Altered Expression of DAAM1 and PREP Induced by Cadmium Toxicity Is Counteracted by Melatonin in the Rat Testis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Evaluation of Testicular CAT and SOD Activities
2.3. Thiobarbituric Acid-Reactive Species (TBARS) Levels Assessment
2.4. Histology and TUNEL Assay
2.5. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.6. Protein Extraction and Western Blotting (WB) Analysis
2.7. Immunofluorescence (IF) Analysis
2.8. Statistical Analysis
3. Results
3.1. Histological Study
3.2. Oxidative Stress Markers
3.2.1. CAT and SOD Activities
3.2.2. Lipid Peroxidation
3.3. Effect of Cd and/or MLT on Apoptosis
3.4. Effect of Cd and/or MLT Testicular Steroidogenesis
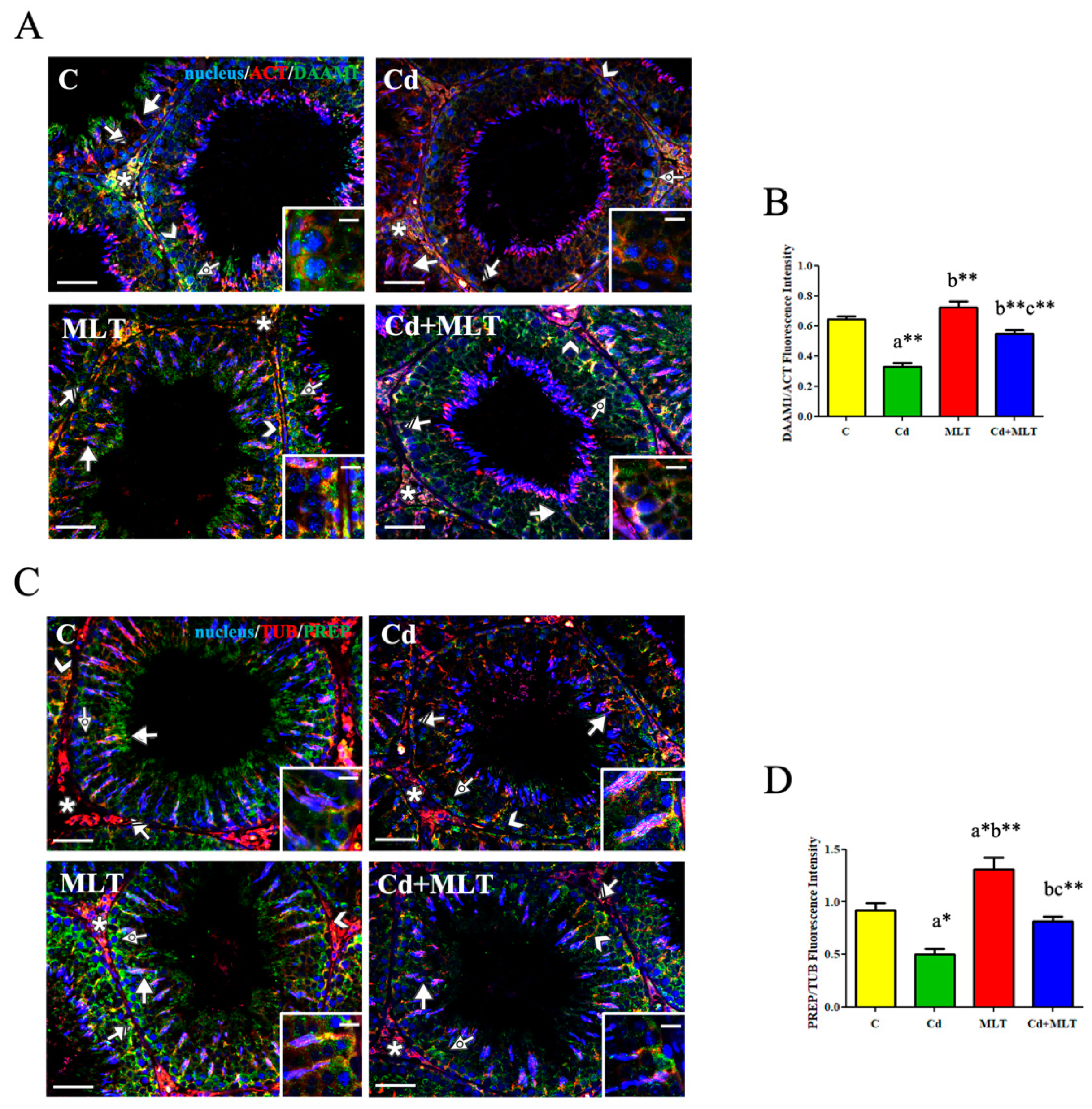
3.5. Effect of Cd and/or MLT on DAAM1 and PREP
3.5.1. RT-PCR and WB Analysis
3.5.2. Immunofluorescence Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 1–9. [Google Scholar] [CrossRef]
- Gabrielsen, J.S.; Tanrikut, C. Chronic exposures and male fertility: The impacts of environment, diet, and drug use on spermatogenesis. Andrology 2016, 4, 648–661. [Google Scholar] [CrossRef]
- Gao, Y.; Mruk, D.D.; Cheng, C.Y. Sertoli cells are the target of environmental toxicants in the testis—A mechanistic and therapeutic insight. Expert Opin. Ther. Targets 2015, 19, 1073–1090. [Google Scholar] [CrossRef]
- Ramgir, S.S.; Abilash, V.G. Genetic and environmental factors involved in human male infertility: A review. Asian J. Pharm. Clin. Res. 2015, 8, 34–43. [Google Scholar]
- Bhardwaj, J.K.; Panchal, H.; Saraf, P. Cadmium as a testicular toxicant: A Review. J. Appl. Toxicol. 2021, 41, 105–117. [Google Scholar] [CrossRef]
- Wirth, J.J.; Mijal, R.S. Adverse Effects of Low Level Heavy Metal Exposure on Male Reproductive Function. Syst. Biol. Reprod. Med. 2010, 56, 147–167. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, C.; Galdiero, M.; Pivonello, C.; Salzano, C.; Gianfrilli, D.; Piscitelli, P.; Lenzi, A.; Colao, A.; Pivonello, R. The environment and male reproduction: The effect of cadmium exposure on reproductive function and its implication in fertility. Reprod. Toxicol. 2017, 73, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Li, X.; Ge, R.-S. Toxicological Effects of Cadmium on Mammalian Testis. Front. Genet. 2020, 11, 527. [Google Scholar] [CrossRef] [PubMed]
