Abstract
Extracellular vesicles (EVs) have attracted interest as mediators of intercellular communication following the discovery that EVs contain RNA molecules, including non-coding RNA (ncRNA). Growing evidence for the enrichment of peculiar RNA species in specific EV subtypes has been demonstrated. ncRNAs, transferred from donor cells to recipient cells, confer to EVs the feature to regulate the expression of genes involved in differentiation, proliferation, apoptosis, and other biological processes. These multiple actions require accuracy in the isolation of RNA content from EVs and the methodologies used play a relevant role. In liver, EVs play a crucial role in regulating cell–cell communications and several pathophysiological events in the heterogeneous liver class of cells via horizontal transfer of their cargo. This review aims to discuss the rising role of EVs and their ncRNAs content in regulating specific aspects of hepatocellular carcinoma development, including tumorigenesis, angiogenesis, and tumor metastasis. We analyze the progress in EV-ncRNAs’ potential clinical applications as important diagnostic and prognostic biomarkers for liver conditions.
1. Extracellular Vesicles
1.1. Definition and Classification
Extracellular vesicles (EVs)are defined as lipid bilayer particles naturally released from cells into the extracellular space. They became attractive in the research field when their potential role in cellular crosstalk was discovered [1].
Several studies highlighted that EVs mediate cell-to-cell communication in various biological processes, recognizing them as an additional class of signal mediators, such as cell-to-cell direct interaction or secretion of soluble molecules, i.e., growth factors, cytokines, metabolites, and hormones [2,3,4]. This intercellular communication mechanism allows the delivery of a particular cargo of messages to EV-accepting cells.
This functional cargo varies according to the cell type of origin and the physiological or pathological status of cells when they package and secrete EVs.
EVs are carriers of different molecules [5], such as proteins [6], bioactive lipids [7], and nucleic acids [8].
General criteria classify EV subpopulations based on their biogenesis, adding other hallmarks such as density, size, shape, internal content, surface molecules, and cellular origin [9].
Based on their origin, EVs are classified into two main types: exosomes (Exs) and microvesicles (MVs). EVs originating from an intracellular endocytic trafficking pathway are called “exosomes”, whereas EVs, which are formed directly by outward budding of the plasma membrane (PM), are defined as “microvesicles”, “ectosomes”, and “microparticles”. Given their nature, Exs have a typically rounded morphology with variable diameter from 50 to 150 nm and a buoyant density of 1.10–1.14 g/mL; in contrast, MVs appear more heterogeneous in shape and size with a diameter that varies from 50 to 500 (up to 1000) nm (Figure 1) [10].
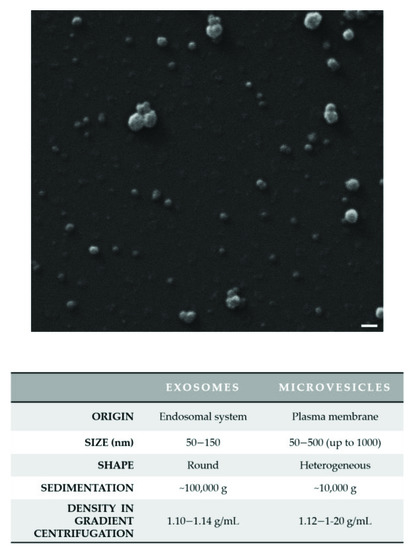
Figure 1.
The heterogeneity of extracellular vesicles (EVs). A representative and original image of small EVs by scanning electron microscopy (SEM) is shown. EVs were isolated from the hepatocarcinoma Mahlavu cell culture medium by differential centrifugation with a final ultracentrifugation step and were characterized by electron microscopy. EVs were fixed with 2.5% glutaraldehyde in filtered PBS, sedimented onto glass coverslips and then allowed to dry at room temperature. SEM images were obtained using a SEM Zeiss EVO 40 (Zeiss; Oberkochen, Germany). EVs display their heterogeneity of size. The main features of the two main EV subtypes (Exs and MVs) are reported in the table. Scale bar: 200 nm.
Newer techniques (such as cryo-TEM) led to the finding that exosomes’ previous ‘‘cup-shaped’’ morphology was an artefact related to fixation for TEM analysis. When observed in a close-to-native state by cryo-electron microscopy (cryo-EM), Exs have a rounded shape [10,11].
Since in some isolation approaches, the nature of EVs according to their biogenesis has not been found, the recommendations of MISEV 2018 (i.e., the main consensus of the largest group of EV experts) have recently proposed referring to EV biophysical characteristics such as their size (“small EVs” (sEVs), “medium/large EVs” (mEVs), and “large EVs” (lEVs)), density (light, medium, or heavy), or their biochemical composition, such as the co-presence of protein markers CD81+/CD9+/CD63+ in EVs [12,13].
In this review, the term “EVs”, including both Exs and MVs, has been used since it is not easy to ascertain EV subtypes using the current purification methods often obtained in mixtures of heterogeneous vesicle subsets [14].
1.2. Biological and Functional Features of EVs
EVs are involved in a plethora of biological processes such as inflammation, immune response, neurological diseases, and cancer.
A study on Exs-mediated activation of T cells revealed a mechanism in which the Treg cells transferred Exs-associated miRNAs to other immune cells, including T-helper 1 cells, with suppression of proliferation and cytokine secretion [15]. In adipose tissue, M1 macrophages released Exs containing miRNA-155 that targeted adipocytes, suppressing the expression of peroxisome proliferator-activated receptor-γ (PPAR-γ), that makes them insulin resistant; in contrast, M2 macrophages secreted Exs containing miR-223 that, renders them sensitive to insulin [16].
Cancer-derived EVs influence both stromal [17] and tumor cells in each phase of cancer development. EVs can induce endothelial proliferation and neovascularization as demonstrated by an in vitro study showing that glioblastoma-derived EVs increase levels of vascular endothelial growth factor (VEGF) in endothelial cells and activate VEGF receptor 2 in an autocrine manner [18].
Moreover, EVs can enhance tumor cell migration and invasion: Wnt11-loaded CD81-positive EVs can induce protrusive behavior of breast cancer cells, migration in vitro, and metastases in vivo [19].
One of the most relevant features of EVs is that, as previously demonstrated, EVs are secreted from almost all cell types and can be retrieved in a wide variety of human body fluids and secretions, such as blood (or serum/plasma), urine, breast milk, saliva, synovial fluid, amniotic fluid, cerebrospinal fluid, ascites, and bronchoalveolar lavage fluids [20].
From these different sources, EVs can be isolated, purified, and then biochemically and functionally characterized [21].
Thanks to their ubiquity and self-replenishing efficiency, EVs are considered reliable biomarkers for diagnostic and disease-monitoring purposes [22].
Another aspect that makes them an ideal biomarker is that the content of these vesicles reflects the pathological state of the cells and tissues of origin, thus representing a valuable tool for monitoring the onset, progression, and prognosis of the disease, as well as providing a system to analyze therapeutic efficacy [23].
Indeed, it has been shown that EVs released by tumor cells during malignant progression contain proteins [24], nucleic acids [25,26], and lipids [27] that can be used as markers of the neoplastic and metastatic phenotype. An example is the identification of caveolin-1, a protein associated with the metastatic behavior of tumors, in plasma EVs. The detection of caveolin-1-associated EVs in plasma can, therefore, be considered a useful tumor biomarker [28].
Cancer-derived EVs can be isolated using membrane-specific proteins from cancer tissues. For example, the presence of fibronectin on the membrane of circulating EVs was revealed as a tumor-specific antigen, to detect breast cancer [29]. In a recent publication reporting the analysis of lipids in EVs isolated from plasma of 20 pancreatic cancer patients and healthy controls, it was revealed that specific lipids, LysoPC 22:0, PC (P-14:0/22:2), and PE (16:0/18:1), are correlated with tumor stage, and, further, PE (16:0/18:1) was associated with a patient’s overall survival [30].
Furthermore, the importance of EVs lies in their ability to influence the phenotype and functions of cells either nearby or distant from the producing cells, modulating their activities also toward the development of pathophysiological conditions. This is possible due to the presence in EVs of nucleic acids [31].
This review summarizes the current knowledge of EV RNA content, with a specific focus on their ability of mediating the communication between normal and pathological liver cells.
2. Extracellular Vesicles as Carriers of RNA Molecules
2.1. Classes of RNA Molecules Retrieved in EVs and Their Biological Function
The discovery that EVs can carry nucleic acids revealed their crucial role in horizontal genetic transfer [31,32].
The circulating RNAs associated with EVs can reach cells other than the originating, both in neighboring cells and in cells located elsewhere in the body, and, once inside, can influence gene expression.
EVs can contain messenger RNAs (mRNAs) [33] and non-coding RNAs (ncRNAs) of different length, including long non-coding RNAs (lncRNAs) [34], microRNAs (miRNAs), and circular RNAs (circRNAs) [35].
Valadi and colleagues (2007) carried out the first study in which the presence of mRNA was investigated in EVs derived from a mouse (MC/9) and human mast-cell lines (HMC-1) and primary bone marrow-derived mouse mast cells (BMMC) [31].
