Alternative Splicing: A Key Mediator of Diabetic Vasculopathy
Abstract
1. Introduction
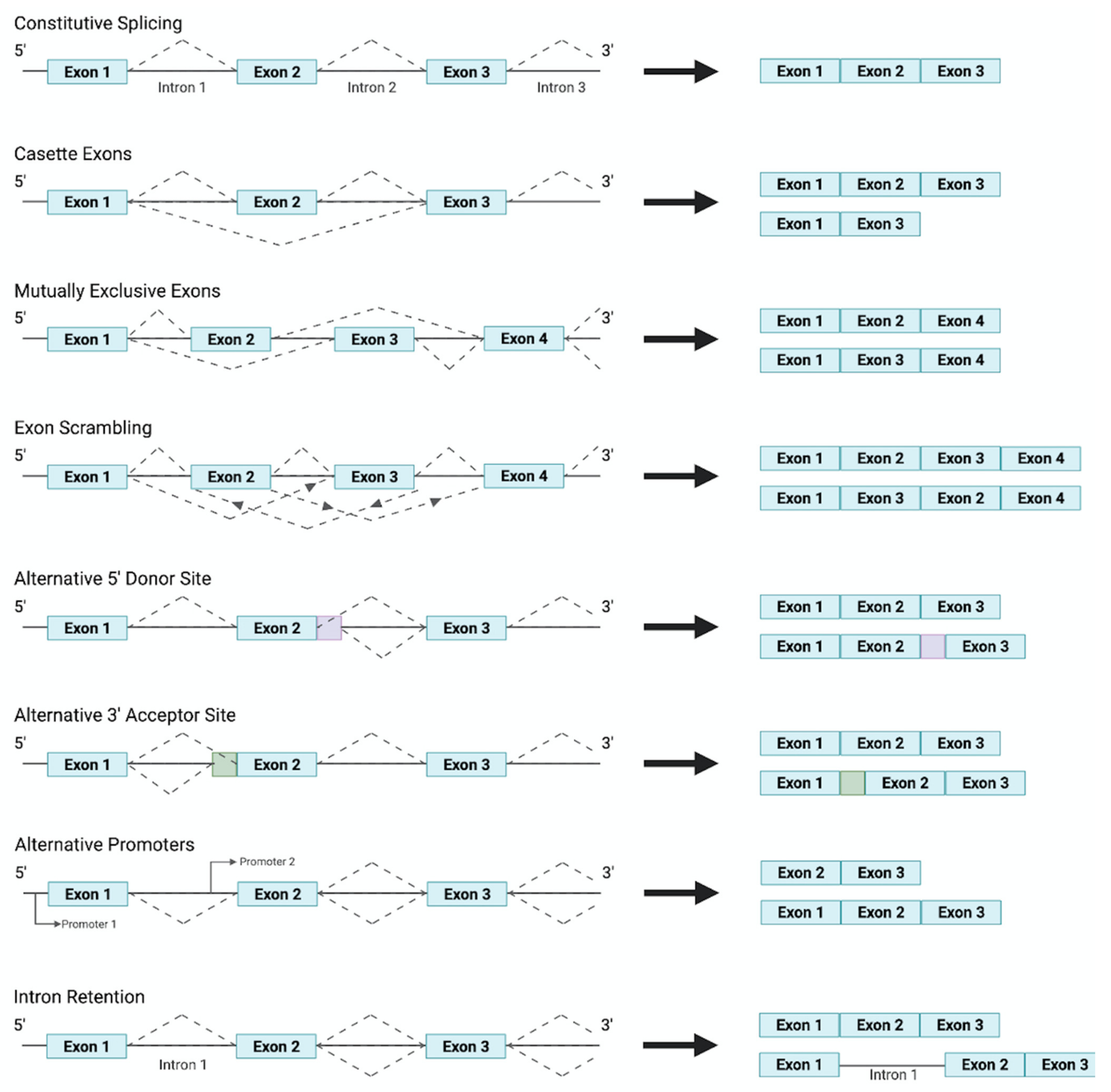
2. Alternative Splicing and the Vasculature
3. Alternative Splicing of QKI and Endothelial Cell Physiology
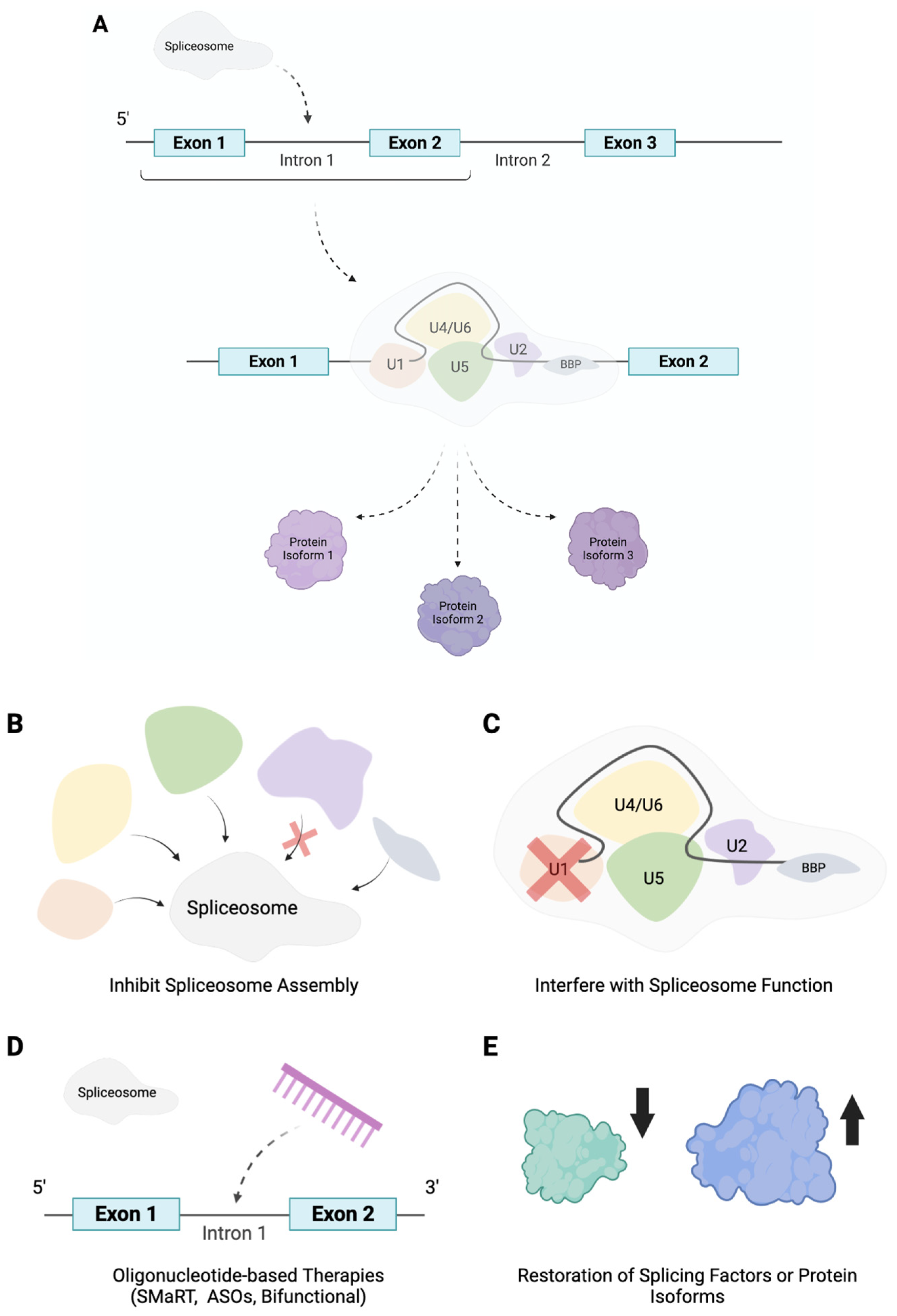
4. Alternative Splicing Based Therapeutic Strategies
5. Conclusions
Funding
Conflicts of Interest
References
- Jiang, W.; Chen, L. Alternative splicing: Human disease and quantitative analysis from high-throughput sequencing. Comput. Struct. Biotechnol. J. 2020, 19, 183–195. [Google Scholar] [CrossRef]
- Dlamini, Z.; Mokoena, F.; Hull, R. Abnormalities in alternative splicing in diabetes: Therapeutic targets. J. Mol. Endocrinol. 2017, 59, R93–R107. [Google Scholar] [CrossRef]
- Mironidou-Tzouveleki, M.; Tsartsalis, S.; Tomos, C. Vascular endothelial growth factor (VEGF) in the pathogenesis of diabetic nephropathy of type 1 diabetes mellitus. Curr. Drug Targets 2011, 12, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, V.A.; Yacoub, A.; Kelaini, S.; Margariti, A. Diabetic endotheliopathy: RNA-binding proteins as new therapeutic targets. Int. J. Biochem. Cell Biol. 2021, 131, 105907. [Google Scholar] [CrossRef] [PubMed]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- American Heart Association. Cardiovascular Disease and Diabetes. 2021. Available online: https://www.heart.org/en/health-topics/diabetes/diabetes-complications-and-risks/cardiovascular-disease--diabetes (accessed on 25 July 2021).