- Cupertino, M.C.; Novaes, R.D.; Santos, E.C.; Neves, A.C.; Silva, E.; Oliveira, J.; Matta, S.L.P. Differential Susceptibility of Germ and Leydig Cells to Cadmium-Mediated Toxicity: Impact on Testis Structure, Adiponectin Levels, and Steroidogenesis. Oxidative Med. Cell. Longev. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Niknafs, B.; Salehnia, M.; Kamkar, M. Induction and determination of apoptotic and necrotic cell death by cad-mium chloride in testis tissue of mouse. J. Reprod. Infertil. 2015, 16, 24–29. [Google Scholar]
- Zhao, L.-L.; Ru, Y.-F.; Liu, M.; Tang, J.-N.; Zheng, J.-F.; Wu, B.; Gu, Y.-H.; Shi, H.-J. Reproductive effects of cadmium on sperm function and early embryonic development in vitro. PLoS ONE 2017, 12, e0186727. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, Y.; Chen, H.; Jesus, T.; Tang, E.; Li, N.; Lian, Q.; Ge, R.-S.; Cheng, C.Y. Cell polarity, cell adhesion, and spermatogenesis: Role of cytoskeletons. F1000Research 2017, 6, 1565. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Tang, E.I.; Xiao, X.; Gao, Y.; Chu, D.S.; Mruk, D.D.; Silvestrini, B.; Cheng, C.Y. Transport of germ cells across the seminiferous epithelium during spermatogenesis-the involvement of both actin- and microtubule-based cytoskele-tons. Tissue Barriers 2016, 4, e1265042-1–e1265042-13. [Google Scholar] [CrossRef] [PubMed]
- Lie, P.P.Y.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Cytoskeletal dynamics and spermatogenesis. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Pariante, P.; Dotolo, R.; Venditti, M.; Ferrara, D.; Donizetti, A.; Aniello, F.; Minucci, S. First Evidence of DAAM1 Localization During the Post-Natal Development of Rat Testis and in Mammalian Sperm. J. Cell. Physiol. 2016, 231, 2172–2184. [Google Scholar] [CrossRef] [PubMed]
- Santillo, A.; Venditti, M.; Minucci, S.; Baccari, G.C.; Falvo, S.; Rosati, L.; Di Fiore, M.M. D-Asp upregulates PREP and GluA2/3 expressions and induces p-ERK1/2 and p-Akt in rat testis. Reproduction 2019, 158, 357–367. [Google Scholar] [CrossRef]
- Venditti, M.; Minucci, S. Subcellular Localization of Prolyl Endopeptidase During the First Wave of Rat Spermatogenesis and in Rat and Human Sperm. J. Histochem. Cytochem. 2019, 67, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Venditti, M.; Santillo, A.; Falvo, S.; Di Fiore, M.M.; Baccari, G.C.; Minucci, S. D-Aspartate Upregulates DAAM1 Protein Levels in the Rat Testis and Induces Its Localization in Spermatogonia Nucleus. Biomolecules 2020, 10, 677. [Google Scholar] [CrossRef]
