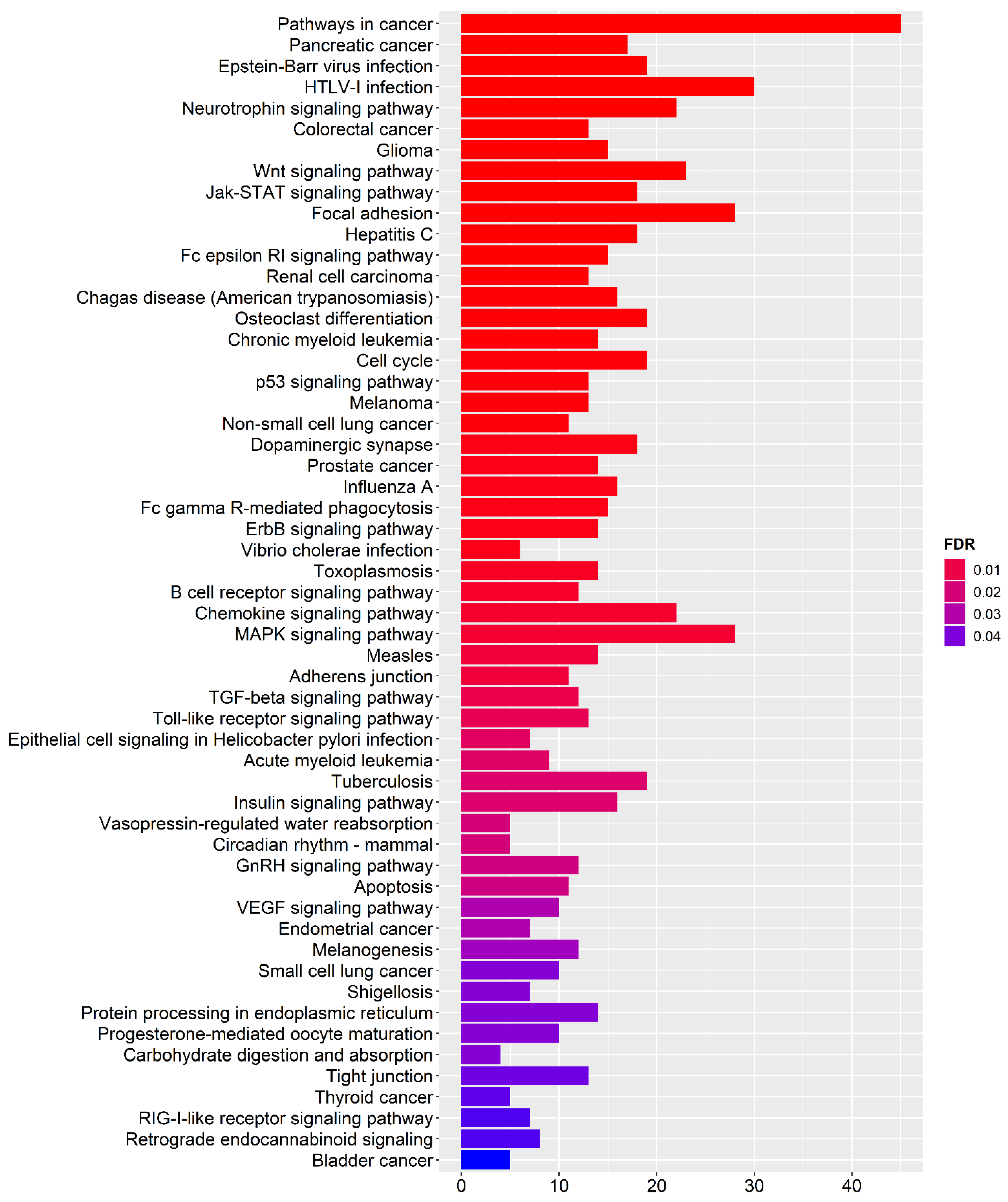
2.3. Functional Enrichment Analysis
3. Results
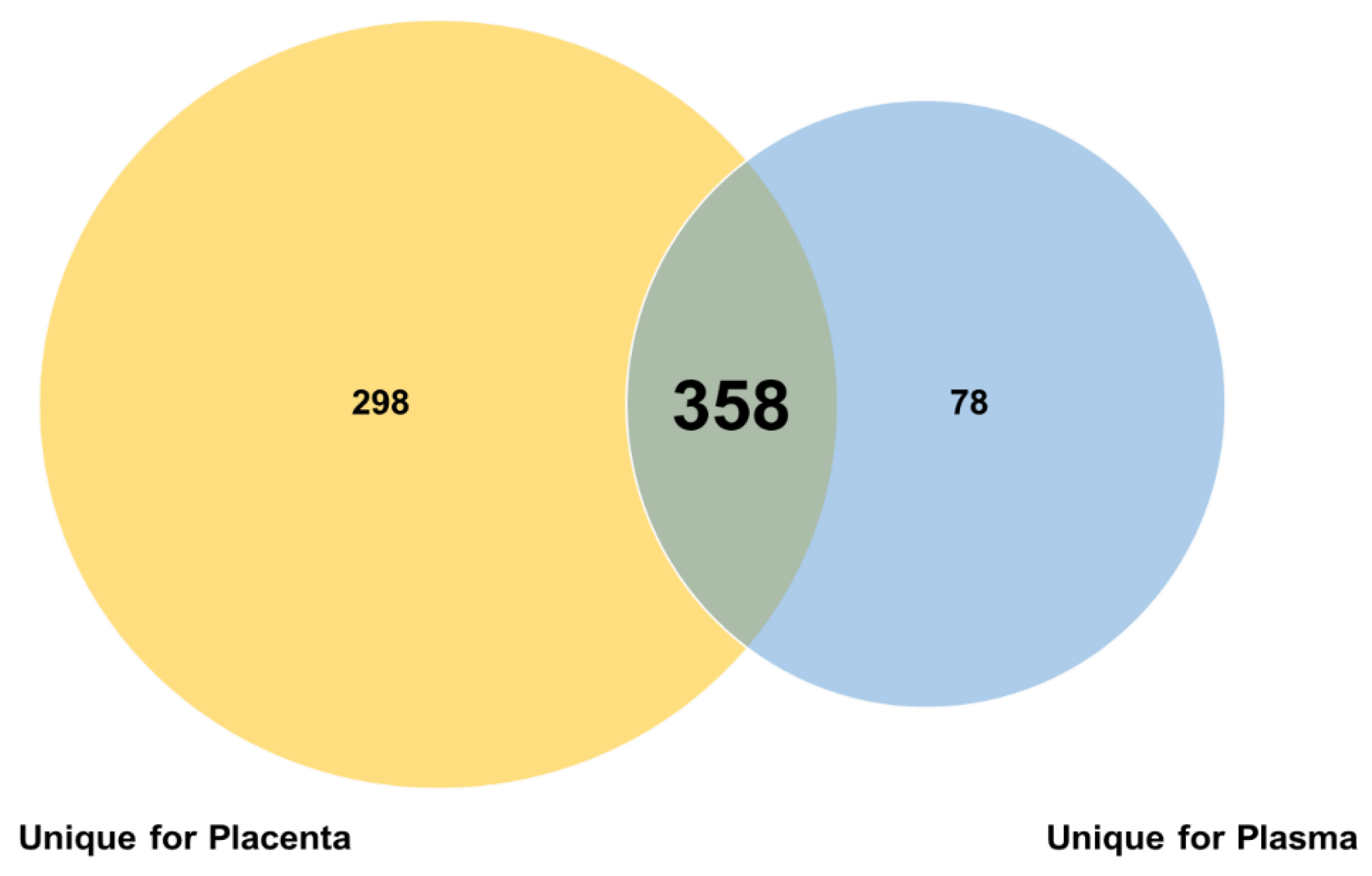
3.1. The Plasma and Placental miRNoma Landscape in Preeclampsia
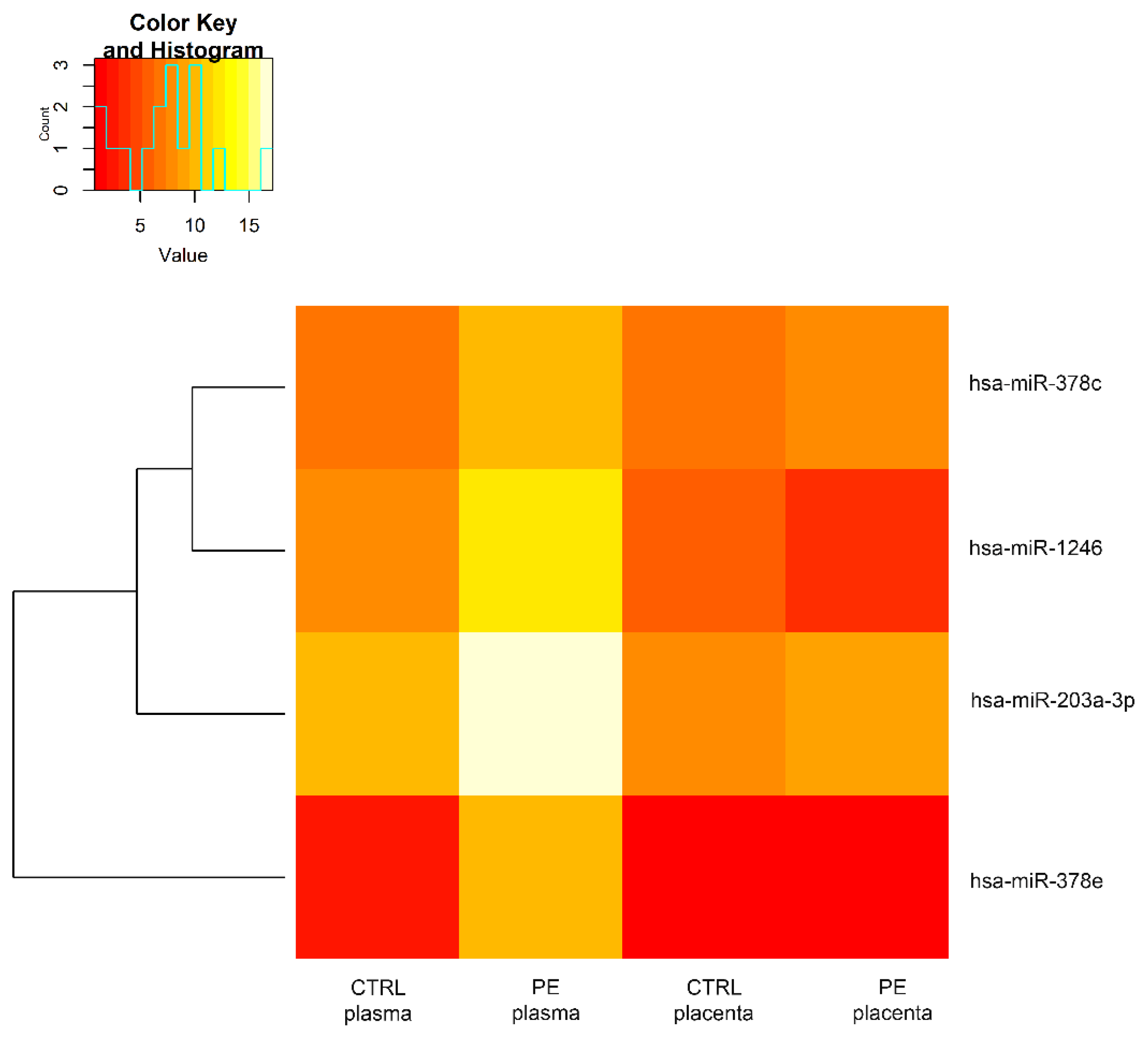
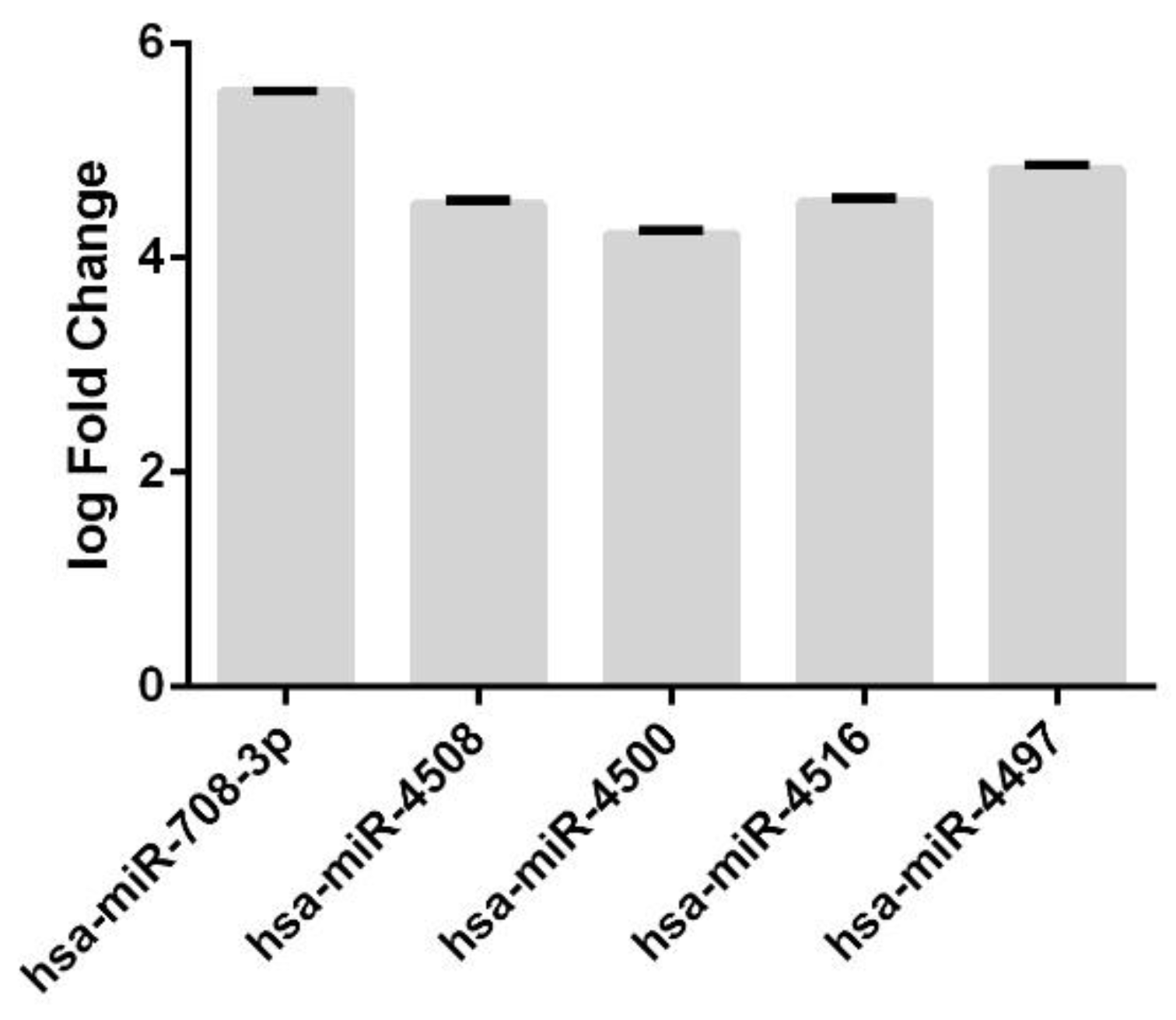
3.2. Comparison of miRNAs Expression Profiles in a Woman with PE
3.3. Annotation of miRNAs Involved in the Pathogenesis of Preeclampsia and Their Targets
3.4. Analysis of the Predicted miRNA Targets
3.5. Functional Enrichment Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Budde, U.; Schneppenheim, R. Von Willebrand factors and von Willebrand disease. Rev. Clin. Exp. Hematol. 2001, 5, 335–368. [Google Scholar] [CrossRef] [PubMed]
- Horan, J.T.; Francis, C.W. Fibrin degradation products, fibrin monomer and soluble fibrin in disseminated intravascular coagulation. Semin. Thromb. Hemost. 2001, 27, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Szpera-Gozdziewicz, A.; Breborowicz, G.H. Endothelial dysfunction in the pathogenesis of pre-eclampsia. Front. Biosci.-Landmark 2014, 19, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Hodgins, S. Pre-eclampsia as Underlying Cause for Perinatal Deaths: Time for Action. Glob. Health Sci. Pract. 2015, 3, 525–527. [Google Scholar] [CrossRef]
- Wagner, L.K. Diagnosis and management of preeclampsia. Am. Fam. Physician 2004, 70, 2317–2324. [Google Scholar] [PubMed]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Petrillo, F.; Iervolino, A.; Angrisano, T.; Jelen, S.; Costanzo, V.; D’Acierno, M.; Cheng, L.; Wu, Q.; Guerriero, I.; Mazzarella, M.C.; et al. Dysregulation of Principal Cell miRNAs Facilitates Epigenetic Regulation of AQP2 and Results in Nephrogenic Diabetes Insipidus. J. Am. Soc. Nephrol. 2021, 32, 1339–1354. [Google Scholar] [CrossRef]
- Skalis, G.; Katsi, V.; Miliou, A.; Georgiopoulos, G.; Papazachou, O.; Vamvakou, G.; Nihoyannopoulos, P.; Tousoulis, D.; Makris, T. MicroRNAs in Preeclampsia. Microrna 2019, 8, 28–35. [Google Scholar]
- Mavreli, D.; Lykoudi, A.; Lambrou, G.; Papaioannou, G.; Vrachnis, N.; Kalantaridou, S.; Papantoniou, N.; Koliale, A. Deep Sequencing Identified Dysregulated Circulating MicroRNAs in Late Onset Preeclampsia. In Vivo 2020, 34, 2317–2324. [Google Scholar] [CrossRef]
