Meta-Analysis of Heifer Traits Identified Reproductive Pathways in Bos indicus Cattle
Abstract
1. Introduction
2. Materials and Methods
2.1. Phenotypes
2.2. Genotypes and Imputation
2.3. Fixed Effects
2.4. Genome-Wide Association Studies
2.5. Genetic and Phenotypic Correlation of the Traits
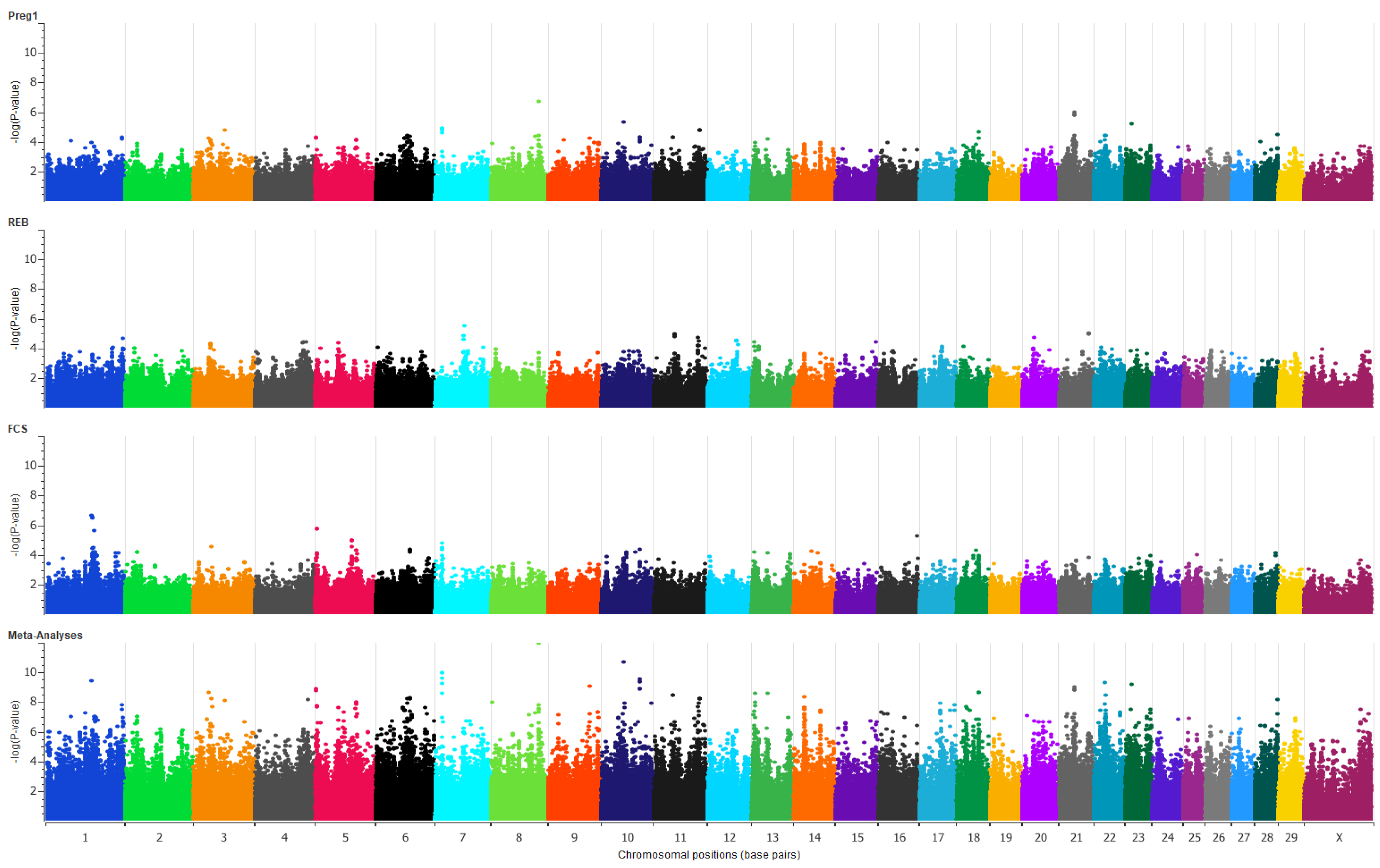
2.6. Multi-Trait Meta-Analysis
2.7. SNP Informed Positional Candidate Gene List
2.8. Functional Pathway Analysis
2.9. Using Information from Transcriptomics and Proteomics Studies Related to Cattle Puberty
2.10. Transcription Factor Analysis
3. Results
4. Discussion
4.1. Genes Located within 0.5 Mb of Peak SNPs
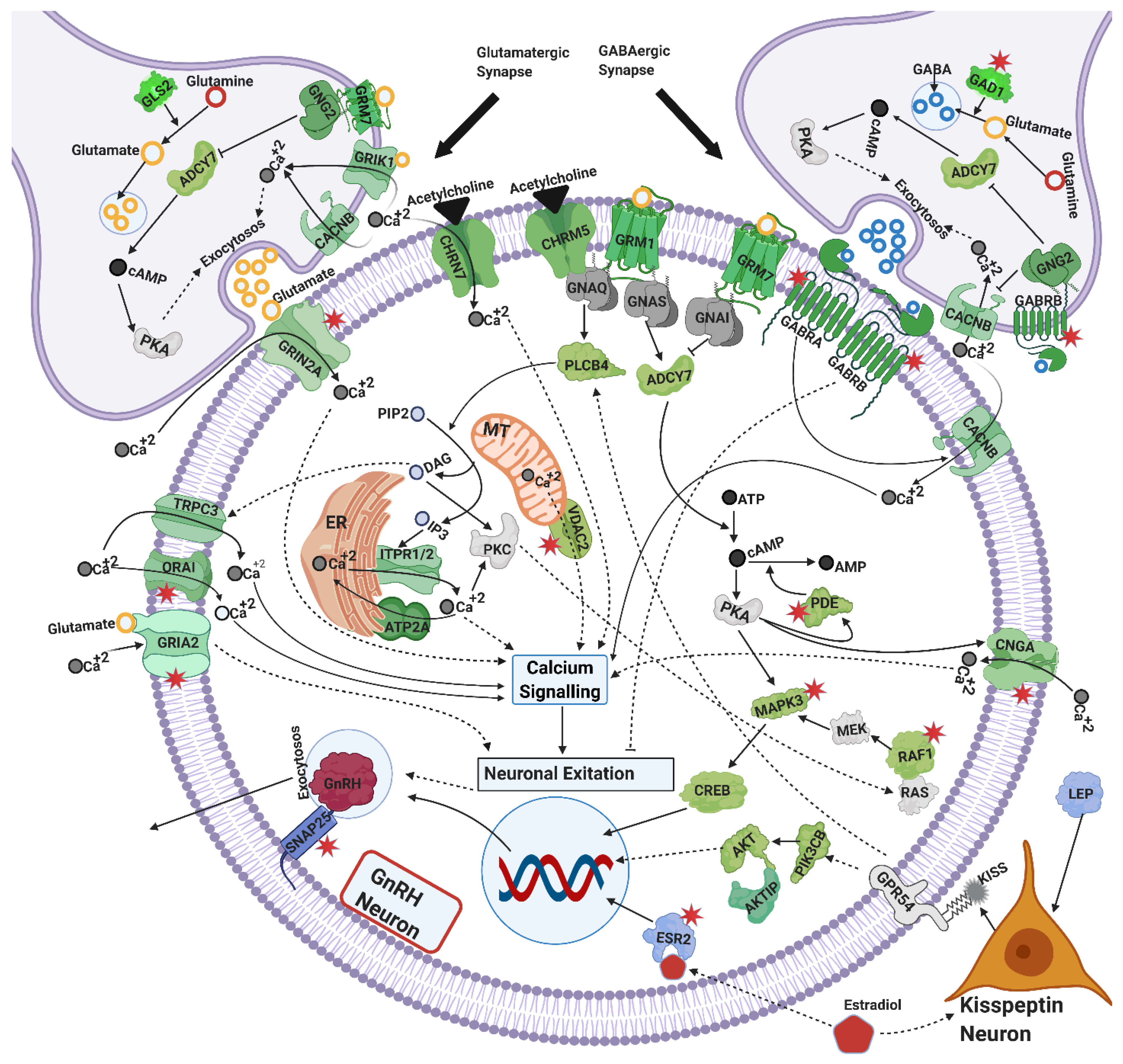
4.2. Exocytosis and GnRH Secretion
4.2.1. Calcium Signaling
4.2.2. Cyclic AMP (cAMP) Signaling
4.2.3. Glutamatergic and GABAergic Synapse
4.2.4. Cholinergic Synapse
4.2.5. Melatonin Signaling
4.2.6. Estrogen Signaling
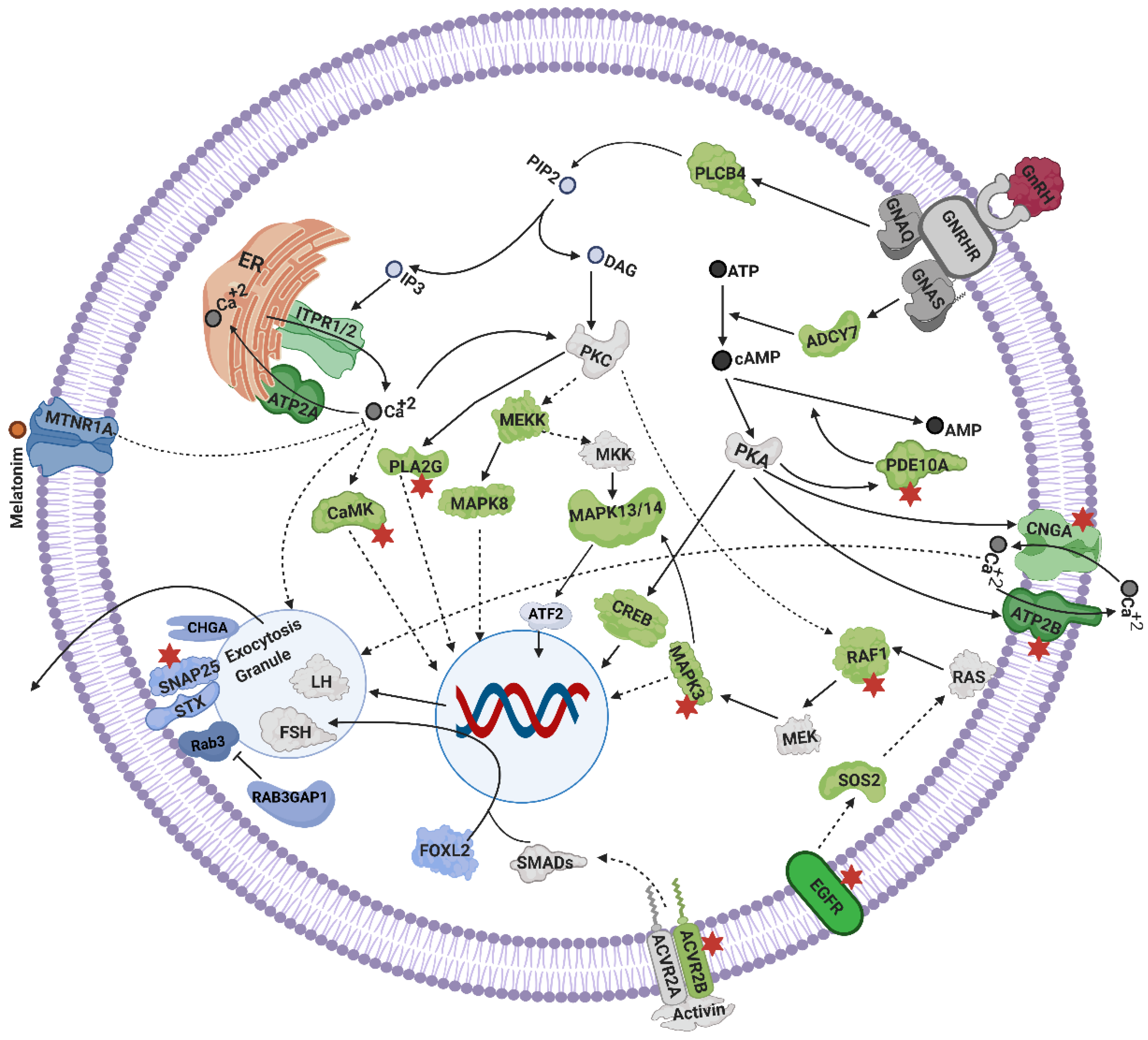
4.3. Mechanisms Linked to Gonadotrophins Secretion by the Pituitary
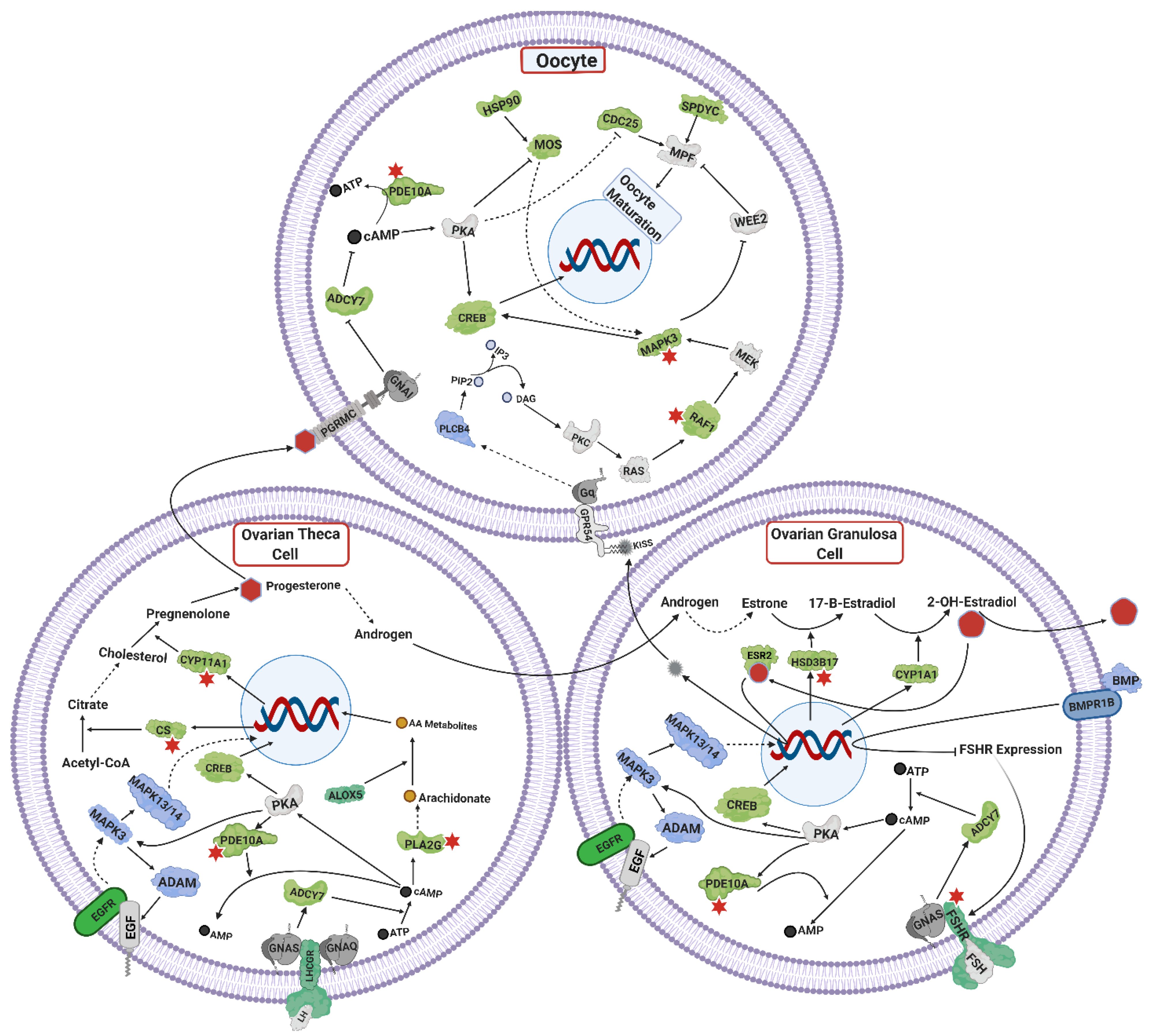
4.4. Ovarian Steroidogenesis, and TGF Signaling
4.5. Oocyte Maturation
4.6. Olfactory Signaling
4.7. Transcription Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diskin, M.; Kenny, D. Optimising reproductive performance of beef cows and replacement heifers. Animals 2014, 8, 27–39. [Google Scholar] [CrossRef]
- Johnston, D.J.; Barwick, S.A.; Fordyce, G.; Holroyd, R.G.; Williams, P.J.; Corbet, N.J.; Grant, T. Genetics of early and lifetime annual reproductive performance in cows of two tropical beef genotypes in northern Australia. Anim. Prod. Sci. 2014, 54, 1–15. [Google Scholar] [CrossRef]
- Chase, C.C.; Chenoweth, P.J.; Larsen, R.E.; Hammond, A.C.; Olson, T.A.; West, R.L.; Johnson, D.D. Growth, puberty, and carcass characteristics of Brahman-, Senepol-, and Tuli-sired F1 Angus bulls. J. Anim. Sci. 2001, 79, 2006–2015. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.P.; Dahl, G.E.; Glover, B.H.; Karsch, F.J. Central regulation of pulsatile gonadotropin-releasing hormone (GnRH) secretion by estradiol during the period leading up to the preovulatory GnRH surge in the ewe. Endocrinology 1994, 134, 1806–1811. [Google Scholar] [CrossRef] [PubMed]
- Dubois, S.L.; Wolfe, A.; Radovick, S.; Boehm, U.; Levine, J.E. Estradiol Restrains Prepubertal Gonadotropin Secretion in Female Mice via Activation of ERα in Kisspeptin Neurons. Endocrinology 2016, 157, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Iremonger, K.J.; Constantin, S.; Liu, X.; Herbison, A.E. Glutamate regulation of GnRH neuron excitability. Brain Res. 2010, 1364, 35–43. [Google Scholar] [CrossRef]
- Krsmanovic, L.Z.; Hu, L.; Leung, P.-K.; Feng, H.; Catt, K.J. The hypothalamic GnRH pulse generator: Multiple regulatory mechanisms. Trends Endocrinol. Metab. 2009, 20, 402–408. [Google Scholar] [CrossRef]
- Clarkson, J.; De Tassigny, X.D.; Moreno, A.S.; Colledge, W.H.; Herbison, A.E. Kisspeptin-GPR54 Signaling Is Essential for Preovulatory Gonadotropin-Releasing Hormone Neuron Activation and the Luteinizing Hormone Surge. J. Neurosci. 2008, 28, 8691–8697. [Google Scholar] [CrossRef]
- Clarkson, J.; Herbison, A.E. Development of GABA and glutamate signaling at the GnRH neuron in relation to puberty. Mol. Cell. Endocrinol. 2006, 254–255, 32–38. [Google Scholar] [CrossRef]
- Arai, Y.; Ishii, H.; Kobayashi, M.; Ozawa, H. Subunit profiling and functional characteristics of acetylcholine receptors in GT1-7 cells. J. Physiol. Sci. 2017, 67, 313–323. [Google Scholar] [CrossRef] [PubMed]
- De La Escalera, G.M. Dopaminergic regulation of the GT1 gonadotropin-releasing hormone (GnRH) neuronal cell lines: Stimulation of GnRH release via D1- receptors positively coupled to adenylate cyclase. Endocrinology 1992, 131, 2965–2971. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Mahesh, V.B.; Bhat, G.K.; Ping, L.; Brann, D.W. Evidence for a role of bradykinin neurons in the control of gonadotropin-releasing hormone secretion. Neuroendocrinology 1998, 67, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Macrae, M.B.; Davidson, J.S.; Millar, R.P.; A Van Der Merwe, P. Cyclic AMP stimulates luteinizing-hormone (lutropin) exocytosis in permeabilized sheep anterior-pituitary cells. Synergism with protein kinase C and calcium. Biochem. J. 1990, 271, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Pawson, A.J.; McNeilly, A.S. The pituitary effects of GnRH. Anim. Reprod. Sci. 2005, 88, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Cammack, K.; Thomas, M.; Enns, R. Reproductive Traits and Their Heritabilities in Beef Cattle. Prof. Anim. Sci. 2009, 25, 517–528. [Google Scholar] [CrossRef]
- Porto-Neto, L.R.; Edwards, S.; Fortes, M.R.S.; Lehnert, S.A.; Reverter, A.; McGowan, M.; Porto-Neto, L. Genome-wide association for the outcome of fixed-time artificial insemination of Brahman heifers in northern Australia. J. Anim. Sci. 2015, 93, 5119–5127. [Google Scholar] [CrossRef] [PubMed]
- Hawken, R.J.; Zhang, Y.D.; Fortes, M.R.S.; Collis, E.; Barris, W.C.; Corbet, N.J.; Williams, P.J.; Fordyce, G.; Holroyd, R.G.; Walkley, J.R.W.; et al. Genome-wide association studies of female reproduction in tropically adapted beef cattle. J. Anim. Sci. 2012, 90, 1398–1410. [Google Scholar] [CrossRef]
- Irano, N.; De Camargo, G.M.F.; Costa, R.B.; Terakado, A.P.N.; Magalhães, A.F.B.; Silva, R.M.D.O.; Dias, M.M.; Bignardi, A.B.; Baldi, F.; Carvalheiro, R.; et al. Genome-Wide Association Study for Indicator Traits of Sexual Precocity in Nellore Cattle. PLoS ONE 2016, 11, e0159502. [Google Scholar] [CrossRef]
- Stahl, E.A.; BIRAC Consortium; Raychaudhuri, S.; Remmers, E.F.; Xie, G.; Eyre, S.; Thomson, B.P.; Li, Y.; Kurreeman, F.A.S.; Zhernakova, A.; et al. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat. Genet. 2010, 42, 508–514. [Google Scholar] [CrossRef]
- Manolio, T.A.; Collins, F.S.; Cox, N.J.; Goldstein, D.B.; Hindorff, L.A.; Hunter, D.J.; McCarthy, M.I.; Ramos, E.M.; Cardon, L.R.; Chakravarti, A.; et al. Finding the missing heritability of complex diseases. Nat. Cell Biol. 2009, 461, 747–753. [Google Scholar] [CrossRef]
- Gibson, G. Hints of hidden heritability in GWAS. Nat. Genet. 2010, 42, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Visscher, P.M.; Hemani, G.; Vinkhuyzen, A.A.E.; Chen, G.-B.; Lee, S.H.; Wray, N.R.; Goddard, M.E.; Yang, J. Statistical Power to Detect Genetic (Co)Variance of Complex Traits Using SNP Data in Unrelated Samples. PLoS Genet. 2014, 10, e1004269. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Santos, E.S.A. Methodological issues and advances in biological meta-analysis. Evol. Ecol. 2012, 26, 1253–1274. [Google Scholar] [CrossRef]