- Chemek, M.; Venditti, M.; Boughamoura, S.; Mimouna, S.B.; Messaoudi, I.; Minucci, S. Involvement of testicular DAAM1 expression in zinc protection against cadmium-induced male rat reproductive toxicity. J. Cell. Physiol. 2018, 233, 630–640. [Google Scholar] [CrossRef]
- Venditti, M.; Chemek, M.; Minucci, S.; Messaoudi, I. Cadmium-induced toxicity increases prolyl endopeptidase (PREP) expression in the rat testis. Mol. Reprod. Dev. 2020, 87, 565–573. [Google Scholar] [CrossRef]
- Knani, L.; Venditti, M.; Kechiche, S.; Banni, M.; Messaoudi, I.; Minucci, S. Melatonin protects bone against cadmium-induced toxicity via activation of Wnt/β-catenin signaling pathway. Toxicol. Mech. Methods 2019, 30, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Kechiche, S.; Venditti, M.; Knani, L.; Jabłońska, K.; Dzięgiel, P.; Messaoudi, I.; Reiter, R.J.; Minucci, S. First evidence of the protective role of melatonin in counteracting cadmium toxicity in the rat ovary via the mTOR pathway. Environ. Pollut. 2021, 270, 116056. [Google Scholar] [CrossRef]
- Reiter, R.; Fraschini, F. Endocrine Aspects of the Mammalian Pineal Gland: A Review. Neuroendocrinology 1969, 5, 219–255. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Sharma, R. Central and peripheral actions of melatonin on reproduction in seasonal and continuous breeding mammals. Gen. Comp. Endocrinol. 2021, 300, 113620. [Google Scholar] [CrossRef] [PubMed]
- Jou, M.-J.; Peng, T.-I.; Reiter, R.J.; Jou, S.-B.; Wu, H.-Y.; Wen, S.-T. Visualization of the antioxidative effects of melatonin at the mitochondrial level during oxidative stress-induced apoptosis of rat brain astrocytes. J. Pineal Res. 2004, 37, 55–70. [Google Scholar] [CrossRef]
- Kopustinskiene, D.; Bernatoniene, J. Molecular Mechanisms of Melatonin-Mediated Cell Protection and Signaling in Health and Disease. Pharmaceutics 2021, 13, 129. [Google Scholar] [CrossRef]
- Loren, P.; Sánchez, R.; Arias, M.-E.; Felmer, R.; Risopatrón, J.; Cheuquemán, C. Melatonin Scavenger Properties against Oxidative and Nitrosative Stress: Impact on Gamete Handling and In Vitro Embryo Production in Humans and Other Mammals. Int. J. Mol. Sci. 2017, 18, 1119. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Manchester, L.C.; Qi, W. Biochemical Reactivity of Melatonin with Reactive Oxygen and Nitrogen Species: A Review of the Evidence. Cell Biophys. 2001, 34, 237–256. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Moniuszko-Jakoniuk, J. Bone metabolism of male rats chronically exposed to cadmium. Toxicol. Appl. Pharmacol. 2005, 207, 195–211. [Google Scholar] [CrossRef]
