High Chromosomal Stability and Immortalized Totipotency Characterize Long-Term Tissue Cultures of Chinese Ginseng (Panax ginseng)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Cytogenetic Assay
2.3. Transcriptome Sequencing
2.4. Analyses of Expression of Genes Respectively Related to CIN and Totipotency
3. Results
3.1. Persistent High Totipotency of the Ginseng Cultures
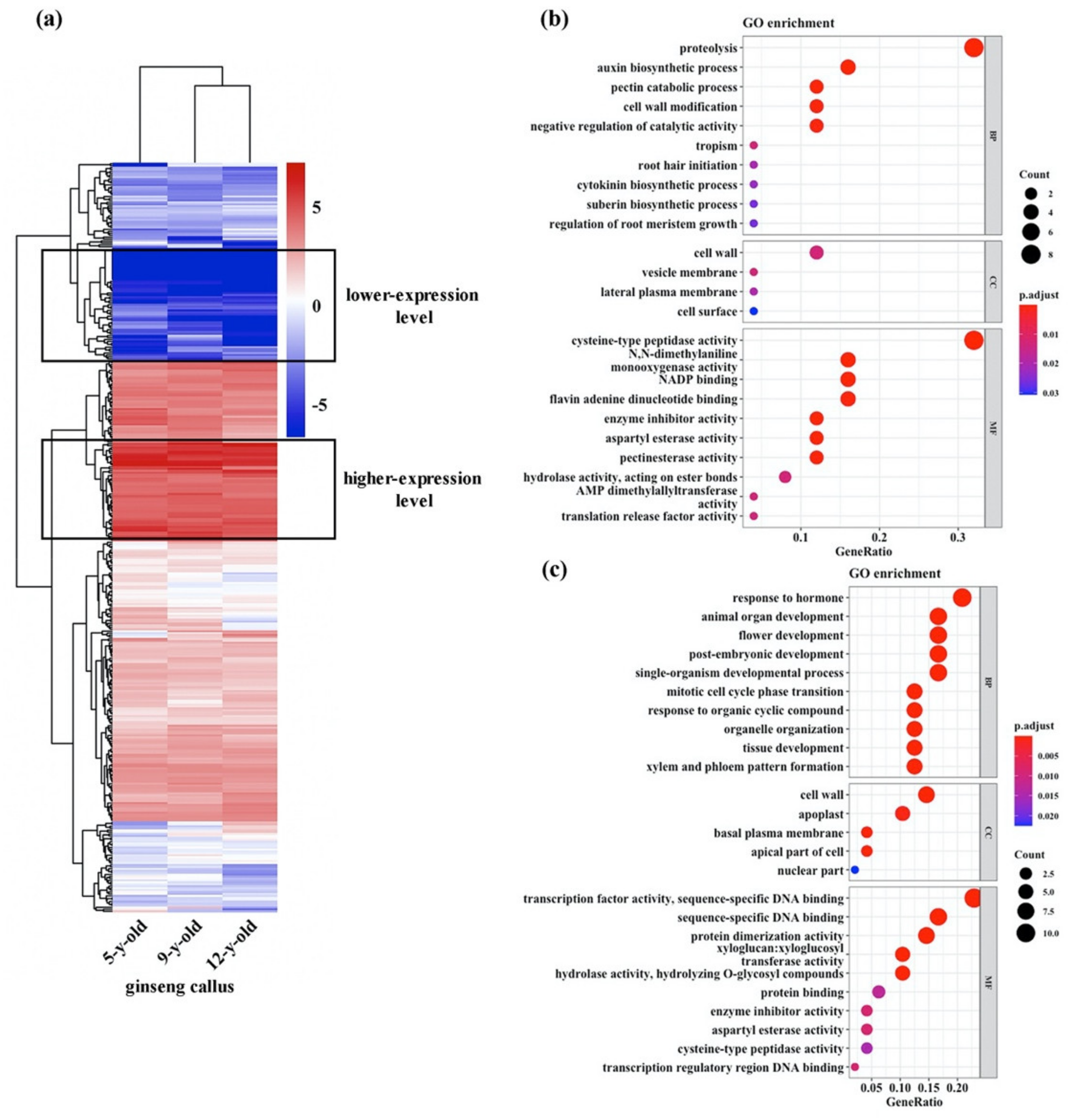
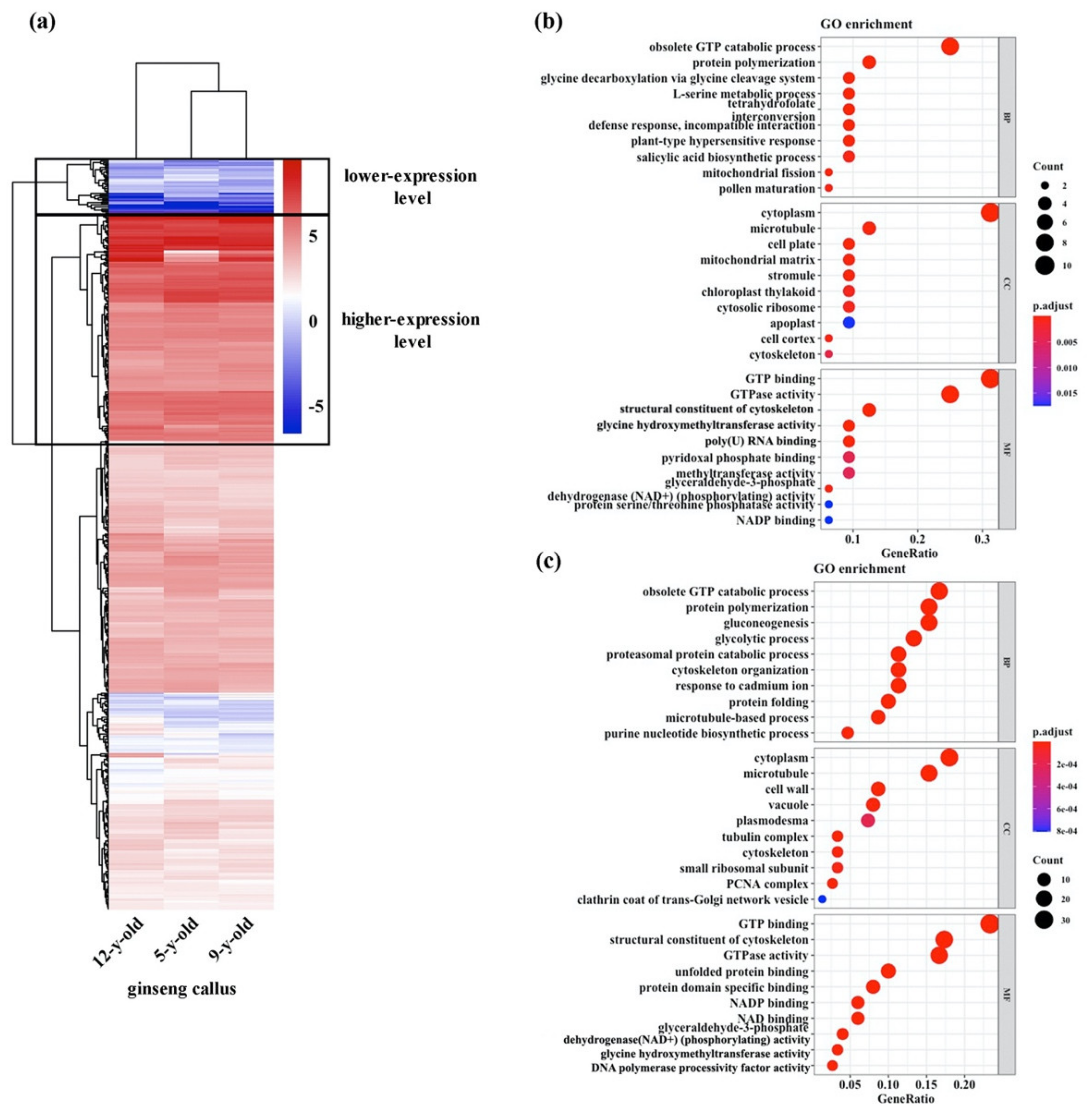
3.2. Expression Profiles of Genes Potentially Related to Maintenance of High Totipotency of the Ginseng Cultures
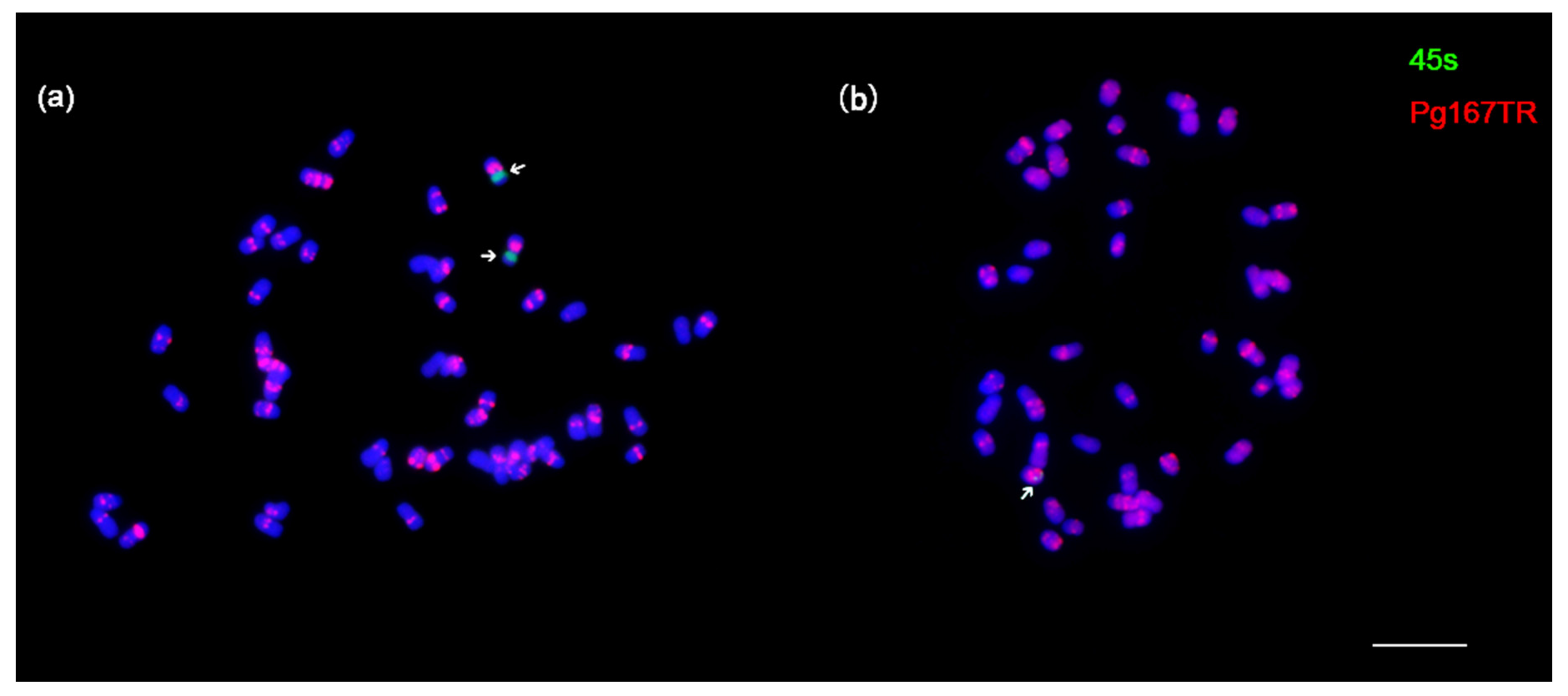
3.3. Remarkable Chromosomal Stability in the Ginseng Cultures
3.4. Expression Profiles of Genes Potentially Involved in Maintenance of Chromosomal Stability of the Ginseng Cultures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otto, S.P. The Evolutionary Consequences of Polyploidy. Cell 2007, 131, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bian, Y.; Gou, X.; Dong, Y.; Rustgi, S.; Zhang, B.; Xu, C.; Li, N.; Qi, B.; Han, F.; et al. Intrinsic karyotype stability and gene copy number variations may have laid the foun-dation for tetraploid wheat formation. Proc. Natl. Acad. Sci. USA 2013, 110, 19466–19471. [Google Scholar] [CrossRef]