- Vashukova, E.S.; Glotov, A.S.; Fedotov, P.V.; Efimova, O.A.; Pakin, V.S.; Mozgovaya, E.V.; Pendina, A.A.; Tikhonov, A.V.; Koltsova, A.S.; Baranov, V.S. Placental microRNA expression in pregnancies complicated by superimposed pre-eclampsia on chronic hypertension. Mol. Med. Rep. 2016, 14, 22–32. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, X. Prediction of functional microRNA targets by integrative modeling of microRNA binding and target expression data. Genome Biol. 2019, 22, 18. [Google Scholar] [CrossRef]
- Miranda, K.C.; Huynh, T.; Tay, Y.; Ang, Y.; Tam, W.; Thomson, A.M.; Lim, B.; Rigoutsos, I. A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell 2006, 126, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- McGeary, S.E.; Lin, K.S.; Shi, C.Y.; Pham, T.M.; Bisaria, N.; Kelley, G.M.; Bartel, D.P. The biochemical basis of microRNA targeting efficacy. Science 2019, 20, 1741. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Bell, G.W.; Nam, J.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, 05005. [Google Scholar] [CrossRef]
- García, D.M.; Baek, D.; Shin, C.; Bell, G.W.; Grimson, A.; Bartel, D.P. Weak Seed-Pairing Stability and High Target-Site Abundance Decrease the Proficiency of lsy-6 and Other miRNAs. Nat. Struct. Mol. Biol. 2011, 8, 1139–1146. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most Mammalian mRNAs Are Conserved Targets of MicroRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Grimson, A.; Farh, K.K.; Johnsto, W.K.; Garrett-Engele, P.; Lim, L.P.; Bartel, D.P. MicroRNA Targeting Specificity in Mammals: Determinants beyond Seed Pairing. Mol. Cell 2007, 27, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved Seed Pairing, Often Flanked by Adenosines, Indicates that Thousands of Human Genes are MicroRNA Targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Zhou, G.; Soufan, O.; Xia, J. miRNet 2.0—Network-based visual analytics for miRNA functional analysis and systems biology. Nucl. Acids Res. 2020, 48, W244–W251. [Google Scholar] [CrossRef]