- Chwełatiuk, E.; Wlostowski, T.; Krasowska, A.; Bonda-Ostaszewska, E. The effect of orally administered melatonin on tissue accumulation and toxicity of cadmium in mice. J. Trace Elements Med. Biol. 2006, 19, 259–265. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase Activity. In Handbook of Methods for Oxygen Radical Research, 1st ed.; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Lama, S.; Vanacore, D.; Diano, N.; Nicolucci, C.; Errico, S.; Dallio, M.; Federico, A.; Loguercio, C.; Stiuso, P. Ameliorative effect of Silybin on bisphenol A induced oxidative stress, cell proliferation and steroid hormones oxidation in HepG2 cell cultures. Sci. Rep. 2019, 9, 3228. [Google Scholar] [CrossRef]
- Pariante, P.; Dotolo, R.; Venditti, M.; Ferrara, D.; Donizetti, A.; Aniello, F.; Minucci, S. Prothymosin α ex-pression and localization during the spermatogenesis of Danio rerio. Zygote 2016, 24, 583–593. [Google Scholar] [CrossRef]
- Ergoli, M.; Venditti, M.; Picillo, E.; Minucci, S.; Politano, L. Study of expression of genes potentially responsible for re-duced fitness in patients with myotonic dystrophy type 1 and identification of new biomarkers of testicular function. Mol. Reprod. Dev. 2020, 87, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Venditti, M.; Fasano, C.; Santillo, A.; Aniello, F.; Minucci, S. First evidence of DAAM1 localization in mouse seminal vesicles and its possible involvement during regulated exocytosis. C. R. Biol. 2018, 341, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.S.; Shakes, D.C. Spermatogenesis. Chem. Biol. Pteridines Folates 2012, 757, 171–203. [Google Scholar] [CrossRef]
- Venditti, M.; Donizetti, A.; Aniello, F.; Minucci, S. EH domain binding protein 1-like 1 (EHBP1L1), a protein with calponin homology domain, is expressed in the rat testis. Zygote 2020, 28, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, Z.; Song, W.; Hong, D.; Huang, L.; Li, Y. A review on Cadmium Exposure in the Population and Intervention Strategies against Cadmium Toxicity. Bull. Environ. Contam. Toxicol. 2021, 106, 65–74. [Google Scholar] [CrossRef]