- Funk, L.C.; Zasadil, L.M.; Weaver, B.A. Living in CIN: Mitotic Infidelity and Its Consequences for Tumor Promotion and Suppression. Dev. Cell 2016, 39, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Choi, Y.L.; Kwon, M.; Park, P.J. Mechanisms and Consequences of Cancer Genome Instability: Lessons from Genome Sequencing Studies. Annu. Rev. Pathol. 2016, 11, 283–312. [Google Scholar] [CrossRef] [PubMed]
- Lengauer, C.; Kinzler, K.W.; Vogelstein, B. Genetic instability in colorectal cancers. Nat. Cell Biol. 1997, 386, 623–627. [Google Scholar] [CrossRef]
- Vasudevan, A.; Schukken, K.M.; Sausville, E.L.; Girish, V.; Adebambo, O.A.; Sheltzer, J.M. Aneuploidy as a promoter and suppressor of malignant growth. Nat. Rev. Cancer 2021, 21, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.J.; Stilgenbauer, L.; Moy, A.; Liu, G.; Heng, H.H. What Is Karyotype Coding and Why Is Genomic Topology Important for Cancer and Evolu-tion? Front. Genet. 2019, 10, 1082. [Google Scholar] [CrossRef]
- Larkin, P.J.; Scowcroft, W.R. Somaclonal variation—A novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 1981, 60, 197–214. [Google Scholar] [CrossRef]
- Phillips, R.L.; Kaeppler, S.M.; Olhoft, P. Genetic instability of plant tissue cultures: Breakdown of normal controls. Proc. Natl. Acad. Sci. USA 1994, 91, 5222–5226. [Google Scholar] [CrossRef] [PubMed]