- Melo, T.P.; Fortes, M.R.S.; Bresolin, T.; Mota, L.F.M.; Albuquerque, L.G.; Carvalheiro, R. Multitrait meta-analysis identified genomic regions associated with sexual precocity in tropical beef cattle. J. Anim. Sci. 2018, 96, 4087–4099. [Google Scholar] [CrossRef]
- Xiang, R.; Berg, I.V.D.; MacLeod, I.M.; Daetwyler, H.D.; Goddard, M.E. Effect direction meta-analysis of GWAS identifies extreme, prevalent and shared pleiotropy in a large mammal. Commun. Biol. 2020, 3, 1–14. [Google Scholar] [CrossRef]
- Loh, P.-R.; Danecek, P.; Palamara, P.F.; Fuchsberger, C.; A Reshef, Y.; Finucane, H.K.; Schoenherr, S.; Forer, S.S.L.; McCarthy, S.; Abecasis, C.F.G.R.; et al. Reference-based phasing using the Haplotype Reference Consortium panel. Nat. Genet. 2016, 48, 1443–1448. [Google Scholar] [CrossRef]
- Das, S.; Forer, L.; Schönherr, S.; Sidore, C.; E Locke, A.; Kwong, A.; I Vrieze, S.; Chew, E.Y.; Levy, S.; McGue, M.; et al. Next-generation genotype imputation service and methods. Nat. Genet. 2016, 48, 1284–1287. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Hawlader, A.A.; Bernardes, P.A.; Lim, D.; Park, B.; Gondro, C. A guide to imputation of low density single nucleotide polymorphism data up to sequence level. J. Anim. Breed. Genomics 2017, 1, 59–68. [Google Scholar]
- Golden Helix, I. SNP & Variation Suite ™ [Software]. [(Version 8.x)]. Available online: http://www.goldenhelix.com (accessed on 15 May 2020).
- Zhang, Y.D.; Johnston, D.J.; Bolormaa, S.; Hawken, R.J.; Tier, B. Genomic selection for female reproduction in Australian tropically adapted beef cattle. Anim. Prod. Sci. 2014, 54, 16–24. [Google Scholar] [CrossRef]
- Sarup, P.; Jensen, J.; Ostersen, T.; Henryon, M.; Sørensen, P. Increased prediction accuracy using a genomic feature model including prior information on quantitative trait locus regions in purebred Danish Duroc pigs. BMC Genet. 2016, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Vanvanhossou, S.F.U.; Scheper, C.; Dossa, L.H.; Yin, T.; Brügemann, K.; König, S. A multi-breed GWAS for morphometric traits in four Beninese indigenous cattle breeds reveals loci associated with conformation, carcass and adaptive traits. BMC Genom. 2020, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bolormaa, S.; Pryce, J.E.; Reverter, A.; Zhang, Y.; Barendse, W.; Kemper, K.; Tier, B.; Savin, K.; Hayes, B.J.; Goddard, M.E. A Multi-Trait, Meta-analysis for Detecting Pleiotropic Polymorphisms for Stature, Fatness and Reproduction in Beef Cattle. PLoS Genet. 2014, 10, e1004198. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Pereira, A.G.T.; Utsunomiya, Y.T.; Milanesi, M.; Torrecilha, R.B.P.; Carmo, A.S.; Neves, H.H.R.; Carvalheiro, R.; Ajmone-Marsan, P.; Sonstegard, T.S.; Sölkner, J.; et al. Pleiotropic Genes Affecting Carcass Traits in Bos indicus (Nellore) Cattle Are Modulators of Growth. PLoS ONE 2016, 11, e0158165. [Google Scholar] [CrossRef]
- McKay, S.D.; Schnabel, R.D.; Murdoch, B.M.; Matukumalli, L.K.; Aerts, J.; Coppieters, W.; Crews, D.; Neto, E.D.; Gill, C.A.; Gao, C.; et al. Whole genome linkage disequilibrium maps in cattle. BMC Genet. 2007, 8, 1–12. [Google Scholar] [CrossRef]
- Rosen, B.D.; Bickhart, D.M.; Schnabel, R.D.; Koren, S.; Elsik, C.G.; Tseng, E.; Rowan, T.N.; Low, W.Y.; Zimin, A.; Couldrey, C.; et al. De novo assembly of the cattle reference genome with single-molecule sequencing. GigaScience 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef]
- Guney, E.; Oliva, B. Exploiting Protein-Protein Interaction Networks for Genome-Wide Disease-Gene Prioritization. PLoS ONE 2012, 7, e43557. [Google Scholar] [CrossRef] [PubMed]
- Fortes, M.R.S.; Nguyen, L.T.; Weller, M.M.D.C.A.; Cánovas, A.; Islas-Trejo, A.; Porto-Neto, L.R.; Reverter, A.; Lehnert, S.A.; Boe-Hansen, G.B.; Thomas, M.G.; et al. Transcriptome analyses identify five transcription factors differentially expressed in the hypothalamus of post-versus prepubertal Brahman heifers. J. Anim. Sci. 2016, 94, 3693–3702. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Reverter, A.; Cánovas, A.; Venus, B.; Islas-Trejo, A.; Porto-Neto, L.R.; Lehnert, S.A.; Medrano, J.F.; Moore, S.S.; Fortes, M.R.S. Global differential gene expression in the pituitary gland and the ovaries of pre- and postpubertal Brahman heifers. J. Anim. Sci. 2017, 95, 599–615. [Google Scholar] [CrossRef][Green Version]
- Fortes, M.R.S.; Zacchi, L.F.; Nguyen, L.T.; Raidan, F.; Weller, M.M.D.C.A.; Choo, J.J.Y.; Reverter, A.; Rego, J.P.A.; Boe-Hansen, G.B.; Porto-Neto, L.R.; et al. Pre- and post-puberty expression of genes and proteins in the uterus of Bos indicus heifers: The luteal phase effect post-puberty. Anim. Genet. 2018, 49, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Roider, H.G.; Manke, T.; O’Keeffe, S.; Vingron, M.; Haas, S.A. PASTAA: Identifying transcription factors associated with sets of co-regulated genes. Bioinformatics 2009, 25, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Roepke, T.A.; Kelly, M.J.; Rønnekleiv, O.K. Kisspeptin Depolarizes Gonadotropin-Releasing Hormone Neurons through Activation of TRPC-Like Cationic Channels. J. Neurosci. 2008, 28, 4423–4434. [Google Scholar] [CrossRef]
- Zhang, X.-B.; Spergel, D.J. Kisspeptin Inhibits High-Voltage Activated Ca2+Channels in GnRH Neurons via Multiple Ca2+Influx and Release Pathways. Neuroendocrinology 2012, 96, 68–80. [Google Scholar] [CrossRef]
- Zhang, C.; Bosch, M.A.; Rønnekleiv, O.K.; Kelly, M.J. Kisspeptin Activation of TRPC4 Channels in Female GnRH Neurons Requires PIP2 Depletion and cSrc Kinase Activation. Endocrinology 2013, 154, 2772–2783. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hemond, P.J.; O’Boyle, M.P.; Roberts, C.B.; Delgado-Reyes, A.; Hemond, Z.; Suter, K.J. Simulated GABA Synaptic Input and L-Type Calcium Channels Form Functional Microdomains in Hypothalamic Gonadotropin-Releasing Hormone Neurons. J. Neurosci. 2012, 32, 8756–8766. [Google Scholar] [CrossRef]
- Padgett, C.L.; Slesinger, P.A. GABAB Receptor Coupling to G-proteins and Ion Channels. In Advances in Pharmacology; Elsevier BV: Amsterdam, The Netherlands, 2010; Volume 58, pp. 123–147. [Google Scholar]
