Dynamic Spatiotemporal Expression Changes in Connexins of the Developing Primate’s Cochlea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimens
2.2. Tissue Preparation
2.3. Immunohistochemistry
2.4. Antibodies
3. Results
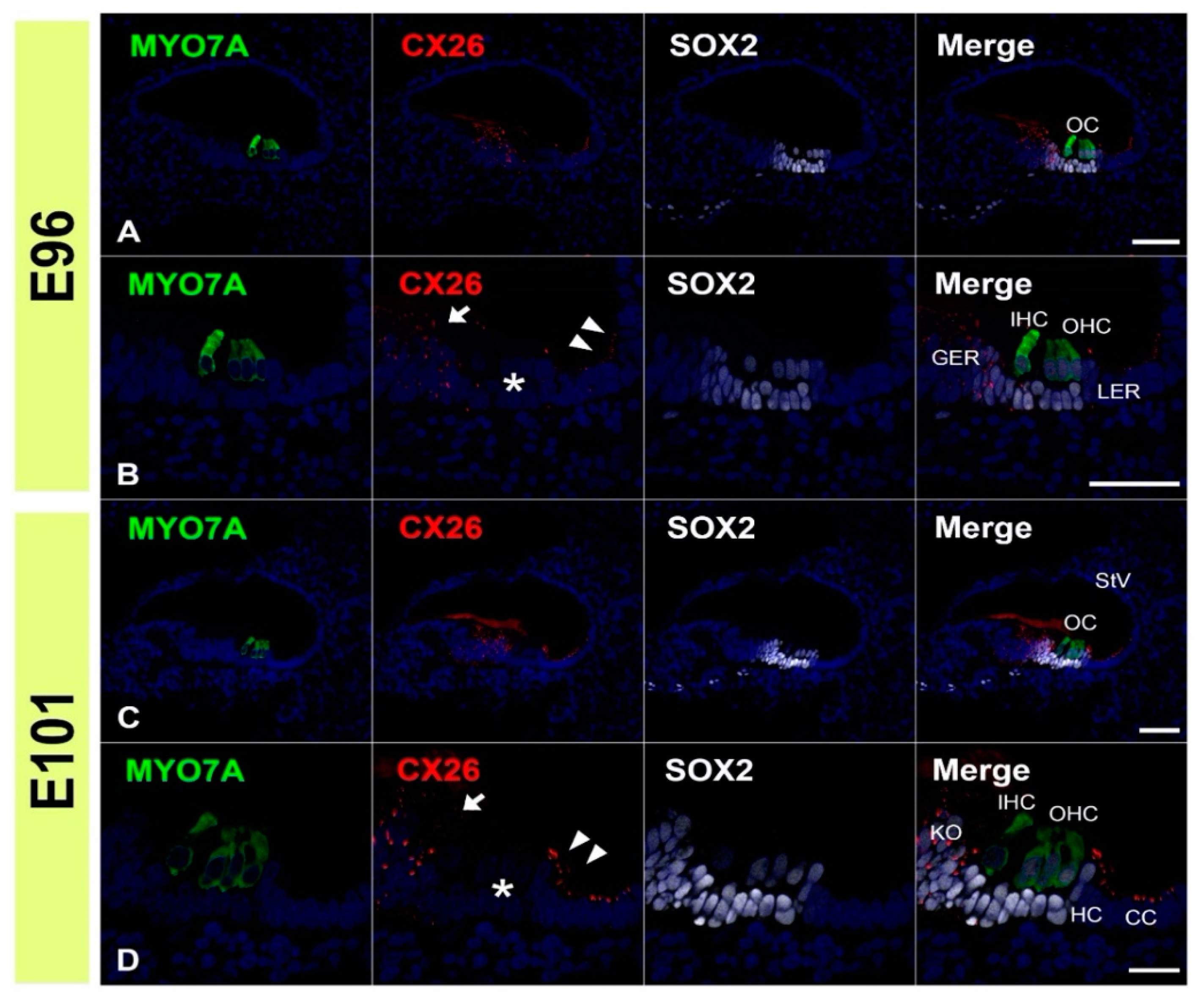
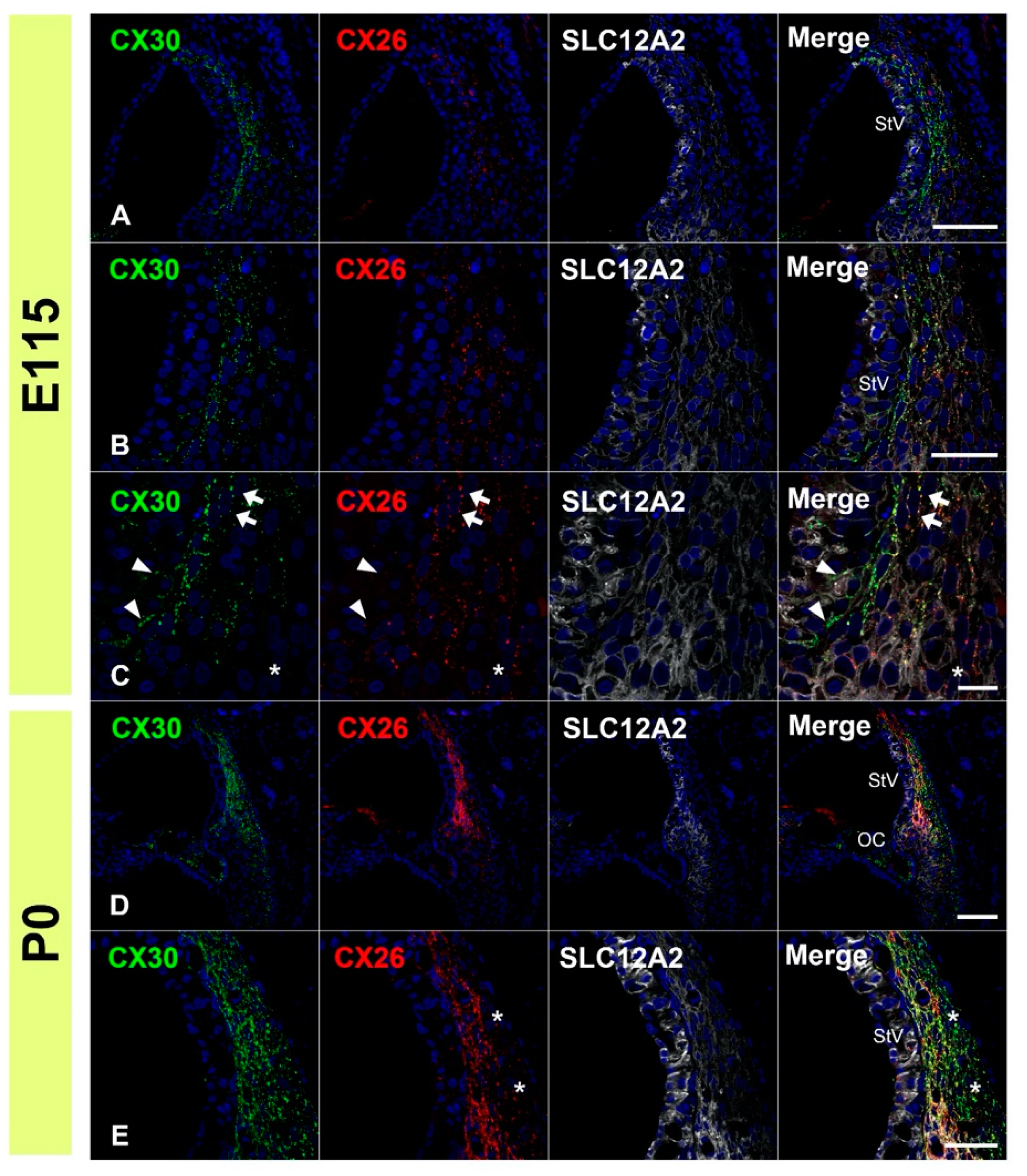
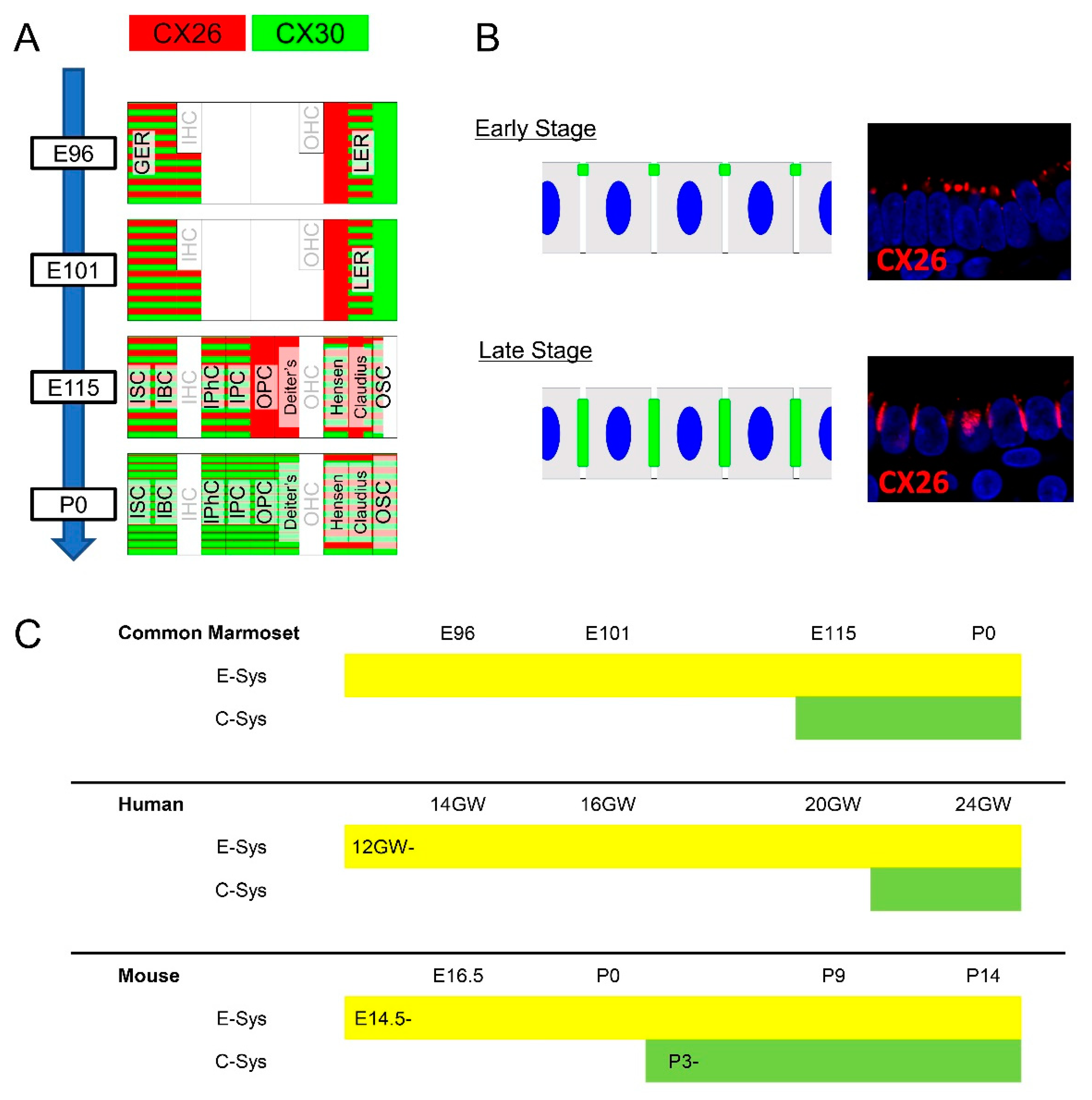
3.1. Expression of CX26
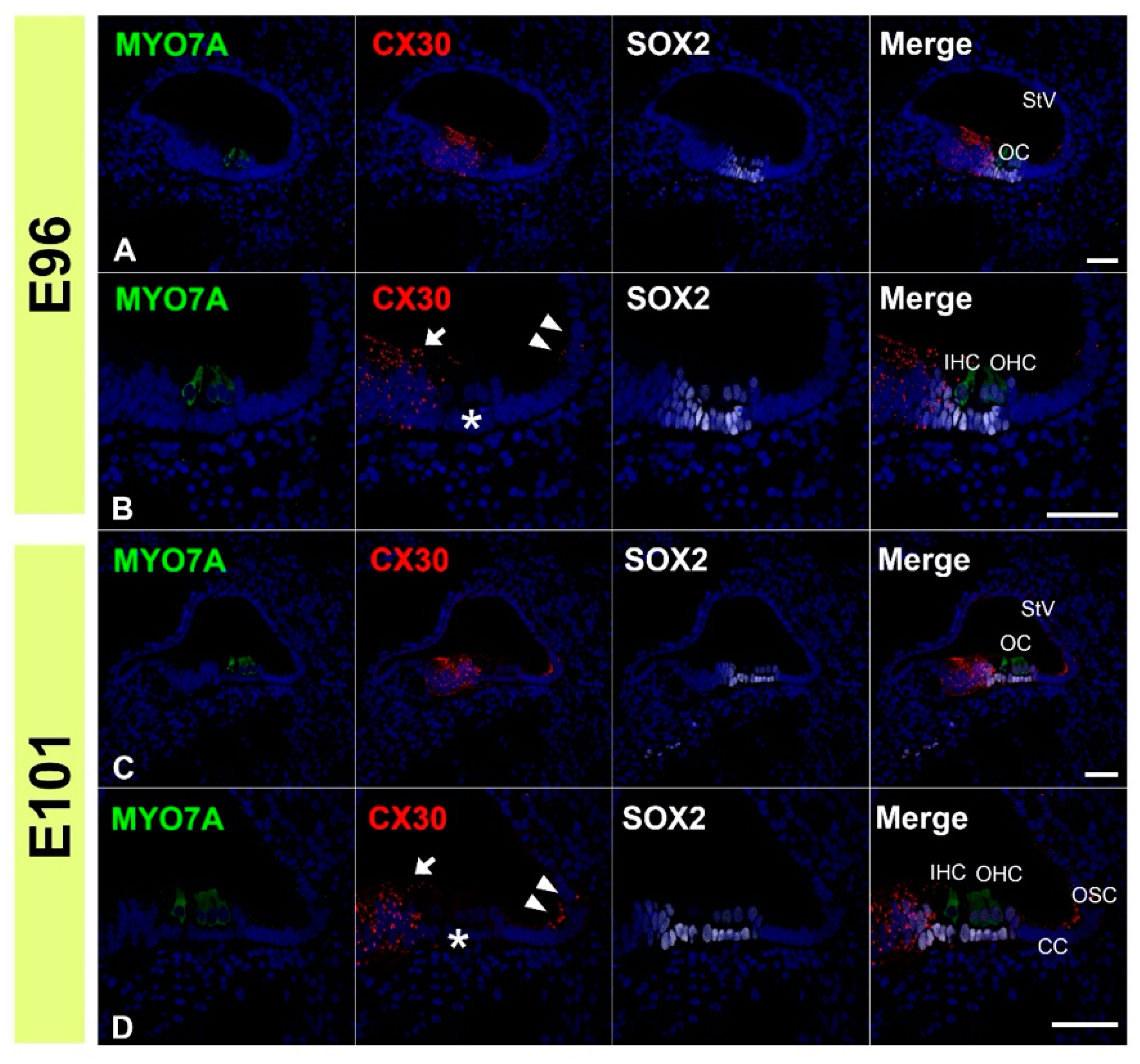
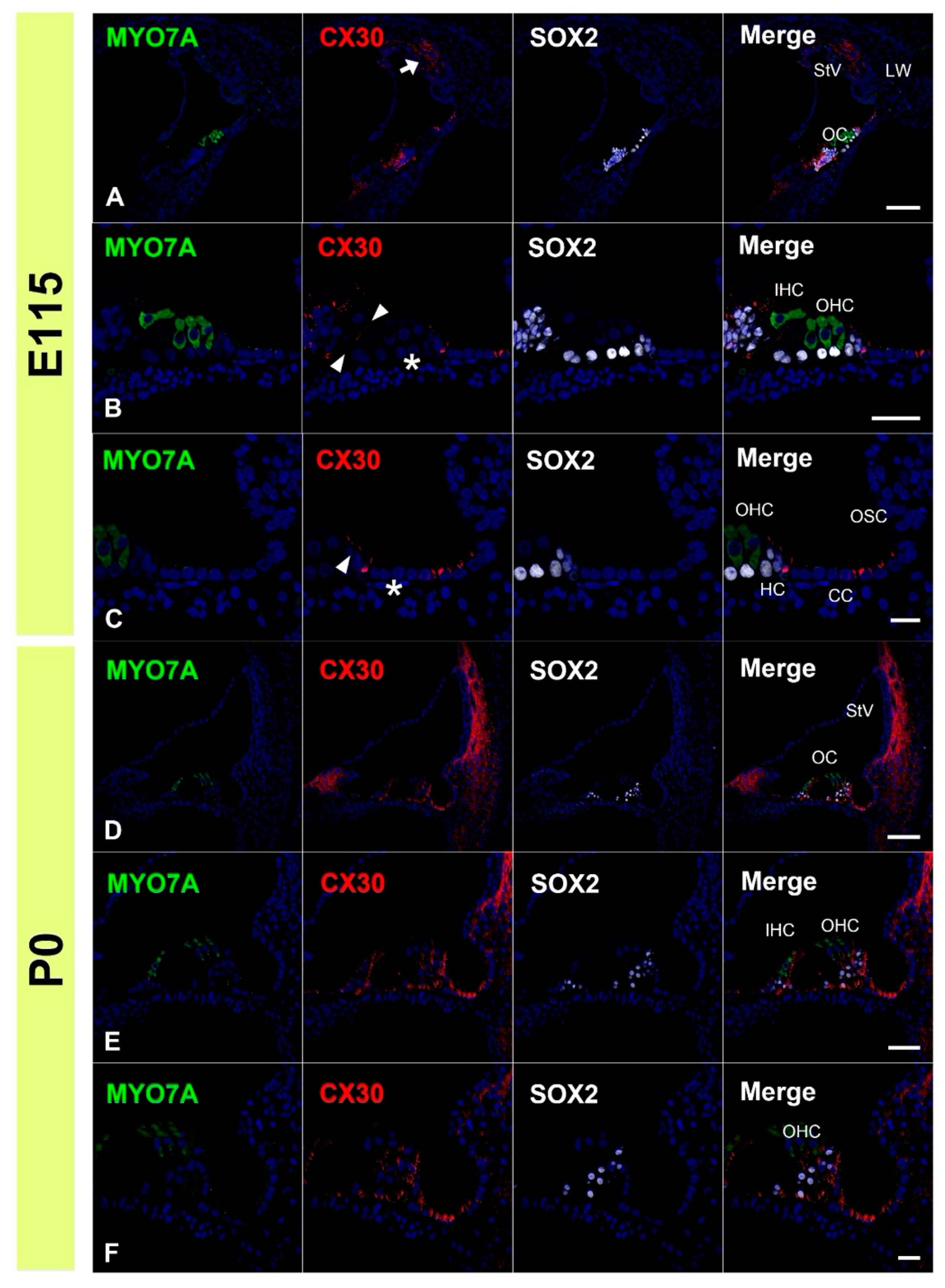
3.2. Expression of CX30
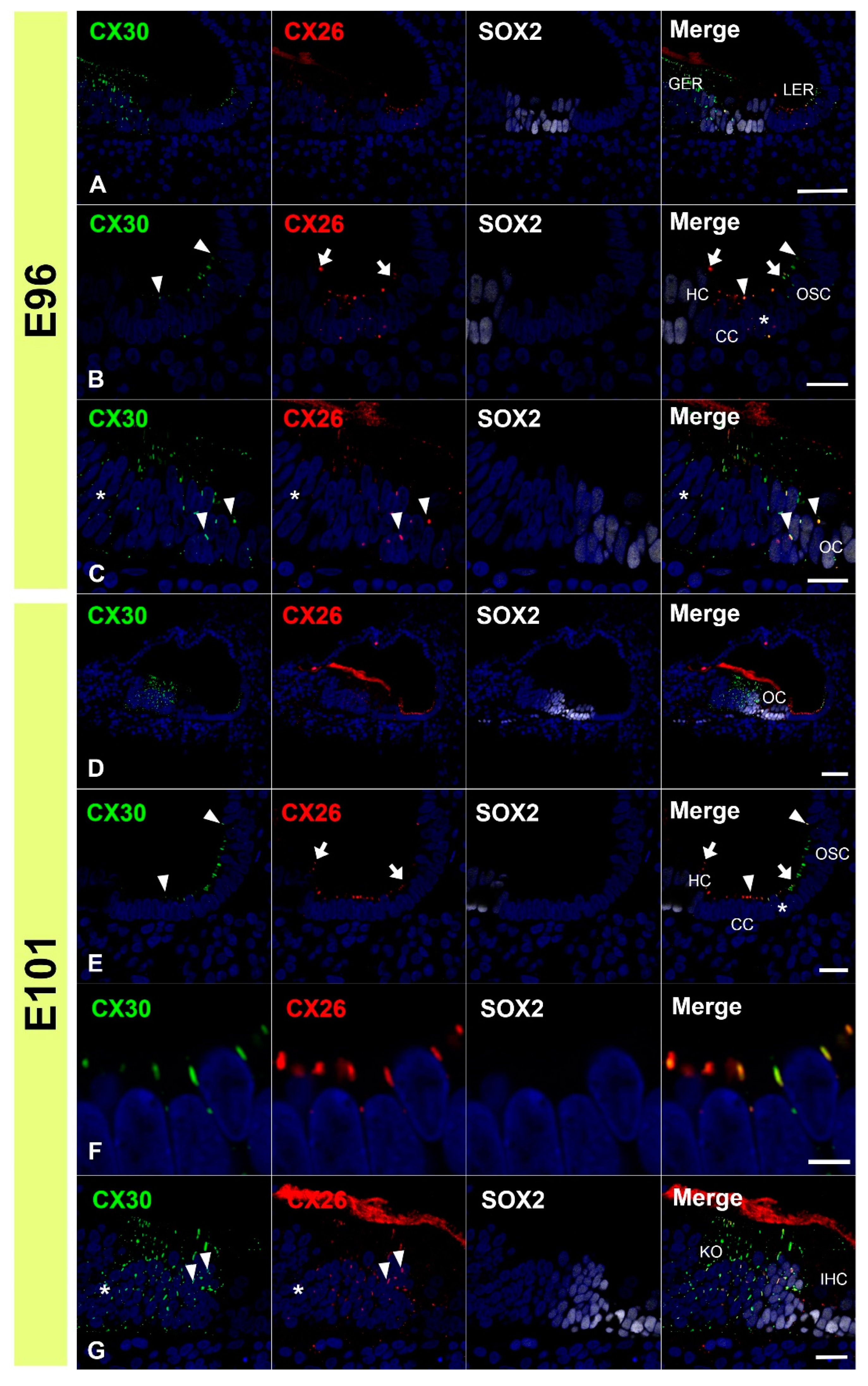
3.3. Comparing the Expression Patterns of CX26 and CX30 in the Sensory Epithelium
3.4. Comparison of the Expression Patterns of CX26 and CX30 in Lateral Wall Fibrocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehl, A.L.; Thomson, V. Newborn hearing screening: The great omission. Pediatrics 1998, 101, E4. [Google Scholar] [CrossRef] [Green Version]
- Mehl, A.L.; Thomson, V. The Colorado newborn hearing screening project, 1992–1999: On the threshold of effective popula-tion-based universal newborn hearing screening. Pediatrics 2002, 109, E7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. World Report on Hearing; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Morton, N.E. Genetic epidemiology of hearing impairment. Ann. N. Y. Acad. Sci. 1991, 630, 16–31. [Google Scholar] [CrossRef]
- Zelante, L.; Gasparini, P.; Estivill, X.; Melchionda, S.; D’Agruma, A.; Govea, N.; Milá, M.; Monica, M.D.; Lutfi, J.; Shohat, M.; et al. Connexin26 mutations associated with the most common form of non-syndromic neurosensory autosomal recessive deafness (DFNB1) in Mediterraneans. Hum. Mol. Genet. 1997, 6, 1605–1609. [Google Scholar] [CrossRef] [Green Version]