- Thompson, J.; Bannigan, J. Cadmium: Toxic effects on the reproductive system and the embryo. Reprod. Toxicol. 2008, 25, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Eybl, V.; Kotyzova, D.; Koutensky, J. Comparative study of natural antioxidants—Curcumin, resveratrol and melatonin—In cadmium-induced oxidative damage in mice. Toxicology 2006, 225, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.-L.; Wang, H.; Meng, C.; Zhao, X.-F.; Zhang, C.; Zhang, Y.; Zhao, M.; Chen, Y.-H.; Meng, X.-H.; Xu, D.-X. Melatonin alleviates cadmium-induced cellular stress and germ cell apoptosis in testes. J. Pineal Res. 2011, 52, 71–79. [Google Scholar] [CrossRef]
- Kara, H.; Cevik, A.; Konar, V.; Dayangac, A.; Yilmaz, M. Protective Effects of Antioxidants Against Cadmium-induced Oxidative Damage in Rat Testes. Biol. Trace Element Res. 2007, 120, 205–211. [Google Scholar] [CrossRef]
- Karbownik, M.; Gitto, E.; Lewinski, A.; Reiter, R. Induction of lipid peroxidation in hamster organs by the carcinogen cadmium: Amelioration by melatonin. Cell Biol. Toxicol. 2001, 17, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Luo, X.; Li, L.; Peng, Q.; Yang, Y.; Zhao, L.; Ma, M.; Hou, Z. The Protective Effects of Melatonin Against Oxidative Stress and Inflammation Induced by Acute Cadmium Exposure in Mice Testis. Biol. Trace Element Res. 2015, 170, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Waisberg, M.; Joseph, P.; Hale, B.; Beyersmann, D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 2003, 192, 95–117. [Google Scholar] [CrossRef]
- Casalino, E.; Calzaretti, G.; Sblano, C.; Landriscina, C. Cadmium-Dependent Enzyme Activity Alteration Is Not Imputable to Lipid Peroxidation. Arch. Biochem. Biophys. 2000, 383, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab. J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef]
- Unsal, V.; Dalkiran, T.; Çiçek, M.; Kölükçü, E. The Role of Natural Antioxidants Against Reactive Oxygen Species Produced by Cadmium Toxicity: A Review. Adv. Pharm. Bull. 2020, 10, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Drevet, J.R. The Importance of Oxidative Stress in Determining the Functionality of Mammalian Spermatozoa: A Two-Edged Sword. Antioxidants 2020, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, Z.; Zou, P.; Zhang, G.; Dong, X.; Ling, X.; Zhang, X.; Liu, J.; Ye, N.; Cao, J.; et al. Induction of DNA Damage and G2 Cell Cycle Arrest by Diepoxybutane through the Activation of the Chk1-Dependent Pathway in Mouse Germ Cells. Chem. Res. Toxicol. 2015, 28, 518–531. [Google Scholar] [CrossRef]