- Kornau, H.C. GABA(B) receptors and synaptic modulation. Cell Tissue Res. 2006, 326, 517–533. [Google Scholar] [CrossRef]
- Jacob, T.C.; Moss, S.J.; Jurd, R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat. Rev. Neurosci. 2008, 9, 331–343. [Google Scholar] [CrossRef]
- Chalifoux, J.R.; Carter, A.G. GABAB receptor modulation of synaptic function. Curr. Opin. Neurobiol. 2011, 21, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Ferraguti, F.; Crepaldi, L.; Nicoletti, F. Metabotropic Glutamate 1 Receptor: Current Concepts and Perspectives. Pharmacol. Rev. 2008, 60, 536–581. [Google Scholar] [CrossRef] [PubMed]
- Palmada, M.; Centelles, J.J. Excitatory amino acid neurotransmission. Pathways for metabolism, storage and reuptake of glutamate in brain. Front. Biosci. 1998, 3, d701–d718. [Google Scholar] [PubMed]
- Schoepp, D.D. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J. Pharmacol. Exp. Ther. 2001, 299, 12–20. [Google Scholar]
- Conti, F.; Weinberg, R.J. Shaping excitation at glutamatergic synapses. Trends Neurosci. 1999, 22, 451–458. [Google Scholar] [CrossRef]
- Novaira, H.J.; Ng, Y.; Wolfe, A.; Radovick, S. Kisspeptin increases GnRH mRNA expression and secretion in GnRH secreting neuronal cell lines. Mol. Cell. Endocrinol. 2009, 311, 126–134. [Google Scholar] [CrossRef]
- Szereszewski, J.M.; Pampillo, M.; Ahow, M.R.; Offermanns, S.; Bhattacharya, M.; Babwah, A.V. GPR54 regulates ERK1/2 activity and hypothalamic gene expression in a Gα q/11 and β-arrestin-dependent manner. PLoS ONE 2010, 5, e12964. [Google Scholar] [CrossRef]
- Caulfield, M.P.; Birdsall, N.J. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol. Rev. 1998, 50, 279–290. [Google Scholar]
- Kaneishi, K.; Sakuma, Y.; Kobayashi, H.; Kato, M. 3′,5′-Cyclic Adenosine Monophosphate Augments Intracellular Ca2+ Concentration and Gonadotropin-Releasing Hormone (GnRH) Release in Immortalized GnRH Neurons in an Na+-Dependent Manner. Endocrinology 2002, 143, 4210–4217. [Google Scholar] [CrossRef][Green Version]
- Sakakibara, H.; Conti, M.; Weiner, R. Role of Phosphodiesterases in the Regulation of Gonadotropin- Releasing Hormone Secretion in GT1 Cells. Neuroendocrinology 1998, 68, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Vitalis, E.A.; Costantin, J.L.; Tsai, P.-S.; Sakakibara, H.; Paruthiyil, S.; Iiri, T.; Martini, J.-F.; Taga, M.; Choi, A.L.H.; Charles, A.C.; et al. Role of the cAMP signaling pathway in the regulation of gonadotropin-releasing hormone secretion in GT1 cells. Proc. Natl. Acad. Sci. USA 2000, 97, 1861–1866. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Martinez, R.; Shorte, S.L.; Faught, W.J.; Leaumont, D.C.; Frawley, L.S.; Boockfor, F.R. Pulsatile exocytosis is functionally associated with GnRH gene expression in immortalized GnRH-expressing cells. Endocrinology 2001, 142, 5364–5370. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rønnekleiv, O.K.; Bosch, M.A.; Zhang, C. Regulation of endogenous conductances in GnRH neurons by estrogens. Brain Res. 2010, 1364, 25–34. [Google Scholar] [CrossRef]
- Biorender. Available online: www.biorender.com.
- Ruf, F.; Sealfon, S.C. Genomics view of gonadotrope signaling circuits. Trends Endocrinol. Metab. 2004, 15, 331–338. [Google Scholar] [CrossRef]
- Naor, Z.; Benard, O.; Seger, R. Activation of MAPK Cascades by G-protein-coupled Receptors: The Case of Gonadotropin-releasing Hormone Receptor. Trends Endocrinol. Metab. 2000, 11, 91–99. [Google Scholar] [CrossRef]
- Kraus, S.; Naor, Z.; Seger, R. Intracellular Signaling Pathways Mediated by the Gonadotropin-Releasing Hormone (GnRH) Receptor. Arch. Med. Res. 2001, 32, 499–509. [Google Scholar] [CrossRef]
- Bliss, S.P.; Navratil, A.M.; Xie, J.; Roberson, M.S. GnRH signaling, the gonadotrope and endocrine control of fertility. Front. Neuroendocr. 2010, 31, 322–340. [Google Scholar] [CrossRef]
- Haisenleder, D.J.; Ferris, H.A.; Shupnik, M.A. The Calcium Component of Gonadotropin-Releasing Hormone-Stimulated Luteinizing Hormone Subunit Gene Transcription Is Mediated by Calcium/Calmodulin-Dependent Protein Kinase Type II. Endocrinology 2003, 144, 2409–2416. [Google Scholar] [CrossRef]
- Hehl, S.; Golard, A.; Hille, B. Involvement of mitochondria in intracellular calcium sequestration by rat gonadotropes. Cell Calcium 1996, 20, 515–524. [Google Scholar] [CrossRef]
- Poulin, B.; Rich, N.; Mitev, Y.; Gautron, J.P.; Kordon, C.; Enjalbert, A.; Drouva, S.V. Differential involvement of calcium channels and protein kinase-C activity in GnRH-induced phospholipase-C, -A2 and -D activation in a gonadotrope cell line (αT3-1). Mol. Cell. Endocrinol. 1996, 122, 33–50. [Google Scholar] [CrossRef]
- Bernard, D.J.; Tran, S. Mechanisms of Activin-Stimulated FSH Synthesis: The Story of a Pig and a Fox. Biol. Reprod. 2013, 88, 78. [Google Scholar] [CrossRef]
- Vanecek, J. Inhibitory effect of melatonin on GnRH-induced LH release. Rev. Reprod. 1999, 4, 67–72. [Google Scholar] [CrossRef]
- Durán-Pastén, M.L.; Fiordelisio, T. GnRH-induced Ca2+ signaling patterns and gonadotropin secretion in pituitary gonadotrophs. Functional adaptations to both ordinary and extraordinary physiological demands. Front. Endocrinol. 2013, 4, 127. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.R.; Strauss, J.F., 3rd. Multiple signal transduction pathways regulate ovarian steroidogenesis. Rev. Endocr. Metab. Disord. 2002, 3, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Young, J.M.; McNeilly, A.S. Theca: The forgotten cell of the ovarian follicle. Reproduction 2010, 140, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Stocco, D.M. Cyclic AMP and arachidonic acid: A tale of two pathways. Mol. Cell. Endocrinol. 1999, 158, 7–12. [Google Scholar] [CrossRef]
- Wang, X.; Shen, C.L.; Dyson, M.T.; Yin, X.; Schiffer, R.B.; Grammas, P.; Stocco, D.M. The involvement of epoxygenase metabolites of arachidonic acid in cAMP-stimulated steroidogenesis and steroidogenic acute regulatory protein gene expression. J. Endocrinol. 2006, 190, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, T.; Otsuka, F.; Inagaki, K.; Otani, H.; Takeda, M.; Suzuki, J.; Goto, J.; Ogura, T.; Makino, H. Differential Regulation of Steroidogenesis by Bone Morphogenetic Proteins in Granulosa Cells: Involvement of Extracellularly Regulated Kinase Signaling and Oocyte Actions in Follicle-Stimulating Hormone-Induced Estrogen Production. Endocrinology 2007, 148, 337–345. [Google Scholar] [CrossRef]