- Denoyelle, F.; Marlin, S.; Weil, D.; Moatti, L.; Chauvin, P.; Garabédian, É.-N.; Petit, C. Clinical features of the prevalent form of childhood deafness, DFNB1, due to a connexin-26 gene defect: Implications for genetic counselling. Lancet 1999, 353, 1298–1303. [Google Scholar] [CrossRef]
- Green, G.E.; Scott, D.A.; McDonald, J.M.; Woodworth, G.G.; Sheffield, V.; Smith, R.J.H. Carrier rates in the midwestern United States for GJB2 mutations causing inherited deafness. JAMA 1999, 281, 2211–2216. [Google Scholar] [CrossRef] [Green Version]
- Abe, S.; Usami, S.-I.; Shinkawa, H.; Kelley, P.M.; Kimberling, W.J. Prevalent connexin 26 gene (GJB2) mutations in Japanese. J. Med. Genet. 2000, 37, 41–43. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Xia, X.; Ke, X.; Ouyang, X.; Du, L.; Liu, Y.; Angeli, S.; Telischi, F.F.; Nance, W.E.; Balkany, T.; et al. The prevalence of connexin 26 (GJB2) mutations in the Chinese population. Hum. Genet. 2002, 111, 394–397. [Google Scholar] [CrossRef]
- Tsukada, K.; Nishio, S.; Usami, S.; The Deafness Gene Study Consortium. A large cohort study of GJB2 mutations in Japanese hearing loss patients. Clin. Genet. 2010, 78, 464–470. [Google Scholar] [CrossRef]
- Cohen-Salmon, M.; Ott, T.; Michel, V.; Jean-Pierre, H.; Perfettini, I.; Eybalin, M.; Wu, T.; Marcus, D.C.; Wangemann, P.; Willecke, K.; et al. Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impair-ment and cell death. Curr. Biol. 2002, 12, 1106–1111. [Google Scholar] [CrossRef]