- McClintock, B. The significance of responses of the genome to challenge. Science 1984, 226, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Haberlandt, G. Culturversuche Mit Isolierten Pflanzenzellen. In Plant Tissue Culture; Metzler, J.B., Ed.; Springer: Vienna, Austria, 2003; Volume 61, pp. 1–24. [Google Scholar]
- Neelakandan, A.K.; Wang, K. Recent progress in the understanding of tissue culture-induced genome level changes in plants and potential applications. Plant Cell Rep. 2011, 31, 597–620. [Google Scholar] [CrossRef]
- Bairu, M.W.; Aremu, A.O.; Van Staden, J. Somaclonal variation in plants: Causes and detection methods. Plant Growth Regul. 2011, 63, 147–173. [Google Scholar] [CrossRef]
- Lee, M.; Phillips, R.L. The Chromosomal Basis of Somaclonal Variation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 413–437. [Google Scholar] [CrossRef]
- Choi, H.-I.; Kim, N.-H.; Lee, J.; Choi, B.S.; Kim, K.D.; Park, J.Y.; Lee, S.-C.; Yang, T.-J. Evolutionary relationship of Panax ginseng and P. quinquefolius inferred from sequencing and comparative analysis of expressed sequence tags. Genet. Resour. Crop Evol. 2012, 60, 1377–1387. [Google Scholar] [CrossRef]
- Shi, F.-X.; Li, M.-R.; Li, Y.-L.; Jiang, P.; Zhang, C.; Pan, Y.-Z.; Liu, B.; Xiao, H.-X.; Li, L.-F. The impacts of polyploidy, geographic and ecological isolations on the diversification of Panax (Araliaceae). BMC Plant Biol. 2015, 15, 297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.C. The Chinese Ginseng; The Shanghai S & T Publishing House: Shanghai, China, 1992. [Google Scholar]