- Shimasaki, S.; Moore, R.K.; Otsuka, F.; Erickson, G.F. The Bone Morphogenetic Protein System in Mammalian Reproduction. Endocr. Rev. 2004, 25, 72–101. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.E. The role of steroids in follicular growth. Reprod. Biol. Endocrinol. 2006, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Nebreda, A.R. Signalling pathways in oocyte meiotic maturation. J. Cell Sci. 2002, 115, 2457–2459. [Google Scholar] [CrossRef] [PubMed]
- Nebreda, A.R.; Ferby, I. Regulation of the meiotic cell cycle in oocytes. Curr. Opin. Cell Biol. 2000, 12, 666–675. [Google Scholar] [CrossRef]
- Andersen, C.B.; Sakaue, H.; Nedachi, T.; Kovacina, K.S.; Clayberger, C.; Conti, M.; Roth, R.A. Protein kinase B/Akt is essential for the insulin- but not progesterone-stimulated resumption of meiosis in Xenopus oocytes. Biochem. J. 2003, 369, 227–238. [Google Scholar] [CrossRef]
- Duckworth, B.C.; Weaver, J.S.; Ruderman, J.V. G2 arrest in Xenopus oocytes depends on phosphorylation of cdc25 by protein kinase A. Proc. Natl. Acad. Sci. USA 2002, 99, 16794–16799. [Google Scholar] [CrossRef]
- Abrieu, A.; Doree, M.; Fisher, D. The interplay between cyclin-B-Cdc2 kinase (MPF) and MAP kinase during maturation of oocytes. J. Cell Sci. 2001, 114, 257–267. [Google Scholar] [CrossRef]
- Cavani, L.; Garcia, D.A.; Carreño, L.O.D.; Ono, R.K.; Pires, M.P.; Farah, M.M.; Ventura, H.T.; Millen, D.D.; Fonseca, R. Estimates of genetic parameters for reproductive traits in Brahman cattle breed. J. Anim. Sci. 2015, 93, 3287–3291. [Google Scholar] [CrossRef]
- Perry, J.R.B.; Stolk, L.; Franceschini, N.; Lunetta, K.L.; Zhai, G.; McArdle, P.F.; Smith, A.V.; Aspelund, T.; Bandinelli, S.; Boerwinkle, E.; et al. Meta-analysis of genome-wide association data identifies two loci influencing age at menarche. Nat. Genet. 2009, 41, 648–650. [Google Scholar] [CrossRef]
- Mills, M.C.; Tropf, F.C.; Brazel, D.M.; Van Zuydam, N.; Vaez, A.; Pers, T.H.; Snieder, H.; Perry, J.R.; Ong, K.K.; Den Hoed, M.; et al. Identification of 370 loci for age at onset of sexual and reproductive behaviour, highlighting common aetiology with reproductive biology, externalizing behaviour and longevity. BioRxiv 2020. [Google Scholar] [CrossRef]
- Cousminer, D.L.; Stergiakouli, E.; Berry, D.J.; Ang, W.; Groen-Blokhuis, M.M.; Körner, A.; Siitonen, N.; Ntalla, I.; Marinelli, M.; Perry, J.R.; et al. Genome-wide association study of sexual maturation in males and females highlights a role for body mass and menarche loci in male puberty. Hum. Mol. Genet. 2014, 23, 4452–4464. [Google Scholar] [CrossRef]
- Weller, J.; Bickhart, D.; Wiggans, G.; Tooker, M.; O’Connell, J.; Jiang, J.; Ron, M.; VanRaden, P. Determination of quantitative trait nucleotides by concordance analysis between quantitative trait loci and marker genotypes of US Holsteins. J. Dairy Sci. 2018, 101, 9089–9107. [Google Scholar] [CrossRef]
- Höglund, J.K.; Buitenhuis, B.; Guldbrandtsen, B.; Lund, M.S.; Sahana, G. Genome-wide association study for female fertility in Nordic Red cattle. BMC Genet. 2015, 16, 1–11. [Google Scholar] [CrossRef]
- Tahir, M.S.; Nguyen, L.T.; Schulz, B.L.; Boe-Hansen, G.A.; Thomas, M.G.; Moore, S.S.; Lau, L.Y.; Fortes, M.R.S. Proteomics Recapitulates Ovarian Proteins Relevant to Puberty and Fertility in Brahman Heifers (Bos indicus L.). Genes 2019, 10, 923. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Perez, J.E.; Herrera, N.; Martinez, R.; Bejarano, D.; Rocham, J.F. Genomic association study for age at first calving and calving interval in Romosinuano and Costeño con Cuernos cattle. Genet. Mol. Res. 2019. [Google Scholar] [CrossRef]
- Tomiyama, H. A Commentary on Axon guidance pathway genes and Parkinson’s disease. J. Hum. Genet. 2010, 56, 102–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xin, Q.; Wang, X.; Wang, S.; Wang, H.; Zhang, W.; Yang, Y.; Zhang, Y.; Zhang, Z.; Wang, C.; et al. Estrogen receptors in granulosa cells govern meiotic resumption of pre-ovulatory oocytes in mammals. Cell Death Dis. 2017, 8, e2662. [Google Scholar] [CrossRef] [PubMed]
- Rumi, M.K.; Singh, P.; Roby, K.F.; Zhao, X.; Iqbal, K.; Ratri, A.; Lei, T.; Cui, W.; Borosha, S.; Dhakal, P.; et al. Defining the Role of Estrogen Receptor beta in the Regulation of Female Fertility. Endocrinology 2017, 158, 2330–2343. [Google Scholar] [CrossRef]
- Lang-Muritano, M.; Sproll, P.; Wyss, S.; Kolly, A.; Hürlimann, R.; Konrad, D.; Biason-Lauber, A. Early-Onset Complete Ovarian Failure and Lack of Puberty in a Woman with Mutated Estrogen Receptor β (ESR2). J. Clin. Endocrinol. Metab. 2018, 103, 3748–3756. [Google Scholar] [CrossRef] [PubMed]
- Antonson, P.; Apolinário, L.M.; Shamekh, M.M.; Humire, P.; Poutanen, M.; Ohlsson, C.; Nalvarte, I.; Gustafsson, J.-Å. Generation of an all-exon Esr2 deleted mouse line: Effects on fertility. Biochem. Biophys. Res. Commun. 2020, 529, 231–237. [Google Scholar] [CrossRef]
- E Irwin, R.; Pentieva, K.; Cassidy, T.; Lees-Murdock, D.J.; McLaughlin, M.; Prasad, G.; McNulty, H.; Walsh, C.P. The interplay between DNA methylation, folate and neurocognitive development. Epigenomics 2016, 8, 863–879. [Google Scholar] [CrossRef]
- Südhof, T.C.; Rizo, J. Synaptic Vesicle Exocytosis. Cold Spring Harb. Perspect. Biol. 2011, 3, a005637. [Google Scholar] [CrossRef]
- Burgoyne, R.D. Secretory Vesicle-Associated Proteins and Their Role in Exocytosis. Annu. Rev. Physiol. 1990, 52, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Krsmanovic, L.Z.; Stojilkovic, S.S.; Balla, T.; Al-Damluji, S.; Weiner, R.I.; Catt, K.J. Receptors and neurosecretory actions of endothelin in hypothalamic neurons. Proc. Natl. Acad. Sci. USA 1991, 88, 11124–11128. [Google Scholar] [CrossRef]
- Han, S.-K.; Gottsch, M.L.; Lee, K.J.; Popa, S.M.; Smith, J.T.; Jakawich, S.K.; Clifton, D.K.; Steiner, R.A.; Herbison, A. Activation of Gonadotropin-Releasing Hormone Neurons by Kisspeptin as a Neuroendocrine Switch for the Onset of Puberty. J. Neurosci. 2005, 25, 11349–11356. [Google Scholar] [CrossRef]