LncRNAs are RNA molecules characterized by a nucleotide size > 200 bp and lack of protein-coding sequences [36]. In 2014, Gezer et al. identified lncRNAs, including MALAT1, HOTAIR, lincRNAp21, GAS5 (growth arrest-specific 5), TUG1 (taurine upregulated gene 1), and ncRNA-CCND1 (cyclin D1) in EVs derived from HeLa and MCF-7 cell lines. The identified expression patterns of lncRNAs were different in EVs compared with their parental cells [37].
miRNAs are small ncRNA molecules with a length of approximately 18–24 nucleotides, which pair with a specific sequence of mRNAs with imperfect binding [38].
Therefore, they can regulate hundreds of transcripts, reducing and/or increasing mRNA degradation, removing specific resident proteins in the cells [39]. miRNA expression has a tissue-specific profile pattern, and their expression is impaired in many diseases, including cancer [40,41,42].
Finally, circRNAs are circular RNA molecules composed of a covalently closed loop structure, lacking a poly-A tail or 5′ to 3′ polarity [35,43]. Growing evidence has shown the presence of circRNAs enriched in EVs, involving various biological processes of cancer, particularly malignant tumor metastasis [44]. A recent study showed that circRNAs increased their level two-fold in EVs released from MHCC-LM3 liver cancer cells when compared to their parental cells [35]. Indeed, this research group showed that the sorting mechanism of circRNAs is a process linked to the regulation of related miRNA levels in parental cells [45].
The circRNA expression profiles were investigated in EVs released from three isogenic colon cancer cell lines diverging for KRAS mutation compared to their parental cells. Dou and colleagues identified that the circRNA levels are higher in EVs than their parental colon–rectal cancer cells [46].
The subcellular localization of RNAs and the EV subtypes positively influences their loading.
Interestingly, a selective sorting process was identified for specific RNAs, sharing a short sequence called hEXO motif during the hepatocytes’ EV formation. The main component of this loading machinery belongs to the synaptotagmin-binding, cytoplasmic RNA-interacting protein (SYNCRIP) complex, which directly binds to some miRNA enriched in EVs [47].
Different studies demonstrated that EVs produced by different cell types presented different RNA content [48], depending on EV subcellular source and cell physio-pathological conditions [49]. An increasing amount of RNA molecules have been found to be aberrantly expressed in human cancers [50].
The up- or downregulation of specific ncRNAs, associated with disruption of cells’ physiological mechanisms, may lead to diseases [51]. Alterations in the action of miRNAs or their biogenesis processes can be used to indicate disease prognosis in a patient.
For example, alterations in miR-122 and miR-33 levels are linked to the development of obesity, hepatic steatosis, and hepatocellular carcinoma (HCC) [37]. miR-33a is an important regulator of cell proliferation and apoptosis by acting on PPARα (peroxisome proliferator-activated receptor α). Functional experiments of miR-33a gain- and loss-of-function demonstrated that its overexpression in Huh7 hepatocarcinoma cells triggers increased proliferation by reducing PPARα levels. In contrast, its inhibition in HepG2 hepatocarcinoma cells reduced cell proliferation and induced apoptosis due to the hyperactivation of PPARα expression. miR-33a is considered a potential prognosis marker for hepatocarcinoma patients; high levels of miR-33a correlate with a shorter survival of 5 years [52].
Based on this information, it is clear that ncRNAs’ biological message is related to tumor cell spreading and oncogenic onset. On the other hand, it is not easy to identify a single ncRNA as a specific disease marker because they act with the principle of cooperation. For example, a single gene targets several miRNAs, just as the same miRNA can act on several genes [53]. Thus, it is more likely to identify a pattern of ncRNAs whose expression is related to a specific alteration.
2.2. Methodological Approaches to Study RNA Molecules Carried by EVs
RNA molecules can be isolated from biological samples (i.e., cell culture medium or blood/plasma) in two ways: RNAs can be obtained by extracting the total RNA from both EV-associated RNA or free and protein-bound RNA. Alternatively, more accurately, EVs can be isolated from biological samples using a differential centrifugation approach, ultracentrifugation, or other methods, such as size exclusion chromatography, and only EV RNA can be isolated [54].
Currently, there is no gold standard technique for EV isolation and, thus, the method should be chosen based on both the type and amount of EVs.
Conventional methodologies for EV isolation suffer from limitations in separation technology. In particular, the detection of EVs is vulnerable to artefacts partly induced by sample collection and the huge heterogeneity of EV populations. Furthermore, the main approaches are based on EVs’ physical properties (density, solubility, or size), and are not able to separate the tumor-derived EVs from total EVs [55].
To overcome this issue, researchers are committed to exploring different immunoaffinity-based approaches to purify tumor-derived EVs. In particular, Sun and colleagues (2020) developed an EV purification system using a multiple marker cocktail to recognize, enrich, and recover HCC EVs secreted from highly heterogeneous HCC [56]. Several biotechnology companies are currently working to develop a quick and easy assay based on precipitation to isolate EVs. These kits often require polyethylene glycol l (PEG1) solutions, that once mixed with samples allow EVs to precipitate at low speed. Precipitation-based isolation is inexpensive, requires no special equipment, and is compatible with both low- and high-sample volumes. Nevertheless, this method suffers from co-isolation of non-EV particles and protein complexes and must be further improved [57].
The analysis of RNA molecules is allowed by different approaches, which include microarrays, quantitative real-time polymerase chain reaction (qRT-PCR), digital PCR (dPCR), NanoString’s nCounter technology, and next-generation sequencing (NGS) [58,59,60]. The main difference between these methods is the sensitivity of the RNA transcript detection.
The most common RNA detection is the microarray analysis because it can detect simultaneously different nucleic acids and can be customized [61,62].
Digital PCR (dPCR) can be considered an alternative to the qPCR approach and provides more accurate data of the nucleic acid target molecule without a standard curve and dependence on amplification efficiency. The hypersensitivity of dPCR allows detecting RNA molecule targets of low abundance below the qPCR’s sensitivity limit [63,64]. This system can easily reveal and quantify the low amount, like EV content, but it is a long procedure and relatively expensive.
The Nanostring platform is a very recent technology to measure RNA expression [65]. The system, formed on a multiplexed probe library, contains two types of probes (capture probe and reporter probe) specific for each nucleic acid molecule to detect. The capture probes are tagged with biotin at the 3′-end, whereas the reporter probes carry a barcode signal at the 5’-end [65]. The probes are mixed with the total RNA, and the hybridized complexes are then immobilized, and digitally detected, thus assessing the level of expression. This technology works without amplification or reverse transcription. Small RNA amounts could be precisely analyzed, and several hundred unique transcripts could be counted in the same reaction because the counts are measured digitally [66]. In a recent study, the RNA content from EVs was analyzed by the nCounter platform, demonstrating this method’s efficacy to detect plasma EV mRNA transcripts [67].
Finally, it was demonstrated that the Nanostring nCounter is a more accurate system than microarrays and comparable in susceptibility to real-time PCR [66].
NGS consists of sequencing technology and is supported by different platforms. It offers the advantage to generate a huge amount of sequence data sets, ranging from megabases to gigabases [68].
4. Extracellular Vesicle-Derived RNAs as Potential Biomarkers in HCC
The need to develop accurate and reliable early diagnostic tools to complement and potentially replace invasive liver biopsy, to perform disease stratification and response monitoring for therapeutic interventions, is increasingly growing [104].
New biomarkers’ discovery in early diagnosis and prognosis requires different processes, starting from basic research and validation to clinical implementation.
The final aim is to create clinically available biomarker tests to guide clinical decision-making and improve patient outcomes (Figure 2).
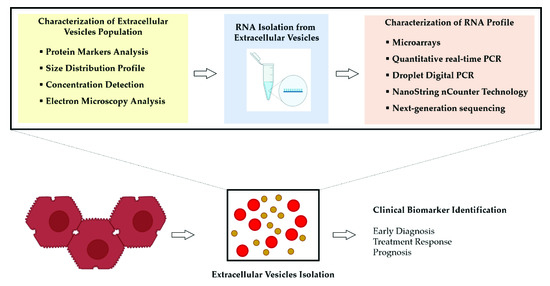
Figure 2.
Overview of the process for the analysis of RNA molecules obtained from extracellular vesicles (EVs) released by hepatocellular carcinoma (HCC) cells. An adequate approach to isolate the EVs and the choice of assay having suitable sensitivity to detect small amounts of RNA represent the main determinants of success to discover disease-specific RNA molecules.
Although liver biopsy remains the “gold standard” method, with limitations either in the absence or presence of cirrhosis [69], “liquid biopsy” is proposed as a novel tool for monitoring HCC development and progression. The liquid biopsy is based on the analysis of different biomarkers, including circulating tumor cells (CTCs), circulating proteins, or cell-free nucleic acids (such as cfDNA) and EVs.
Circulating EVs containing nucleic acids (DNA and RNA) have several advantages as disease biomarkers [105,106].
In plasma/serum, the number of EVs is considerably higher than CTCs, EVs are more stable in the bloodstream, and their cargo is well-protected within the double-leaflet membrane concerning the cfDNAs.
Considering the EV content, they contain various pieces of information, and they cannot be considered as single biomarkers but as a heterogeneous complex of potential biomarkers.
Several studies have focused on EV-associated RNAs as potential diagnostic or prognostic biomarkers [107,108,109].
Most of the studies considered serum as the primary liquid biopsy source for HCC and only one study employed urine as a potential EV source associated with HCC diagnosis.