- Migliaccio, V.; Sica, R.; Scudiero, R.; Simoniello, P.; Putti, R.; Lionetti, L. Physiological Adaptation to Simultaneous Chronic Exposure to High-Fat Diet and Dichlorodipheniletylhene (DDE) in Wistar Rat Testis. Cells 2019, 8, 443. [Google Scholar] [CrossRef]
- Naumova, N.; Šachl, R. Regulation of Cell Death by Mitochondrial Transport Systems of Calcium and Bcl-2 Proteins. Membranes 2020, 10, 299. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, L.; Dong, X.; Wang, H.; He, L.; Zhong, S. Downregulation of vdac2 inhibits spermatogenesis via JNK and P53 signalling in mice exposed to cadmium. Toxicol. Lett. 2020, 326, 114–122. [Google Scholar] [CrossRef]
- Plunk, E.C.; Richards, S.M. Endocrine-Disrupting Air Pollutants and Their Effects on the Hypothalamus-Pituitary-Gonadal Axis. Int. J. Mol. Sci. 2020, 21, 9191. [Google Scholar] [CrossRef]
- Lafuente, A.; Márquez, N.; Pérez-Lorenzo, M.; Pazo, D.; Esquifino, A.I. Cadmium effects on hypothalamic-pituitary-testicular axis in male rats. Exp. Biol. Med. 2001, 226, 605–611. [Google Scholar] [CrossRef]
- Lafuente, A. The hypothalamic–pituitary–gonadal axis is target of cadmium toxicity. An update of recent studies and potential therapeutic approaches. Food Chem. Toxicol. 2013, 59, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Venditti, M.; Romano, M.Z.; Aniello, F.; Minucci, S. Preliminary Investigation on the Ameliorative Role Exerted by D-Aspartic Acid in Counteracting Ethane Dimethane Sulfonate (EDS) Toxicity in the Rat Testis. Animals 2021, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, M.; Wong, C.K.C.; Ge, R.; Wu, X.; Sun, F.; Cheng, C.Y. Microtubule-associated proteins (MAPs) in microtubule cytoskeletal dynamics and spermatogenesis. Histol. Histopathol. 2021, 36, 249–265. [Google Scholar] [PubMed]
- Venditti, M.; Fasano, C.; Minucci, S.; Serino, I.; Sinisi, A.A.; Dale, B.; Di Matteo, L. DAAM1 and PREP are involved in human spermatogenesis. Reprod. Fertil. Dev. 2020, 32, 484. [Google Scholar] [CrossRef] [PubMed]
- Dotolo, R.; Kim, J.D.; Pariante, P.; Minucci, S.; Diano, S. Prolyl Endopeptidase (PREP) is Associated with Male Reproductive Functions and Gamete Physiology in Mice. J. Cell. Physiol. 2015, 231, 551–557. [Google Scholar] [CrossRef]
