Simple summary
Having a new and more precise definition of BrS, based on its cardiomyopathic component, may be crucial to meliorate the current clinical management of patients, at (i) diagnostic, (ii) prognostic, and (iii) therapeutic levels: (i) diagnostic, since specific tests may be added to the current standards of BrS to identify associated arrhythmogenic substrates; (ii) prognostic, since multiple factors from an extended diagnostic workup may be associated with an increased arrhythmic risk (as already demonstrated in many cardiomyopathies), subsequently improving the patient selection for a primary prevention ICD implant; (iii) at therapeutic levels, since the identification of unexpected substrates may turn into a significant change in the current treatment practice.
Abstract
Brugada syndrome (BrS) is an inherited autosomal dominant genetic disorder responsible for sudden cardiac death from malignant ventricular arrhythmia. The term “channelopathy” is nowadays used to classify BrS as a purely electrical disease, mainly occurring secondarily to loss-of-function mutations in the α subunit of the cardiac sodium channel protein Nav1.5. In this setting, arrhythmic manifestations of the disease have been reported in the absence of any apparent structural heart disease or cardiomyopathy. Over the last few years, however, a consistent amount of evidence has grown in support of myocardial structural and functional abnormalities in patients with BrS. In detail, abnormal ventricular dimensions, either systolic or diastolic dysfunctions, regional wall motion abnormalities, myocardial fibrosis, and active inflammatory foci have been frequently described, pointing to alternative mechanisms of arrhythmogenesis which challenge the definition of channelopathy. The present review aims to depict the status of the art of concealed arrhythmogenic substrates in BrS, often resulting from an advanced and multimodal diagnostic workup, to foster future preclinical and clinical research in support of the cardiomyopathic nature of the disease.
1. Brugada Syndrome: Definition and Current Classification
Brugada syndrome (BrS) is an inherited autosomal dominant genetic disorder, first described in 1992 [1], which combines typical electrocardiographic findings with an increased risk of malignant ventricular arrhythmias. Its prevalence is estimated from 1 in 5000 to 1 in 2000 cases, with a strong male predominance [2].
Current international guidelines [3,4] agree in defining BrS in presence of a type 1 Brugada electrocardiogram (ECG) pattern, i.e., a persistent ST-segment elevation ≥2 mm followed by a negative T-wave in ≥1 of the right precordial leads V1 to V2, occurring either spontaneously or following a sodium channel blocker test (Figure 1). However, according to the Shanghai score system of 2016 [5], if the type 1 pattern is unmasked during a sodium channel blocker test, then clinical history, family history, and a genetic test need to be evaluated to meet the diagnostic criteria.
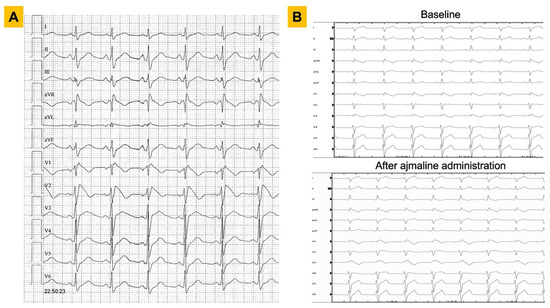
Figure 1.
ECG findings in patients with Brugada syndrome. Representative examples of diagnostic ECGs for Brugada syndrome are shown. Panel (A). A 25-year-old male with a spontaneous type 1 pattern on 12-lead ECG. Panel (B). A 36-year-old male with right bundle branch block pattern on baseline ECG (upper panel) and subsequent unmasking of a type 1 Brugada ECG pattern after administration of ajmaline at 1 mg/kg (lower panel). ECG = electrocardiogram.
Despite recent advances, the pathogenetic mechanisms of the disease remain not fully understood. BrS had been initially proposed to be a primary disease functionally involving impairments in the electric potential transmission. BrS was defined as a channelopathy, due to the association of the disease genotype with loss-of-function mutations in genes encoding subunits of the cardiac ion channels [6]. A consistent amount of attention was invested in mutations in the SCN5A gene, encoding the α subunit of the cardiac sodium channel protein Nav1.5, responsible for the initial upstroke of the action potential [7]. This had been thought to happen in the absence of ischemia, electrolyte disturbance, or structural heart disease, as supported by silent imaging and post-mortem pathology [8].
Nevertheless, several studies suggested that subtle structural or microscopic abnormalities may actually take place in BrS, including dilation of the right ventricular outflow tract (RVOT), localized inflammation, and fibrosis [9,10]. These observations lead to a rethink of the context of the disease, referring it to apparently normal hearts instead of structurally normal hearts, paving the way for a controversial overlap between BrS and cardiomyopathies [11,12]. Indeed, case reports and case series exploring the presence of concealed substrates in BrS are still preliminary.
The disclosure of concealed substrate abnormalities in BrS may be the answer to the perception of BrS as more than a pure channelopathy, potentially enabling an improvement in the current diagnostic, prognostic, and therapeutic workflow. The present review aims at exploring this concept while providing an updated description of cardiomyopathic changes associated with the disease, from pathophysiological, diagnostic, and prognostic viewpoints.
2. Inheritance and Genetic Bases of Brugada Syndrome: The State of The Art
At the time that the first genetic alteration in the SCN5A gene underlying BrS was reported in 1998 [6], highlighting an autosomal dominant inheritance, two other BrS genetic hallmarks had already been recognized: incomplete penetrance and variable expressivity. To date, more than 150 loss-of-function alterations have been described in the SCN5A gene [13], leading to a decrease in the I-Na+ current and a consequent shortening of the depolarization phase of the action potential [7].
Some studies suggested a role for SCN5A alterations in the prediction of patients’ arrhythmic risk. Indeed, carriers of a deleterious variant in the SCN5A gene show a spontaneous BrS ECG [14] and a more aggressive arrhythmic phenotype; however, this feature needs to be further investigated [15].
Overall, about 20% of cases are caused by rare coding variants in the SCN5A gene [16,17], which still remains the only gene with definitive evidence of an association with BrS and is clinically actionable [18,19,20]. Currently, more than 20 candidate BrS genes have been proposed [6,17,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43], but their causality in BrS pathogenesis is widely debated [15]. The current knowledge about genetics in BrS is summarized in Table 1. To date, however, most patients do not carry causative mutations on the panel of BrS genes, highlighting the need for a better characterization of the molecular basis of this disorder.

Table 1.
Genetics of Brugada syndrome.
The limited number of BrS cases with a clear monogenic inheritance has pointed toward new hypotheses of a more complex genetic architecture of the disease, involving multigenic inheritance and a polygenic risk score that can influence penetrance and risk stratification [44]. Recently, studies exploiting the genome-wide association study (GWAS) approach suggested that common genetic variations can modulate the phenotypic expression of BrS, providing evidence for a model of inheritance more complex than previously thought [17,43].
Indeed, polygenic risk score analyses based on several susceptibility variants demonstrate a cumulative contribution of common risk alleles among different BrS patients, as well as genetic associations with cardiac electrical traits in the general population, thus supporting the concept of “genomic arrhythmia” [43].
Moreover, the recent findings also highlight that genes encoding structural proteins or cardiac transcription factors are associated with the BrS phenotype, thus strengthening the hypothesis of overlap with structural cardiomyopathies [43,45].
Clinical BrS manifestations are more common in adults, and despite autosomal inheritance, they are eightfold more frequent in males than in females [4]. To date, gender differences in BrS phenotype manifestation are widely recognized: female patients less frequently display a type 1 Brugada ECG pattern and exhibit lower inducibility rates. But the underlying causality remains unclear and needs to be further investigated [46]. Recently, a higher prevalence of pathogenic variants in SCN5A has been published in symptomatic female patients with BrS compared with male patients, and an even higher prevalence in females with BrS with arrhythmic events [47] suggesting that pathogenic variants in SCN5A in women may be a risk factor, perhaps by overcoming a “protective” environment [1].
Overall, although different genetic approaches have been adopted, the characterization of BrS molecular bases remains limited. The identification of new candidate genes and risk factors can lead to a better definition of BrS pathogenic mechanisms, allowing an increase in diagnostic sensitivity and the improvement of family and clinical management and risk stratification.
3. Imaging Abnormalities
Cardiomyopathies are uniformly characterized by the identification of either structural or functional myocardial abnormalities via imaging techniques. Although most patients with BrS display no remarkable alterations on a transthoracic echocardiogram (TTE) or via cardiac magnetic resonance (CMR) imaging [1], some ECG findings have been suggested as possible indicators for underlying anatomical arrhythmogenic substrates [48] (Table 2). For instance, a correlation between patients with a spontaneous type 1 ECG pattern and a lower right ventricular ejection fraction (RVEF) has been described [49], as well as focal mechanical abnormalities in the RVOT [50].