- Muralimanoharan, S.; Kwak, Y.T.; Mendelson, C.R. Redox-Sensitive Transcription Factor NRF2 Enhances Trophoblast Differentiation via Induction of miR-1246 and Aromatase. Endocrinology 2018, 159, 2022–2033. [Google Scholar] [CrossRef]
- de Los Santos-Jiménez, J.; Campos-Sandoval, J.A.; Márquez-Torres, C.; Urbano-Polo, N.; Brøndegaard, D.; Martín-Rufián, M.; Lobo, C.; Peñalver, A.; Gómez-García, M.C.; Martín-Campos, J.; et al. Glutaminase isoforms expression switches microRNA levels and oxidative status in glioblastoma cells. J. Biomed. Sci. 2021, 28, 14. [Google Scholar] [CrossRef]
- Ma, H.Y.; Cu, W.; Sun, Y.H.; Chen, X. MiRNA-203a-3p inhibits inflammatory response in preeclampsia through regulating IL24. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5223–5230. [Google Scholar]
- Chen, Z.; Zhang, W.; Wu, M.; Huang, H.; Zou, L.; Luo, Q. Pathogenic mechanisms of preeclampsia with severe features implied by the plasma exosomal mirna profile. Bioengineered 2021, 12, 9140–9149. [Google Scholar] [CrossRef]
- Menon, R.; Debnath, C.; Lai, A.; Guanzon, D.; Bhatnagar, S.; Kshetrapal, P.K.; Sheller-Miller, S.; Salomon, C. ù Circulating Exosomal miRNA Profile During Term and Preterm Birth Pregnancies: A Longitudinal Study. Endocrinology 2019, 160, 249–275. [Google Scholar] [CrossRef]
- Gu, Y.; Sun, J.; Groome, L.J.; Wang, Y. Differential miRNA expression profiles between the first and third trimester human placentas. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E836–E843. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.X.; Xia, S.H.; Wu, Y.H.; Zhang, J.H.; Wang, L.Y.; Zhu, W.P. Comprehensive analysis of the differential expression profile of microRNAs in missed abortion. Kaohsiung J. Med. Sci. 2020, 36, 114–121. [Google Scholar] [CrossRef]
- Chen, P.; Li, T.; Guo, Y.; Jia, L.; Wang, Y.; Fang, C. Construction of Circulating MicroRNAs-Based Non-invasive Prediction Models of Recurrent Implantation Failure by Network Analysis. Front. Genet. 2021, 12, 712150. [Google Scholar] [CrossRef] [PubMed]