- Clarke, I.J. Control of GnRH secretion: One step back. Front. Neuroendocr. 2011, 32, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Belsham, D.D. Melatonin receptor activation regulates GnRH gene expression and secretion in GT1–7 GnRH neurons signal transduction mechanisms. J. Biol. Chem. 2002, 277, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Bootman, M.D.; Collins, T.J.; Peppiatt, C.M.; Prothero, L.S.; MacKenzie, L.; De Smet, P.; Travers, M.; Tovey, S.C.; Seo, J.T.; Berridge, M.J.; et al. Calcium signalling—An overview. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Liu, X.; Lee, K.; Herbison, A.E. Kisspeptin Excites Gonadotropin-Releasing Hormone Neurons through a Phospholipase C/Calcium-Dependent Pathway Regulating Multiple Ion Channels. Endocrinology 2008, 149, 4605–4614. [Google Scholar] [CrossRef]
- Krsmanovic, L.Z.; Stojilkovic, S.S.; Merelli, F.; Dufour, S.M.; Virmani, M.A.; Catt, K.J. Calcium signaling and episodic secretion of gonadotropin-releasing hormone in hypothalamic neurons. Proc. Natl. Acad. Sci. USA 1992, 89, 8462–8466. [Google Scholar] [CrossRef]
- Spergel, D.J.; Krsmanović, L.Z.; Stojilkovic, S.S.; Catt, K.J. L-Type Ca2+ Channels Mediate Joint Modulation by Gamma-Amino-butyric Acid and Glutamate of [Ca2+]i and Neuropeptide Secretion in Immortalized Gonadodropin-Releasing Hormone Neurons. Neuroendocrinology 1995, 61, 499–508. [Google Scholar] [CrossRef]
- Sun, Y.; Sukumaran, P.; Bandyopadhyay, B.C.; Singh, B.B. Physiological Function and Characterization of TRPCs in Neurons. Cells 2014, 3, 455–475. [Google Scholar] [CrossRef]
- Dalmazzo, S.; Antoniotti, S.; Ariano, P.; Gilardino, A.; Lovisolo, D. Expression and localisation of TRPC channels in immortalised GnRH neurons. Brain Res. 2008, 1230, 27–36. [Google Scholar] [CrossRef]
- Salido, G.M.; Jardín, I.; Rosado, J.A. The TRPC ion Channels: Association with Orai1 and STIM1 Proteins and Participation in Capacitative and Non-Capacitative Calcium Entry. In Transient Receptor Potential Channels; Springer: Berlin, Germany, 2011; pp. 413–433. [Google Scholar]
- Kelly, M.J.; Qiu, J.; Rønnekleiv, O.K. TRPCing around the hypothalamus. Front. Neuroendocr. 2018, 51, 116–124. [Google Scholar] [CrossRef]
- Naghdi, S.; Hajnóczky, G. VDAC2-specific cellular functions and the underlying structure. Biochim. Biophys. Acta Bioenerg. 2016, 1863, 2503–2514. [Google Scholar] [CrossRef] [PubMed]
- Martínez de la Escalera, G.; Choi, A.L.H.; Weiner, R.I. Signaling Pathways Involved in GnRH Secretion in GT1 Cells. Neuroendocrinology 1995, 61, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Weiner, R.I.; Charles, A. Regulation of gonadotropinmreleasing hormone release by cyclic AMP signalling pathways. Growth Horm. Igf Res. 2001, 11, S9–S15. [Google Scholar] [CrossRef]
- Barrachina, M.; Martín, M.; Ciruela, F.; Ferrer, F.C.A.I. Epigenetic Modulation of Adenosine A2A Receptor: A Putative Therapeutical Tool for the Treatment of Parkinson’s Disease. New Ther. Parkinson’s Dis. 2011, 295–312. [Google Scholar] [CrossRef]
- Vastagh, C.; Rodolosse, A.; Solymosi, N.; Liposits, Z. Altered Expression of Genes Encoding Neurotransmitter Receptors in GnRH Neurons of Proestrous Mice. Front. Cell. Neurosci. 2016, 10, 230. [Google Scholar] [CrossRef]
- Mohammadi, A.; Alijani, S.; Rafat, S.A.; Abdollahi-Arpanahi, R. Genome-Wide Association Study and Pathway Analysis for Female Fertility Traits in Iranian Holstein Cattle. Ann. Anim. Sci. 2020, 20, 825–851. [Google Scholar] [CrossRef]
- Spergel, D.J.; Krsmanovic, L.Z.; Stojilkovic, S.S.; Catt, K.J. Glutamate Modulates [Ca2+]i and Gonadotropin-Releasing Hormone Secretion in Immortalized Hypothalamic GT1-7 Neurons. Neuroendocrinology 1994, 59, 309–317. [Google Scholar] [CrossRef]
- O’Connor, V.; El Far, O.; Bofill-Cardona, E.; Nanoff, C.; Freissmuth, M.; Karschin, A.; Airas, J.M.; Betz, H.; Boehm, S. Calmodulin Dependence of Presynaptic Metabotropic Glutamate Receptor Signaling. Science 1999, 286, 1180–1184. [Google Scholar] [CrossRef]
- Mahesh, V.B.; Zamorano, P.; De Sevilla, L.; Lewis, D.; Brann, D.W. Characterization of ionotropic glutamate receptors in rat hypothalamus, pituitary and immortalized gonadotropin-releasing hormone (GnRH) neurons (GT1-7 cells). Neuroendocrinology 1999, 69, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Sortino, M.A.; Aleppo, G.; Copani, A.; Casabona, G.; Nicoletti, F.; Ventra, C.; Kuhn, R.; Knöpfel, T.; Malitschek, B.; Canonico, P.L. Immortalized Hypothalamic Neurons Express Metabotropic Glutamate Receptors Positively Coupled to Cyclic AMP Formation. Eur. J. Neurosci. 1996, 8, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Fukuda, A.; Nabekura, J. The role of GABA in the regulation of GnRH neurons. Front. Neurosci. 2014, 8, 387. [Google Scholar] [CrossRef]
- Peters, A.; Riley, G. Is the Cow a Seasonal Breeder? Br. Veter J. 1982, 138, 533–537. [Google Scholar] [CrossRef]
- Revel, F.G.; Masson-Pévet, M.; Pévet, P.; Mikkelsen, J.D.; Simonneaux, V. Melatonin Controls Seasonal Breeding by a Network of Hypothalamic Targets. Neuroendocrinology 2009, 90, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.J.; Qiu, J. Estrogen signaling in hypothalamic circuits controling reproduction. Brain Res. 2010, 1364, 44–52. [Google Scholar] [CrossRef]
- Rønnekleiv, O.K.; Kelly, M.J. Kisspeptin Signaling in Reproductice Biology. In Advances in Experimental Medicine and Biology; Kauffman, A.S., Jeremy, S., Eds.; Springer: New York, NY, USA, 2013; pp. 113–131. [Google Scholar]
- Ábrahám, I.M.; Han, S.K.; Todman, M.G.; Korach, K.S.; Herbison, A.E. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J. Neurosci. 2003, 23, 5771–5777. [Google Scholar] [CrossRef]
- Hu, L.; Gustofson, R.L.; Feng, H.; Leung, P.K.; Mores, N.; Krsmanovic, L.Z.; Catt, K.J. Converse Regulatory Functions of Estrogen Receptor-α and -β Subtypes Expressed in Hypothalamic Gonadotropin-Releasing Hormone Neurons. Mol. Endocrinol. 2008, 22, 2250–2259. [Google Scholar] [CrossRef][Green Version]
- Amstalden, M.; Williams, G. Kisspeptin neuronal networks in pubertal development of domestic female ruminants. In Bioscientifica Proceedings; Bioscientifica: Bristol, UK, 2019; pp. 127–140. [Google Scholar]
- Dedes, I. Kisspeptins and the control of gonadotrophin secretion. Syst. Biol. Reprod. Med. 2012, 58, 121–128. [Google Scholar] [CrossRef]
- Kanaya, M.; Higo, S.; Ozawa, H. Neurochemical Characterization of Neurons Expressing Estrogen Receptor β in the Hypothalamic Nuclei of Rats Using in Situ Hybridization and Immunofluorescence. Int. J. Mol. Sci. 2019, 21, 115. [Google Scholar] [CrossRef]
- Papaoiconomou, E.; Msaouel, P.; Makri, A.; Diamanti-Kandarakis, E.; Koutsilieris, M. The role of kisspeptin/GPR54 in the reproductive system. In Vivo 2011, 25, 343–354. [Google Scholar] [PubMed]