4.1. EV-Derived miRNAs as Prognostic and Diagnostic Markers for HCC
miR-93. Considering the important role of EV-derived miRNA in HCC tumorigenesis and that miR-93 increases cancer cell growth via modulating PTEN in several types of cancer [110,111], Xue and colleagues (2018) investigated the role of EV-derived miR-93 as a new diagnostic and prognostic biomarker in HCC [112].
In EVs of tumor sera, they found high expression of miR-93 and its expression level in HCC patients can be considered a diagnostic marker in association with tumor size and TNM (tumor, node, metastasis) stage. Further, the upregulation of EV-derived miR-93 predicts poor prognosis for patients with HCC [112].
miR-21. Wang et al. (2014) analyzed the miRNA profile from serum EVs in HCC, identifying miR-21 as a candidate biomarker to discriminate patients with liver cancer from those with chronic hepatitis B (CHB) or healthy subjects [78]. In fact, the expression level of serum EV-derived miR-21 was significantly higher in patients with HCC than those with CHB or healthy volunteers [78].
Using gene expression arrays to profile miRNAs in tumor and healthy tissue and EVs from HCC patients has been performed. It showed the upregulation of miR-21 in tumor tissue and the plasma EVs with a positive correlation between serum EVs and HCC tissue miR-21 expression, suggesting that miR-21 moved from tissue to bloodstream via EVs [113].
Tian and co-authors (2019) showed that the overexpression of miR-21 and miR-10b in EVs from HCC is induced by the acidic microenvironment in HCC, demonstrating that this oncogenic event promotes the proliferation and metastasis of HCC cells. Further, their finding indicated that EV-derived miR-21 and miR-10b can be used as prognostic molecular markers and therapeutic targets of HCC [114].
Another study highlighted that EV-associated miR-18a, miR-221, miR-222, and miR-224 were significantly higher in the serum of patients with HCC than in those with CHB or LC. Serum levels of EV-associated miR-101, miR-106b, miR-122, and miR-195 were lower in HCC than in CHB, whereas there was no apparent difference in miR-21 and miR-93 levels between the three groups [115].
A comparative analysis of miRNA level expression between serum-circulating miRNA and EV-associated miRNA in each patient group was also performed in this study. The results show a high correlation of serum-circulating miRNAs and serum EV-associated miR-221, miR-222, and miR-224 in the HCC and LC groups; however, the differences detected in serum miRNA levels were lower than those detected inside the EVs. The data obtained highlight an interesting aspect regarding the possibility of better-discriminating HCC from CHB or LC using serum EV-associated miRNAs when compared to serum circulating miRNAs [115].
miR-9-3p. The study of Tang and colleagues (2018) investigated the diagnostic use of EV-derived miR-9-3p in HCC, showing lower serum levels of EV-derived miR-9-3p in HCC patients than in healthy donors. Further, the overexpression of EV-derived miR-9-3p reduced the viability and proliferation of HCC cells and additionally reduced ERK1/2 expression, suggesting a potential mechanism for miR-9-3p action [116].
miR-224. The expression level of serum EV-derived miR-224 was increased in HCC patients compared to healthy controls, as determined by qPCR, thus suggesting its ability to stimulate the proliferation and invasion of HCC cells [117].
EV-derived miR-224 was tested as a biomarker to distinguish HCC patients from healthy controls. The expression of serum EV-derived miR-224 was higher in patients with larger tumors and advanced stages. The correlation between the expression of EV-derived miR-224 and the overall survival of patients has been analyzed. The results demonstrate that the higher the expression level of serum EV-derived miR-224, the shorter the patient’s overall survival, suggesting serum EV-derived miR-224 as a prognostic factor in HCC patients [117].
Currently, detection of early-stage HCC using a serum marker in patients at high risk of developing HCC is challenging. To improve the prognosis of patients with HCC, a reliable serum biomarker for diagnosing early-stage HCC is fundamental [118].
In a recent study, the analysis of serum samples from 28 healthy individuals, 60 CLD patients, and 90 HCC patients was carried out. It was found that serum EV-derived miR-10b-5p displayed a relevant diagnostic efficiency in detecting early-stage HCC and serum EV-derived miR-215-5p was recognized as a biomarker for predicting prognosis in HCC patients [118]. In this study, the expression of the two serum EV-derived miRNAs was much higher than that of the circulating serum miR form.
miR-429. Li and colleagues (2015) reported that miR-429 is overexpressed in HCC tissue and primary liver tumor-initiating cells (T-ICs). This overexpression is paralleled with HCC-derived circulating EVs and, therefore, may be proposed as a prognostic factor in HCC patients [119]. The high amount and the internalization ability of miR-429 in HCC cells, specifically in epithelial cell adhesion molecule (EPCAM) + T-ICs, contribute to promoting and developing tumor features such as self-renewal, tumorigenicity, malignant proliferation, chemoresistance, and progression [119]. These EVs shuttling and spreading miR-429 into their surrounded target cells induced a novel functional axis with Rb-binding protein 4 (RBBP4) and the transcriptional function of E2F1, POU class 5 homeobox 1 (POU5F1) expression [119].
In particular, miR-429 is able to enhance the transcriptional activity of E2F1 by directly targeting RBBP4, a known tumor suppressor protein, which is downregulated.
The molecular mechanism regulating miR-429 expression is an epigenetic event involving four abnormal hypomethylated sites upstream of the miR-200b/miR-200a/miR-429 cluster. The EV-derived miR-429 could potentially inactivate T-ICs, thus providing a novel strategy for HCC prevention and treatment [119].
miR-125b. Liu et al. (2017) identified the EV-associated miR-125b as a useful prognostic marker for disease recurrence and survival of HCC patients. EVs were isolated from serum samples and divided into three groups: HCC, CHB, and LC. The authors found that miR-125b levels were significantly increased in HCC-derived EVs compared to those in serum from patients with CHB or LC. Therefore, the enrichment of miR-125b in EVs can help to establish the efficacy of treatment regimens and the survival of HCC patients [120].
miR-718. EV-derived miR-718 can be considered a biomarker to predict HCC relapse after liver transplantation (LT). The association between the expression level of the potential miR-718 target genes in the primary HCC and post-operative prognosis was examined.
The study showed that the expression level of EV-associated miR-718 isolated from patients with HCC recurrence after LT decreased when compared to those without HCC recurrence. Additionally, in this clinical study, the downregulation of miR-718 level was directly correlated with the oncogenic homeobox protein 8 (HOX-B8) overexpression, resulting in tumorigenesis and tumor invasion.
The upregulation of HOXB8 expression affected the poor overall and recurrence-free survivals of HCC patients, with statistical significance [121].
miR-122. Another study analyzed the importance of serum EV-miRNA expression levels in HCC patients that underwent transarterial chemoembolization (TACE). Based on the relative expression of miR-122 before or after TACE, patients with a higher ratio had remarkably longer disease-specific survival than those with a lower miR-122 ratio. As a result, serum EV-derived miR-122 was confirmed as a predictive biomarker in TACE-treated HCC patients [122].
miR-638. The study from Shi and colleagues (2018) revealed that the serum EV derived-miR-638 not only affects the initiation, but also the progression of HCC, thus worsening the prognosis of HCC patients. The EV-derived miR-638 was downregulated in serum samples from patients with HCC compared to healthy donors. Levels of serum EV derived-miR-638 are decreased in HCC patients with larger tumor size (>5 cm) or at later TNM stage (III/IV), suggesting that the downregulation of mir-638 predicts poor prognosis in HCC patients [123].
4.2. Other EV-Derived RNA Molecules as Biomarkers for HCC
Recently, in a clinical study that included a large cohort comprising 159 healthy individuals, 150 patients with five cancer types, and 43 patients with other diseases, more than 10,000 EV-RNA were analyzed from human plasma.
mRNAs represented most of the total mapped reads, and the data also showed that short RNAs and circRNAs were enriched in EVs. It was analyzed whether certain EV-derived RNA could diagnose a specific tumor type, such as HCC.
Finally, eight EV-RNA molecules resulted as biomarkers for HCC diagnosis with high diagnostic efficiency [124].
Different EV-associated lncRNAs, including lncRNA-HEIH, LINC02394, LINC0635, LINC00161, and JPX, were considered diagnostic biomarkers for HCC [125,126,127,128]. LINC00853 was the last lncRNA found as a possible diagnostic biomarker of both all-stage HCC and early HCC [129].
However, the diagnostic performance of EV-derived lncRNAs in HCC still suffers the limitation of analyzing small sample sizes with a still unsatisfactory diagnostic efficiency for early-stage HCC.
In Table 1, the clinical significance of EV-derived RNAs in HCC is summarized.

Table 1.
Extracellular Vesicle-derived RNA molecules are detected as biomarkers for early diagnosis and prognosis of hepatocellular carcinoma (HCC).
Table 1.