- Kudo, T.; Kure, S.; Ikeda, K.; Xia, A.-P.; Katori, Y.; Suzuki, M.; Kojima, K.; Ichinohe, A.; Suzuki, Y.; Aoki, Y.; et al. Transgenic expression of a dominant-negative connexin26 causes degeneration of the organ of Corti and non-syndromic deafness. Hum. Mol. Genet. 2003, 12, 995–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Chang, Q.; Tang, W.; Sun, Y.; Zhou, B.; Li, H.; Lin, X. Targeted connexin26 ablation arrests postnatal development of the organ of Corti. Biochem. Biophys. Res. Commun. 2009, 385, 33–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Chen, J.; Zhu, Y.; Liang, C.; Zhao, H.-B. Deafness induced by Connexin 26 (GJB2) deficiency is not determined by endocochlear potential (EP) reduction but is associated with cochlear developmental disorders. Biochem. Biophys. Res. Com-mun. 2014, 448, 28–32. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Liang, C.; Chen, J.; Zong, L.; Chen, G.-D.; Zhao, H.-B. Active cochlear amplification is dependent on supporting cell gap junctions. Nat. Commun. 2013, 4, 1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teubner, B.; Michel, V.; Pesch, J.; Lautermann, J.; Cohen-Salmon, M.; Söhl, G.; Jahnke, K.; Winterhager, E.; Herberhold, C.; Hardelin, J.-P.; et al. Connexin30 (Gjb6)-deficiency causes severe hearing impairment and lack of endocochlear potential. Hum. Mol. Genet. 2003, 12, 13–21. [Google Scholar] [CrossRef]
- Lautermann, J.; Cate, W.-J.F.T.; Altenhoff, P.; Grümmer, R.; Traub, O.; Frank, H.-G.; Jahnke, K.; Winterhager, E. Expression of the gap-junction connexins 26 and 30 in the rat cochlea. Cell Tissue Res. 1998, 294, 415–420. [Google Scholar] [CrossRef]
- Lautermann, J.; Frank, H.-G.; Jahnke, K.; Traub, O.; Winterhager, E. Developmental expression patterns of connexin26 and -30 in the rat cochlea. Dev. Genet. 1999, 25, 306–311. [Google Scholar] [CrossRef]