- Rivellese, A.A.; Riccardi, G.; Vaccaro, O. Cardiovascular risk in women with diabetes. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 474–480. [Google Scholar] [CrossRef]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109, Iii27-32. [Google Scholar] [CrossRef] [PubMed]
- Sena, C.M.; Pereira, A.M.; Seiça, R. Endothelial dysfunction—A major mediator of diabetic vascular disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 2216–2231. [Google Scholar] [CrossRef]
- Duffy, A.; Liew, A.; O’Sullivan, J.; Avalos, G.; Samali, A.; O’Brien, T. Distinct Effects of High-Glucose Conditions on Endothelial Cells of Macrovascular and Microvascular Origins. Endothelium 2006, 13, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.J.-Y.; Tsai, C.-H.; Su, C.-H.; Wu, Y.-J.; Chen, S.-J.; Chiu, J.-J.; Shiao, M.-S.; Yeh, H.-I. Diabetes Reduces Aortic Endothelial Gap Junctions in ApoE-deficient Mice: Simvastatin Exacerbates the Reduction. J. Histochem. Cytochem. 2008, 56, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, O.; Niessen, P.M.; Haenen, G.; Miyata, T.; Brownlee, M.; Stehouwer, C.D.; De Mey, J.G.; Schalkwijk, C.G. Hyperglycaemia-induced impairment of endothelium-dependent vasorelaxation in rat mesenteric arteries is mediated by intracellular methylglyoxal levels in a pathway dependent on oxidative stress. Diabetologia 2010, 53, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Karbach, S.; Jansen, T.; Horke, S.; Heeren, T.; Scholz, A.; Coldewey, M.; Karpi, A.; Hausding, M.; Kröller-Schön, S.; Oelze, M.; et al. Hyperglycemia and oxidative stress in cultured endothelial cells—A comparison of primary endothelial cells with an immortalized endothelial cell line. J. Diabetes Its Complicat. 2012, 26, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kemeny, S.F.; Figueroa, D.S.; Clyne, A.M. Hypo- and hyperglycemia impair endothelial cell actin alignment and nitric oxide synthase activation in response to shear stress. PLoS ONE 2013, 8, e66176. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, indicators, risk factors and new hopes. Int. J. Prev. Med. 2014, 5, 927–946. [Google Scholar] [PubMed]
- Moradipoor, S.; Ismail, P.; Etemad, A.; Wan Sulaiman, W.A.; Ahmadloo, S. Expression Profiling of Genes Related to Endothelial Cells Biology in Patients with Type 2 Diabetes and Patients with Prediabetes. Biomed. Res. Int. 2016, 2016, 1845638. [Google Scholar] [CrossRef]
- Foundation, B.H. Facts and Figures. 2021. Available online: https://www.bhf.org.uk/what-we-do/news-from-the-bhf/contact-the-press-office/facts-and-figures (accessed on 25 July 2021).