- Elias, C.F.; Purohit, D. Leptin signaling and circuits in puberty and fertility. Cell. Mol. Life Sci. 2012, 70, 841–862. [Google Scholar] [CrossRef]
- Magni, P.; Vettor, R.; Pagano, C.; Calcagno, A.; Beretta, E.; Messi, E.; Zanisi, M.; Martini, L.; Motta, M. Expression of a leptin receptor in immortalized gonadotropin-releasing hormone-secreting neurons. Endocrinology 1999, 140, 1581–1585. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gamba, M.; Pralong, F.P. Control of GnRH neuronal activity by metabolic factors: The role of leptin and insulin. Mol. Cell. Endocrinol. 2006, 254–255, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garrido, M.A.; Tena-Sempere, M. Metabolic control of puberty: Roles of leptin and kisspeptins. Horm. Behav. 2013, 64, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, D.; Pujol-Gualdo, N.; Arnoldussen, I.A.; Kiliaan, A.J. Adipokines: A gear shift in puberty. Obes. Rev. 2020, 21, e13005. [Google Scholar] [CrossRef]
- Berridge, M.J. Inositol trisphosphate and calcium signalling. Nat. Cell Biol. 1993, 361, 315–325. [Google Scholar] [CrossRef]
- Tse, A.; Tse, F.W.; Almers, W.; Hille, B. Rhythmic exocytosis stimulated by GnRH-induced calcium oscillations in rat gonadotropes. Science 1993, 260, 82–84. [Google Scholar] [CrossRef]
- Fukui, K.; Sasaki, T.; Imazumi, K.; Matsuura, Y.; Nakanishi, H.; Takai, Y. Isolation and Characterization of a GTPase Activating Protein Specific for the Rab3 Subfamily of Small G Proteins. J. Biol. Chem. 1997, 272, 4655–4658. [Google Scholar] [CrossRef]
- Senthilkumaran, B.; Yoshikuni, M.; Nagahama, Y. A shift in steroidogenesis occurring in ovarian follicles prior to oocyte maturation. Mol. Cell. Endocrinol. 2004, 215, 11–18. [Google Scholar] [CrossRef]
- Stocco, C. Aromatase expression in the ovary: Hormonal and molecular regulation. Steroids 2008, 73, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Stocco, D.M.; Wang, X.; Jo, Y.; Manna, P.R. Multiple Signaling Pathways Regulating Steroidogenesis and Steroidogenic Acute Regulatory Protein Expression: More Complicated than We Thought. Mol. Endocrinol. 2005, 19, 2647–2659. [Google Scholar] [CrossRef]
- Cochran, S.D.; Cole, J.B.; Null, D.J.; Hansen, P.J. Discovery of single nucleotide polymorphisms in candidate genes associated with fertility and production traits in Holstein cattle. BMC Genet. 2013, 14, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-C.; Li, S.-J.; Tang, K.-Q.; Hua, G.-H.; Zhang, C.-Y.; Yu, J.-N.; Han, L.; Yang, L.-G. Polymorphisms in the 5′ upstream region of the FSH receptor gene, and their association with superovulation traits in Chinese Holstein cows. Anim. Reprod. Sci. 2010, 119, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Guay, C.; Madiraju, S.M.; Aumais, A.; Joly, É.; Prentki, M. A Role for ATP-Citrate Lyase, Malic Enzyme, and Pyruvate/Citrate Cycling in Glucose-induced Insulin Secretion. J. Biol. Chem. 2007, 282, 35657–35665. [Google Scholar] [CrossRef] [PubMed]
- Glister, C.; Kemp, C.F.; Knight, P.G. Bone morphogenetic protein (BMP) ligands and receptors in bovine ovarian follicle cells: Actions of BMP-4, -6 and -7 on granulosa cells and differential modulation of Smad-1 phosphorylation by follistatin. Reproduction 2004, 127, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Knight, P.G.; Glister, C. TGF-beta superfamily members and ovarian follicle development. Reproduction 2006, 132, 191–206. [Google Scholar] [CrossRef]
- Otsuka, F.; Yamamoto, S.; Erickson, G.F.; Shimasaki, S. Bone Morphogenetic Protein-15 Inhibits Follicle-stimulating Hormone (FSH) Action by Suppressing FSH Receptor Expression. J. Biol. Chem. 2001, 276, 11387–11392. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.; Wu, X.-Y.; Juengel, J.L.; Ross, I.K.; Lumsden, J.M.; Lord, E.A.; Dodds, K.G.; Walling, G.A.; McEwan, J.C.; O’Connell, A.R.; et al. Highly Prolific Booroola Sheep Have a Mutation in the Intracellular Kinase Domain of Bone Morphogenetic Protein IB Receptor (ALK-6) That Is Expressed in Both Oocytes and Granulosa Cells. Biol. Reprod. 2001, 64, 1225–1235. [Google Scholar] [CrossRef]
- Bornstein, S.; Rutkowski, H.; Vrezas, I. Cytokines and steroidogenesis. Mol. Cell. Endocrinol. 2004, 215, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V. Cytokines: Signalling molecules controlling ovarian functions. Int. J. Biochem. Cell Biol. 2011, 43, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Bevers, M.; Dieleman, S.; Hurk, R.V.D.; Izadyar, F. Regulation and modulation of oocyte maturation in the bovine. Theriogenology 1997, 47, 13–22. [Google Scholar] [CrossRef]
- Hansen, P. Embryonic mortality in cattle from the embryo’s perspective. J. Anim. Sci. 2002, 80 (Suppl. 2), E33–E44. [Google Scholar] [CrossRef]
- Yamashita, Y.; ShiMADA, M. The release of EGF domain from EGF-like factors by a specific cleavage enzyme activates the EGFR-MAPK3/1 pathway in both granulosa cells and cumulus cells during the ovulation process. J. Reprod. Deve. 2012, 58, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.; Thao, K.; Conti, M. Genetic Dissection of Epidermal Growth Factor Receptor Signaling during Luteinizing Hormone-Induced Oocyte Maturation. PLoS ONE 2011, 6, e21574. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.; Ze’ev, A.R. ATF2–at the crossroad of nuclear and cytosolic functions. J. Cell Sci. 2012, 125, 2815–2824. [Google Scholar] [CrossRef] [PubMed]
- Villa-Diaz, L.G.; Miyano, T. Activation of p38 MAPK During Porcine Oocyte Maturation. Biol. Reprod. 2004, 71, 691–696. [Google Scholar] [CrossRef]
- Jamnongjit, M.; Gill, A.; Hammes, S.R. Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc. Natl. Acad. Sci. USA 2005, 102, 16257–16262. [Google Scholar] [CrossRef]
- Kume, S.; Endo, T.; Nishimura, Y.; Kano, K.; Naito, K. Porcine SPDYA2 (RINGO A2) Stimulates CDC2 Activity and Accelerates Meiotic Maturation of Porcine Oocytes. Biol. Reprod. 2007, 76, 440–447. [Google Scholar] [CrossRef]
- Dupré, A.; Haccard, O.; Jessus, C. Mos in the Oocyte: How to Use MAPK Independently of Growth Factors and Transcription to Control Meiotic Divisions. J. Signal. Transduct. 2011, 2011, 1–15. [Google Scholar] [CrossRef]
- Lonergan, P.; Carolan, C.; Van Langendonckt, A.; Donnay, I.; Khatir, H.; Mermillod, P. Role of Epidermal Growth Factor in Bovine Oocyte Maturation and Preimplantation Embryo Development in Vitro. Biol. Reprod. 1996, 54, 1420–1429. [Google Scholar] [CrossRef]