Extracellular Vesicle-derived RNA molecules are detected as biomarkers for early diagnosis and prognosis of hepatocellular carcinoma (HCC).
| Clinical Significance | EV-Derived RNA | Expression Level | Patients | Source | EV Isolation Method | Clinical Relevance | Ref. |
|---|---|---|---|---|---|---|---|
| Detection and Diagnosis | miR-21 | Upregulated | 30 HCC 30 CHB 30 HV | Serum | Total Exosome Isolation Kit (Invitrogen, Carlsbad, CA, USA). | Discrimination between HCC and CHB or LC | [78] |
| miR-93 | Upregulated | 85 HCC 23 HV | Serum | Total Exosome Isolation Kit (Thermo Fisher Scientific, Waltham, MA, USA) | Biomarker for both diagnosis and prognosis in HCC. | [112] | |
| miR-224 | Upregulated | 89 HCC 50 HV | Serum | Total Exosome Isolation Kit (Thermo Fisher Scientific) | Biomarker of diagnosis and prognosis of HCC patient | [117] | |
| miR-718 | Downregulated | 59 HCC | Serum | Differential Centrifugation with a Final Ultracentrifugation Step | Predicting biomarker for recurrence after LT | [121] | |
| miR-18a miR-221 miR-222 miR-224 miR-101 miR-106b miR-122 miR-195 | Upregulated Downregulated | 20 HCC 20 LC 20 CHB | Serum | ExoQuick Exosome Precipitation Solution (System Biosciences, Mountain View, CA, USA) | Discrimination between HCC and CHB or LC | [115] | |
| miR-10b-5p miR-18a-5p miR-215-5p miR-940 | Upregulated | 90 HCC 60 CLD 28 HV | Serum | Differential Centrifugation with a Final Ultracentrifugation Step | miR-10b-5p as a diagnostic biomarker for early stage HCC | [118] | |
| miRNA-26a miRNA-29c miRNA-21 | Downregulated | 72 HCC 72 LC 72 HBV | Serum | ExoQuick Exosome Precipitation Solution (System Biosciences, Mountain View, CA, USA) | Diagnostic biomarkers for patients with HCC | [130] | |
| miR-122 miR-148a miR-1246 | Upregulated | 5 HCC 5 LC | Serum | 8% Polyethylene glycol (PEG) 6000 (Sigma-Aldrich, St Louis, MO, USA) | Diagnostic biomarker for patients with HCC | [131] | |
| lncRNA-HEIH | Upregulated | 35 CHC 22 HCV 10 HCC | Serum | Total Exosome Isolation reagent (GS0301; Guangzhou 141 Geneseed Biotech Co., Guangzhou, China) with a final centrifugation passage | Biomarker in the HCV-related hepatocellular carcinoma | [128] | |
| LINC00853 | Upregulated | 90 HCC 28 CH 35 LC 29 HV | Serum | ExoQuick Exosome Precipitation Solution (System Biosciences, Mountain View, CA, USA) | Diagnostic biomarker discriminating both all-stage HCC and early HCC | [129] | |
| LINC00161 | Upregulated | 56 HCC 56 HV 15 HCC 15 HV | SerumUrine | Total Exosome Isolation Kit (Invitrogen, USA) | Diagnostic biomarker for patients with HCC | [126] | |
| ENSG00000258332.1 LINC00635 | Upregulated | 60 HCC 85 LC 96 CHB 60 HV | Serum | Total Exosome Isolation Kit (Thermo Fisher Scientific) | Biomarker of diagnosis and prognosis of HCC patients | [127] | |
| Jpx | Upregulated | 74 HCC 26 LC 34 CHB 72 HV | Serum | ExoQuick Exosome Precipitation Solution (System Biosciences, Mountain View, CA, USA) | Biomarkers for diagnosis of female patients with HCC | [125] | |
| 8 EV-ncRNA (circRNA and lncRNA) | Upregulated | 71 HCC (early stage, n = 45; advanced stage, n = 26) 94 HV 18 benign HCC 11 CHB 8 LC | Serum | exoRNeasy Serum/Plasma kit (Qiagen, Hilden, Germany) | Biomarkers for hepatocellular carcinoma (HCC) diagnosis | [124] | |
| Detection and Prognosis | miR-125b | Upregulated | 158 HCC 30 CHB 30 LC | Serum | ExoQuick Exosome Precipitation Solution (System Biosciences, Palo Alto, CA, USA) | Predicting biomarker for recurrence and survival | [120] |
| miR-638 | Downregulated | 126 HCC 21 HV | Serum | Total exosome isolation kit (Invitrogen, Carlsbad, CA, USA). | Poor prognosis marker for patients with HCC | [123] | |
| miR-215-5p | Upregulated | 90 HCC 60 CLD 28 HV | Serum | Differential Centrifugation with a Final Ultracentrifugation Step | miR-215-5p: prognostic biomarker for HCC | [118] | |
| miR-744 | Downregulated | 68 HCC 52 normal liver tissue samples | Serum | Differential Centrifugation with a Final Ultracentrifugation Step | Inhibition of Proliferation and chemoresistance | [132] | |
| miR-224 | Upregulated | 89 HCC 50 HV | Serum | Total Exosome Isolation Kit (Thermo Fisher Scientific) | Biomarker of diagnosis and prognosis of HCC patient | [117] | |
| miR-21 miR-10b | Upregulated | 124 HCC N.A. HV | Serum | ExoQuick-TC exosome precipitation solution (System Biosciences, CA, USA) | Prognostic molecular markers and therapeutic targets for HCC. | [114] | |
| miR-9-3p | Downregulated | N.A. HCC N.A. HV | Serum | Differential Centrifugation with a Final Ultracentrifugation Step | Potential therapeutic target for HCC. | [116] | |
| circPTGR1 | Upregulated | 82 HCC 47 HV | Serum | ExoQuick-TC exosome precipitation solution (System Biosciences, CA, USA) | Prognostic biomarker and therapeutic target in HCC | [95] |
HCC, hepatocellular carcinoma; CLD, chronic liver disease; LC, liver cirrhosis; CHB, chronic hepatitis B; HBV, hepatitis B virus; HCV, hepatitis C virus; CHC, chronic hepatitis C; CH, chronic hepatitis; HV, healthy volunteers.
5. Conclusions
Research of nucleic acids within EVs to identify a panel of biomarkers has the ability to provide new biological knowledge and support diagnosis and therapeutic monitoring in HCC.
Despite the exponential interest in the EV field and the recent advances in isolation/characterization methods, the challenge of acquiring EV samples with high yield and purity and standardizing RNA processing is still open.
It is now well known that RNA molecules are stable within EVs, since the lipid bilayer conserves them from the enzymatic activity of RNases. Therefore, to evaluate EV-derived RNAs as potential biomarkers in HCC diagnosis and prognosis, blood samples are appropriate.
Further advancement in the isolation and detection of EV-derived RNAs is required, defining the different sources from which EV-derived RNAs are obtained.
A larger cohort of HCC patients with a control cohort of healthy subjects is also necessary, considering not only the evolution of HCC progression but also other risk factors such as chronic HBV or HCV infection, alcohol abuse, LC, and aflatoxin exposure.
An important step forward will be taken when tumor properties, such as tumor differentiation stage or the post-surgery tumor relapse, including the presence of microvascular invasion, are associated with the expression level of specific EV-derived RNA molecules.
Finally, for a more relevant clinical use, a pattern of biomarkers associated with EVs in the progression of HCC may be considered. Correlation panels among RNA content and proteins and lipids associated with EVs could be set up. We are increasingly convinced that EVs contain rich information; therefore, to consider only a part of their content would be reductive from a diagnostic and prognostic perspective.