- Kasiappan, R.; Rajarajan, D. Role of MicroRNA Regulation in Obesity-Associated Breast Cancer: Nutritional Perspectives. Adv. Nutr. 2017, 8, 868–888. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, R.; Zhang, J.; Meng, C.; Zhang, J.; Song, X.; Lv, C. MicroRNA-708-3p as a potential therapeutic target via the ADAM17-GATA/STAT3 axis in idiopathic pulmonary fibrosis. Exp. Mol. Med. 2018, 50, e465. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Kawasaki, M.; Hirai, T.; Yoshida, Y.; Tsushima, H.; Fujishiro, M.; Ikeda, K.; Morimoto, S.; Takamori, K.; Sekigawa, I. MicroRNA-766-3p Contributes to Anti-Inflammatory Responses through the Indirect Inhibition of NF-κB Signaling. Int. J. Mol. Sci. 2019, 20, 809. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cheng, Y.; Lin, L.; Liu, Z.; Du, S.; Ma, L.; Li, J.; Peng, Z.; Yan, J. Global Analysis of miRNA Signature Differentially Expressed in Insulin-resistant Human Hepatocellular Carcinoma Cell Line. Int. J. Med. Sci. 2020, 17, 664–677. [Google Scholar] [CrossRef]
- Yu, F.Y.; Tu, Y.; Deng, Y.; Guo, C.; Ning, J.; Zhu, Y.; Lv, X.; Ye, H. MiR-4500 is epigenetically downregulated in colorectal cancer and functions as a novel tumor suppressor by regulating HMGA2. Cancer Biol. Ther. 2016, 17, 1149–1157. [Google Scholar] [CrossRef]
- Li, S.; Mai, H.; Zhu, Y.; Li, G.; Sun, J.; Li, G.; Liang, B.; Chen, S. MicroRNA-4500 Inhibits Migration, Invasion, and Angiogenesis of Breast Cancer Cells via RRM2-Dependent MAPK Signaling Pathway. Mol. Ther. Nucleic Acids 2020, 21, 278–289. [Google Scholar] [CrossRef]
- Huang, R.; Yu, T.; Li, Y.; Hu, J. Upregulated has-miR-4516 as a potential biomarker for early diagnosis of dust-induced pulmonary fibrosis in patients with pneumoconiosis. Toxicol. Res. 2018, 7, 415–422. [Google Scholar] [CrossRef]
- Chowdhari, S.; Saini, N. hsa-miR-4516 mediated downregulation of STAT3/CDK6/UBE2N plays a role in PUVA induced apoptosis in keratinocytes. J. Cell Physiol. 2014, 229, 1630–1638. [Google Scholar] [CrossRef]
- Chowdhari, S.; Sardana, K.; Saini, N. miR-4516, a microRNA downregulated in psoriasis inhibits keratinocyte motility by targeting fibronectin/integrin α9 signaling. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 3142–3152. [Google Scholar] [CrossRef]