- Chiasson, J.-L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M.; STOP-NIDDM Trial Research Group. Acarbose Treatment and the Risk of Cardiovascular Disease and Hypertension in Patients With Impaired Glucose ToleranceThe STOP-NIDDM Trial. JAMA 2003, 290, 486–494. [Google Scholar] [CrossRef]
- Hanefeld, M.; Cagatay, M.; Petrowitsch, T.; Neuser, D.; Petzinna, D.; Rupp, M. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: Meta-analysis of seven long-term studies. Eur. Heart J. 2004, 25, 10–16. [Google Scholar] [CrossRef]
- Bergers, G.; Song, S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005, 7, 452–464. [Google Scholar] [CrossRef]
- Shi, Y.; Vanhoutte, P.M. Macro- and microvascular endothelial dysfunction in diabetes. J. Diabetes 2017, 9, 434–449. [Google Scholar] [CrossRef]
- Eelen, G.; de Zeeuw, P.; Simons, M.; Carmeliet, P. Endothelial cell metabolism in normal and diseased vasculature. Circ. Res. 2015, 116, 1231–1244. [Google Scholar] [CrossRef] [PubMed]
- Failla, C.M.; Carbo, M.; Morea, V. Positive and Negative Regulation of Angiogenesis by Soluble Vascular Endothelial Growth Factor Receptor-1. Int. J. Mol. Sci. 2018, 19, 1306. [Google Scholar] [CrossRef]
- Xin, H.; Zhong, C.; Nudleman, E.; Ferrara, N. Evidence for Pro-angiogenic Functions of VEGF-Ax. Cell 2016, 167, 275–284.e6. [Google Scholar] [CrossRef]
- Hagedorn, M.; Balke, M.; Schmidt, A.; Bloch, W.; Kurz, H.; Javerzat, S.; Rousseau, B.; Wilting, J.; Bikfalvi, A. VEGF coordinates interaction of pericytes and endothelial cells during vasculogenesis and experimental angiogenesis. Dev. Dyn. 2004, 230, 23–33. [Google Scholar] [CrossRef]
- Bowler, E.; Oltean, S. Alternative Splicing in Angiogenesis. Int. J. Mol. Sci. 2019, 20, 2067. [Google Scholar] [CrossRef]
- Mamer, S.B.; Wittenkeller, A.; Imoukhuede, P.I. VEGF-A splice variants bind VEGFRs with differential affinities. Sci. Rep. 2020, 10, 14413. [Google Scholar] [CrossRef] [PubMed]
- Krilleke, D.; DeErkenez, A.; Schubert, W.; Giri, I.; Robinson, G.S.; Ng, Y.S.; Shima, D.T. Molecular mapping and functional characterization of the VEGF164 heparin-binding domain. J. Biol. Chem. 2007, 282, 28045–28056. [Google Scholar] [CrossRef]
- Shiying, W.; Boyun, S.; Jianye, Y.; Wanjun, Z.; Ping, T.; Jiang, L.; Hongyi, H. The Different Effects of VEGFA121 and VEGFA165 on Regulating Angiogenesis Depend on Phosphorylation Sites of VEGFR2. Inflamm. Bowel. Dis. 2017, 23, 603–616. [Google Scholar] [CrossRef][Green Version]
- Poltorak, Z.; Cohen, T.; Sivan, R.; Kandelis, Y.; Spira, G.; Vlodavsky, I.; Keshet, E.; Neufeld, G. VEGF145, a secreted vascular endothelial growth factor isoform that binds to extracellular matrix. J. Biol. Chem. 1997, 272, 7151–7158. [Google Scholar] [CrossRef] [PubMed]
- Neagoe, P.E.; Lemieux, C.; Sirois, M.G. Vascular endothelial growth factor (VEGF)-A165-induced prostacyclin synthesis requires the activation of VEGF receptor-1 and -2 heterodimer. J. Biol. Chem. 2005, 280, 9904–9912. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, G.; Cohen, T.; Gengrinovitch, S.; Poltorak, Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999, 13, 9–22. [Google Scholar] [CrossRef]
- Nakamura, M.; Abe, Y.; Tokunaga, T. Pathological significance of vascular endothelial growth factor A isoform expression in human cancer. Pathol. Int. 2002, 52, 331–339. [Google Scholar] [CrossRef]
- Hoar, F.J.; Lip, G.Y.; Belgore, F.; Stonelake, P.S. Circulating levels of VEGF-A, VEGF-D and soluble VEGF-A receptor (sFIt-1) in human breast cancer. Int. J. Biol. Markers 2004, 19, 229–235. [Google Scholar] [CrossRef]
- Eymin, B.; Boudria, A.; Abou-Faycal, C. VEGF-A Splice Variants: Do They Play a Role in Tumor Responses to Anti-angiogenic Therapies? In Molecular Mechanisms of Angiogenesis: From Ontogenesis to Oncogenesis; Feige, J.-J., Pagès, G., Soncin, F., Eds.; Springer Paris: Paris, France, 2014; pp. 421–442. [Google Scholar] [CrossRef]