Table 2.
Concealed substrates in Brugada syndrome.
Although the classical echocardiography parameters have a limited yield in BrS, new techniques including strain and speckle tracking [65,66] have led to a more accurate evaluation of the systolic and diastolic functions in BrS. In addition, the TEI index, which evaluates both systolic and diastolic time intervals to assess global cardiac dysfunction, has been used to differentiate BrS and non-BrS patients through a sodium channel blocking test: only the former ones showed prolonged PQ intervals and a decreased biventricular function at the TEI index [51,52]. Evaluation of the RV longitudinal strain with 2D speckle tracking quantifies regional myocardial deformation, with high spatial resolution speckle tracking not being affected by angle dependency or translation or tethering from the surrounding tissue [67]. The RV longitudinal strain has shown a significant reduction in BrS patients [51]. Moreover, speckle tracking echocardiography may help in differentiating BrS from the right bundle branch block (RBBB), as it was shown to track slower conduction through free wall segments which are found in RBBB but not in BrS [66].
CMR is an accurate and reproducible tool for estimating both left ventricular (LV) and RV volumes and is now considered the gold standard technique for cardiomyopathies [3,4]. Although controversial [68], anatomical involvement in BrS has been demonstrated in the literature. In detail, greater RV volumes and reduced RV function have been described [69], especially at RVOT [55]. In addition, some BrS patients display a midwall stria of late gadolinium enhancement within the LV consistent with an underlying cardiomyopathic process [69]. These findings lend further support to the presence of subtle structural abnormalities in BrS, with a possible evolution toward a cardiomyopathic phenotype over time [69]. Examples are shown in Figure 2. Additional morphofunctional abnormalities were recently reported: for instance, a direct correlation was shown between the LV/RV dilation and SCN5A mutation, with wider involvement of the RV than the LV [54] as observed in the classic arrhythmogenic right ventricular cardiomyopathy (ARVC). A recent study [70] allowed a more accurate localization of the aforementioned abnormalities, which appear to be more extended than RVOT as the ajmaline test had them localized both in the upper anterior wall but also in the antero-inferior wall, leading to an increased arrhythmogenic risk. In the same study, a significant correlation was observed between the RV dilation/dysfunction and SCN5A mutations [70]. In particular, the regional RV contractility abnormalities were found to be dynamic and functionally related to the expansion of the electrical substrate after ajmaline [70], accounting for the limited diagnostic value of baseline CMR.
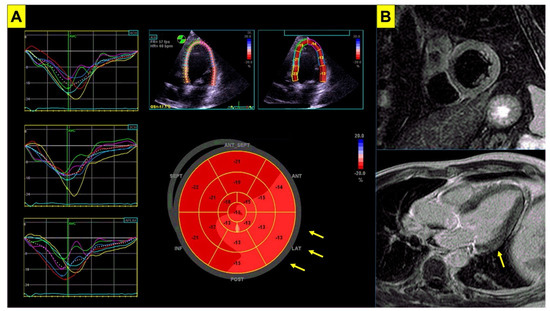
Figure 2.
Imaging abnormalities in Brugada syndrome. Subtle imaging abnormalities associated with BrS are shown. Panel (A) echocardiogram of a patient with genetically proven BrS. Despite normal left ventricular systolic function (LVEF = 62%), impairment in global longitudinal strain is shown (GLS = −16%, nv < −20%) mainly involving the lateral wall (arrows). Panel (B) cardiac magnetic resonance in the same patient shows slight hyperintensity in T2-weighted short tau inversion recovery sequences (STIR, upper panel) involving the inferolateral basal segment of the left ventricular wall, and focal late gadolinium enhancement (LGE, lower panel) involving the basal segment of the lateral wall (arrow). BrS = Brugada syndrome; LVEF = left ventricular ejection fraction.
4. Histopathological Findings
From the first characterizations of the ECG pattern, structural alterations, such as fibrosis, fibrolipomatosis, and RV cardiomyopathic changes, were described in patients with apparent idiopathic ventricular fibrillation (VF) [53]. As recently described for desmoplakin cardiomyopathy [71], cardiac inflammation might represent a “hot-phase” in BrS and lead to the natural progression of the disease [72,73].
Lymphocytic myocarditis (Figure 3) with inflammatory infiltrates and focal necrosis, with or without microaneurysms, was found in endomyocardial biopsies from the RV, and the LV as well, in patients with symptomatic BrS [56]. Among BrS patients, those who were carriers of SCN5A mutations displayed more cardiomyopathic changes. Remarkably, many patients were positive for intracardiac viral genomes. The authors suggest that the classic BrS ECG pattern is not a marker of a specific syndrome, but rather an electrical expression of RV structural abnormalities which may be the outcome of genetic, infective, and inflammatory conditions. In another study, RVOT endomyocardial biopsy, guided by a three-dimensional voltage map, showed that myocardial inflammation at histology correlated with a higher prevalence of abnormal bipolar map and greater bipolar low-voltage area extension in patients with BrS [10]. Notably, parvovirus B19 was associated with myocarditis-induced VF in many patients with BrS [57,58,59]. On the other hand, critical SCN5A variants can be found in patients with arrhythmic myocarditis, even in the absence of the BrS ECG pattern [74]. These findings support the role of myocardial inflammation as a possible arrhythmogenic substrate [75,76].
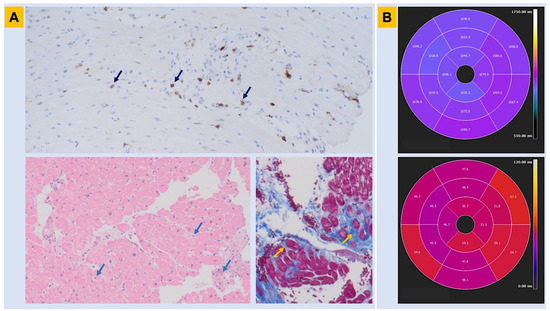
Figure 3.
Histopathologic findings in Brugada syndrome. Myocardial tissue abnormalities associated with BrS are shown. Panel (A). Endomyocardial biopsy obtained from the right ventricle in a patient with genetically proven BrS shows lymphocytic inflammatory infiltrate with a CD3+ T-cell count consistent with myocarditis (immunohistochemistry assay—upper panel; hematoxylin-eosin assay—lower left panel; arrows). In the same patient, trichrome assay identifies areas of interstitial and replacement-type fibrosis, in blue color (lower right panel, arrows). Panel (B). Cardiac magnetic resonance obtained in the same patient before the automated cardioverter defibrillator implant shows abnormalities in parametric mapping involving the inferolateral left ventricular wall in both T1 and T2 sequences (n.v. for parametric mapping: T1 < 1045 ms; T2 < 50 ms). BrS = Brugada syndrome.
However, other studies failed to confirm definite myocarditis in biopsies from the RV, by showing only moderate myocardial hypertrophy, moderate fibrosis, and fatty replacement of the myocardium, with hypokinetic RV and RV trabeculae [77]. A genetically positive BrS patient who underwent a heart transplantation for recurrent VF episodes showed RV hypertrophy and fibrosis with epicardial fatty infiltration, which were deemed as the origins of ECG alterations. Specifically, the RVOT endocardium showed activation slowing due to interstitial fibrosis and was the origin of VF, without a transmural repolarization gradient, and with normal conduction in the LV [60]. Another patient with compound heterozygosity for a nonsense and a missense mutation in SCN5A revealed changes consistent with a dilated cardiomyopathy and advanced degeneration of the electrical conduction system with severe sodium channel dysfunction [78]. Even asymptomatic family members with BrS and SCN5A gene mutation showed histological abnormalities [79], and up to 33% of the families of patients suffering from unexplained sudden cardiac deaths with idiopathic fibrosis and/or hypertrophy received a post-mortem diagnosis of BrS [80].
Epicardial surface and interstitial fibrosis were described in BrS, along with increased collagen throughout the heart and a reduction in the expression of gap junctions in the RVOT. There was a correlation between structural abnormalities and abnormal potentials, and their ablation abolished the BrS phenotype and malignant arrhythmias [9]. Another group confirmed that BrS is associated with increased collagen content throughout the RV and the LV, but irrespective of sampling location or myocardial layer in patients experiencing sudden cardiac death [61]. Based on the data provided above, an endomyocardial biopsy could become a new diagnostic tool for the research of concealed morphological abnormalities in BrS, as well as for the identification of dynamic arrhythmogenic substrates [75,81,82].
5. Electroanatomical Substrates
Electroanatomical mapping (EAM) is an invasive method to visualize intracardiac electrical activation [83]. Low voltage and prolonged or fragmented ventricular signals reflect the arrhythmogenic substrate in BrS patients undergoing EAM [84] (Figure 4). Initial studies with endocardial mapping localized the electroanatomical substrate in the RVOT [10,84,85]. However, recent studies could demonstrate that the electroanatomical substrate is located most often on the epicardial surface of the RVOT [62,63,64].