- Ding, J.; Foxcroft, G.R. Epidermal growth factor enhances oocyte maturation in pigs. Mol. Reprod. Dev. 1994, 39, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ouyang, H.; Xia, G. The signal pathway of gonadotrophins-induced mammalian oocyte meiotic resumption. Mol. Hum. Reprod. 2009, 15, 399–409. [Google Scholar] [CrossRef]
- Chakravarthi, V.P.; Ghosh, S.; Housami, S.M.; Wang, H.; Roby, K.F.; Wolfe, M.W.; Kinsey, W.H.; Rumi, M.K. ERβ regulated ovarian kisspeptin plays an important role in oocyte maturation. Mol. Cell. Endocrinol. 2021, 527, 111208. [Google Scholar] [CrossRef]
- Gupta, A.; Tiwari, M.; Prasad, S.; Chaube, S.K. Role of Cyclic Nucleotide Phosphodiesterases During Meiotic Resumption from Diplotene Arrest in Mammalian Oocytes. J. Cell. Biochem. 2016, 118, 446–452. [Google Scholar] [CrossRef]
- Jäger, R.; Russwurm, C.; Schwede, F.; Genieser, H.-G.; Koesling, D.; Russwurm, M. Activation of PDE10 and PDE11 Phosphodiesterases*. J. Biol. Chem. 2012, 287, 1210–1219. [Google Scholar] [CrossRef]
- Conti, M.; Hsieh, M.; Zamah, A.M.; Oh, J.S. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol. Cell. Endocrinol. 2012, 356, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Haccard, O.; Jessus, C. Oocyte Maturation, Mos and Cyclins—A Matter of Synthesis: Two Functionally Redundant Ways to Induce Meiotic Maturation. Cell Cycle 2006, 5, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Kawamura, K.; Sasaki, M.; Kawamura, N.; Groenen, P.; Gelpke, M.D.S.; Rauch, R.; Hsueh, A.J.; Tanaka, T. Kit ligand promotes first polar body extrusion of mouse preovulatory oocytes. Reprod. Biol. Endocrinol. 2009, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Lowther, K.M.; Mehlmann, L.M. Embryonic Poly(A)-Binding Protein Is Required During Early Stages of Mouse Oocyte Development for Chromatin Organization, Transcriptional Silencing, and Meiotic Competencebiol. Biol. Reprod. 2015, 93, 43. [Google Scholar] [CrossRef]
- Izard, M.K.; Vandenbergh, J.G. The Effects of Bull Urine on Puberty and Calving Date in Crossbred Beef Heifers. J. Anim. Sci. 1982, 55, 1160–1168. [Google Scholar] [CrossRef]
- Vyas, S.; Briant, C.; Chemineau, P.; Le Danvic, C.; Nagnan-Le Meillour, P. Oestrus pheromones in farm mammals, with special reference to cow. Indian J. Anim. Sci. 2012, 82, 256–267. [Google Scholar]
- Yoon, H.; Enquist, L.; Dulac, C. Olfactory Inputs to Hypothalamic Neurons Controlling Reproduction and Fertility. Cell 2005, 123, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.B.; Camargo, G.M.; Diaz, I.D.; Irano, N.; Dias, M.M.; Carvalheiro, R.; Boligon, A.A.; Baldi, F.; Oliveira, H.N.; Tonhati, H.; et al. Genome-wide association study of reproductive traits in Nellore heifers using Bayesian inference. Genet. Sel. Evol. 2015, 47, 67. [Google Scholar] [CrossRef]
- Melo, T.P.D.; De Camargo, G.M.F.; De Albuquerque, L.G.; Carvalheiro, R. Genome-wide association study provides strong evidence of genes affecting the reproductive performance of Nellore beef cows. PLoS ONE 2017, 12, e0178551. [Google Scholar] [CrossRef] [PubMed]
- Ushizawa, K.; Takahashi, T.; Hosoe, M.; Ishiwata, H.; Kaneyama, K.; Kizaki, K.; Hashizume, K. Global gene expression analysis and regulation of the principal genes expressed in bovine placenta in relation to the transcription factor AP-2 family. Reprod. Biol. Endocrinol. 2007, 5, 17. [Google Scholar] [CrossRef]
- Kramer, P.R.; Krishnamurthy, R.; Mitchell, P.J.; Wray, S. Transcription Factor Activator Protein-2 Is Required for Continued Luteinizing Hormone-Releasing Hormone Expression in the Forebrain of Developing Mice. Endocrinology 2000, 141, 1823–1838. [Google Scholar] [CrossRef] [PubMed]
- Fortes, M.R.S.; Lehnert, S.A.; Bolormaa, S.; Reich, C.; Fordyce, G.; Corbet, N.J.; Whan, V.; Hawken, R.J.; Reverter, A. Finding genes for economically important traits: Brahman cattle puberty. Anim. Prod. Sci. 2012, 52, 143–150. [Google Scholar] [CrossRef]
- Li, R.; Wu, S.-P.; Zhou, L.; Nicol, B.; Lydon, J.P.; Yao, H.H.-C.; DeMayo, F.J. Increased FOXL2 expression alters uterine structures and functions. Biol. Reprod. 2020, 103, 951–965. [Google Scholar] [CrossRef]
- Matagne, V.; Kim, J.G.; Ryu, B.J.; Hur, M.K.; Kim, M.S.; Kim, K.; Park, B.S.; Damante, G.; Smiley, G.; Lee, B.J.; et al. Thyroid Transcription Factor 1, a Homeodomain Containing Transcription Factor, Contributes to Regulating Periodic Oscillations in GnRH Gene Expression. J. Neuroendocr. 2012, 24, 916–929. [Google Scholar] [CrossRef] [PubMed]
- Daigneault, B.W.; Rajput, S.; Smith, G.W.; Ross, P.J. Embryonic POU5F1 is Required for Expanded Bovine Blastocyst Formation. Sci. Rep. 2018, 8, 7753. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Fernandez-Rhodes, L.; Brzyski, R.G.; Carlson, C.S.; Chen, Z.; Heiss, G.; North, K.E.; Woods, N.F.; Rajkovic, A.; Kooperberg, C.; et al. Replication of loci influencing ages at menarche and menopause in Hispanic women: The Women’s Health Initiative SHARe Study. Hum. Mol. Genet. 2011, 21, 1419–1432. [Google Scholar] [CrossRef] [PubMed]
- Mesbah-Uddin, M. Genome-Wide Association Study with Imputed Whole-Genome Sequence Variants Including Large Deletions for Female Fertility in Three Nordic Dairy Breeds. Ph.D. Thesis, Aarhus University, Aarhus, Denmark, 2019; p. 84. [Google Scholar]
- Gioia, L.; Barboni, B.; Turriani, M.; Capacchietti, G.; Pistilli, M.G.; Berardinelli, P.; Mattioli, M. The capability of reprogramming the male chromatin after fertilization is dependent on the quality of oocyte maturation. Reproduction 2005, 130, 29–39. [Google Scholar] [CrossRef] [PubMed]
| No. | Trait | Score | Scoring Criteria |
|---|---|---|---|
| 1 | PREG1 | 1 | Not pregnant as a result of the first mating opportunity (n = 600) |
| 2 | Pregnant as a result of the first mating opportunity (n = 1719) | ||
| 2 | FCS | 1 | Never conceived up to 36 months of age (n = 429) |
| 2 | Conceived between 29 and 36 months of age (n = 436) | ||
| 3 | Conceived before 29 months of age (n = 1150) | ||
| 3 | REB | 1 | Not pregnant as a result of the first two mating opportunities (n = 153) |
| 2 | Pregnant as a result of the second mating opportunity, but not the first (n = 550) | ||
| 2.5 | Pregnant as a result of the first mating opportunity, not the second (n = 506) | ||
| 3.5 | Pregnant twice, as a result of the first two mating opportunities (n = 326) |
| Traits | PREG1 | FCS | REB |
|---|---|---|---|
| PREG1 | 0.17 (0.03) | 0.839 (0.06) | 0.799 (0.07) |