- Umair, Z.; Baek, M.O.; Song, J.; An, S.; Chon, S.J.; Yoon, M.S. MicroRNA-4516 in Urinary Exosomes as a Biomarker of Premature Ovarian Insufficiency. Cells 2022, 11, 2797. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ma, Q.; Wang, Y.; Tang, Z. Clinical application of exosomes and circulating microRNAs in the diagnosis of pregnancy complications and foetal abnormalities. J. Transl. Med. 2020, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, L.; Tang, S. MicroRNA-4497 functions as a tumor suppressor in laryngeal squamous cell carcinoma via negatively modulation the GBX2. Auris Nasus Larynx 2019, 46, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.H.; Kaur, S.; Nielsen, L.B.; Størling, J.; Yarani, R.; Roursgaard, M.; Mathiesen, E.R.; Damm, P.; Svare, J.; Mortensen, H.B.; et al. Breast Milk-Derived Extracellular Vesicles Enriched in Exosomes from Mothers with Type 1 Diabetes Contain Aberrant Levels of microRNAs. Front. Immunol. 2019, 10, 2543. [Google Scholar] [CrossRef]
- Wen, H.; Chen, L.; He, J.; Lin, J. MicroRNA expression profiles and networks in placentas complicated with selective intrauterine growth restriction. Mol. Med. Rep. 2017, 16, 6650–6673. [Google Scholar] [CrossRef]
- Gathiram, P.; Moodley, J. Pre-eclampsia: Its pathogenesis and pathophysiolgy. Cardiovasc. J. Afr. 2016, 27, 71–78. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, Y.; Lin, Y.; Lin, H.; Li, H.; Su, X.; Fang, Z.; Wang, J.; Wei, Q.; Teng, J.; et al. Potential miRNA biomarkers for the diagnosis and prognosis of esophageal cancer detected by a novel absolute quantitative RT-qPCR method. Sci. Rep. 2020, 10, 20065. [Google Scholar] [CrossRef]
- Buhagiar, A.; Seria, E.; Borg, M.; Borg, J.; Ayers, D. Overview of microRNAs as liquid biopsy biomarkers for colorectal cancer sub-type profiling and chemoresistance. Cancer Drug Resist 2021, 4, 934–945. [Google Scholar] [CrossRef]
- Ragozzino, E.; Brancaccio, M.; Di Costanzo, A.; Scalabrì, F.; Andolfi, G.; Wanderlingh, L.G.; Patriarca, E.J.; Minchiotti, G.; Altamura, S.; Summa, V.; et al. 6-Bromoindirubin-3′-oxime intercepts GSK3 signaling to promote and enhance skeletal muscle differentiation affecting miR-206 expression in mice. Sci. Rep. 2019, 9, 18091. [Google Scholar] [CrossRef]