- Forge, A.; Becker, D.; Casalotti, S.; Edwards, J.; Marziano, N.; Nevill, G. Gap junctions in the inner ear: Comparison of dis-tribution patterns in different vertebrates and assessement of connexin composition in mammals. J. Comp. Neurol. 2003, 467, 207–231. [Google Scholar] [CrossRef]
- del Castillo, I.; Villamar, M.; Moreno-Pelayo, M.A.; del Castillo, F.J.; Álvarez, A.; Tellería, D.; Menéndez, I.; Moreno, F. A deletion involving the connexin 30 gene in nonsyndromic hearing impairment. N. Engl. J. Med. 2002, 346, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Chen, J.; Zong, L.; Zhu, Y.; Liang, C.; Jones, O.; Zhao, H.-B. A deafness mechanism of digenic Cx26 (GJB2) and Cx30 (GJB6) mutations: Reduction of endocochlear potential by impairment of heterogeneous gap junctional function in the cochlear lateral wall. Neurobiol. Dis. 2017, 108, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, Y.; Guan, J.; Lan, L.; Yang, J.; Xiong, W.; Zhao, C.; Xie, L.; Yu, L.; Wang, D.; et al. Phenotypic Heterogeneity of Post-lingual and/or Milder Hearing Loss for the Patients With the GJB2 c.235delC Homozygous Mutation. Front. Cell Dev. Biol. 2021, 9, 647240. [Google Scholar] [CrossRef]
- Hosoya, M.; Fujioka, M.; Sone, T.; Okamoto, S.; Akamatsu, W.; Ukai, H.; Ueda, H.R.; Ogawa, K.; Matsunaga, T.; Okano, H. Cochlear Cell Modeling Using Disease-Specific iPSCs Unveils a Degenerative Phenotype and Suggests Treatments for Congenital Progressive Hearing Loss. Cell Rep. 2017, 18, 68–81. [Google Scholar] [CrossRef]