| FCS | 0.86 (0.01) | 0.11 (0.03) | 0.756 (0.1) |
| REB | 0.73 (0.02) | 0.65 (0.02) | 0.28 (0.05) |
| SNP | Gene | BTA | Location of SNP | p-Value | Function | Overall SNP Effect |
|---|---|---|---|---|---|---|
| BovineHD1100009366 | LHCGR | 11 | 31339285 | 7.8 × 10−6 | Steroid Synthesis | 0.057 |
| BovineHD1100009366 | FSHR | 11 | 31339285 | 7.8 × 10−6 | Steroid Synthesis | 0.057 |
| BovineHD4100003128 | LEP | 4 | 92253894 | 4.0 × 10−6 | GnRH Secretion | 0.053 |
| BovineHD1400007251 | MOS | 14 | 23304037 | 1.8 × 10−7 | Oocyte Maturation | 0.059 |
| BovineHD2200014848 | CDC25A | 22 | 51689566 | 4.6 × 10−6 | Oocyte Maturation | −0.050 |
| BovineHD2200003516 | AVCR2B | 22 | 11918372 | 4.6 × 10−7 | TGF-β Signaling | −0.056 |
| BovineHD2200000211 | EGFR | 22 | 878627 | 8.0 × 10−6 | GnRH Signaling | −0.052 |
| BovineHD2500007459 | MAPK3 | 25 | 26160282 | 3.4 × 10−6 | GnRH Signaling | 0.060 |
| BovineHD1000021917 | ESR2 | 10 | 76586616 | 3.0 × 10−10 | Estrogen Signaling | −0.114 |
| BovineHD0900023775 | GRM1 | 9 | 83806867 | 9.5 × 10−10 | Glutamate Signaling | −0.066 |
| BovineHD1700011908 | GRIA2 | 17 | 41973761 | 3.0 × 10−6 | Glutamate Synapse | 0.098 |
| BovineHD2500002242 | GRIN2A | 25 | 8381736 | 4.2 × 10−6 | Glutamate Synapse | 0.057 |
| BovineHD2200005404 | GRM7 | 22 | 18702200 | 4.4 × 10−6 | Glutamate Synapse | 0.070 |
| BovineHD0600018549 | GABRA4 | 6 | 65504186 | 3.1 × 10−6 | GABAergic Synapse | −0.050 |
| BovineHD0200007364 | GAD1 | 2 | 25614206 | 7.9 × 10−6 | GABAergic Synapse | −0.053 |
| BovineHD0600018549 | GABRB1 | 6 | 65504186 | 3.1 × 10−6 | GABAergic Synapse | −0.050 |
| BovineHD0600018311 | GABRA2 | 6 | 64738586 | 3.3 × 10−6 | GABAergic Synapse | −0.048 |
| BovineHD0600018311 | GABRG1 | 6 | 64738586 | 3.3 × 10−6 | GABAergic Synapse | −0.048 |
| BovineHD1300000677 | PLCB4 | 13 | 2565300 | 9.7 × 10−6 | Calcium Signaling | 0.047 |
| BovineHD2200006328 | ITPR1 | 22 | 21699681 | 5.2 × 10−10 | Calcium Signaling | −0.092 |
| BovineHD0400018696 | CREB5 | 4 | 67587933 | 3.8 × 10−6 | cAMP Signaling | −0.082 |
| BovineHD1800005855 | ADCY7 | 18 | 18675150 | 4.5 × 10−7 | cAMP Signaling | 0.059 |
| BovineHD0600018878 | CNGA1 | 6 | 66763069 | 7.2 × 10−7 | cAMP Signaling | 0.062 |
| BovineHD0700033604 | PDE4A | 7 | 15081779 | 2.2 × 10−7 | cAMP Signaling | 0.064 |
| BovineHD0300002075 | HSD17B7 | 3 | 6617455 | 1.3 × 10−6 | Steroid Synthesis | −0.052 |
| BovineHD2100009894 | CYP11A | 21 | 34099081 | 4.2 × 10−7 | Steroid Synthesis | −0.052 |
| BovineHD1400007252 | PLAG1 | 14 | 23313248 | 1.8 × 10−7 | Transcription Regulation | 0.059 |
| BovineHD2300013198 | TFAP2A | 23 | 45590544 | 1.7 × 10−6 | Transcription Regulation | 0.058 |
| BovineHD1100029888 | TTF1 | 11 | 102587601 | 3.2 × 10−6 | Transcription Regulation | 0.046 |
| BovineHD2100008703 | CHRNA7 | 21 | 29677844 | 1.9 × 10−7 | Cholinergic Synapse | −0.063 |
| BovineHD2700004441 | MTNR1A | 27 | 16259785 | 1.1 × 10−6 | Melatonin Receptor | −0.048 |
| Pathway | Gene Count | Gene Names | Adj. p-Value * |
|---|---|---|---|
| GnRH Signaling | 16 | RAF1, SOS2, ADCY7, CAMK2A, CAMK2D, EGFR, ITPR1, ITPR2, MAPK13, MAPK3, MAPK8, MAP3K1, PLA2G4D, PLA2G4E, PLA2G4B, PLCB4 | 1.2 × 10−3 |
| Progesterone Mediated Oocyte Maturation | 13 | BRAF, RAF1, ADCY7, CDC25A, HSP90AA1, HSP90AB1, MAPK10, MAPK13, MAPK3, MAPK8, PIK3CB, SPDYC, MOS | 5.6 × 10−3 |
| Estrogen Signaling | 17 | FKBP5, RAF1, ADCY7, CREB3L2, ESR2, SOS2, GRM1, HSP90AA1, HSP90AB1, ITPR1, EGFR, ITPR2, MAPK3, OPRM1, PIK3CB, PLCB4, AKT1 | 1.2 × 10−3 |
| Glutamatergic Synapse | 15 | GNG2, ADCY7, GRIA2, GRIN2A, GRIK1, GRM7, GRM1, GLS2, ITPR1, ITPR2, MAPK3, PLA2G4B, PLA2G4E, PLA2G4D, PLCB4 | 7.0 × 10−2 |
| Regulation of Actin Cytoskeleton | 25 | BRAF, MRAS, LIMK2, RAF1, ARHGEF1, CHRM5, CYFIP2, ENAH, FGFR3, ITGA4, ITGA8, ITGAL, ITGB2, MAPK3, MYLPF, PAK2, PAK5, PIK3CB, PDGFC, PDGFRA, PDGFRB, MOS, VAV3, ITG5, ITGB2 | 1.2 × 10−3 |
| Cholinergic Synapse | 17 | AKT1, GNG2, ADCY7, CREB3L2, CAMK2A, CAMK2D, CHRM5, CHRNA3, CHRNB4, CHRNA7, ITPR1, ITPR2, MAPK3, PIK3CB, PLCB4, SLC5A7, KCNQ4 | 9.3 × 10−4 |
| cAMP Signaling | 24 | AKT1, ATP2B3, BRAF, FXYD1, RAF1, RAPGEF4, ADCY7, CREB3L2, CAMK2D, CNGA1, FSHR, GLP1R, GRIA2, GRIN2A, MAPK3, MAPK8, NPR1, OXTR, PIK3CB, PDE4A, VAV3, ORAI1, RELA, ADORA2A, CAMK2A. | 9.6 × 10−4 |
| Calcium Signaling | 26 | ATP2B3, ATP2A1, ORAI1, ORAI3, ADCY7, AGTR1, CAMK2A, CAMK2D, CHRM5, CHRNA7, EGFR, GRPR, GRIN2A, GRM1, ITPR1, ITPR2, ITPKA, LHCGR, OXTR, PLCB4, PLCD1, PHKG2, PDGFRA, PDGFRB, SLC25A4, VDAC2, ADORA2A | 3.6 × 10−5 |
| Focal Adhesion | 25 | AKT1, BRAF, RAF1, SOS2, CAPN2, COL4A3, COL4A5, COL4A6, ITGA4, ITGA8, ITGAB5, KDR, LAMC3, MAPK3, MAPK8, MYLPF, PAK2, PAK5, PIK3CB, PDGFC, PDGFRA, CAV3, PDGFRB, EGFR, VAV3, VWF, ZYX | 5.6 × 10−4 |
| PI3K-Akt Signaling | 40 | AKT1, CD19, GNG2, RBL2, RELA, RAF1, SOS2, CREB3L2, CASP9, CDC37, COL4A3, COL4A5, COL4A6, CSF1, CSF1R, CDK6, CDKN1A, EGFR, FGF19, FGFR3, GHR, HSP90AA1, HSP90AB1, ITGA4, ITGA8, ITGB5, IL7, KDR, LAMC3, LPAR1, LPAR3, MAPK3, PIK3CB, PDGFC, PDGFRA, PDGFRB, PPP2R5E, PPP2R2C, TSC1, VWF | 1.6 × 10−3 |
| Ovarian Steroidogenesis | 10 | HSD17B7, ADCY7, ALOX5, CYP11A1, CYP1A1, FSHR, LHCGR, PLA2G4B, PLA2G4D, PLA2G4E | 4.2 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahir, M.S.; Porto-Neto, L.R.; Gondro, C.; Shittu, O.B.; Wockner, K.; Tan, A.W.L.; Smith, H.R.; Gouveia, G.C.; Kour, J.; Fortes, M.R.S. Meta-Analysis of Heifer Traits Identified Reproductive Pathways in Bos indicus Cattle. Genes 2021, 12, 768. https://doi.org/10.3390/genes12050768
Tahir MS, Porto-Neto LR, Gondro C, Shittu OB, Wockner K, Tan AWL, Smith HR, Gouveia GC, Kour J, Fortes MRS. Meta-Analysis of Heifer Traits Identified Reproductive Pathways in Bos indicus Cattle. Genes. 2021; 12(5):768. https://doi.org/10.3390/genes12050768
Chicago/Turabian StyleTahir, Muhammad S., Laercio R. Porto-Neto, Cedric Gondro, Olasege B. Shittu, Kimberley Wockner, Andre W. L. Tan, Hugo R. Smith, Gabriela C. Gouveia, Jagish Kour, and Marina R. S. Fortes. 2021. "Meta-Analysis of Heifer Traits Identified Reproductive Pathways in Bos indicus Cattle" Genes 12, no. 5: 768. https://doi.org/10.3390/genes12050768
APA StyleTahir, M. S., Porto-Neto, L. R., Gondro, C., Shittu, O. B., Wockner, K., Tan, A. W. L., Smith, H. R., Gouveia, G. C., Kour, J., & Fortes, M. R. S. (2021). Meta-Analysis of Heifer Traits Identified Reproductive Pathways in Bos indicus Cattle. Genes, 12(5), 768. https://doi.org/10.3390/genes12050768