- Beazley-Long, N.; Hua, J.; Jehle, T.; Hulse, R.P.; Dersch, R.; Lehrling, C.; Bevan, H.; Qiu, Y.; Lagrèze, W.A.; Wynick, D.; et al. VEGF-A165b is an endogenous neuroprotective splice isoform of vascular endothelial growth factor A in vivo and in vitro. Am. J. Pathol. 2013, 183, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, A.L.; Rennel, E.S.; Hua, J.; Bevan, H.S.; Beazley Long, N.; Lehrling, C.; Gammons, M.; Floege, J.; Harper, S.J.; Agostini, H.T.; et al. VEGF-A165b is cytoprotective and antiangiogenic in the retina. Invest. Ophthalmol. Vis. Sci. 2010, 51, 4273–4281. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.T.M.; Farb, M.G.; Kikuchi, R.; Karki, S.; Tiwari, S.; Bigornia, S.J.; Bates, D.O.; LaValley, M.P.; Hamburg, N.M.; Vita, J.A.; et al. Antiangiogenic Actions of Vascular Endothelial Growth Factor-A165b, an Inhibitory Isoform of Vascular Endothelial Growth Factor-A, in Human Obesity. Circulation 2014, 130, 1072–1080. [Google Scholar] [CrossRef]
- Chi, Y.-I.; Frantz, J.D.; Oh, B.-C.; Hansen, L.; Dhe-Paganon, S.; Shoelson, S.E. Diabetes Mutations Delineate an Atypical POU Domain in HNF-1α. Mol. Cell 2002, 10, 1129–1137. [Google Scholar] [CrossRef]
- Giampietro, C.; Deflorian, G.; Gallo, S.; Di Matteo, A.; Pradella, D.; Bonomi, S.; Belloni, E.; Nyqvist, D.; Quaranta, V.; Confalonieri, S.; et al. The alternative splicing factor Nova2 regulates vascular development and lumen formation. Nat. Commun. 2015, 6, 8479. [Google Scholar] [CrossRef]
- Gortan Cappellari, G.; Barazzoni, R.; Cattin, L.; Muro, A.F.; Zanetti, M. Lack of Fibronectin Extra Domain A Alternative Splicing Exacerbates Endothelial Dysfunction in Diabetes. Sci. Rep. 2016, 6, 37965. [Google Scholar] [CrossRef]
- Cackowski, F.C.; Xu, L.; Hu, B.; Cheng, S.Y. Identification of two novel alternatively spliced Neuropilin-1 isoforms. Genomics 2004, 84, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, C.; Dubail, J.; Brohée, L.; Delforge, Y.; Colige, A.; Deroanne, C. A Novel Physiological Glycosaminoglycan-Deficient Splice Variant of Neuropilin-1 Is Anti-Tumorigenic In Vitro and In Vivo. PLoS ONE 2016, 11, e0165153. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ye, Q.; Chen, M.; Li, A.; Mi, W.; Fang, Y.; Zaytseva, Y.Y.; O’Connor, K.L.; Vander Kooi, C.W.; Liu, S.; et al. N-glycosylation-defective splice variants of neuropilin-1 promote metastasis by activating endosomal signals. Nat. Commun. 2019, 10, 3708. [Google Scholar] [CrossRef]
- Parker, M.W.; Linkugel, A.D.; Goel, H.L.; Wu, T.; Mercurio, A.M.; Vander Kooi, C.W. Structural basis for VEGF-C binding to neuropilin-2 and sequestration by a soluble splice form. Structure 2015, 23, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Horie, S.; Suzuki, Y.; Kobayashi, M.; Kadonosono, T.; Kondoh, S.; Kodama, T.; Sato, Y. Distinctive role of vasohibin-1A and its splicing variant vasohibin-1B in tumor angiogenesis. Cancer Gene Ther. 2016, 23, 133–141. [Google Scholar] [CrossRef]
- Sato, Y.; Sonoda, H. The vasohibin family: A negative regulatory system of angiogenesis genetically programmed in endothelial cells. Arter. Thromb. Vasc. Biol. 2007, 27, 37–41. [Google Scholar] [CrossRef]