- Goldstein, B. Ginseng: Its history, dispersion, and folk tradition. Am. J. Chin. Med. 1975, 3, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhao, Y.Q.; Liang, X.J. Current Evaluation of the Millennium Phytomedicine—Ginseng (II): Collected Chemical Entities, Modern Pharmacology, and Clinical Applications Emanated from Traditional Chinese Medicine. Curr. Med. Chem. 2009, 16, 2924–2942. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, S.; Sun, C.; Lin, Y.; Yin, R.; Wang, Y.; Zhang, M. The Spatial and Temporal Transcriptomic Landscapes of Ginseng, Panax ginseng C. A. Meyer. Sci. Rep. 2015, 5, 18283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, Z.; Wang, N.; Gao, Y.; Liu, Y.; Wu, Y.; Bai, Y.; Zhang, Z.; Lin, X.; Dong, Y.; et al. Tissue Culture-Induced Heritable Genomic Variation in Rice, and Their Phenotypic Implications. PLoS ONE 2014, 9, e96879. [Google Scholar] [CrossRef] [PubMed]
- Waminal, N.E.; Choi, H.-I.; Kim, N.-H.; Jang, W.; Lee, J.; Park, J.Y.; Kim, H.H.; Yang, T.-J. A refined Panax ginseng karyotype based on an ultra-high copy 167-bp tandem repeat and ribosomal DNAs. J. Ginseng Res. 2017, 41, 469–476. [Google Scholar] [CrossRef]
- Gordon, A.; Hannon, G. FASTX-Toolkit, FASTQ/A Short-Reads Pre-Processing Tools. 2010. Available online: http://hannonlab.cshl.edu/fastx_toolkit/ (accessed on 5 January 2014).