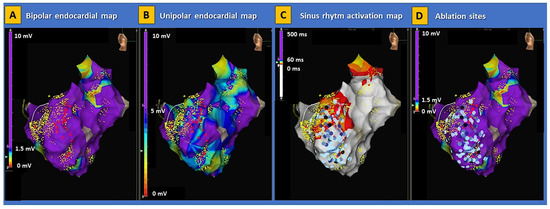
Figure 4.
Electroanatomical substrate of Brugada syndrome. Examples of electroanatomical map abnormalities involving the right ventricle are shown in a patient with genetically proven BrS. The disproportion between low-voltage areas in bipolar (panel (A)) and unipolar (panel (B)) endocardial maps indicates the presence of a deep arrhythmogenic substrate consistent with classic arrhythmogenic right ventricular cardiomyopathy. The activation map during sinus rhythm (panel (C)) shows an extensive area of late potentials within the basal lateral segment of the right ventricle. In this patient, radiofrequency energy was extensively delivered (panel (D)), aimed at the complete abolishment of abnormal potentials in the right ventricle. No ventricular arrhythmias were induced on post-procedural programmed ventricular stimulation. BrS = Brugada syndrome.
In their landmark paper, Nademanee et al. were the first group that performed endocardial and epicardial mapping of the RVOT in a case series of nine patients with a type 1 BrS ECG pattern. They demonstrated that the underlying mechanism is delayed depolarization over the anterior aspect of the RVOT epicardium [63]. The issue is relevant since the RVOT has distinct electrophysiological properties as compared to the surrounding myocardium [86]. Catheter ablation of the substrate resulted in the normalization of the BrS ECG pattern and the non-inducibility of VF/ventricular tachycardia (VT) in most patients [63]. Furthermore, ablation was associated with an event-free follow-up of 20 ± 6 months in all patients [63]. The largest study of endocardial and epicardial EAM with subsequent ablation in BrS patients (n = 135) was performed by Pappone et al. [64]. Combined endo-epicardial mapping localized the substrate exclusively on the anterior RVOT and RV anterior free wall of the epicardium. Ajmaline administration increased the area of the epicardial substrate and catheter ablation resulted in the normalization of the type 1 BrS ECG pattern and non-inducibility of VT/VF [64]. The substrate size correlates with the arrhythmia inducibility during the electrophysiologic study [87]. A cutoff of > 4 cm2 of the abnormal electrophysiological substrate on EAM was described as an independent predictor of inducible ventricular arrhythmias (VT/VF) during programmed ventricular stimulation [88]. Radiofrequency catheter ablation of ventricular arrhythmias can reduce the burden of VT/VF and is now recommended for patients with recurrent ICD shocks or patients who are not suitable or decline an ICD according to current US guidelines (class I indication, level of evidence B from non-randomized trials) [4]. In the recent HRS/EHRA/APHRS/LAHRS expert consensus statement [83], catheter ablation was assigned a class IIa indication (level of evidence B from non-randomized trials) for patients with recurrent sustained ventricular arrhythmias or implantable cardioverter defibrillator (ICD) therapies. The ablation strategy has shifted away from targeting premature ventricular complex-triggered VF in BrS patients [89] and toward directly targeting the substrate on the epicardial aspect of the RVOT [90]. In a systematic review of 233 patients from 11 case series and 11 case reports, it has been demonstrated that endocardial mapping alone does not identify the electroanatomic substrate in 93% of cases and that epicardial substrate modification via catheter ablation is more effective than an endocardial-only approach [90].
6. Critical Review of Arrhythmic Risk Stratification
The identification of high-risk BrS patients remains a pivotal issue for the prevention of sudden cardiac death (SCD). Although almost every author agrees on the importance of symptoms and a spontaneous type 1 pattern [3,4], some other risk markers are controversial. An aborted SCD or documented VT/VF are clear recommendations [3] for ICD implantation, giving the burden of recurrences as high as 8–10% per year [91]. Patients diagnosed after syncope are still at a high risk irrespective of a spontaneous ECG pattern (1.9% per year if a type-1-induced pattern vs. 2.3% per year if a spontaneous type 1, statistically nonsignificant), provided vasovagal etiology has been excluded [92]. Defining the etiology of every syncope is often challenging, and a great effort should be directed toward history collection to improve patient selection for ICD implants.
A spontaneous type 1 BrS pattern is consistently associated with a higher event rate, even when asymptomatic (1.2% per year vs. 0.4% per year in drug-induced type 1, p = 0.049) [92]. However, since the longitudinal variation in the ST-segment in the right precordial leads is well described [93,94], a structured follow-up must be considered by employing 12-lead ECG Holter recordings [95].
Considering the high psychological and physical impact of an ICD in a young population, in the last two decades, other features have been proposed for better stratification of the arrhythmic risk in BrS. So far, little evidence corroborates the hypothesis of a strong association between a specific gene mutation and a worse prognosis [1]. Therefore, multimodal prognostic workup should also include clinical, electrocardiographic, and electrophysiological parameters.
Among the clinical parameters, age and the number of familial cases of SCD can help to define the individual risk [96,97]. Although few data are available for younger (<12 years old) and older (>60 years old) BrS patients, the event risk seems lower in elderly patients [98]. Males are largely predominant in all BrS groups, including SCD and syncope patients, driving a threefold increase in event risk. For female patients, a PQ interval greater than 200 ms as well as sinus node dysfunction have been proposed as risk factors [99], defining a strong role for the hormonal balance on the sodium channels pathophysiology.
As for the electrocardiographic parameters, QRS fragmentation and duration [100], late potentials [101], and the aVR sign [97] have been proposed. In particular, QRS fragmentation is associated with a twofold to ninefold increase in events, depending on filtering and recording modalities in different studies [3]. Furthermore, the aVR sign [97] establishes a link with the pathophysiology of the disease, analyzing right ventricle outflow tract involvement in some severe arrhythmic phenotypes. The extent of the ST alterations was in turn linked to the severity of the arrhythmic risk. A BrS type 1 pattern in the peripheral leads [102] and early repolarization pattern in the inferior leads [103] were linked to an increased arrhythmic risk. Instead, a prolonged (>200 ms) T peak–T end interval was not confirmed in different studies [104,105]. A recent paper focused on the depolarization delay shown by the r’ wave morphology. The authors found a strong correlation between the dST-Tiso interval and the VT/VF inducibility during the EPS [106]. Nevertheless, further evidence is needed to use this marker as an independent risk factor.
Albeit controversial, the prognostic value of VF/VT induction during the electrophysiological test (EPS) remains a cornerstone in clinical practice. The latest ESC guidelines [3] assign a class IIb to the ICD implantation after a positive EPS in asymptomatic patients with a spontaneous type 1 BrS ECG. No universal agreement exists about the stimulation protocol, but a standardized right apex and outflow tract stimulation, a drive train [S1-S1] of eight beats at 600 and 400 ms, and three extrastimuli [S2-S3-S4] with a minimum coupling interval of 200 ms have been suggested [107]. Inducibility is confirmed if sustained VT or VF are recorded [107]. Nonetheless, the association between a positive EPS and subsequent clinical events was confuted by many authors [108,109]. Metanalysis and large observational studies [110,111] found a more than twofold (hazard ratio 2.55) augmented risk of spontaneous VF after a positive EPS. Furthermore, an induction with a single extrastimulus or a ventricular refractory period < 200 ms [3] are valuable elements of vulnerability to consider in a multiparameter assessment.
An overview of the prognostic factors for BrS is provided in Table 3. Given the multiple and controversial candidate prognostic factors, different risk scores have been proposed in the last few years to improve the SCD risk stratification in BrS [5,112]. However, these scores showed good results for low- and high-risk patients, but poor performances in the large grey zone of the intermediate-risk patients [113]. However, since most evidence to date is based on small case series or isolated reports, it is still not possible to define a hierarchy of prognostic factors as well. Dedicated studies with uniform design and advanced diagnostic workup are needed for improving it.

Table 3.
Known and candidate prognostic factors for Brugada syndrome.
In this setting, the identification of novel prognostic signs from concealed structural abnormalities may considerably improve the patient selection for ICD implants, especially with a primary prevention indication.