- Tribolet, L.; Kerr, E.; Cowled, C.; Bean, A.G.D.; Stewart, C.R.; Dearnley, M.; Farr, R.J. MicroRNA Biomarkers for Infectious Diseases: From Basic Research to Biosensing. Front. Microbiol. 2020, 11, 1197. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, M.; Natale, F.; Falco, G.; Angrisano, T. Cell-Free DNA Methylation: The New Frontiers of Pancreatic Cancer Biomarkers’ Discovery. Genes 2019, 11, 14. [Google Scholar] [CrossRef]
- Brancaccio, M.; Mennitti, C.; Calvanese, M.; Gentile, A.; Musto, R.; Gaudiello, G.; Scamardella, G.; Terracciano, D.; Frisso, G.; Pero, R.; et al. Diagnostic and Therapeutic Potential for HNP-1, HBD-1 and HBD-4 in Pregnant Women with COVID-19. Int. J. Mol. Sci. 2022, 23, 3450. [Google Scholar] [CrossRef]
- Pero, R.; Brancaccio, M.; Mennitti, C.; Gentile, L.; Franco, A.; Laneri, S.; De Biasi, M.G.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; et al. HNP-1 and HBD-1 as Biomarkers for the Immune Systems of Elite Basketball Athletes. Antibiotics 2020, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.; Storm, C.S.; Makarious, M.B.; Bandres-Ciga, S. Genetic and Transcriptomic Biomarkers in Neurodegenerative Diseases: Current Situation and the Road Ahead. Cells 2021, 10, 1030. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Xie, H.; Zhang, Y. Predicting Diagnostic Gene Biomarkers Associated with Immune Infiltration in Patients with Acute Myocardial Infarction. Front. Cardiovasc. Med. 2020, 7, 586871. [Google Scholar] [CrossRef]
- Ruggieri, V.; Russi, S.; Zoppoli, P.; La Rocca, F.; Angrisano, T.; Falco, G.; Calice, G.; Laurino, S. The Role of MicroRNAs in the Regulation of Gastric Cancer Stem Cells: A Meta-Analysis of the Current Status. J. Clin. Med. 2019, 8, 639. [Google Scholar] [CrossRef]
- Chang, G.; Mouillet, J.F.; Mishima, T.; Chu, T.; Sadovsky, E.; Coyne, C.B.; Parks, W.T.; Surti, U.; Sadovsky, Y. Expression and trafficking of placental microRNAs at the feto-maternal interface. FASEB J. 2017, 31, 2760–2770. [Google Scholar] [CrossRef]