- Chen, M.; Manley, J.L. Mechanisms of alternative splicing regulation: Insights from molecular and genomics approaches. Nat. Rev. Mol. Cell Biol. 2009, 10, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, D.; Storkebaum, E.; Morimoto, M.; Del-Favero, J.; Desmet, F.; Marklund, S.L.; Wyns, S.; Thijs, V.; Andersson, J.; van Marion, I.; et al. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat. Genet. 2003, 34, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.G.; Woolard, J.; Amin, E.M.; Konopatskaya, O.; Saleem, M.A.; Churchill, A.J.; Ladomery, M.R.; Harper, S.J.; Bates, D.O. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J. Cell Sci. 2008, 121, 3487–3495. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.G.; Cherry, J.; Williams, K.; Turner, S.; Bates, D.O.; Churchill, A.J. Splicing factor polymorphisms, the control of VEGF isoforms and association with angiogenic eye disease. Curr. Eye Res. 2011, 36, 328–335. [Google Scholar] [CrossRef]
- Ye, X.; Abou-Rayyah, Y.; Bischoff, J.; Ritchie, A.; Sebire, N.J.; Watts, P.; Churchill, A.J.; Bates, D.O. Altered ratios of pro- and anti-angiogenic VEGF-A variants and pericyte expression of DLL4 disrupt vascular maturation in infantile haemangioma. J. Pathol. 2016, 239, 139–151. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, J. Role of alternative splicing of VEGF-A in the development of atherosclerosis. Aging 2018, 10, 2695–2708. [Google Scholar] [CrossRef] [PubMed]
- Camaré, C.; Pucelle, M.; Nègre-Salvayre, A.; Salvayre, R. Angiogenesis in the atherosclerotic plaque. Redox Biol. 2017, 12, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Insull, W. The Pathology of Atherosclerosis: Plaque Development and Plaque Responses to Medical Treatment. Am. J. Med. 2009, 122, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.I.; Mills, K.; Ye, X.; Blakely, B.; Min, J.; Kong, W.; Zhang, N.; Gou, L.; Regmi, A.; Hu, S.Q.; et al. Association between the expression of vascular endothelial growth factors and metabolic syndrome or its components: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2018, 10, 62. [Google Scholar] [CrossRef]
- Giannarelli, C.; Alique, M.; Rodriguez, D.T.; Yang, D.K.; Jeong, D.; Calcagno, C.; Hutter, R.; Millon, A.; Kovacic, J.C.; Weber, T.; et al. Alternatively spliced tissue factor promotes plaque angiogenesis through the activation of hypoxia-inducible factor-1α and vascular endothelial growth factor signaling. Circulation 2014, 130, 1274–1286. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, R.; Nakamura, K.; MacLauchlan, S.; Ngo, D.T.-M.; Shimizu, I.; Fuster, J.J.; Katanasaka, Y.; Yoshida, S.; Qiu, Y.; Yamaguchi, T.P.; et al. An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat. Med. 2014, 20, 1464–1471. [Google Scholar] [CrossRef]
- Oltean, S.; Qiu, Y.; Ferguson, J.K.; Stevens, M.; Neal, C.; Russell, A.; Kaura, A.; Arkill, K.P.; Harris, K.; Symonds, C.; et al. Vascular Endothelial Growth Factor-A165b Is Protective and Restores Endothelial Glycocalyx in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2015, 26, 1889. [Google Scholar] [CrossRef]
- Zacchigna, S.; Lambrechts, D.; Carmeliet, P. Neurovascular signalling defects in neurodegeneration. Nat. Rev. Neurosci. 2008, 9, 169–181. [Google Scholar] [CrossRef]
- Joutel, A.; Corpechot, C.; Ducros, A.; Vahedi, K.; Chabriat, H.; Mouton, P.; Alamowitch, S.; Domenga, V.; Cécillion, M.; Maréchal, E.; et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature 1996, 383, 707–710. [Google Scholar] [CrossRef]
- Kachamakova-Trojanowska, N.; Stepniewski, J.; Dulak, J. Human iPSCs-Derived Endothelial Cells with Mutation in HNF1A as a Model of Maturity-Onset Diabetes of the Young. Cells 2019, 8, 1440. [Google Scholar] [CrossRef]
- Balamurugan, K.; Bjørkhaug, L.; Mahajan, S.; Kanthimathi, S.; Njølstad, P.R.; Srinivasan, N.; Mohan, V.; Radha, V. Structure-function studies of HNF1A (MODY3) gene mutations in South Indian patients with monogenic diabetes. Clin. Genet. 2016, 90, 486–495. [Google Scholar] [CrossRef]
- Ellard, S.; Colclough, K. Mutations in the genes encoding the transcription factors hepatocyte nuclear factor 1 alpha (HNF1A) and 4 alpha (HNF4A) in maturity-onset diabetes of the young. Hum. Mutat. 2006, 27, 854–869. [Google Scholar] [CrossRef] [PubMed]