7. Conclusive Remarks and Future Directions
We hereby showed that BrS sometimes displays concealed substrates that may be identified via advanced diagnostic techniques, including CMR, EMB, and EAM. In this setting, diagnostic criteria for cardiomyopathy may be met more frequently than expected following genetics and simple tests such as ECGs. Waiting for further research and big data analysis, many parameters derived from advanced myocardial imaging, electroanatomical mapping, and histology may be included in a multimodal score to significantly improve the arrhythmic risk stratification of BrS. For instance, LGE [114], replacement fibrosis [115], low-voltage areas [116], and myocardial inflammation [117] are recognized risk factors for many cardiomyopathies. In the setting of BrS, major beneficial effects are expected from a multimodal assessment, in particular for the majority of patients currently classified at intermediate risk for SCD [113] and with no clear indications for a primary prevention ICD implant [3,4].
Author Contributions
Conceptualization, G.P. (Giovanni Peretto) and C.D.R.; methodology, G.P. (Giovanni Peretto); software, Gianluca P.; validation, C.D.R., S.S. and S.B.; formal analysis, C.D.R., J.B., A.V., M.M., Gianluca P, F.F. and S.S.; investigation, C.D.R., J.B., A.V., M.M., Giovanni P, F.F., and S.S.; resources, C.D.R.; data curation, G.P. (Giovanni Peretto) and C.D.R.; writing—original draft preparation, C.D.R., J.B., A.V., M.M., Giovanni P, F.F., R.T. and S.S.; writing—review and editing, G.P. (Giovanni Peretto), C.D.R. and S.B.; visualization, C.D.R., J.B., A.V., M.M., G.P. (Giovanni Peretto), F.F., S.S., G.P. (Gianluca Piliand), and S.B.; supervision, S.S. and S.B.; project administration, G.P. (Giovanni Peretto) and C.D.R.; funding acquisition, C.D.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Thanks to Ministero della Salute (GR-2016-02362316).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brugada, J.; Campuzano, O.; Arbelo, E.; Sarquella-Brugada, G.; Brugada, R. Present Status of Brugada Syndrome. J. Am. Coll. Cardiol. 2018, 72, 1046–1059. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.-Q.; Li, S.; Liu, R.; Zheng, K.; Wu, X.-F.; Tang, Q. A meta-analytic review of prevalence for Brugada ECG patterns and the risk for death. Medicine 2016, 95, e5643. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, ehac262. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. J. Am. Coll. Cardiol. 2018, 72, e91–e220. [Google Scholar] [PubMed]
- Kawada, S.; Morita, H.; Antzelevitch, C.; Morimoto, Y.; Nakagawa, K.; Watanabe, A.; Nishii, N.; Nakamura, K.; Ito, H. Shanghai Score System for Diagnosis of Brugada Syndrome: Validation of the Score System and System and Reclassification of the Patients. JACC Clin. Electrophysiol. 2018, 4, 724–730. [Google Scholar] [CrossRef]
- Chen, Q.; Kirsch, G.E.; Zhang, D.; Brugada, R.; Brugada, J.; Brugada, P.; Potenza, D.; Moya, A.; Borggrefe, M.; Breithardt, G.; et al. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature 1998, 392, 293–296. [Google Scholar] [CrossRef]
- Wilde, A.A.; Amin, A.S. Clinical Spectrum of SCN5A Mutations. JACC Clin. Electrophysiol. 2018, 4, 569–579. [Google Scholar] [CrossRef]
- Basso, C.; Carturan, E.; Pilichou, K.; Rizzo, S.; Corrado, D.; Thiene, G. Sudden cardiac death with normal heart: Molecular autopsy. Cardiovasc. Pathol. 2010, 19, 321–325. [Google Scholar] [CrossRef]
- Nademanee, K.; Raju, H.; de Noronha, S.V.; Papadakis, M.; Robinson, L.; Rothery, S.; Makita, N.; Kowase, S.; Boonmee, N.; Vitayakritsirikul, V.; et al. Fibrosis, Connexin-43, and Conduction Abnormalities in the Brugada Syndrome. J. Am. Coll. Cardiol. 2015, 66, 1976–1986. [Google Scholar] [CrossRef]
- Pieroni, M.; Notarstefano, P.; Oliva, A.; Campuzano, O.; Santangeli, P.; Coll, M.; Nesti, M.; Carnevali, A.; Fraticelli, A.; Iglesias, A.; et al. Electroanatomic and Pathologic Right Ventricular Outflow Tract Abnormalities in Patients with Brugada Syndrome. J. Am. Coll. Cardiol. 2018, 72, 2747–2757. [Google Scholar] [CrossRef]
- Barry, J.M.; Jeffrey, A.T.; Gaetano, T.; Charles, A.; Domenico, C.; Donna, A.; James, B.Y. Contemporary definitions and classification of the cardiomyopathies. Circulation 2006, 113, 1807–1816. [Google Scholar] [CrossRef]
- Ben-Haim, Y.; Asimaki, A.; Behr, E.R. Brugada syndrome and arrhythmogenic cardiomyopathy: Overlapping disorders of the connexome. In Europace; Oxford University Press: Oxford, UK, 2021; Volume 23, pp. 653–664. [Google Scholar] [CrossRef]
- Behr, E.R. The genomic architecture of the Brugada syndrome. Heart Rhythm. 2021, 18, 1707–1708. [Google Scholar] [CrossRef] [PubMed]
- Sommariva, E.; Pappone, C.; Boneschi, F.M.; Di Resta, C.; Carbone, M.R.; Salvi, E.; Vergara, P.; Sala, S.; Cusi, D.; Ferrari, M.; et al. Genetics can contribute to the prognosis of Brugada syndrome: A pilot model for risk stratification. Eur. J. Hum. Genet. 2013, 21, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Krahn, A.D.; Behr, E.R.; Hamilton, R.; Probst, V.; Laksman, Z.; Han, H.C. Brugada Syndrome. JACC Clin. Electrophysiol. 2022, 8, 386–405. [Google Scholar] [CrossRef]
- Le Scouarnec, S.; Karakachoff, M.; Gourraud, J.-B.; Lindenbaum, P.; Bonnaud, S.; Portero, V.; Duboscq-Bidot, L.; Daumy, X.; Simonet, F.; Teusan, R.; et al. Testing the burden of rare variation in arrhythmia-susceptibility genes provides new insights into molecular diagnosis for Brugada syndrome. Hum. Mol. Genet. 2015, 24, 2757–2763. [Google Scholar] [CrossRef]
- Bezzina, C.R.; Barc, J.; Mizusawa, Y.; Remme, C.A.; Gourraud, J.-B.; Simonet, F.; Verkerk, A.O.; Schwartz, P.J.; Crotti, L.; Dagradi, F.; et al. Common variants at SCN5A-SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death. Nat. Genet. 2013, 45, 1044–1049. [Google Scholar] [CrossRef]
- ClinGen Resource. Available online: https://clinicalgenome.org/ (accessed on 10 August 2022).