- Ishihara, K.; Okuyama, S.; Kumano, S.; Iida, K.; Hamana, H.; Murakoshi, M.; Kobayashi, T.; Usami, S.; Ikeda, K.; Haga, Y.; et al. Salicylate restores transport function and anion exchanger activity of missense pendrin mutations. Hear. Res. 2010, 270, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, A.; Colbert, B.M.; Nisenbaum, E.; El-Amraoui, A.; Dykxhoorn, D.M.; Koehler, K.R.; Chen, Z.-Y.; Liu, X.Z. Stem Cells and Gene Therapy in Progressive Hearing Loss: The State of the Art. J. Assoc. Res. Otolaryngol. 2021, 22, 95–105. [Google Scholar]
- Takeda, H.; Hosoya, M.; Fujioka, M.; Saegusa, C.; Saeki, T.; Miwa, T.; Okano, H.; Minoda, R. Engraftment of Human Plu-ripotent Stem Cell-derived Progenitors in the Inner Ear of Prenatal Mice. Sci Rep. 2018, 8, 1941. [Google Scholar] [CrossRef] [Green Version]
- Minoda, R.; Miwa, T.; Ise, M.; Takeda, H. Potential treatments for genetic hearing loss in humans: Current conundrums. Gene Ther. 2015, 22, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, H.; Shibata, S.B.; Ranum, P.T.; Moteki, H.; Smith, R.J.H. Targeted Allele Suppression Prevents Progressive Hearing Loss in the Mature Murine Model of Human TMC1 Deafness. Mol. Ther. 2019, 27, 681–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akil, O.; Seal, R.P.; Burke, K.; Wang, C.; Alemi, A.; During, M.; Edwards, R.H.; Lustig, L.R. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron 2012, 75, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Akil, O.; Dyka, F.; Calvet, C.; Emptoz, A.; Lahlou, G.; Nouaille, S.; de Monvel, J.B.; Hardelin, J.