- Teplova, M.; Hafner, M.; Teplov, D.; Essig, K.; Tuschl, T.; Patel, D.J. Structure-function studies of STAR family Quaking proteins bound to their in vivo RNA target sites. Genes Dev. 2013, 27, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Noveroske, J.K.; Lai, L.; Gaussin, V.; Northrop, J.L.; Nakamura, H.; Hirschi, K.K.; Justice, M.J. Quaking is essential for blood vessel development. Genesis 2002, 32, 218–230. [Google Scholar] [CrossRef]
- van Mil, A.; Grundmann, S.; Goumans, M.-J.; Lei, Z.; Oerlemans, M.I.; Jaksani, S.; Doevendans, P.A.; Sluijter, J.P.G. MicroRNA-214 inhibits angiogenesis by targeting Quaking and reducing angiogenic growth factor release. Cardiovasc. Res. 2012, 93, 655. [Google Scholar] [CrossRef]
- de Bruin, R.G.; van der Veer, E.P.; Prins, J.; Lee, D.H.; Dane, M.J.; Zhang, H.; Roeten, M.K.; Bijkerk, R.; de Boer, H.C.; Rabelink, T.J.; et al. The RNA-binding protein quaking maintains endothelial barrier function and affects VE-cadherin and beta-catenin protein expression. Sci. Rep. 2016, 6, 21643. [Google Scholar] [CrossRef]
- Cochrane, A.; Kelaini, S.; Tsifaki, M.; Bojdo, J.; Vila-Gonzalez, M.; Drehmer, D.; Caines, R.; Magee, C.; Eleftheriadou, M.; Hu, Y.; et al. Quaking Is a Key Regulator of Endothelial Cell Differentiation, Neovascularization, and Angiogenesis. Stem. Cells 2017, 35, 952–966. [Google Scholar] [CrossRef] [PubMed]
- Caines, R.; Cochrane, A.; Kelaini, S.; Vila-Gonzalez, M.; Yang, C.; Eleftheriadou, M.; Moez, A.; Stitt, A.W.; Zeng, L.; Grieve, D.J.; et al. The RNA-binding protein QKI controls alternative splicing in vascular cells, producing an effective model for therapy. J. Cell Sci. 2019, 132. [Google Scholar] [CrossRef]
- Yang, C.; Eleftheriadou, M.; Kelaini, S.; Morrison, T.; González, M.V.; Caines, R.; Edwards, N.; Yacoub, A.; Edgar, K.; Moez, A.; et al. Targeting QKI-7 in vivo restores endothelial cell function in diabetes. Nat. Commun. 2020, 11, 3812. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Bray, W.; Smith, A.J.; Zhou, W.; Calaoagan, J.; Lagisetti, C.; Sambucetti, L.; Crews, P.; Lokey, R.S.; Webb, T.R. An exon skipping screen identifies antitumor drugs that are potent modulators of pre-mRNA splicing, suggesting new therapeutic applications. PLoS ONE 2020, 15, e0233672. [Google Scholar] [CrossRef]
- Kaida, D.; Motoyoshi, H.; Tashiro, E.; Nojima, T.; Hagiwara, M.; Ishigami, K.; Watanabe, H.; Kitahara, T.; Yoshida, T.; Nakajima, H.; et al. Spliceostatin A targets SF3b and inhibits both splicing and nuclear retention of pre-mRNA. Nat. Chem. Biol. 2007, 3, 576–583. [Google Scholar] [CrossRef]
- Kotake, Y.; Sagane, K.; Owa, T.; Mimori-Kiyosue, Y.; Shimizu, H.; Uesugi, M.; Ishihama, Y.; Iwata, M.; Mizui, Y. Splicing factor SF3b as a target of the antitumor natural product pladienolide. Nat. Chem. Biol. 2007, 3, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Maire, S.; Gaillard, M.C.; Sahel, J.A.; Hantraye, P.; Bemelmans, A.P. mRNA trans-splicing in gene therapy for genetic diseases. Wiley Interdiscip. Rev. RNA 2016, 7, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Havens, M.A.; Hastings, M.L. Splice-switching antisense oligonucleotides as therapeutic drugs. Nucleic Acids Res. 2016, 44, 6549–6563. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, U.; Hsieh, W.-C.; Yan, H.; Guo, Z.-F.; Shaikh, A.Y.; Soltani, A.; Song, Y.; Ly, D.H.; Liang, F.-S. Bifunctional small molecule-oligonucleotide hybrid as microRNA inhibitor. Bioorganic Med. Chem. 2020, 28, 115394. [Google Scholar] [CrossRef]