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Favero, D.S.; Sakamoto, Y.; Iwase, A.; Coleman, D.; Rymen, B.; Sugimoto, K. Molecular Mechanisms of Plant Regeneration. Annu. Rev. Plant Biol. 2019, 70, 377–406. [Google Scholar] [CrossRef] [PubMed]
- Rohrberg, J.; Van de Mark, D.; Amouzgar, M.; Lee, J.V.; Taileb, M.; Corella, A.; Kilinc, S.; Williams, J.; Jokisch, M.-L.; Camarda, R.; et al. MYC Dysregulates Mitosis, Revealing Cancer Vulnerabilities. Cell Rep. 2020, 30, 3368–3382.e7. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Li, N.; Zhang, Z.; Meng, X.; Dong, Q.; Xu, C.; Gong, L.; Liu, B. CG hypomethylation leads to complex changes in DNA methylation and transpositional burst of diverse transposable elements in callus cultures of rice. Plant J. 2019, 101, 188–203. [Google Scholar] [CrossRef]
- Su, Y.H.; Tang, L.P.; Zhao, X.Y.; Zhang, X.S. Plant cell totipotency: Insights into cellular reprogramming. J. Integr. Plant Biol. 2021, 63, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Duesberg, P.; Rausch, C.; Rasnick, D.; Hehlmann, R. Genetic instability of cancer cells is proportional to their degree of aneuploidy. Proc. Natl. Acad. Sci. USA 1998, 95, 13692–13697. [Google Scholar] [CrossRef]
- Gordon, D.J.; Resio, B.; Pellman, D. Causes and consequences of aneuploidy in cancer. Nat. Rev. Genet. 2012, 13, 189–203. [Google Scholar] [CrossRef]
- Passerini, V.; Ozeri-Galai, E.; De Pagter, M.S.; Donnelly, N.; Schmalbrock, S.; Kloosterman, W.P.; Kerem, B.; Storchová, Z. The presence of extra chromosomes leads to genomic instability. Nat. Commun. 2016, 7, 10754. [Google Scholar] [CrossRef] [PubMed]
- Sheltzer, J.M.; Blank, H.M.; Pfau, S.J.; Tange, Y.; George, B.M.; Humpton, T.J.; Brito, I.L.; Hiraoka, Y.; Niwa, O.; Amon, A. Aneuploidy Drives Genomic Instability in Yeast. Science 2011, 333, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Targa, A.; Rancati, G. Cancer: A CINful evolution. Curr. Opin. Cell Biol. 2018, 52, 136–144. [Google Scholar] [CrossRef]
- Ngezahayo, F.; Wang, X.; Yu, X.; Jiang, L.; Chu, Y.; Shen, B.; Yan, Z.; Liu, B. Habitat-induced reciprocal transformation in the root phenotype of Oriental ginseng is associated with alteration in DNA methylation. Chin. Sci. Bull. 2011, 56, 1685–1689. [Google Scholar] [CrossRef][Green Version]
- Goldrosen, M.H.; Straus, S.E. Complementary and alternative medicine: Assessing the evidence for immunological benefits. Nat. Rev. Immunol. 2004, 4, 912–921. [Google Scholar] [CrossRef]
- Chester, M.; Gallagher, J.P.; Symonds, V.V.; Da Silva, A.V.C.; Mavrodiev, E.V.; Leitch, A.R.; Soltis, P.S.; Soltis, D.E. Extensive chromosomal variation in a recently formed natural allopolyploid species, Tragopogon miscellus (Asteraceae). Proc. Natl. Acad. Sci. USA 2012, 109, 1176–1181. [Google Scholar] [CrossRef]
- Deng, X.; Sha, Y.; Lv, Z.; Wu, Y.; Zhang, A.; Wang, F.; Liu, B. The Capacity to Buffer and Sustain Imbalanced D-Subgenome Chromosomes by the BBAA Component of Hexaploid Wheat Is an Evolved Dominant Trait. Front. Plant Sci. 2018, 9, 1149. [Google Scholar] [CrossRef]