Author Contributions
E.C. and L.M.N.: idealization, intellectual input; E.C., C.S. and C.B.: literature search and writing the initial version of the manuscript; G.V. and I.C.: manuscript editing; L.M.N.: manuscript editing and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by “Local Research grants” from the University of Ferrara, Italy (FIR and FAR to L.M.N. and FAR to C.S.) and by Fondazione Di Bella Onlus (L.M.N.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
The authors are grateful to Paola Boldrini (LTTA–Electron Microscopy Center, University of Ferrara, Italy) for helpful collaboration.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Lo Cicero, A.; Stahl, P.D.; Raposo, G. Extracellular Vesicles Shuffling Intercellular Messages: For Good or for Bad. Curr. Opin. Cell Biol. 2015, 35, 69–77. [Google Scholar] [CrossRef]
- Greening, D.W.; Simpson, R.J. Understanding Extracellular Vesicle Diversity–Current Status. Expert. Rev. Proteom. 2018, 15, 887–910. [Google Scholar] [CrossRef]
- Kalra, H.; Gangoda, L.; Fonseka, P.; Chitti, S.V.; Liem, M.; Keerthikumar, S.; Samuel, M.; Boukouris, S.; Al Saffar, H.; Collins, C.; et al. Extracellular Vesicles Containing Oncogenic Mutant β-Catenin Activate Wnt Signalling Pathway in the Recipient Cells. J. Extracell. Vesicles 2019, 8, 1690217. [Google Scholar] [CrossRef]
- Sagini, K.; Costanzi, E.; Emiliani, C.; Buratta, S.; Urbanelli, L. Extracellular Vesicles as Conveyors of Membrane-Derived Bioactive Lipids in Immune System. Int. J. Mol. Sci. 2018, 19, 1227. [Google Scholar] [CrossRef]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA Delivery by Extracellular Vesicles in Mammalian Cells and Its Applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, É.; Pap, E.; Kittel, Á.; et al. Membrane Vesicles, Current State-of-the-Art: Emerging Role of Extracellular Vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Noble, J.M.; Roberts, L.D.M.; Vidavsky, N.; Chiou, A.E.; Fischbach, C.; Paszek, M.J.; Estroff, L.A.; Kourkoutis, L.F. Direct Comparison of Optical and Electron Microscopy Methods for Structural Characterization of Extracellular Vesicles. J. Struct. Biol. 2020. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic Comparison Defines Novel Markers to Characterize Heterogeneous Populations of Extracellular Vesicle Subtypes. Proc. Natl. Acad. Sci. USA 2016. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Théry, C. Extracellular Vesicles or Exosomes? On Primacy, Precision, and Popularity Influencing a Choice of Nomenclature. J. Extracell. Vesicles 2019, 8, 1648167. [Google Scholar] [CrossRef]
- Okoye, I.S.; Coomes, S.M.; Pelly, V.S.; Czieso, S.; Papayannopoulos, V.; Tolmachova, T.; Seabra, M.C.; Wilson, M.S. MicroRNA-Containing T-Regulatory-Cell-Derived Exosomes Suppress Pathogenic T Helper 1 Cells. Immunity 2014, 41, 89–103. [Google Scholar] [CrossRef]
- Ying, W.; Riopel, M.; Bandyopadhyay, G.; Dong, Y.; Birmingham, A.; Seo, J.B.; Ofrecio, J.M.; Wollam, J.; Hernandez-Carretero, A.; Fu, W.; et al. Adipose Tissue Macrophage-Derived Exosomal MiRNAs Can Modulate In vivo and In Vitro Insulin Sensitivity. Cell 2017, 171, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Webber, J.P.; Spary, L.K.; Sanders, A.J.; Chowdhury, R.; Jiang, W.G.; Steadman, R.; Wymant, J.; Jones, A.T.; Kynaston, H.; Mason, M.D.; et al. Differentiation of Tumour-Promoting Stromal Myofibroblasts by Cancer Exosomes. Oncogene 2015, 34, 319–333. [Google Scholar] [CrossRef]
- Al-Nedawi, K.; Meehan, B.; Kerbel, R.S.; Allison, A.C.; Rak, A. Endothelial Expression of Autocrine VEGF upon the Uptake of Tumor-Derived Microvesicles Containing Oncogenic EGFR. Proc. Natl. Acad. Sci. USA 2009, 106, 3794–3799. [Google Scholar] [CrossRef]
- Luga, V.; Zhang, L.; Viloria-Petit, A.M.; Ogunjimi, A.A.; Inanlou, M.R.; Chiu, E.; Buchanan, M.; Hosein, A.N.; Basik, M.; Wrana, J.L. Exosomes Mediate Stromal Mobilization of Autocrine Wnt-PCP Signaling in Breast Cancer Cell Migration. Cell 2012, 151, 1542–1556. [Google Scholar] [CrossRef]
- Ma, C.; Jiang, F.; Ma, Y.; Wang, J.; Li, H.; Zhang, J. Isolation and Detection Technologies of Extracellular Vesicles and Application on Cancer Diagnostic. Dose Response 2019, 17, 1559325819891004. [Google Scholar] [CrossRef]
- Keller, S.; Ridinger, J.; Rupp, A.K.; Janssen, J.W.G.; Altevogt, P. Body Fluid Derived Exosomes as a Novel Template for Clinical Diagnostics. J. Transl. Med. 2011. [Google Scholar] [CrossRef] [PubMed]
- Urbanelli, L.; Buratta, S.; Sagini, K.; Ferrara, G.; Lanni, M.; Emiliani, C. Exosome-Based Strategies for Diagnosis and Therapy. Recent Patents CNS Drug Discov. 2015. [Google Scholar] [CrossRef] [PubMed]
- Merchant, M.L.; Rood, I.M.; Deegens, J.K.J.; Klein, J.B. Isolation and Characterization of Urinary Extracellular Vesicles: Implications for Biomarker Discovery. Nat. Rev. Nephrol. 2017, 13, 731–749. [Google Scholar] [CrossRef] [PubMed]
- Zöller, M. Tetraspanins: Push and Pull in Suppressing and Promoting Metastasis. Nat. Rev. Cancer. 2009, 9, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.F.; Patel, T.; Wong, D.; Das, S.; Freedman, J.E.; Laurent, L.C.; Carter, B.S.; Hochberg, F.; Keuren-Jensen, K.V.; Huentelman, M.; et al. Extracellular RNAs: Development as Biomarkers of Human Disease. J. Extracell. Vesicles 2015. [Google Scholar] [CrossRef]
- Ragusa, M.; Barbagallo, C.; Cirnigliaro, M.; Battaglia, R.; Brex, D.; Caponnetto, A.; Barbagallo, D.; Di Pietro, C.D.; Purrello, M. Asymmetric RNA Distribution among Cells and Their Secreted Exosomes: Biomedical Meaning and Considerations on Diagnostic Applications. Front. Mol. Biosci. 2017, 4, 1–14. [Google Scholar] [CrossRef]
- Ferreri, C.; Sansone, A.; Buratta, S.; Urbanelli, L.; Costanzi, E.; Emiliani, C.; Chatgilialoglu, C. The n-10 Fatty Acids Family in the Lipidome of Human Prostatic Adenocarcinoma Cell Membranes and Extracellular Vesicles. Cancers 2020, 12, 900. [Google Scholar] [CrossRef]
- Fais, S.; Logozzi, M. A New Method to Measure and Characterize Microvesicles in the Human Body Fluids. WO Patent 2009092386, 30 July 2009. [Google Scholar]
- Moon, P.G.; Lee, J.E.; Cho, Y.E.; Lee, S.J.; Chae, Y.S.; Jung, J.H.; Kim, I.S.; Park, H.Y.; Baek, M.C. Fibronectin on Circulating Extracellular Vesicles as a Liquid Biopsy to Detect Breast Cancer. Oncotarget 2016, 7, 40189–40199. [Google Scholar] [CrossRef]
- Tao, L.; Zhou, J.; Yuan, C.; Zhang, L.; Li, D.; Si, D.; Xiu, D.; Zhong, L. Metabolomics Identifies Serum and Exosomes Metabolite Markers of Pancreatic Cancer. Metabolomics 2019, 15, 86. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007. [Google Scholar] [CrossRef]
- Di Liegro, C.M.; Schiera, G.; Di Liegro, I. Extracellular Vesicle-Associated RNA as a Carrier of Epigenetic Information. Genes 2017, 8, 240. [Google Scholar] [CrossRef]
- Iraci, N.; Leonardi, T.; Gessler, F.; Vega, B.; Pluchino, S. Focus on Extracellular Vesicles: Physiological Role and Signalling Properties of Extracellular Membrane Vesicles. Int. J. Mol. Sci. 2016, 17, 171. [Google Scholar] [CrossRef]
- Fatima, F.; Nawaz, M. Vesiculated Long Non-Coding RNAs: Offshore Packages Deciphering Trans-Regulation between Cells, Cancer Progression and Resistance to Therapies. Noncoding RNA 2017, 3, 10. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Q.; Bao, C.; Li, S.; Guo, W.; Zhao, J.; Chen, D.; Gu, J.; He, X.; Huang, S. Circular RNA Is Enriched and Stable in Exosomes: A Promising Biomarker for Cancer Diagnosis. Cell Res. 2015, 25, 981–984. [Google Scholar] [CrossRef]