- Fuchs, A.; Riegler, S.; Ayatollahi, Z.; Cavallari, N.; Giono, L.E.; Nimeth, B.A.; Mutanwad, K.V.; Schweighofer, A.; Lucyshyn, D.; Barta, A.; et al. Targeting alternative splicing by RNAi: From the differential impact on splice variants to triggering artificial pre-mRNA splicing. Nucleic Acids Res. 2021, 49, 1133–1151. [Google Scholar] [CrossRef] [PubMed]
- Vancheri, C.; Morini, E.; Prandi, F.R.; Alkhoury, E.; Celotto, R.; Romeo, F.; Novelli, G.; Amati, F. Two RECK Splice Variants (Long and Short) Are Differentially Expressed in Patients with Stable and Unstable Coronary Artery Disease: A Pilot Study. Genes 2021, 12, 939. [Google Scholar] [CrossRef] [PubMed]
| Gene | Alternatively Spliced Isoform | Function |
|---|---|---|
| VEGF-A | VEGF-Axxxa | |
| VEGF-A111 | Pro-angiogenic. [26,27], missing exons 6 and 7, readily diffusible [28] | |
| VEGF-A121 | Pro-angiogenic. Regulates two different phosphorylating sites of VEGFR2, Tyr (1175) and Tyr (1214) [29], missing exons 6 and 7, readily diffusible [28] | |
| VEGF-A145 | Pro-angiogenic. Binds to ECM and KDR/flk-1 receptor of ECs [30] | |
| VEGF-A165 | Pro-angiogenic. Most abundant isoform and most potent initiator of angiogenesis. Activates receptor phosphorylation of VEGFR2 and NRP-1. Promotes the release of NO and prostacyclin [31], binds KDR/flk-1 with VEGF-A145 [30] | |
| VEGF-A183 | Pro-angiogenic. Least abundant [32] | |
| VEGF-A189 | Pro-angiogenic. Linked to tumorigenesis [33] | |
| VEGF-A206 | Pro-angiogenic. Strongly binds to ECM [34] | |
| VEGF-Axxxb | ||
| VEGF-A121b | Anti-angiogenic. Inhibits migration of ECs. Reduces xenografted tumor growth [35] | |
| VEGF-A145b | Anti-angiogenic | |
| VEGF-A165b | Anti-angiogenic. Neuroprotective and cytoprotective properties [36,37]. Similar binding affinity of VEGF-A165a but does not activate phosphorylation of VEGFR2 and NRP-1 [38] | |
| VEGF-A189b | Anti-angiogenic | |
| HNF1A | Transcriptional activator that regulates insulin production and stimulates the transcription of other liver-specific genes CYP1A2, CYP2E1 and CYP3A11 [39] | |
| NOVA2 | Pro-angiogenic. Controls the organization of the endothelial lumen. Is also present in neural cells and important for the development of the nervous system [40] | |
| Fibronectin | EDA-FN, EDB-FN | Pro-angiogenic. Component of ECM, involved in vascular remodelling, and inhibits oxidative stress [41] |
| NRPS | NRP-1 | |
| s11NRP1, s12NRP1, sIIINRP1 | Anti-angiogenic. Lacks transmembrane domain and cytoplasmic tail [42] | |
| & sIVNRP1 | ||
| NRP1-∆7 | Anti-angiogenic. Deletion of seven amino acids in exon 11. Impairs glycosylation of NRP-1 [43] | |
| NRP1-∆E4 & NRP1-∆E5 | Anti-angiogenic. Altered glycosylation and endocytic movement [44] | |
| NRP-2 | ||
| s9NRP2 | Results from intron 9 retention. Inhibits VEGF-C/NRP2 oncogenic signaling [45] | |
| Vasohibins | Vasohibin-1 | |
| VASH1A | Anti-angiogenic. Linked to tumorigenesis [46] | |
| VASHA1B | Anti-angiogenic. Lacks exons 6–8. Involved in heparin binding [46] | |
| Vasohibin-2 | ||
| 290aa & 355aa | Anti-angiogenic. Full function unclear [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornelius, V.A.; Fulton, J.R.; Margariti, A. Alternative Splicing: A Key Mediator of Diabetic Vasculopathy. Genes 2021, 12, 1332. https://doi.org/10.3390/genes12091332
Cornelius VA, Fulton JR, Margariti A. Alternative Splicing: A Key Mediator of Diabetic Vasculopathy. Genes. 2021; 12(9):1332. https://doi.org/10.3390/genes12091332
Chicago/Turabian StyleCornelius, Victoria A., Jenna R. Fulton, and Andriana Margariti. 2021. "Alternative Splicing: A Key Mediator of Diabetic Vasculopathy" Genes 12, no. 9: 1332. https://doi.org/10.3390/genes12091332
APA StyleCornelius, V. A., Fulton, J. R., & Margariti, A. (2021). Alternative Splicing: A Key Mediator of Diabetic Vasculopathy. Genes, 12(9), 1332. https://doi.org/10.3390/genes12091332