-P.; Hauswirth, W.; Avan, P.; et al. Dual AAV-mediated gene therapy restores hearing in a DFNB9 mouse model. Proc. Natl. Acad. Sci. USA 2019, 116, 4496–4501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miwa, T.; Minoda, R.; Ise, M.; Yamada, T.; Yumoto, E. Mouse otocyst transuterine gene transfer restores hearing in mice with connexin 30 deletion-associated hearing loss. Mol. Ther. 2013, 21, 1142–1150. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Wang, Y.; Chang, Q.; Wang, J.; Gong, S.; Li, H.; Lin, X. Virally expressed connexin26 restores gap junction function in the cochlea of conditional Gjb2 knockout mice. Gene Ther. 2014, 21, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Iizuka, T.; Kamiya, K.; Gotoh, S.; Sugitani, Y.; Suzuki, M.; Noda, T.; Minowa, O.; Ikeda, K. Perinatal Gjb2 gene transfer res-cues hearing in a mouse model of hereditary deafness. Hum. Mol. Genet. 2015, 24, 3651–3661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crispino, G.; Di Pasquale, G.; Scimemi, P.; Rodriguez, L.; Ramírez, F.G.; De Siati, R.D.; Santarelli, R.M.; Arslan, E.; Bortoloz-zi, M.; Chiorini, J.A.; et al. BAAV Mediated GJB2 Gene Transfer Restores Gap Junction Coupling in Cochlear Organotypic Cultures from Deaf Cx26Sox10Cre Mice. PLoS ONE 2011, 6, e23279. [Google Scholar] [CrossRef] [Green Version]
- Hosoya, M.; Fujioka, M.; Murayama, A.Y.; Okano, H.; Ogawa, K. The common marmoset as suitable nonhuman alternative for the analysis of primate cochlear development. FEBS J. 2021, 288, 325–353. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, M.; Fujioka, M.; Ogawa, K.; Okano, H. Distinct Expression Patterns Of Causative Genes Responsible For Hereditary Progressive Hearing Loss In Non-Human Primate Cochlea. Sci. Rep. 2016, 6, 