- Yu, F.-J.; Zheng, J.-J.; Dong, P.-H.; Fan, X.-M. Long Non-Coding RNAs and Hepatocellular Carcinoma. Mol. Clin. Oncol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Gezer, U.; Özgür, E.; Cetinkaya, M.; Isin, M.; Dalay, N. Long Non-Coding RNAs with Low Expression Levels in Cells Are Enriched in Secreted Exosomes. Cell Biol. Int. 2014, 38, 1076–1079. [Google Scholar] [CrossRef]
- Conti, I.; Varano, G.; Simioni, C.; Laface, I.; Milani, D.; Rimondi, E.; Neri, L.M. MiRNAs as Influencers of Cell-Cell Communication in Tumor Microenvironment. Cells 2020, 9, 220. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.; Chen, C.Z. Micromanagers of Gene Expression: The Potentially Widespread Influence of Metazoan MicroRNAs. Nat. Rev. Genet 2004, 5, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.M.; Newman, M.; Parker, J.S.; Morin-Kensicki, E.M.; Wright, T.; Hammond, S.M. Extensive Post-Transcriptional Regulation of MicroRNAs and Its Implications for Cancer. Genes Dev. 2006. [Google Scholar] [CrossRef]
- Fabbri, M.; Garzon, R.; Andreeff, M.; Kantarjian, H.M.; Garcia-Manero, G.; Calin, G.A. MicroRNAs and Noncoding RNAs in Hematological Malignancies: Molecular, Clinical and Therapeutic Implications. Leukemia 2008, 22, 1095–1105. [Google Scholar] [CrossRef]
- Calin, G.A.; Croce, C.M. Genomics of Chronic Lymphocytic Leukemia MicroRNAs as New Players with Clinical Significance. Semin. Oncol. 2006, 33, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Ebert, M.S.; Sharp, P.A. MicroRNA Sponges: Progress and Possibilities. RNA 2010, 16, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, B.; Feng, X.; Xu, Y.; Lu, K.; Sun, M. CircRNAs and Exosomes: A Mysterious Frontier for Human Cancer. Mol. Ther. Nucleic Acids 2020, 19, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Lyu, D.; Huang, S. Circular RNA Expands Its Territory. Mol. Cell. Oncol. 2015, 3, E1084443. [Google Scholar] [CrossRef]
- Dou, Y.; Cha, D.J.; Franklin, J.L.; Higginbotham, J.N.; Jeppesen, D.K.; Weaver, A.M.; Prasad, N.; Levy, S.; Coffey, R.J.; Patton, J.G.; et al. Circular RNAs Are down-Regulated in KRAS Mutant Colon Cancer Cells and Can Be Transferred to Exosomes. Sci. Rep. 2016, 6, 37982. [Google Scholar] [CrossRef]
- Santangelo, L.; Giurato, G.; Cicchini, C.; Montaldo, C.; Mancone, C.; Tarallo, R.; Battistelli, C.; Alonzi, T.; Weisz, A.; Tripodi, M. The RNA-Binding Protein SYNCRIP Is a Component of the Hepatocyte Exosomal Machinery Controlling MicroRNA Sorting. Cell Rep. 2016. [Google Scholar] [CrossRef]
- Guduric-Fuchs, J.; O’Connor, A.; Camp, B.; O’Neill, C.L.; Medina, R.J.; Simpson, D.A. Selective Extracellular Vesicle-Mediated Export of an Overlapping Set of MicroRNAs from Multiple Cell Types. BMC Genom. 2012. [Google Scholar] [CrossRef]
- Corbeil, D.; Santos, M.F.; Karbanová, J.; Kurth, T.; Rappa, G.; Lorico, A. Uptake and Fate of Extracellular Membrane Vesicles: Nucleoplasmic Reticulum-Associated Late Endosomes as a New Gate to Intercellular Communication. Cells 2020, 9, 1931. [Google Scholar] [CrossRef]
- Esteller, M. Non-Coding RNAs in Human Disease. Nat. Rev. Genet 2011, 12, 861–874. [Google Scholar] [CrossRef]
- Flynt, A.S.; Lai, E.C. Biological Principles of MicroRNA-Mediated Regulation: Shared Themes amid Diversity. Nat. Rev. Genet 2008, 9, 831–842. [Google Scholar] [CrossRef]
- Chang, W.; Zhang, L.; Xian, Y.; Yu, Z. MicroRNA-33a Promotes Cell Proliferation and Inhibits Apoptosis by Targeting PPARα in Human Hepatocellular Carcinoma. Exp. Ther. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, U.; Lai, X.; Winter, F.; Wolkenhauer, O.; Vera, J.; Gupta, S.K. Cooperative Gene Regulation by MicroRNA Pairs and Their Identification Using a Computational Workflow. Nucleic Acids Res. 2014. [Google Scholar] [CrossRef]
- Patel, T. Extracellular Vesicle Noncoding RNA: New Players in the Diagnosis and Pathogenesis of Cholangiocarcinoma. Hepatology 2014, 60, 782–784. [Google Scholar] [CrossRef]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.E.E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef]
- Sun, N.; Lee, Y.T.; Zhang, R.Y.; Kao, R.; Teng, P.C.; Yang, Y.; Yang, P.; Wang, J.J.; Smalley, M.; Chen, P.J.; et al. Purification of HCC-Specific Extracellular Vesicles on Nanosubstrates for Early HCC Detection by Digital Scoring. Nat. Commun. 2020, 11, 4489. [Google Scholar] [CrossRef] [PubMed]
- Konoshenko, M.Y.; Lekchnov, E.A.; Bryzgunova, O.E.; Kiseleva, E.; Pyshnaya, I.A.; Laktionov, P.P. Isolation of Extracellular Vesicles from Biological Fluids via the Aggregation-Precipitation Approach for Downstream MiRNAs Detection. Diagnostics 2021, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Segundo-Val, I.S.; Sanz-Lozano, C.S. Introduction to the Gene Expression Analysis. Methods Mol. Biol. 2016, 1434, 29–43. [Google Scholar] [CrossRef]
- Narrandes, S.; Xu, W. Gene Expression Detection Assay for Cancer Clinical Use. J. Cancer 2018, 9, 2249–2265. [Google Scholar] [CrossRef]
- Mohankumar, S.; Patel, T. Extracellular Vesicle Long Noncoding RNA as Potential Biomarkers of Liver Cancer. Brief Funct. Genom. 2016, 15, 249–256. [Google Scholar] [CrossRef]
- Zhao, B.; Ding, S.; Li, W.; Jin, Y. Hybridization Kinetics Analysis of an Oligonucleotide Microarray for MicroRNA Detection. Acta Biochim. Biophys. Sin. 2011, 43, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Moya, J.M.; Vilella, F.; Simón, C. MicroRNA: Key Gene Expression Regulators. Fertil. Steril. 2014, 101, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Jing, F.; Li, G.; Wu, Z.; Cheng, Z.; Zhang, J.; Zhang, H.; Jia, C.; Jin, Q.; Mao, H.; et al. Absolute Quantification of Lung Cancer Related MicroRNA by Droplet Digital PCR. Biosen. Bioelectron. 2015, 74, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yan, I.K.; Kim, C.; Kim, J.; Patel, T. Analysis of Extracellular RNA by Digital PCR. Front. Oncol. 2014. [Google Scholar] [CrossRef]
- Shukla, N.; Yan, I.K.; Patel, T. Multiplexed Detection and Quantitation of Extracellular Vesicle RNA Expression Using NanoString. Methods Mol. Biol. 2018, 1740, 177–185. [Google Scholar] [CrossRef]
- Geiss, G.K.; Bumgarner, R.E.; Birditt, B.; Dahl, T.; Dowidar, N.; Dunaway, D.L.; Fell, H.P.; Ferree, S.; George, R.D.; Grogan, T.; et al. Direct Multiplexed Measurement of Gene Expression with Color-Coded Probe Pairs. Nat. Biotechnol. 2008, 26, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Bracht, J.W.; Gimenez-Capitan, A.; Huang, C.Y.; Potie, N.; Pedraz-Valdunciel, C.; Warren, S.; Rosell, R.; Molina-Vila, M.A. Analysis of Extracellular Vesicle MRNA Derived from Plasma Using the NCounter Platform. Sci. Rep. 2021, 11, 3712. [Google Scholar] [CrossRef]
- Lopez, J.P.; Cruceanu, C.; Fiori, L.M.; Laboissiere, S.; Guillet, I.; Fontaine, J.; Ragoussis, J.; Benes, V.; Turecki, G.; Ernst, C. Biomarker Discovery: Quantification of MicroRNAs and Other Small Non-Coding RNAs Using Next Generation Sequencing. BMC Med. Genom. 2015, 8, 35. [Google Scholar] [CrossRef]
- Craig, A.J.; von Felden, J.; Garcia-Lezana, T.; Sarcognato, S.; Villanueva, A. Tumour Evolution in Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 139–152. [Google Scholar] [CrossRef]
- Cervello, M.; Emma, M.R.; Augello, G.; Cusimano, A.; Giannitrapani, L.; Soresi, M.; Akula, S.M.; Abrams, S.L.; Steelman, L.S.; Gulino, A.; et al. New Landscapes and Horizons in Hepatocellular Carcinoma Therapy. Aging 2020, 12, 3053–3094. [Google Scholar] [CrossRef]
- D’Agnano, I.; Berardi, A.C. Extracellular Vesicles, A Possible Theranostic Platform Strategy for Hepatocellular Carcinoma-An Overview. Cancers 2020, 12, 261. [Google Scholar] [CrossRef]