- Venditti, M.; Aniello, F.; Santillo, A.; Minucci, S. Study on PREP localization in mouse seminal vesicles and its possible involve-ment during regulated exocytosis. Zygote 2019, 27, 160–165. [Google Scholar] [CrossRef]
- Lafuente, A.; Márquez, N.; `pérez-Lorenzo, M.; Pazo, D.; Esquifino, A. Pubertal and postpubertal cadmium exposure differentially affects the hypothalamic–pituitary–testicular axis function in the rat. Food Chem. Toxicol. 2000, 38, 913–923. [Google Scholar] [CrossRef]
- Kato, T.; Nakano, T.; Kojima, K.; Nagatsu, T.; Sakakibara, S. Changes in Prolyl Endopeptidase during Maturation of Rat Brain and Hydrolysis of Substance P by the Purified Enzyme. J. Neurochem. 1980, 35, 527–535. [Google Scholar] [CrossRef]
- Agirregoitia, N.; Bizet, P.; Agirregoitia, E.; Boutelet, I.; Peralta, L.; Vaudry, H.; Jégou, S. Prolyl endopeptidase mRNA expression in the central nervous system during rat development. J. Chem. Neuroanat. 2010, 40, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Hannula, M.J.; Männistö, P.T.; Myöhänen, T.T. Sequential Expression, Activity and Nuclear Localization of Prolyl Oligopeptidase Protein in the Developing Rat Brain. Dev. Neurosci. 2011, 33, 38–47. [Google Scholar] [CrossRef]
- Benitez-King, G. Melatonin as a cytoskeletal modulator: Implications for cell physiology and disease. J. Pineal Res. 2006, 40, 1–9. [Google Scholar] [CrossRef]
- Gardiner, J.; Overall, R.; Marc, J. The Nervous System Cytoskeleton under Oxidative Stress. Diseases 2013, 1, 36–50. [Google Scholar] [CrossRef]
- Go, Y.-M.; Orr, M.; Jones, D.P. Actin cytoskeleton redox proteome oxidation by cadmium. Am. J. Physiol. Cell. Mol. Physiol. 2013, 305, L831–L843. [Google Scholar] [CrossRef]
- Hu, W.-G.; Lu, Q.-P. Impact of oxidative stress on the cytoskeleton of pancreatic epithelial cells. Exp. Ther. Med. 2014, 8, 1438–1442. [Google Scholar] [CrossRef][Green Version]
- Wilson, C.; González-Billault, G. Regulation of cytoskeletal dynamics by redox signaling and oxidative stress: Im-plications for neuronal development and trafficking. Front Cell Neurosci. 2015, 9, 381. [Google Scholar] [CrossRef] [PubMed]
- Babkova, K.; Korabecny, J.; Soukup, O.; Nepovimova, E.; Jun, D.; Kuca, K. Prolyl oligopeptidase and its role in the organism: Attention to the most promising and clinically relevant inhibitors. Future Med. Chem. 2017, 9, 1015–1038. [Google Scholar] [CrossRef]