- Hosseini, S.M.; Kim, R.; Udupa, S.; Costain, G.; Jobling, R.; Liston, E.; Jamal, S.M.; Szybowska, M.; Morel, C.F.; Bowdin, S.; et al. Reappraisal of reported genes for sudden arrhythmic death: Evidence-based evaluation of gene validity for Brugada syndrome. Circulation 2018, 138, 1195–1205. [Google Scholar] [CrossRef]
- Wilde, A.A.M.; Semsarian, C.; Márquez, M.F.; Sepehri, S.A.; Ackerman, M.J.; Ashley, E.A.; Sternick, E.B.; Barajas-Martinez, H.; Behr, E.R.; Bezzina, C.R.; et al. Expert Consensus Statement on the State of Genetic Testing for Cardiac Diseases; Europace: Azemmour, Morocco, 2022. [Google Scholar]
- London, B.; Michalec, M.; Mehdi, H.; Zhu, X.; Kerchner, L.; Sanyal, S.; Viswanathan, P.C.; Pfahnl, A.E.; Shang, L.L.; Madhusudanan, M.; et al. Mutation in glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias. Circulation 2007, 116, 2260–2268. [Google Scholar] [CrossRef]
- Antzelevitch, C.; Pollevick, G.D.; Cordeiro, J.; Casis, O.; Sanguinetti, M.C.; Aizawa, Y.; Guerchicoff, A.; Pfeiffer, R.; Oliva, A.; Wollnik, B.; et al. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT inter- vals, and sudden cardiac death. Circulation 2007, 115, 442–449. [Google Scholar] [CrossRef]
- Watanabe, H.; Koopmann, T.T.; Le Scouarnec, S.; Yang, T.; Ingram, C.R.; Schott, J.-J.; Demolombe, S.; Probst, V.; Anselme, F.; Escande, D.; et al. Sodium channel β1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J. Clin. Investig. 2008, 118, 2260–2268. [Google Scholar] [CrossRef]
- Delpón, E.; Cordeiro, J.M.; Núñez, L.; Thomsen, P.E.B.; Guerchicoff, A.; Pollevick, G.D.; Wu, Y.; Larsen, C.T.; Hofman-Bang, J.; Burashnikov, E.; et al. Functional effects of KCNE3 mutation and its role in the develop- ment of Brugada syndrome. Circ. Arrhythm. Electrophysiol. 2008, 1, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Barajas-Martinez, H.; Burashnikov, E.; Springer, M.; Wu, Y.; Varro, A.; Pfeiffer, R.; Koopmann, T.T.; Cordeiro, J.; Guerchicoff, A.; et al. A mutation in the β3 subunit of the cardiac sodium channel associated with Brugada ECG phenotype. Circ. Cardiovasc. Genet. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Hirano, Y.; Higashiuesato, Y.; Aizawa, Y.; Hayashi, T.; Inagaki, N.; Tana, T.; Ohya, Y.; Takishita, S.; Muratani, H.; et al. Role of HCN4 channel in preventing ventricular arrhythmia. J. Hum. Genet. 2009, 54, 115–121. [Google Scholar] [CrossRef]
- Giudicessi, J.R.; Ye, D.; Tester, D.J.; Crotti, L.; Mugione, A.; Nesterenko, V.V.; Albertson, R.M.; Antzelevitch, C.; Schwartz, P.J.; Ackerman, M.J. Transient outward current (I(to)) gain-of-function mutations in the KCND3- encoded Kv4.3 potassium channel and Brugada syndrome. Heart Rhythm 2011, 8, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-Domingo, A.; Tan, B.-H.; Crotti, L.; Tester, D.J.; Eckhardt, L.; Cuoretti, A.; Kroboth, S.; Song, C.; Zhou, Q.; Kopp, D.; et al. Gain-of-function mutation S422L in the KCNJ8-encoded cardiac KATP channel Kir6.1 as a pathogenic substrate for J-wave syndromes. Heart Rhythm 2010, 7, 1466–1471. [Google Scholar] [CrossRef]
- Burashnikov, E.; Pfeiffer, R.; Barajas-Martinez, H.; Delpón, E.; Hu, D.; Desai, M.; Borggrefe, M.; Häissaguerre, M.; Kanter, R.; Pollevick, G.D.; et al. Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm 2010, 7, 1872–1882. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Zankov, D.P.; Ding, W.-G.; Itoh, H.; Makiyama, T.; Doi, T.; Shizuta, S.; Hattori, T.; Miyamoto, A.; Naiki, N.; et al. KCNE5 (KCNE1L) variants are novel modulators of Brugada syndrome and idio- pathic ventricular fibrillation. Circ. Arrhythm. Electrophysiol. 2011, 4, 352–361. [Google Scholar] [CrossRef]
- Olesen, M.S.; Jensen, N.F.; Holst, A.G.; Nielsen, J.B.; Tfelt-Hansen, J.; Jespersen, T.; Sajadieh, A.; Haunsø, S.; Lund, J.T.; Calloe, K.; et al. A novel nonsense variant in Nav1.5 cofactor MOG1 eliminates its sodium current increasing effect and may increase the risk of arrhythmias. Can. J. Cardiol. 2011, 27, e17–e23. [Google Scholar] [CrossRef]
- Perrin, M.J.; Adler, A.; Green, S.; Al-Zoughool, F.; Doroshenko, P.; Orr, N.; Uppal, S.; Healey, J.S.; Birnie, D.; Sanatani, S.; et al. Evaluation of genes encoding for the transient outward current (Ito) identifies the KCND2 gene as a cause of J-wave syndrome associated with sudden cardiac death. Circ. Cardiovasc. Genet. 2014, 7, 782–789. [Google Scholar] [CrossRef]
- Liu, H.; Chatel, S.; Simard, C.; Syam, N.; Salle, L.; Probst, V.; Morel, J.; Millat, G.; Lopez, M.; Abriel, H.; et al. Molecular genetics and functional anomalies in a series of 248 Brugada cases with 11 mutations in the TRPM4 channel. PLoS ONE 2013, 8, e54131. [Google Scholar] [CrossRef]
- Riuró, H.; Beltran-Alvarez, P.; Tarradas, A.; Selga, E.; Campuzano, O.; Vergés, M.; Pagans, S.; Iglesias, A.; Brugada, J.; Brugada, P.; et al. A missense mutation in the sodium channel β2 subunit reveals SCN2B as a new candidate gene for Brugada syndrome. Hum. Mutat. 2013, 34, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Cerrone, M.; Lin, X.; Zhang, M.; Agullo-Pascual, E.; Pfenniger, A.; Chkourko Gusky, H.; Novelli, V.; Kim, C.; Tirasawadichai, T.; Judge, D.P.; et al. Missense mutations in plakophilin-2 cause sodium current deficit and associate with a Brugada syndrome phenotype. Circulation 2014, 129, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Barajas-Martínez, H.; Terzic, A.; Park, S.; Pfeiffer, R.; Burashnikov, E.; Wu, Y.; Borggrefe, M.; Veltmann, C.; Schimpf, R.; et al. ABCC9 is a novel Brugada and early repolarization syndrome susceptibility gene. Int. J. Cardiol. 2014, 171, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Sato, A.; Marcou, C.A.; Tester, D.J.; Ackerman, M.J.; Crotti, L.; Schwartz, P.J.; Keun On, Y.; Park, J.-E.; Nakamura, K.; et al. A novel disease gene for Brugada syndrome: Sarcolemmal membrane-asso- ciated protein gene mutations impair intracellular trafficking of hNav1.5. Circ. Arrhythm. Electrophysiol. 2012, 5, 1098–1107. [Google Scholar] [CrossRef]
- Wang, Q.; Ohno, S.; Ding, W.-G.; Fukuyama, M.; Miyamoto, A.; Itoh, H.; Makiyama, T.; Wu, J.; Bai, J.; Hasegawa, K.; et al. Gain-of-function KCNH2 mutations in patients with Brugada syndrome. J. Cardiovasc. Electrophysiol. 2014, 25, 522–530. [Google Scholar] [CrossRef]
- Hu, D.; Barajas-Martínez, H.; Pfeiffer, R.; Dezi, F.; Pfeiffer, J.; Buch, T.; Betzenhauser, M.J.; Belardinelli, L.; Kahlig, K.M.; Rajamani, S.; et al. Mutations in SCN10A are responsible for a large fraction of cases of Brugada syndrome. J. Am. Coll. Cardiol. 2014, 64, 66–79. [Google Scholar] [CrossRef]
- Behr, E.R.; Savio-Galimberti, E.; Barc, J.; Holst, A.G.; Petropoulou, E.; Prins, B.P.; Jabbari, J.; Torchio, M.; Berthet, M.; Mizusawa, Y.; et al. Role of common and rare variants in SCN10A: Results from the Brugada syndrome QRS locus gene discovery collaborative study. Cardiovasc. Res. 2015, 106, 520–529. [Google Scholar] [CrossRef]
- Hennessey, J.A.; Marcou, C.A.; Wang, C.; Wei, E.Q.; Wang, C.; Tester, D.J.; Torchio, M.; Dagradi, F.; Crotti, L.; Schwartz, P.J.; et al. FGF12 is a candidate Brugada syndrome locus. Heart Rhythm 2013, 10, 1886–1894. [Google Scholar] [CrossRef]