22250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, N.; Hosoya, M.; Oishi, N.; Okano, H.; Fujioka, M.; Ogawa, K. Expression pattern of wolframin, the WFS1 (Wolfram syndrome-1 gene) product, in common marmoset (Callithrix jacchus) cochlea. NeuroReport 2016, 27, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Hosoya, M.; Okano, H.; Fujioka, M.; Ogawa, K. Expression pattern of EYA4 in the common marmoset (Callithrix jacchus) cochlea. Neurosci. Lett. 2018, 662, 185–188. [Google Scholar] [CrossRef]
- Kikuchi, T.; Adams, J.C.; Miyabe, Y.; So, E.; Kobayashi, T. Potassium ion recycling pathway via gap junction systems in the mammalian cochlea and its interruption in hereditary nonsyndromic deafness. Med. Electron. Microsc. 2000, 33, 51–56. [Google Scholar] [CrossRef]
- Sun, J.; Ahmad, S.; Chen, S.; Tang, W.; Zhang, Y.; Chen, P.; Lin, X. Cochlear gap junctions coassembled from Cx26 and 30 show faster intercellular Ca2+ signaling than homomeric counterparts. Am. J. Physiol. Cell Physiol. 2005, 288, C613–C623. [Google Scholar] [CrossRef] [Green Version]
- Locher, H.; De Groot, J.C.; Van Iperen, L.; Huisman, M.A.; Frijns, J.H.; Chuva de Sousa Lopes, S.M. Development of the stria vascularis and potassium regulation in the human fetal cochlea: Insights into hereditary sensorineural hearing loss. Dev. Neurobiol. 2015, 75, 1219–1240. [Google Scholar] [CrossRef] [Green Version]
- Kammen-Jolly, K.; Ichiki, H.; Scholtz, A.W.; Gsenger, M.; Kreczy, A.; Schrott-Fischer, A. Connexin 26 in human fetal devel-opment of the inner ear. Hear. Res. 2001, 160, 15–21. [Google Scholar] [CrossRef]