- Hirsova, P.; Ibrahim, S.H.; Verma, V.K.; Morton, L.A.; Shah, V.H.; LaRusso, N.F.; Gores, G.J.; Malhi, H. Extracellular Vesicles in Liver Pathobiology: Small Particles with Big Impact. Hepatology 2016, 64, 2219–2233. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Qin, H.; Poon, T.C.W.; Sze, S.C.; Ding, X.; Co, N.N.; Ngai, S.M.; Chan, T.F.; Wong, N. Hepatocellular Carcinoma-Derived Exosomes Promote Motility of Immortalized Hepatocyte through Transfer of Oncogenic Proteins and RNAs. Carcinogenesis 2015. [Google Scholar] [CrossRef] [PubMed]
- Kogure, T.; Yan, I.K.; Lin, W.L.; Patel, T. Extracellular Vesicle-Mediated Transfer of a Novel Long Noncoding RNA TUC339: A Mechanism of Intercellular Signaling in Human Hepatocellular Cancer. Genes Cancer 2013. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Takeshita, F.; Matsuki, Y.; Ochiya, T. Secretory Mechanisms and Intercellular Transfer of MicroRNAs in Living Cells. J. Biol. Chem. 2010. [Google Scholar] [CrossRef]
- Meng, F.; Henson, R.; Wehbe-Janek, H.; Ghoshal, K.; Jacob, S.T.; Patel, T. MicroRNA-21 Regulates Expression of the PTEN Tumor Suppressor Gene in Human Hepatocellular Cancer. Gastroenterology 2007, 133, 647–658. [Google Scholar] [CrossRef]
- Liao, Q.; Han, P.; Huang, Y.; Wu, Z.; Chen, Q.; Li, S.; Ye, J.; Wu, X. Potential Role of Circulating MicroRNA-21 for Hepatocellular Carcinoma Diagnosis: A Meta-Analysis. PLoS ONE 2015, 10, e0130677. [Google Scholar] [CrossRef]
- Wang, H.; Hou, L.; Li, A.; Duan, Y.; Gao, H.; Song, X. Expression of Serum Exosomal MicroRNA-21 in Human Hepatocellular Carcinoma. BioMed Res. Int. 2014. [Google Scholar] [CrossRef]
- Shi, J. Considering Exosomal MiR-21 as a Biomarker for Cancer. J. Clin. Med. 2016, 42. [Google Scholar] [CrossRef]
- Feng, Y.H.; Tsao, C.J. Emerging Role of MicroRNA-21 in Cancer. Biomed. Rep. 2016, 5, 395–402. [Google Scholar] [CrossRef]
- Cao, L.Q.; Yang, X.W.; Chen, Y.B.; Zhang, D.W.; Jiang, X.F.; Xue, P. Exosomal MiR-21 Regulates the TETs/PTENp1/PTEN Pathway to Promote Hepatocellular Carcinoma Growth. Mol. Cancer. 2019, 18, 148, Erratum in 2020, 19, 59. [Google Scholar] [CrossRef]
- Bettermann, K.; Vucur, M.; Haybaeck, J.; Koppe, C.; Janssen, J.; Heymann, F.; Weber, A.; Weiskirchen, R.; Liedtke, C.; Gassler, N.; et al. TAK1 Suppresses a NEMO-Dependent but NF-KappaB-Inde-Pendent Pathway to Liver Cancer. Cancer Cell 2010, 17, 481–496. [Google Scholar] [CrossRef]
- Inokuchi, S.; Aoyama, T.; Miura, K.; Österreicher, C.H.; Kodama, Y.; Miyai, K.; Akira, S.; Brenner, D.A.; Seki, E. Disruption of TAK1 in Hepatocytes Causes Hepatic Injury, Inflammation, FIbrosis, and Carcinogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 844–849. [Google Scholar] [CrossRef]
- Besse, A.; Lamothe, B.; Campos, A.D.; Webster, W.K.; Maddineni, U.; Lin, S.C.; Wu, H.; Darnay, B.G. TAK1-Dependent Signaling Requires Functional Interaction with TAB2/TAB3. J. Biol. Chem. 2007. [Google Scholar] [CrossRef]
- Roh, Y.S.; Song, J.; Seki, E. TAK1 Regulates Hepatic Cell Survival and Carcinogenesis. J. Gastroenterol. 2014, 49, 185–194. [Google Scholar] [CrossRef]
- Lin, X.J.; Fang, J.H.; Yang, X.J.; Zhang, C.; Yuan, Y.; Zheng, L.; Zhuang, S.M. Hepatocellular Carcinoma Cell-Secreted Exosomal MicroRNA-210 Promotes Angiogenesis In Vitro and In Vivo. Mol. Ther. Nucleic Acids 2018. [Google Scholar] [CrossRef]
- Fang, J.H.; Zhang, Z.J.; Shang, L.R.; Luo, Y.W.; Lin, Y.F.; Yuan, Y.; Zhuang, S.M. Hepatoma Cell-Secreted Exosomal MicroRNA-103 Increases Vascular Permeability and Promotes Metastasis by Targeting Junction Proteins. Hepatology 2018. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Lv, H.; Lv, G.; Li, T.; Wang, C.; Han, Q.; Yu, L.; Su, B.; Guo, L.; Huang, S.; et al. Tumor-Derived Exosomal MiR-1247-3p Induces Cancer-Associated Fibroblast Activation to Foster Lung Metastasis of Liver Cancer. Nat. Commun. 2018. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yan, I.K.; Haga, H.; Patel, T. Modulation of Hypoxia-Signaling Pathways by Extracellular Linc-RoR. J. Cell Sci. 2014. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yan, I.K.; Wood, J.; Haga, H.; Patel, T. Involvement of Extracellular Vesicle Long Noncoding RNA (Linc-VLDLR) in Tumor Cell Responses to Chemotherapy. Mol. Cancer Res. 2014. [Google Scholar] [CrossRef]
- Braconi, C.; Valeri, N.; Kogure, T.; Gasparini, P.; Huang, N.; Nuovo, G.J.; Terracciano, L.; Croce, C.M.; Patel, T. Expression and Functional Role of a Transcribed Noncoding RNA with an Ultraconserved Element in Hepatocellular Carcinoma. Proc. Natl. Acad. Sci. USA 2011, 108, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lei, Y.; Wu, M.; Li, N. Regulation of Macrophage Activation and Polarization by HCC-Derived Exosomal LncRNA TUC339. Int. J. Mol. Sci. 2018, 2958. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, A.; Ho, T.T.; Zhang, Z.; Zhou, N.; Ding, X.; Zhang, X.; Xu, M.; Mo, Y.Y. Linc-RoR Promotes c-Myc Expression through HnRNP i and AUF1. Nucleic Acids Res. 2015. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, T.; Ge, S.; Liu, Y.; Bai, M.; Zhu, K.; Fan, Q.; Li, J.; Ning, T.; Tian, F.; et al. Exosome CircRNA Secreted from Adipocytes Promotes the Growth of Hepatocellular Carcinoma by Targeting Deubiquitination-Related USP7. Oncogene 2019, 38, 2844–2859. [Google Scholar] [CrossRef]
- Wang, G.; Liu, W.; Zou, Y.; Wang, G.; Deng, Y.; Luo, J.; Zhang, Y.; Li, H.; Zhang, Q.; Yang, Y.; et al. Three Isoforms of Exosomal CircPTGR1 Promote Hepatocellular Carcinoma Metastasis via the MiR449a–MET Pathway. EBioMedicine 2019. [Google Scholar] [CrossRef] [PubMed]
- Takaki, Y.; Saito, Y.; Takasugi, A.; Toshimitsu, K.; Yamada, S.; Muramatsu, T.; Kimura, M.; Sugiyama, K.; Suzuki, H.; Arai, E.; et al. Silencing of MicroRNA-122 Is an Early Event during Hepatocarcinogenesis from Non-Alcoholic Steatohepatitis. Cancer Sci. 2014, 105, 1254–1260. [Google Scholar] [CrossRef]
- Esau, C.; Davis, S.; Murray, S.F.; Yu, X.X.; Pandey, S.K.; Pear, M.; Watts, L.; Booten, S.L.; Graham, M.; McKay, R.; et al. MiR-122 Regulation of Lipid Metabolism Revealed by in vivo Antisense Targeting. Cell Metab. 2006. [Google Scholar] [CrossRef]
- Povero, D.; Eguchi, A.; Li, H.; Johnson, C.D.; Papouchado, B.G.; Wree, A.; Messer, K.; Feldstein, A.E. Circulating Extracellular Vesicles with Specific Proteome and Liver MicroRNAs Are Potential Biomarkers for Liver Injury in Experimental Fatty Liver Disease. PLoS ONE 2014. [Google Scholar] [CrossRef] [PubMed]
- Povero, D.; Eguchi, A.; Niesman, I.R.; Andronikou, N.; de Mollerat Du Jeu, X.; Mulya, A.; Berk, M.; Lazic, M.; Thapaliya, S.; Parola, M.; et al. Lipid-Induced Toxicity Stimulates Hepatocytes to Release Angiogenic Microparticles That Require Vanin-1 for Uptake by Endothelial Cells. Sci. Signal. 2013, 6, Ra88. [Google Scholar] [CrossRef]
- Csak, T.; Bala, S.; Lippai, D.; Satishchandran, A.; Catalano, D.; Kodys, K.; Szabo, G. MicroRNA-122 Regulates Hypoxia-Inducible Factor-1 and Vimentin in Hepatocytes and Correlates with Fibrosis in Diet-Induced Steatohepatitis. Liver Int. 2015, 35, 532–541. [Google Scholar] [CrossRef]
- Basu, S.; Bhattacharyya, S.N. Insulin-like Growth Factor-1 Prevents MiR-122 Production in Neighbouring Cells to Curtail Its Intercellular Transfer to Ensure Proliferation of Human Hepatoma Cells. Nucleic Acids Res. 2014. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Ohashi, K.; Suzuki, K.; Munetsuna, E.; Ando, Y.; Yamazaki, M.; Ishikawa, H.; Ichino, N.; Teradaira, R.; Hashimoto, S. Longitudinal Study of Circulating MiR-122 in a Rat Model of Non-Alcoholic Fatty Liver Disease. Clin. Chim. Acta Int. J. Clin. Chem. 2015, 446, 267–271. [Google Scholar] [CrossRef]