- Boczek, N.J.; Ye, D.; Johnson, E.K.; Wang, W.; Crotti, L.; Tester, D.J.; Dagradi, F.; Mizusawa, Y.; Torchio, M.; Alders, M.; et al. Characterization of SEMA3A -Encoded Semaphorin as a Naturally Occurring K v 4.3 Protein Inhibitor and its Contribution to Brugada Syndrome. Circ. Res. 2014, 115, 460–469. [Google Scholar] [CrossRef]
- Barc, J.; Tadros, R.; Glinge, C.; Chiang, D.Y.; Jouni, M.; Simonet, F.; Jurgens, S.J.; Baudic, M.; Nicastro, M.; Potet, F.; et al. Genome-wide association analyses identify new Brugada syndrome risk loci and highlight a new mechanism of sodium channel regulation in disease susceptibility. Nat. Genet. 2022, 54, 232–239. [Google Scholar] [CrossRef]
- Cerrone, M.; Costa, S.; Delmar, M. The Genetics of Brugada Syndrome. Annu. Rev. Genomics Hum. Genet. 2022, 23, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Di Resta, C.; Pietrelli, A.; Sala, S.; Della Bella, P.; De Bellis, G.; Ferrari, M.; Bordoni, R.; Benedetti, S. High-throughput genetic characterization of a cohort of Brugada syndrome patients. Hum. Mol. Genet. 2015, 24, 5828–5835. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martínez-Barrios, E.; Arbelo, E.; Cesar, S.; Cruzalegui, J.; Fiol, V.; Díez-Escuté, N.; Hernández, C.; Brugada, R.; Brugada, J.; Campuzano, O.; et al. Brugada Syndrome in Women: What Do We Know After 30 Years? Front. Cardiovasc. Med. 2022, 9, 874992. [Google Scholar] [CrossRef] [PubMed]
- Milman, A.; Gourraud, J.-B.; Andorin, A.; Postema, P.G.; Sacher, F.; Mabo, P.; Conte, G.; Giustetto, C.; Sarquella-Brugada, G.; Hochstadt, A.; et al. Gender differences in patients with Brugada syndrome and arrhythmic events: Data from a survey on arrhythmic events in 678 patients. Heart Rhythm 2018, 15, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Pappone, C.; Mecarocci, V.; Manguso, F.; Ciconte, G.; Vicedomini, G.; Sturla, F.; Votta, E.; Mazza, B.; Pozzi, P.; Borrelli, V.; et al. New electromechanical substrate abnormalities in high-risk patients with Brugada syndrome. Heart Rhythm 2019, 17, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Gray, B.; Gnanappa, G.K.; Bagnall, R.D.; Femia, G.; Yeates, L.; Ingles, J.; Burns, C.; Puranik, R.; Grieve, S.M.; Semsarian, C.; et al. Relations between right ventricular morphology and clinical, electrical and genetic parameters in Brugada Syndrome. PLoS ONE 2018, 13, e0195594. [Google Scholar] [CrossRef]
- Zhang, J.; Sacher, F.; Hoffmayer, K.; O’Hara, T.; Strom, M.; Cuculich, P.; Silva, J.; Cooper, D.; Faddis, M.; Hocini, M.; et al. Cardiac Electrophysiological Substrate Underlying the ECG Phenotype and Electrogram Abnormalities in Brugada Syndrome Patients. Circulation 2015, 131, 1950–1959. [Google Scholar] [CrossRef]
- Murata, K.; Ueyama, T.; Tanaka, T.; Nose, Y.; Wada, Y.; Matsuzaki, M. Right ventricular dysfunction in patients with Brugada-like electrocardiography: A two dimensional strain imaging study. Cardiovasc. Ultrasound 2011, 9, 30. [Google Scholar] [CrossRef]
- Tukkie, R.; Sogaard, P.; Vleugels, J.; de Groot, I.K.L.M.; Wilde, A.A.M.; Tan, H.L. Delay in right ventricular activation contributes to Brugada syndrome. Circulation 2004, 109, 1272–1277. [Google Scholar] [CrossRef]
- Martini, B.; Nava, A.; Thiene, G.; Buja, G.F.; Canciani, B.; Scognamiglio, R.; Dalla Volta, S. Ventricular fibrillation without apparent heart disease: Description of six cases. Am. Heart J. 1989, 118, 1203–1209. [Google Scholar] [CrossRef]
- Van Hoorn, F.; Campian, M.E.; Spijkerboer, A.; Blom, M.T.; Planken, R.N.; Van Rossum, A.C.; De Bakker, J.M.T.; Wilde, A.A.M.; Groenink, M.; Tan, H.L. SCN5A Mutations in Brugada Syndrome Are Associated with Increased Cardiac Dimensions and Reduced Contractility. PLoS ONE 2012, 7, e42037. [Google Scholar] [CrossRef] [PubMed]
- Papavassiliu, T.; Wolpert, C.; Flüchter, S.; Schimpf, R.; Neff, W.; Haase, K.K.; Düber, C.; Borggrefe, M. Magnetic Resonance Imaging Findings in Patients with Brugada Syndrome. J. Cardiovasc. Electrophysiol. 2004, 15, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Priori, S.G.; Pieroni, M.; Chimenti, C.; Napolitano, C.; Rivolta, I.; Russo, M.A. Cardiac histological substrate in patients with clinical phenotype of Brugada syndrome. Circulation 2005, 112, 3680–3687. [Google Scholar] [CrossRef] [PubMed]
- Buob, A.; Siaplaouras, S.; Janzen, I.; Schwaab, B.; Hammer, B.; Schneider, G.; Jung, J. Focal parvovirus B19 myocarditis in a patient with Brugada syndrome. Cardiol. Rev. 2003, 11, 45–49. [Google Scholar] [CrossRef]
- Salerno, F.; Girerd, N.; Chalabreysse, L.; Billaud, G.; Lina, B.; Chevalier, P. Myocarditis and cardiac channelopathies: A deadly association? Int. J. Cardiol. 2011, 147, 468–470. [Google Scholar] [CrossRef]
- Juhasz, Z.; Tiszlavicz, L.; Kele, B.; Terhes, G.; Deak, J.; Rudas, L.; Kereszty, E. Sudden cardiac death from parvovirus B19 myocarditis in a young man with Brugada syndrome. J. Forensic Leg. Med. 2014, 25, 8–13. [Google Scholar] [CrossRef]
- Coronel, R.; Casini, S.; Koopmann, T.T.; Wilms-Schopman, F.J.; Verkerk, A.O.; de Groot, J.R.; de Bakker, J.M. Right ventricular fibrosis and conduction delay in a patient with clinical signs of Brugada syndrome: A combined electrophysiological, genetic, histopathologic, and computational study. Circulation 2005, 112, 2769–2777. [Google Scholar] [CrossRef]
- Miles, C.; Asimaki, A.; Ster, I.C.; Papadakis, M.; Gray, B.; Westaby, J.; Behr, E.R. Biventricular Myocardial Fibrosis and Sudden Death in Patients with Brugada Syndrome. J. Am. Coll. Cardiol. 2021, 78, 1511–1521. [Google Scholar] [CrossRef]
- Nagase, S.; Kusano, K.F.; Morita, H.; Fujimoto, Y.; Kakishita, M.; Nakamura, K.; Emori, T.; Matsubara, H.; Ohe, T. Epicardial electrogram of the right ventricular outflow tract in patients with the Brugada syndrome: Using the epicardial lead. J. Am. Coll. Cardiol. 2002, 39, 1992–1995. [Google Scholar] [CrossRef]
- Nademanee, K.; Veerakul, G.; Chandanamattha, P.; Chaothawee, L.; Ariyachaipanich, A.; Jirasirirojanakorn, K.; Likittanasombat, K.; Bhuripanyo, K.; Ngarmukos, T. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation 2011, 123, 1270–1279. [Google Scholar] [CrossRef]
- Pappone, C.; Brugada, J.; Vicedomini, G.; Ciconte, G.; Manguso, F.; Saviano, M.; Vitale, R.; Cuko, A.; Giannelli, L.; Calovic, Z.; et al. Electrical Substrate Elimination in 135 Consecutive Patients with Brugada Syndrome. Circ. Arrhythm. Electrophysiol. 2017, 10, e005053. [Google Scholar] [CrossRef] [PubMed]
- Iacoviello, M.; Forleo, C.; Puzzovivo, A.; Nalin, I.; Guida, P.; Anaclerio, M.; Marangelli, V.; Sorrentino, S.; Monitillo, F.; Ciccone, M.M.; et al. Altered two-dimensional strain measures of the right ventricle in patients with Brugada syndrome and arrhythmogenic right ventricular dysplasia/cardiomyopathy. Eur. J. Echocardiogr. 2011, 12, 773–781. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; La Garza, M.S.-D.; Anselmi, F.; Nunno, L.; Arbelo, E.; Jordà, P.; Marzotti, T.; Aprile, F.; Piu, P.; Natali, B.M.; et al. Electromechanical delay by speckle-tracking echocardiography: A novel tool to distinguish between Brugada syndrome and isolated right bundle branch block. Int. J. Cardiol. 2020, 320, 161–167. [Google Scholar] [CrossRef]