- Xu, P.; Ma, Y.; Wu, H.; Wang, Y.L. Placenta-Derived MicroRNAs in the Pathophysiology of Human Pregnancy. Front. Cell Dev. Biol. 2021, 9, 646326. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Vickers, M.H.; Taylor, R.S.; Jones, B.; Fraser, M.; McCowan, L.M.E.; Baker, P.N.; Perry, J.K. Maternal serum IGF-1, IGFBP-1 and 3, and placental growth hormone at 20weeks’ gestation in pregnancies complicated by preeclampsia. Pregnancy Hypertens 2017, 10, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Ingec, M.; Gursoy, H.G.; Yildiz, L.; Kumtepe, Y.; Kadanali, S. Serum levels of insulin, IGF-1, and IGFBP-1 in pre-eclampsia and eclampsia. Int. J. Gynaecol. Obstet. 2004, 84, 214–219. [Google Scholar] [CrossRef]
- Vatten, L.J.; Nilsen, T.I.; Juul, A.; Jeansson, S.; Jenum, P.A.; Eskild, A. Changes in circulating level of IGF-I and IGF-binding protein-1 from the first to second trimester as predictors of preeclampsia. Eur. J. Endocrinol. 2008, 158, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Halhali, A.; Tovar, A.R.; Torres, N.; Bourges, H.; Garabedian, M.; Larrea, F. Preeclampsia is associated with low circulating levels of insulin-like growth factor I and 1,25-dihydroxyvitamin D in maternal and umbilical cord compartments. J. Clin. Endocrinol. Metab. 2000, 85, 1828–1833. [Google Scholar] [PubMed]
- Wang, Y.; Cheng, K.; Zhou, W.; Liu, H.; Yang, T.; Hou, P.; Li, X. miR-141-5p regulate ATF2 via effecting MAPK1/ERK2 signaling to promote preeclampsia. Biomed. Pharmacother. 2019, 115, 108953. [Google Scholar] [CrossRef] [PubMed]
- Marwa, B.A.; Raguema, N.; Zitouni, H.; Feten, H.B.; Olfa, K.; Elfeleh, R.; Almawi, W.; Mahjoub, T. FGF1 and FGF2 mutations in preeclampsia and related features. Placenta 2016, 43, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.Y.; Wang, X.M.; Xie, C.; Zhao, B.; Niu, Z.; Fan, L.; Hivert, M.F.; Chen, W.Q. Placental surface area mediates the association between FGFR2 methylation in placenta and full-term low birth weight in girls. Clin. Epigenet. 2018, 22, 39. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.R.; Weber, T. Correlations between PRL and chloride, sodium, potassium and calcium in amniotic fluid. Acta Obstet. Gynecol. Scand. 1985, 64, 227–229. [Google Scholar] [CrossRef]
- Lyall, F.; Simpson, H.; Bulmer, J.N.; Barber, A.; Robson, S.C. Transforming growth factor-β expression in human placenta and placental bed in third trimester normal pregnancy, preeclampsia, and fetal growth restriction. Am. J. Pathol. 2001, 159, 1827–1838. [Google Scholar] [CrossRef]