- Gou, X.; Bian, Y.; Zhang, A.; Zhang, H.; Wang, B.; Lv, R.; Li, J.; Zhu, B.; Gong, L.; Liu, B. Transgenerationally Precipitated Meiotic Chromosome Instability Fuels Rapid Karyotypic Evolution and Phenotypic Diversity in an Artificially Constructed Allotetraploid Wheat (AADD). Mol. Biol. Evol. 2018, 35, 1078–1091. [Google Scholar] [CrossRef]
- Xiong, Z.; Gaeta, R.T.; Pires, J.C. Homoeologous shuffling and chromosome compensation maintain genome balance in resynthesized allopolyploid Brassica napus. Proc. Natl. Acad. Sci. USA 2011, 108, 7908–7913. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Shumakova, O.A.; Tchernoded, G.K. Mutation of Panax ginseng genes during long-term cultivation of ginseng cell cultures. J. Plant Physiol. 2011, 168, 1280–1285. [Google Scholar] [CrossRef] [PubMed]

| Subculture (year) | No. Calli Tested | Totipotency (%) * | Regeneration Efficacy † |
|---|---|---|---|
| 2005 | 200 | 197 (98) | 10 ± 2 |
| 2006 | 200 | 195 (97) | 9 ± 1 |
| 2007 | 200 | 197(98) | 9 ± 2 |
| 2008 | 200 | 198 (99) | 8 ± 1 |
| 2009 | 200 | 194 (95) | 8 ± 2 |
| 2010 | 200 | 195 (97) | 10 ± 2 |
| 2011 | 200 | 195 (97) | 9 ± 3 |
| 2012 | 200 | 194 (95) | 10 ± 1 |
| 2013 | 200 | 198 (99) | 8 ± 2 |
| 2014 | 200 | 198 (99) | 8 ± 1 |
| 2015 | 200 | 198 (99) | 10 ± 3 |
| 2016 | 200 | 196 (98) | 8 ± 1 |
| Subculture (year) | No. Metaphase Cells Examined | No. and % Euploid Cells | No. and % Aneuploid Cells | No. and % Cells with Structural Variation * |
|---|---|---|---|---|
| 2005 | 230 | 219 (95.2) | 11 (4.8) | 2 (0.9) |
| 2006 | 201 | 192 ( 95.5) | 9 (4.5) | 1 (0.5) |
| 2007 | 205 | 195 (95.1) | 10 (4.9) | 2 (1.0) |
| 2008 | 200 | 191 (95.5) | 9 (4.5) | 2 (1.0) |
| 2009 | 190 | 183 (96.3) | 7 (3.7) | 2 (1.1) |
| 2010 | 189 | 183 (96.8) | 6 (3.2) | 1 (0.5) |
| 2011 | 187 | 179 (95.7) | 8 (4.3) | 2 (1.1) |
| 2012 | 200 | 191 (95.5) | 9 (4.5) | 2 (1.0) |
| 2013 | 185 | 178 (96.2) | 7 (3.8) | 3 (1.6) |
| 2014 | 190 | 180 (94.7) | 10 (5.3) | 1 (0.5) |
| 2015 | 201 | 192 (95.5) | 9 (4.5) | 3 (1.5) |
| 2016 | 188 | 180 (95.7) | 8 (4.3) | 2 (1.1) |
| Total/average | 2366 | 2163/(95.7) | 103/(4.3) | 23/(1.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhao, J.; Liu, Y.; Li, N.; Wang, Z.; Wang, X.; Liu, X.; Jiang, L.; Liu, B.; Fu, X.; et al. High Chromosomal Stability and Immortalized Totipotency Characterize Long-Term Tissue Cultures of Chinese Ginseng (Panax ginseng). Genes 2021, 12, 514. https://doi.org/10.3390/genes12040514
Liu S, Zhao J, Liu Y, Li N, Wang Z, Wang X, Liu X, Jiang L, Liu B, Fu X, et al. High Chromosomal Stability and Immortalized Totipotency Characterize Long-Term Tissue Cultures of Chinese Ginseng (Panax ginseng). Genes. 2021; 12(4):514. https://doi.org/10.3390/genes12040514
Chicago/Turabian StyleLiu, Sitong, Jing Zhao, Yutong Liu, Ning Li, Zhenhui Wang, Xinfeng Wang, Xiaodong Liu, Lili Jiang, Bao Liu, Xueqi Fu, and et al. 2021. "High Chromosomal Stability and Immortalized Totipotency Characterize Long-Term Tissue Cultures of Chinese Ginseng (Panax ginseng)" Genes 12, no. 4: 514. https://doi.org/10.3390/genes12040514
APA StyleLiu, S., Zhao, J., Liu, Y., Li, N., Wang, Z., Wang, X., Liu, X., Jiang, L., Liu, B., Fu, X., Li, X., & Li, L. (2021). High Chromosomal Stability and Immortalized Totipotency Characterize Long-Term Tissue Cultures of Chinese Ginseng (Panax ginseng). Genes, 12(4), 514. https://doi.org/10.3390/genes12040514