- Amundsen, B.H.; Helle-Valle, T.; Edvardsen, T.; Torp, H.; Crosby, J.; Lyseggen, E.; Støylen, A.; Ihlen, H.; Lima, J.A.; Smiseth, O.A.; et al. Noninvasive Myocardial Strain Measurement by Speckle Tracking Echocardiography. J. Am. Coll. Cardiol. 2006, 47, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Tessa, C.; Del Meglio, J.; Ottonelli, A.G.; Diciotti, S.; Salvatori, L.; Magnacca, M.; Chioccioli, M.; Lera, J.; Vignali, G.; Casolo, J. Evaluation of Brugada syndrome by cardiac magnetic resonance. Int. J. Cardiovasc. Imaging 2012, 28, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Bastiaenen, R.; Cox, A.T.; Castelletti, S.; Wijeyeratne, Y.; Colbeck, N.; Pakroo, N.; Ahmed, H.; Bunce, N.; Anderson, L.; Moon, J.; et al. Late gadolinium enhancement in Brugada syndrome: A marker for subtle underlying cardiomyopathy? Heart Rhythm 2017, 14, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Pappone, C.; Santinelli, V.; Mecarocci, V.; Tondi, L.; Ciconte, G.; Manguso, F.; Sturla, F.; Vicedomini, G.; Micaglio, E.; Anastasia, L.; et al. Brugada Syndrome: New Insights from Cardiac Magnetic Resonance and Electroanatomical Imaging. Circ. Arrhythmia Electrophysiol. 2021, 14, e010004. [Google Scholar] [CrossRef]
- Bariani, R.; Cipriani, A.; Rizzo, S.; Celeghin, R.; Bueno Marinas, M.; Giorgi, B.; Bauce, B. “Hot phase” clinical presentation in arrhythmogenic cardiomyopathy. Europace 2021, 23, 907–917. [Google Scholar] [CrossRef]
- Artico, J.; Merlo, M.; Delcaro, G.; Cannatà, A.; Gentile, P.; De Angelis, G.; Sinagra, G. Lymphocytic Myocarditis: A Genetically Predisposed Disease? J. Am. Coll. Cardiol. 2020, 75, 3098–3100. [Google Scholar] [CrossRef]
- Peretto, G.; Sala, S.; Della Bella, P.; Basso, C.; Cooper, L.T. Reply: Genetic Basis for Acute Myocarditis Presenting with Ventricular Arrhythmias? J. Am. Coll. Cardiol. 2020, 76, 126–128. [Google Scholar] [CrossRef]
- Poller, W.; Escher, F.; Haas, J.; Heidecker, B.; Schultheiss, H.P.; Attanasio, P.; Klaassen, S. Missense Variant E1295K of Sodium Channel SCN5A Associated with Recurrent Ventricular Fibrillation and Myocardial Inflammation. JACC Case Rep. 2022, 4, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Peretto, G.; Sala, S.; Rizzo, S.; De Luca, G.; Campochiaro, C.; Sartorelli, S.; Benedetti, G.; Palmisano, A.; Esposito, A.; Tresoldi, M.; et al. Arrhythmias in myocarditis: State of the art. Heart Rhythm 2018, 16, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Peretto, G.; Sala, S.; Rizzo, S.; Palmisano, A.; Esposito, A.; De Cobelli, F.; Campochiaro, C.; De Luca, G.; Foppoli, L.; Dagna, L.; et al. Ventricular Arrhythmias in Myocarditis. J. Am. Coll. Cardiol. 2020, 75, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Zumhagen, S.; Spieker, T.; Rolinck, J.; Baba, H.A.; Breithardt, G.; Böcker, W.; Schulze-Bahr, E. Absence of pathognomonic or inflammatory patterns in cardiac biopsies from patients with Brugada syndrome. Circ. Arrhythm. Electrophysiol. 2009, 2, 16–23. [Google Scholar] [CrossRef]
- Bezzina, C.R.; Rook, M.B.; Groenewegen, W.A.; Herfst, L.J.; van der Wal, A.C.; Lam, J.; Mannens, M.M. Compound heterozygosity for mutations (W156X and R225W) in SCN5A associated with severe cardiac conduction disturbances and degenerative changes in the conduction system. Circ. Res. 2003, 92, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Russo, M.A.; Chimenti, C. Structural myocardial abnormalities in asymptomatic family members with Brugada syndrome and SCN5A gene mutation. Eur. Heart J. 2009, 30, 1763. [Google Scholar] [CrossRef]
- Papadakis, M.; Raju, H.; Behr, E.R.; De Noronha, S.V.; Spath, N.; Kouloubinis, A.; Sharma, S. Sudden cardiac death with autopsy findings of uncertain significance: Potential for erroneous interpretation. Circ. Arrhythm. Electrophysiol. 2013, 6, 588–596. [Google Scholar] [CrossRef]
- Campuzano, O.; Brugada, R. Age, genetics, and fibrosis in the Brugada syndrome. J. Am. Coll. Cardiol. 2015, 66, 1987–1989. [Google Scholar] [CrossRef][Green Version]
- Peretto, G.; Sala, S.; De Luca, G.; Marcolongo, R.; Campochiaro, C.; Sartorelli, S.; Tresoldi, M.; Foppoli, L.; Palmisano, A.; Esposito, A.; et al. Immunosuppressive Therapy and Risk Stratification of Patients with Myocarditis Presenting with Ventricular Arrhythmias. JACC Clin. Electrophysiol. 2020, 6, 1221–1234. [Google Scholar] [CrossRef]
- Cronin, E.M.; Bogun, F.M.; Maury, P.; Peichl, P.; Chen, M.; Namboodiri, N.; Aguinaga, L.; Leite, L.R.; Al-Khatib, S.M.; Anter, E.; et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias: Executive summary. Heart Rhythm 2020, 17, e155–e205. [Google Scholar] [CrossRef]
- Postema, P.G.; van Dessel, P.F.; de Bakker, J.M.; Dekker, L.R.; Linnenbank, A.C.; Hoogendijk, M.G.; Coronel, R.; Tijssen, J.G.; Wilde, A.A.; Tan, H.L. Slow and discontinuous conduction conspire in Brugada syndrome: A right ventricular mapping and stimulation study. Circ. Arrhythm. Electrophysiol. 2008, 1, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, P.D.; Ahmed, A.K.; Ciaccio, E.J.; Brugada, R.; Lizotte, E.; Chaubey, S.; Ben-Simon, R.; Chow, A.W.; Lowe, M.D.; McKenna, W.J. High-density substrate mapping in Brugada syndrome: Combined role of conduction and repolarization heterogeneities in arrhythmogenesis. Circulation 2009, 120, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Elizari, M.V.; Levi, R.; Acunzo, R.S.; Chiale, P.A.; Civetta, M.M.; Ferreiro, M.; Sicouri, S. Abnormal expression of cardiac neural crest cells in heart development: A different hypothesis for the etiopathogenesis of Brugada syndrome. Heart Rhythm 2007, 4, 359–365. [Google Scholar] [CrossRef]
- Letsas, K.P.; Vlachos, K.; Conte, G.; Efremidis, M.; Nakashima, T.; Duchateau, J.; Bazoukis, G.; Frontera, A.; Mililis, P.; Tse, G.; et al. Right ventricular outflow tract electroanatomical abnormalities in asymptomatic and high-risk symptomatic patients with Brugada syndrome: Evidence for a new risk stratification tool? J. Cardiovasc. Electrophysiol. 2021, 32, 2997–3007. [Google Scholar] [CrossRef] [PubMed]
- Pappone, C.; Ciconte, G.; Manguso, F.; Vicedomini, G.; Mecarocci, V.; Conti, M.; Giannelli, L.; Pozzi, P.; Borrelli, V.; Menicanti, L.; et al. Assessing the Malignant Ventricular Arrhythmic Substrate in Patients with Brugada Syndrome. J. Am. Coll. Cardiol. 2018, 71, 1631–1646. [Google Scholar] [CrossRef] [PubMed]
- Haïssaguerre, M.; Extramiana, F.; Hocini, M.; Cauchemez, B.; Jaïs, P.; Cabrera, J.A.; Farré, J.; Leenhardt, A.; Sanders, P.; Scavée, C.; et al. Mapping and ablation of ventricular fibrillation associated with long-QT and Brugada syndromes. Circulation 2003, 108, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.C.; Fernandes, A.; Cardoso, R.; Nasi, G.; Rivera, M.; Mitrani, R.D.; Goldberger, J.J. Ablation strategies for the management of symptomatic Brugada syndrome: A systematic review. Heart Rhythm 2018, 15, 1140–1147. [Google Scholar] [CrossRef]
- Probst, V.; Veltmann, C.; Eckardt, L.; Meregalli, P.G.; Gaita, F.; Tan, H.L.; Babuty, D.; Sacher, F.; Giustetto, C.; Schulze-Bahr, E.; et al. Long-term prognosis of patients diagnosed with Brugada syndrome: Results from the FINGER Brugada Syndrome Registry. Circulation 2010, 121, 635–643. [Google Scholar] [CrossRef]
- Sieira, J.; Ciconte, G.; Conte, G.; de Asmundis, C.; Chierchia, G.B.; Baltogiannis, G.; Di Giovanni, G.; Saitoh, Y.; Casado-Arroyo, R.; Juliá, J.; et al. Long-term prognosis of drug-induced Brugada syndrome. Heart Rhythm 2017, 14, 1427–1433. [Google Scholar] [CrossRef]