- Maynard, S.E.; Karumanchi, S.A. Angiogenic factors and preeclampsia. Semin. Nephrol. 2011, 31, 33–46. [Google Scholar] [CrossRef]
- Cirkovic, A.; Stanisavljevic, D.; Milin-Lazovic, J.; Rajovic, N.; Pavlovic, V.; Milicevic, O.; Savic, M.; Kostic Peric, J.; Aleksic, N.; Milic, N.; et al. Preeclamptic Women Have Disrupted Placental microRNA Expression at the Time of Preeclampsia Diagnosis: Meta-Analysis. Front. Bioeng. Biotechnol. 2021, 9, 782845. [Google Scholar] [CrossRef]

| miRNA | Target | References |
|---|---|---|
| hsa-mir-1246 |
| [24,25] |
| hsa-mir-203a-3p |
| [26,27,28] |
| hsa-mir-378c |
| [29,30] |
| hsa-mir-378e |
| [31] |
| miRNA | Target | References |
|---|---|---|
| hsa-miR-708-3p |
| [32,33] |
| hsa-miR-4508 |
| [34,35] |
| hsa-miR-4500 |
| [28,36,37] |
| hsa-miR-4516 |
| [38,39,40,41,42] |
| hsa-miR-4497 |
| [43,44,45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brancaccio, M.; Giachino, C.; Iazzetta, A.M.; Cordone, A.; De Marino, E.; Affinito, O.; Vivo, M.; Calabrò, V.; Pollice, A.; Angrisano, T. Integrated Bioinformatics Analysis Reveals Novel miRNA as Biomarkers Associated with Preeclampsia. Genes 2022, 13, 1781. https://doi.org/10.3390/genes13101781
Brancaccio M, Giachino C, Iazzetta AM, Cordone A, De Marino E, Affinito O, Vivo M, Calabrò V, Pollice A, Angrisano T. Integrated Bioinformatics Analysis Reveals Novel miRNA as Biomarkers Associated with Preeclampsia. Genes. 2022; 13(10):1781. https://doi.org/10.3390/genes13101781
Chicago/Turabian StyleBrancaccio, Mariarita, Caterina Giachino, Assunta Maria Iazzetta, Antonio Cordone, Elena De Marino, Ornella Affinito, Maria Vivo, Viola Calabrò, Alessandra Pollice, and Tiziana Angrisano. 2022. "Integrated Bioinformatics Analysis Reveals Novel miRNA as Biomarkers Associated with Preeclampsia" Genes 13, no. 10: 1781. https://doi.org/10.3390/genes13101781
APA StyleBrancaccio, M., Giachino, C., Iazzetta, A. M., Cordone, A., De Marino, E., Affinito, O., Vivo, M., Calabrò, V., Pollice, A., & Angrisano, T. (2022). Integrated Bioinformatics Analysis Reveals Novel miRNA as Biomarkers Associated with Preeclampsia. Genes, 13(10), 1781. https://doi.org/10.3390/genes13101781