- Naulin, P.A.; Lozano, B.; Fuentes, C.; Liu, Y.; Schmidt, C.; Contreras, J.E.; Barrera, N.P. Polydisperse molecular architecture of connexin 26/30 heteromeric hemichannels revealed by atomic force microscopy imaging. J. Biol. Chem. 2020, 295, 16499–16509. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Chen, S.; Sun, J.; Lin, X. Connexins 26 and 30 are co-assembled to form gap junctions in the cochlea of mice. Bio-chem. Biophys. Res. Commun. 2003, 307, 362–368. [Google Scholar] [CrossRef]
- Cottrell, G.T.; Burt, J.M. Functional consequences of heterogeneous gap junction channel formation and its influence in health and disease. Biochim. Biophys. Acta Biomembr. 2005, 1711, 126–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosoya, M.; Fujioka, M.; Murayama, A.Y.; Ogawa, K.; Okano, H.; Ozawa, H. Dynamic Spatiotemporal Expression Changes in Connexins of the Developing Primate’s Cochlea. Genes 2021, 12, 1082. https://doi.org/10.3390/genes12071082
Hosoya M, Fujioka M, Murayama AY, Ogawa K, Okano H, Ozawa H. Dynamic Spatiotemporal Expression Changes in Connexins of the Developing Primate’s Cochlea. Genes. 2021; 12(7):1082. https://doi.org/10.3390/genes12071082
Chicago/Turabian StyleHosoya, Makoto, Masato Fujioka, Ayako Y. Murayama, Kaoru Ogawa, Hideyuki Okano, and Hiroyuki Ozawa. 2021. "Dynamic Spatiotemporal Expression Changes in Connexins of the Developing Primate’s Cochlea" Genes 12, no. 7: 1082. https://doi.org/10.3390/genes12071082
APA StyleHosoya, M., Fujioka, M., Murayama, A. Y., Ogawa, K., Okano, H., & Ozawa, H. (2021). Dynamic Spatiotemporal Expression Changes in Connexins of the Developing Primate’s Cochlea. Genes, 12(7), 1082. https://doi.org/10.3390/genes12071082