- Daw, J.M.; Chahal, C.A.A.; Arkles, J.S.; Callans, D.J.; Dixit, S.; Epstein, A.E.; Frankel, D.S.; Garcia, F.C.; Hyman, M.C.; Kumareswaran, R.; et al. Longitudinal electrocardiographic assessment in Brugada syndrome. Heart Rhythm 2022, 3, 233–240. [Google Scholar] [CrossRef]
- Richter, S.; Sarkozy, A.; Veltmann, C.; Chierchia, G.B.; Boussy, T.; Wolpert, C.; Schimpf, R.; Brugada, J.; Brugada, R.; Borggrefe, M.; et al. Variability of the diagnostic ECG pattern in an ICD patient population with Brugada syndrome. J. Cardiovasc. Electrophysiol. 2009, 20, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Gray, B.; Kirby, A.; Kabunga, P.; Freedman, S.B.; Yeates, L.; Kanthan, A.; Medi, C.; Keech, A.; Semsarian, C.; Sy, R.W. Twelve-lead ambulatory electrocardiographic monitoring in Brugada syndrome: Potential diagnostic and prognostic implications. Heart Rhythm 2017, 14, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Andorin, A.; Behr, E.R.; Denjoy, I.; Crotti, L.; Dagradi, F.; Jesel, L.; Sacher, F.; Petit, B.; Mabo, P.; Maltret, A.; et al. Impact of clinical and genetic findings on the management of young patients with Brugada syndrome. Heart Rhythm 2016, 13, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Calò, L.; Giustetto, C.; Martino, A.; Sciarra, L.; Cerrato, N.; Marziali, M.; Rauzino, J.; Carlino, G.; de Ruvo, E.; Guerra, F.; et al. A New Electrocardiographic Marker of Sudden Death in Brugada Syndrome: The S-Wave in Lead I. J. Am. Coll. Cardiol. 2016, 67, 1427–1440. [Google Scholar] [CrossRef]
- Conte, G.; De Asmundis, C.; Sieira, J.; Levinstein, M.; Chierchia, G.B.; Di Giovanni, G.; Baltogiannis, G.; Ciconte, G.; Saitoh, Y.; Casado-Arroyo, R.; et al. Clinical characteristics, management, and prognosis of elderly patients with Brugada syndrome. J. Cardiovasc. Electrophysiol. 2014, 25, 514–519. [Google Scholar] [CrossRef]
- Benito, B.; Sarkozy, A.; Mont, L.; Henkens, S.; Berruezo, A.; Tamborero, D.; Arzamendi, D.; Berne, P.; Brugada, R.; Brugada, P.; et al. Gender differences in clinical manifestations of Brugada syndrome. J. Am. Coll. Cardiol. 2008, 52, 1567–1573. [Google Scholar] [CrossRef]
- Tokioka, K.; Kusano, K.F.; Morita, H.; Miura, D.; Nishii, N.; Nagase, S.; Nakamura, K.; Kohno, K.; Ito, H.; Ohe, T. Electrocardiographic parameters and fatal arrhythmic events in patients with Brugada syndrome: Combination of depolarization and repolarization abnormalities. J. Am. Coll. Cardiol. 2014, 63, 2131–2138. [Google Scholar] [CrossRef]
- Takagi, A.; Nakazawa, K.; Sakurai, T.; Nanke, T.; Miyake, F. Prolongation of LAS40 (duration of the low amplitude electric potential component (<40 microV) of the terminal portion of the QRS) induced by isoproterenol in 11 patients with Brugada syndrome. Circ. J. 2002, 66, 1101–1104. [Google Scholar] [CrossRef]
- Rollin, A.; Sacher, F.; Gourraud, J.B.; Pasquié, J.L.; Raczka, F.; Duparc, A.; Mondoly, P.; Cardin, C.; Delay, M.; Chatel, S.; et al. Prevalence, characteristics, and prognosis role of type 1 ST elevation in the peripheral ECG leads in patients with Brugada syndrome. Heart Rhythm 2013, 10, 1012–1018. [Google Scholar] [CrossRef]
- Letsas, K.P.; Sacher, F.; Probst, V.; Weber, R.; Knecht, S.; Kalusche, D.; Haïssaguerre, M.; Arentz, T. Prevalence of early repolarization pattern in inferolateral leads in patients with Brugada syndrome. Heart Rhythm 2008, 5, 1685–1689. [Google Scholar] [CrossRef]
- Maury, P.; Sacher, F.; Gourraud, J.B.; Pasquié, J.L.; Raczka, F.; Bongard, V.; Duparc, A.; Mondoly, P.; Sadron, M.; Chatel, S.; et al. Increased Tpeak-Tend interval is highly and independently related to arrhythmic events in Brugada syndrome. Heart Rhythm 2015, 12, 2469–2476. [Google Scholar] [CrossRef]
- Junttila, M.J.; Brugada, P.; Hong, K.; Lizotte, E.; DEZutter, M.; Sarkozy, A.; Brugada, J.; Benito, B.; Perkiomaki, J.S.; Mäkikallio, T.H.; et al. Differences in 12-lead electrocardiogram between symptomatic and asymptomatic Brugada syndrome patients. J. Cardiovasc. Electrophysiol. 2008, 19, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Iacopino, S.; Chierchia, G.B.; Sorrenti, P.; Pesce, F.; Colella, J.; Fabiano, G.; Campagna, G.; Petretta, A.; Placentino, F.; Filannino, P.; et al. dST-Tiso Interval, a Novel Electrocardiographic Marker of Ventricular Arrhythmia Inducibility in Individuals with Ajmaline-Induced Brugada Type I Pattern. Am. J. Cardiol. 2021, 159, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Brugada, P.; Geelen, P.; Brugada, R.; Mont, L.; Brugada, J. Prognostic value of electrophysiologic investigations in Brugada syndrome. J. Cardiovasc. Electrophysiol. 2001, 12, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Brugada, J.; Brugada, R.; Brugada, P. Electrophysiologic testing predicts events in Brugada syndrome patients. Heart Rhythm 2011, 8, 1595–1597. [Google Scholar] [CrossRef]
- Wilde, A.A.; Viskin, S. EP testing does not predict cardiac events in Brugada syndrome. Heart Rhythm 2011, 8, 1598–1600. [Google Scholar] [CrossRef]
- Fauchier, L.; Isorni, M.A.; Clementy, N.; Pierre, B.; Simeon, E.; Babuty, D. Prognostic value of programmed ventricular stimulation in Brugada syndrome according to clinical presentation: An updated meta-analysis of worldwide published data. Int. J. Cardiol. 2013, 168, 3027–3029. [Google Scholar] [CrossRef]
- Sroubek, J.; Probst, V.; Mazzanti, A.; Delise, P.; Hevia, J.C.; Ohkubo, K.; Zorzi, A.; Champagne, J.; Kostopoulou, A.; Yin, X.; et al. Programmed Ventricular Stimulation for Risk Stratification in the Brugada Syndrome: A Pooled Analysis. Circulation 2016, 133, 622–630. [Google Scholar] [CrossRef]
- Sieira, J.; Conte, G.; Ciconte, G.; Chierchia, G.B.; Casado-Arroyo, R.; Baltogiannis, G.; Di Giovanni, G.; Saitoh, Y.; Juliá, J.; Mugnai, G.; et al. A score model to predict risk of events in patients with Brugada Syndrome. Eur. Heart J. 2017, 38, 1756–1763. [Google Scholar] [CrossRef]
- Probst, V.; Goronflot, T.; Anys, S.; Tixier, R.; Briand, J.; Berthome, P.; Geoffroy, O.; Clementy, N.; Mansourati, J.; Jesel, L.; et al. Robustness and relevance of predictive score in sudden cardiac death for patients with Brugada syndrome. Eur. Heart J. 2021, 42, 1687–1695. [Google Scholar] [CrossRef]
- Peretto, G.; Sala, S.; Lazzeroni, D.; Palmisano, A.; Gigli, L.; Esposito, A.; De Cobelli, F.; Camici, P.G.; Mazzone, P.; Basso, C.; et al. Septal Late Gadolinium Enhancement and Arrhythmic Risk in Genetic and Acquired Non-Ischaemic Cardiomyopathies. Hear. Lung Circ. 2019, 29, 1356–1365. [Google Scholar] [CrossRef]
- Peretto, G.; Sala, S.; Basso, C.; Della Bella, P. Programmed ventricular stimulation in patients with active vs previous arrhythmic myocarditis. J. Cardiovasc. Electrophysiol. 2020, 31, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Oloriz, T.; Wellens, H.J.; Santagostino, G.; Trevisi, N.; Silberbauer, J.; Peretto, G.; Maccabelli, G.; Della Bella, P. The value of the 12-lead electrocardiogram in localizing the scar in non-ischaemic cardiomyopathy. Europace 2016, 18, 1850–1859. [Google Scholar] [CrossRef] [PubMed]
- Peretto, G.; Sala, S.; Basso, C.; Rizzo, S.; Radinovic, A.; Frontera, A.; Della Bella, P. Inflammation as a Predictor of Recurrent Ventricular Tachycardia After Ablation in Patients with Myocarditis. J. Am. Coll. Cardiol. 2020, 76, 1644–1656. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).