- Xu, P.; Bao, R.; Zhang, Y.; Lu, E.; Feng, F.; Zhang, L.; Li, J.; Wang, J.; Tan, X.; Tang, M.; et al. Prolyl oligopeptidase regulates progesterone secretion via the ERK signaling pathway in murine luteal cells. Mol. Reprod. Dev. 2019, 86, 714–726. [Google Scholar] [CrossRef] [PubMed]
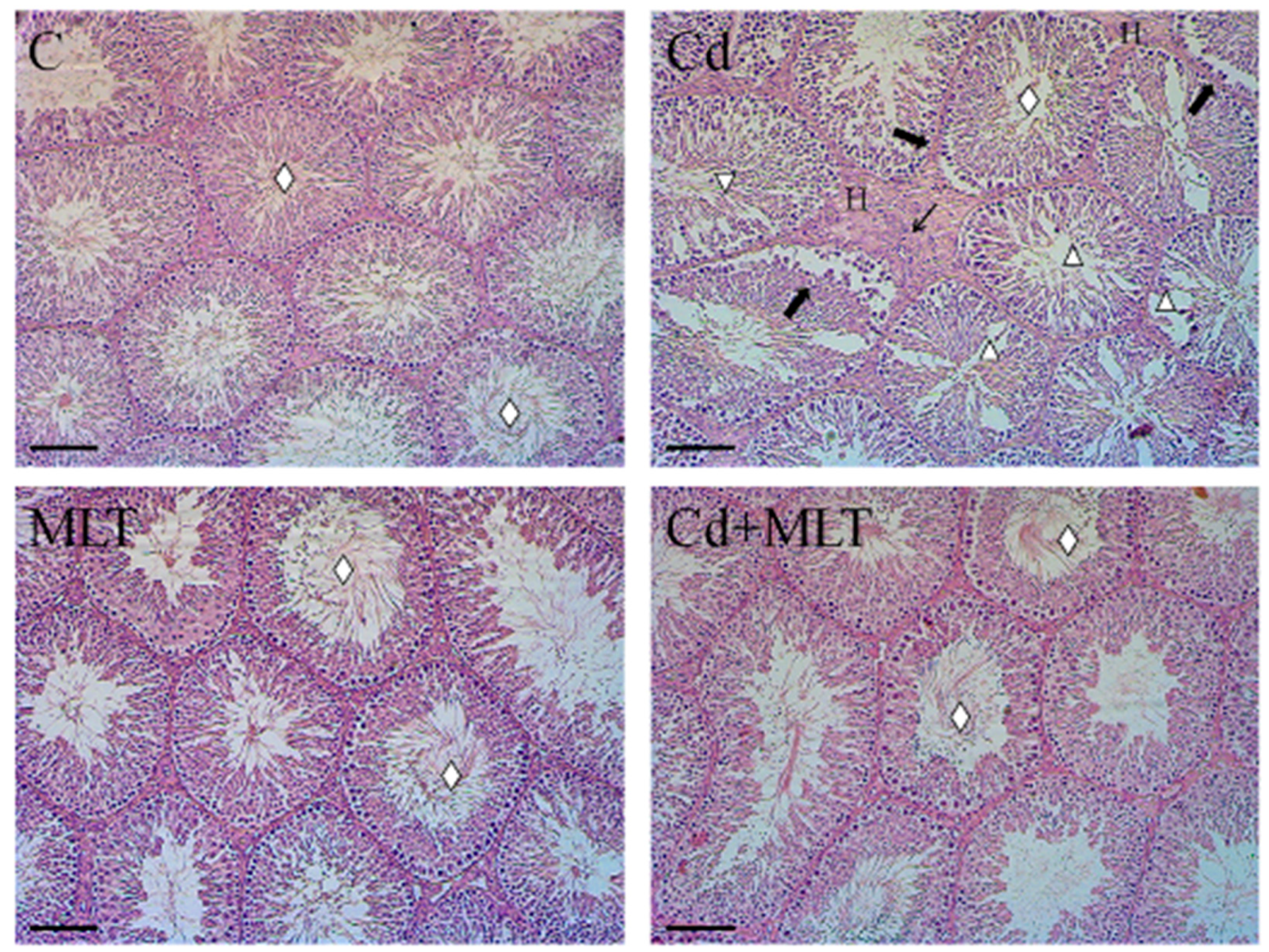
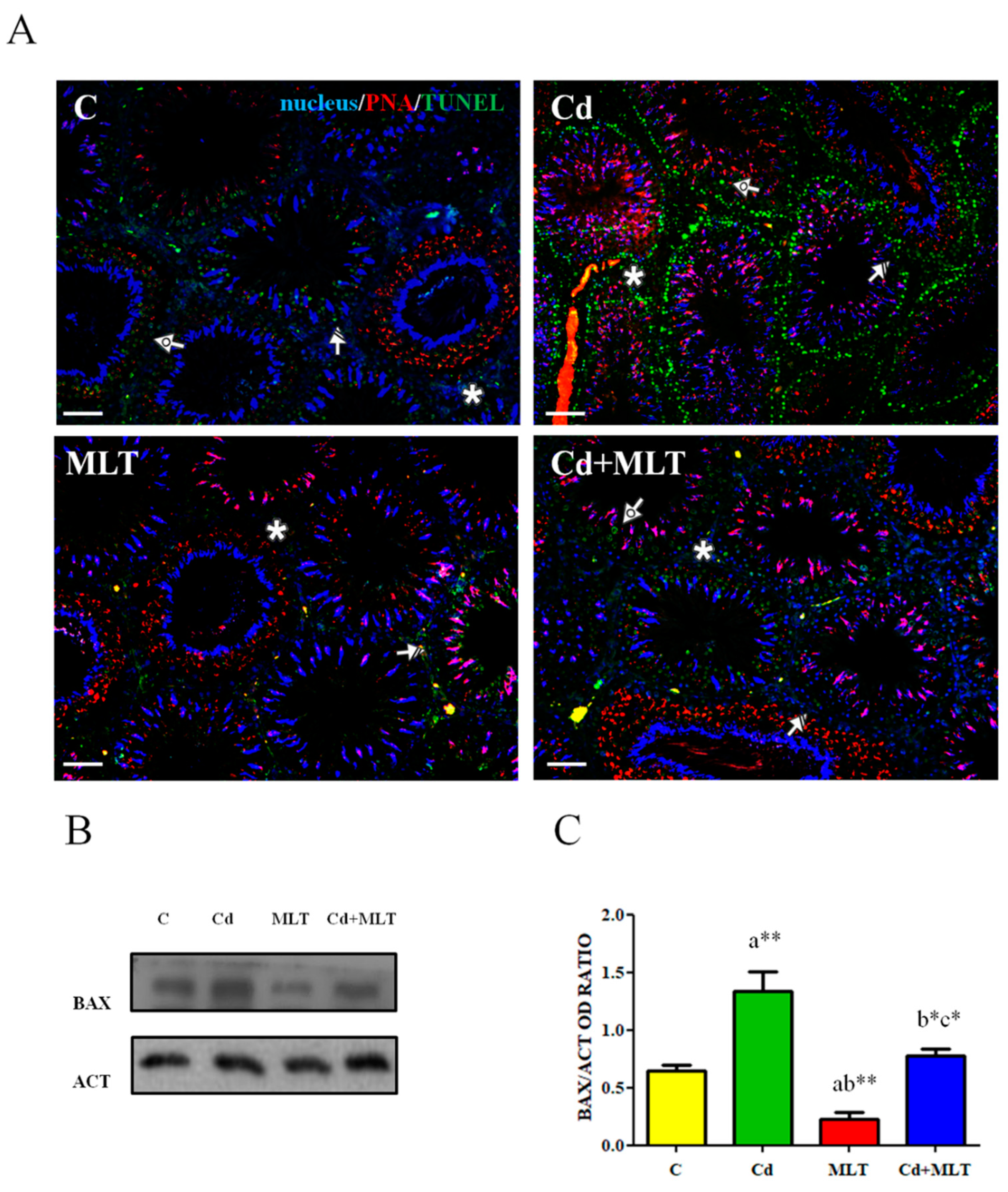
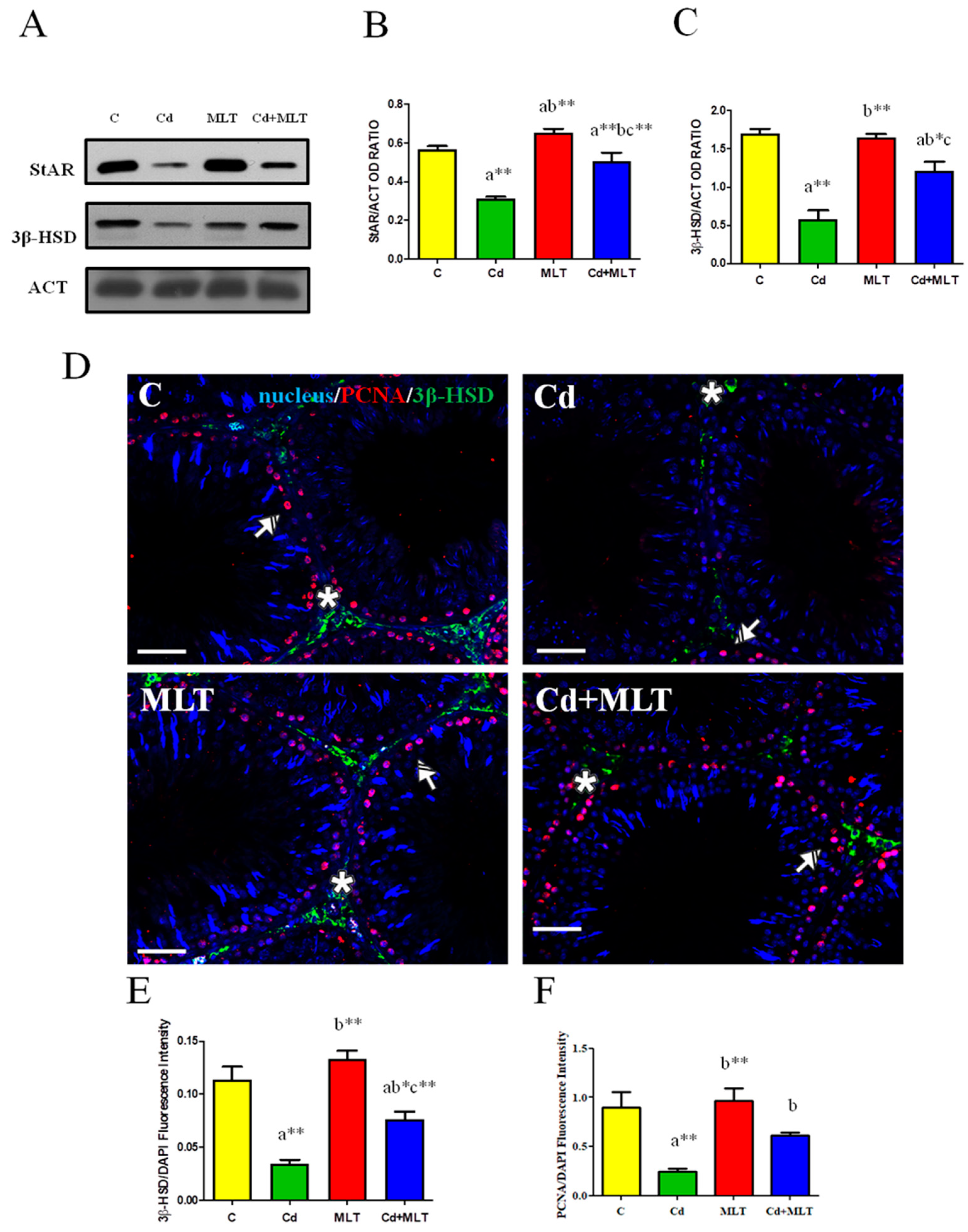
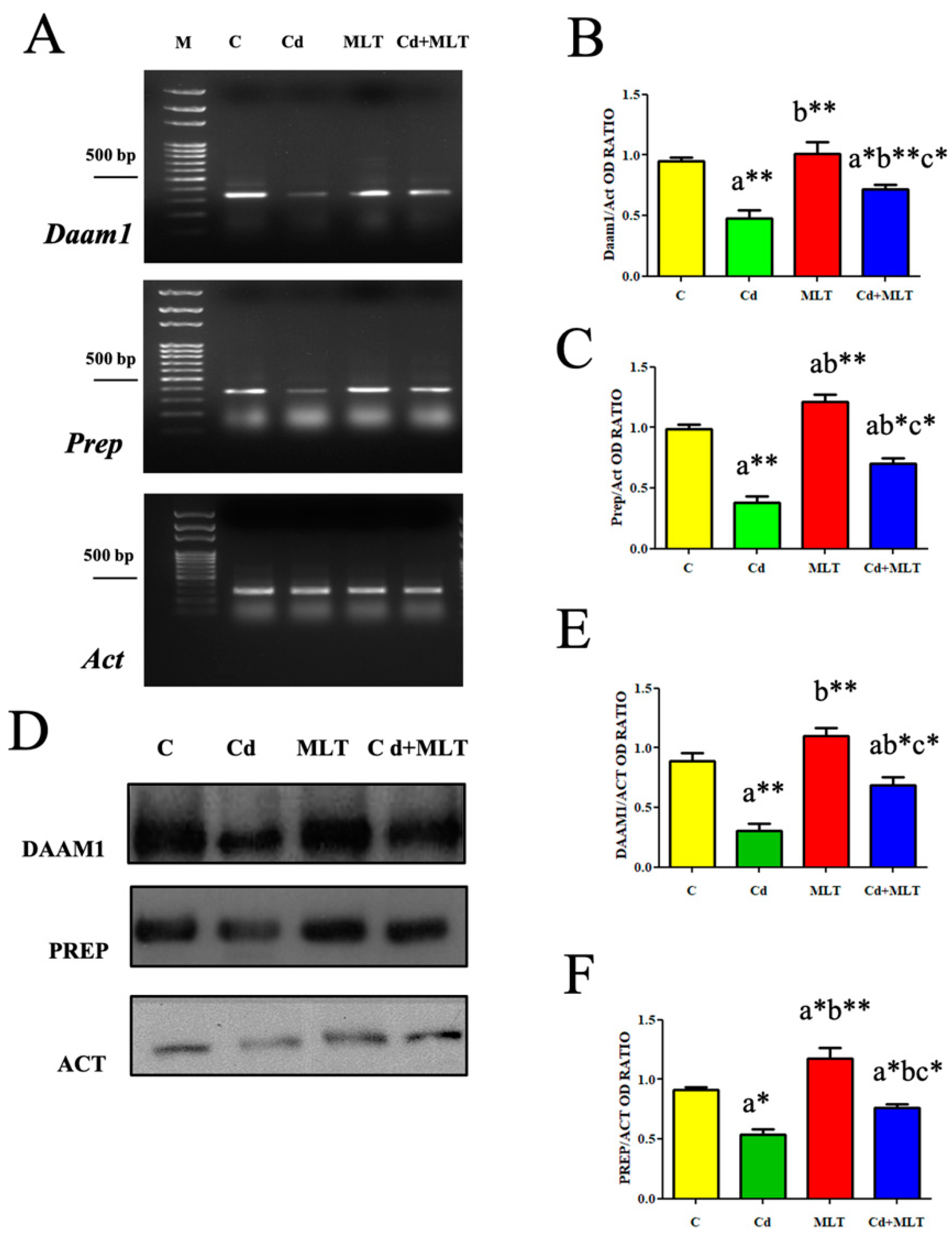
| Groups | C | Cd | MLT | Cd-MLT |
|---|---|---|---|---|
| Tubules Diameter (µm) | 168.19 ± 29.81 | 126.69 ± 21.52 a** | 162.22 ± 38.26 b** | 145.67 ± 19.38 a*b**c** |
| Epithelium Thickness (µm) | 46.01 ± 16.31 | 31.66 ± 9.74 a** | 48.29 ± 12.22 b** | 44.58 ± 13.95 b** |
| Empty Lumen (%) | 56.1 ± 1.75 | 77 ± 2.2 a** | 57.9 ± 3.6 b** | 61.8 ± 4.9 b |
| Groups | C | Cd | MLT | Cd-MLT |
|---|---|---|---|---|
| CAT Activity (U/mg of proteins) | 37.52 ± 1.07 | 24.46 ± 7.25 a** | 49.64 ± 0.88 a**b** | 33.77 ± 1.18 b**c** |
| SOD Activity (U/mg of proteins) | 15.17 ± 0.07 | 8.86 ± 0.13 a** | 16.38 ± 0.08 b** | 12.58 ± 0.05 bc |
| [TBARS] (µM/µg of proteins) | 0.1092 ± 0.002 | 0.1578 ± 0.006 a** | 0.0894 ± 0.004 ab** | 0.1306 ± 0.003 ab*c** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venditti, M.; Ben Rhouma, M.; Romano, M.Z.; Messaoudi, I.; Reiter, R.J.; Minucci, S. Altered Expression of DAAM1 and PREP Induced by Cadmium Toxicity Is Counteracted by Melatonin in the Rat Testis. Genes 2021, 12, 1016. https://doi.org/10.3390/genes12071016
Venditti M, Ben Rhouma M, Romano MZ, Messaoudi I, Reiter RJ, Minucci S. Altered Expression of DAAM1 and PREP Induced by Cadmium Toxicity Is Counteracted by Melatonin in the Rat Testis. Genes. 2021; 12(7):1016. https://doi.org/10.3390/genes12071016
Chicago/Turabian StyleVenditti, Massimo, Mariem Ben Rhouma, Maria Zelinda Romano, Imed Messaoudi, Russel J. Reiter, and Sergio Minucci. 2021. "Altered Expression of DAAM1 and PREP Induced by Cadmium Toxicity Is Counteracted by Melatonin in the Rat Testis" Genes 12, no. 7: 1016. https://doi.org/10.3390/genes12071016
APA StyleVenditti, M., Ben Rhouma, M., Romano, M. Z., Messaoudi, I., Reiter, R. J., & Minucci, S. (2021). Altered Expression of DAAM1 and PREP Induced by Cadmium Toxicity Is Counteracted by Melatonin in the Rat Testis. Genes, 12(7), 1016. https://doi.org/10.3390/genes12071016