- Wei, J.X.; Lv, L.H.; Wan, Y.L.; Cao, Y.; Li, G.L.; Lin, H.M.; Zhou, R.; Shang, C.Z.; Cao, J.; He, H.; et al. Vps4A Functions as a Tumor Suppressor by Regulating the Secretion and Uptake of Exosomal MicroRNAs in Human Hepatoma Cells. Hepatology 2015, 61, 1284–1294. [Google Scholar] [CrossRef]
- Mann, J.; Reeves, H.L.; Feldstein, A.E. Liquid Biopsy for Liver Diseases. Gut 2018. [Google Scholar] [CrossRef]
- De Rubis, G.; Rajeev Krishnan, S.; Bebawy, M. Liquid Biopsies in Cancer Diagnosis, Monitoring, and Prognosis. Trends Pharmacol. Sci. 2019, 40, 172–186. [Google Scholar] [CrossRef]
- Pardini, B.; Sabo, A.A.; Birolo, G.; Calin, G.A. Noncoding RNAs in Extracellular Fluids as Cancer Biomarkers: The New Frontier of Liquid Biopsies. Cancers 2019, 11, 1170. [Google Scholar] [CrossRef]
- Lee, Y.R.; Kim, G.; Tak, W.Y.; Jang, S.Y.; Kweon, Y.O.; Park, J.G.; Lee, H.W.; Han, Y.S.; Chun, J.M.; Park, S.Y.; et al. Circulating Exosomal Noncoding RNAs as Prognostic Biomarkers in Human Hepatocellular Carcinoma. Int. J. Cancer 2019. [Google Scholar] [CrossRef]
- Afonso, M.; Rodrigues, P.; Simão, A.; Castro, R. Circulating MicroRNAs as Potential Biomarkers in Non-Alcoholic Fatty Liver Disease and Hepatocellular Carcinoma. J. Clin. Med. 2016, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, S.; Liu, B. Diagnostic and Prognostic Values of Serum Exosomal MicroRNA-21 in Children with Hepatoblastoma: A Chinese Population-Based Study. Pediatr. Surg. Int. 2016. [Google Scholar] [CrossRef]
- Li, N.; Miao, Y.; Shan, Y.; Liu, B.; Li, Y.; Zhao, L.; Jia, L. MiR-106b and MiR-93 Regulate Cell Progression by Suppression of PTEN via PI3K/Akt Pathway in Breast Cancer. Cell Death Dis. 2017, 8, E2796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Guo, Y.; Ma, Z.; Ma, G.; Xue, Q.; Li, F.; Liu, L. Long Non-Coding RNA PTENP1 Functions as a CeRNA to Modulate PTEN Level by Decoying MiR-106b and MiR-93 in Gastric Cancer. Oncotarget 2017, 8, 26079–26089. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Wang, X.; Zhao, Y.; Hu, R.; Qin, L. Exosomal MiR-93 Promotes Proliferation and Invasion in Hepatocellular Carcinoma by Directly Inhibiting TIMP2/TP53INP1/CDKN1A. Biochem. Biophys. Res. Commun. 2018, 502, 515–521. [Google Scholar] [CrossRef]
- Mjelle, R.; Dima, S.O.; Bacalbasa, N.; Chawla, K.; Sorop, A.; Cucu, D.; Herlea, V.; Sætrom, P.; Popescu, I. Comprehensive Transcriptomic Analyses of Tissue, Serum, and Serum Exosomes from Hepatocellular Carcinoma Patients. BMC Cancer 2019. [Google Scholar] [CrossRef]
- Tian, X.P.; Wang, C.Y.; Jin, X.H.; Li, M.; Wang, F.W.; Huang, W.J.; Yun, J.P.; Xu, R.H.; Cai, Q.Q.; Xie, D. Acidic Microenvironment Up-Regulates Exosomal MiR-21 and MiR-10b in Early-Stage Hepatocellular Carcinoma to Promote Cancer Cell Proliferation and Metastasis. Theranostics 2019, 9, 1965–1979. [Google Scholar] [CrossRef]
- Sohn, W.; Kim, J.; Kang, S.H.; Yang, S.R.; Cho, J.Y.; Cho, H.C.; Shim, S.G.; Paik, Y.H. Serum Exosomal MicroRNAs as Novel Biomarkers for Hepatocellular Carcinoma. Exp. Mol. Med. 2015. [Google Scholar] [CrossRef]
- Tang, J.; Li, Y.; Liu, K.; Zhu, Q.; Yang, W.H.; Xiong, L.K.; Guo, D.L. Exosomal MiR- 9-3p Suppresses HBGF-5 Expression and Is a Functional Biomarker in Hepatocellular Carcinoma. Minerva Med. 2018, 109, 15–23. [Google Scholar] [PubMed]
- Cui, Y.; Xu, H.-F.; Liu, M.-Y.; Xu, Y.-J.; He, J.-C.; Zhou, Y.; Cang, S.-D. Mechanism of Exosomal MicroRNA-224 in Development of Hepatocellular Carcinoma and Its Diagnostic and Prognostic Value. World J. Gastroenterol. 2019, 25, 1890–1898. [Google Scholar] [CrossRef]
- Cho, H.J.; Eun, J.W.; Baek, G.O.; Seoù, C.W.; Ahn, H.R.; Kim, S.S.; Cho, S.W.; Cheong, J.Y. Serum Exosomal MicroRNA, MiR-10b-5p, as a Potential Diagnostic Biomarker for Early-Stage Hepatocellular Carcinoma. J. Clin. Med. 2020, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tang, J.; Zhang, B.; Yang, W.; LiuGao, M.; Wang, R.; Tan, Y.; Fan, J.; Chang, Y.; Fu, J.; et al. Epigenetic Modification of MiR-429 Promotes Liver Tumour-Initiating Cell Properties by Targeting Rb Binding Protein 4. Gut 2015. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hu, J.; Zhou, K.; Chen, F.; Wang, Z.; Liao, B.; Dai, Z.; Cao, Y.; Fan, J.; Zhou, J. Serum Exosomal MiR-125b Is a Novel Prognostic Marker for Hepatocellular Carcinoma. OncoTargets Ther. 2017. [Google Scholar] [CrossRef]
- Sugimachi, K.; Matsumura, T.; Hirata, H.; Uchi, R.; Ueda, M.; Ueo, H.; Shinden, Y.; Iguchi, T.; Eguchi, H.; Shirabe, K.; et al. Identification of a Bona Fide MicroRNA Biomarker in Serum Exosomes That Predicts Hepatocellular Carcinoma Recurrence after Liver Transplantation. Br. J. Cancer 2015. [Google Scholar] [CrossRef] [PubMed]
- Suehiro, T.; Miyaaki, H.; Shibata, H.; Honda, T.; Ozawa, E.; Miuma, S.; Taura, N.; Nakao, K. Significance of Serum Exosomal MiR-122 and MiR-21 as a Predictive Biomarker in Hepatocellular Carcinoma Patients Who Underwent Transarterial Chemoembolization. J. Hepatol. 2017. [Google Scholar] [CrossRef]
- Shi, M.; Jiang, Y.; Yang, L.; Yan, S.; Wang, Y.-G.; Lu, X.-J. Decreased Levels of Serum Exosomal MiR-638 Predict Poor Prognosis in Hepatocellular Carcinoma. J. Cell. Biochem. 2018, 119, 4711–4716. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Yu, S.; Wang, Z.; He, X.; Su, Y.; Guo, T.; Sheng, H.; Chen, J.; Zheng, Q.; et al. Extracellular Vesicles Long RNA Sequencing Reveals Abundant MRNA, CircRNA, and LncRNA in Human Blood as Potential Biomarkers for Cancer Diagnosis. Clin. Chem. 2019, 65, 798–808. [Google Scholar] [CrossRef]
- Ma, X.; Yuan, T.; Yang, C.; Wang, Z.; Zang, Y.; Wu, L.; Zhuang, L. X-Inactive-Specific Transcript of Peripheral Blood Cells Is Regulated by Exosomal Jpx and Acts as a Biomarker for Female Patients with Hepatocellular Carcinoma. Ther. Adv. Med. Oncol. 2017, 9, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Su, Y.; Liu, X.; Xu, M.; Chen, X.; Zhu, Y.; Guo, Z.; Bai, T.; Dong, L.; Wei, C.; et al. Serum and Exosome Long Non Coding RNAs as Potential Biomarkers for Hepatocellular Carcinoma. J. Cancer 2018, 9, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, Y.; Dong, X.; Wang, X. Serum Exosomal Long Noncoding RNAs ENSG00000258332.1 and LINC00635 for the Diagnosis and Prognosis of Hepatocellular Carcinoma. Cancer Epidemiol. Biomark. Prev. 2018, 27, 710–716. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, X.; Qi, Q.; Gao, Y.; Wei, Q.; Han, S. LncRNA-HEIH in Serum and Exosomes as a Potential Biomarker in the HCV-Related Hepatocellular Carcinoma. Cancer Biomark. 2018, 21, 651–659. [Google Scholar] [CrossRef]
- Kim, S.S.; Baek, G.O.; Ahn, H.R.; Sung, S.; Seo, C.W.; Cho, H.J.; Nam, S.W.; Cheong, J.Y.; Eun, J.W. Serum Small ExtracellularVesicle-derived LINC00853 as a Novel Diagnostic Marker for Early Hepatocellular Carcinoma. Mol. Oncol. 2020, 14, 2646–2659. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, Z. Diagnostic Value of a MicroRNAsignature Panel in Exosomes for Patients with Hepatocellular Carcinoma. Int. J. Clin. Exp. Pathol. 2019, 12, 1478–1487. [Google Scholar] [PubMed]
- Wang, Y.; Zhang, C.; Zhang, P.; Guo, G.; Jiang, T.; Zhao, X.; Jiang, J.; Huang, X.; Tong, H.; Tian, Y. Serum Exosomal MicroRNAs Combined with Alpha-Fetoprotein as Diagnostic Markers of Hepatocellular Carcinoma. Cancer Med. 2018, 7, 1670–1679. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, W.; Wang, H.; Qiu, G.; Jiang, Z.; Wei, G.; Li, X. Exosomal MiR-744 Inhibits Proliferation and Sorafenib Chemoresistance in Hepatocellular Carcinoma by Targeting PAX2. Med. Sci. Monit. 2019, 25, 7209–7217. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).