Analysis of Peripheral Blood Mononuclear Cells Gene Expression Highlights the Role of Extracellular Vesicles in the Immune Response following Hematopoietic Stem Cell Transplantation in Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Data Collection
2.3. Molecular Analysis (Microarrays)
2.4. GO Functional Enrichment Analysis and KEGG Pathways Analysis
2.5. PPI Analysis
2.6. Statistical Analysis
3. Results
3.1. Clinical Data
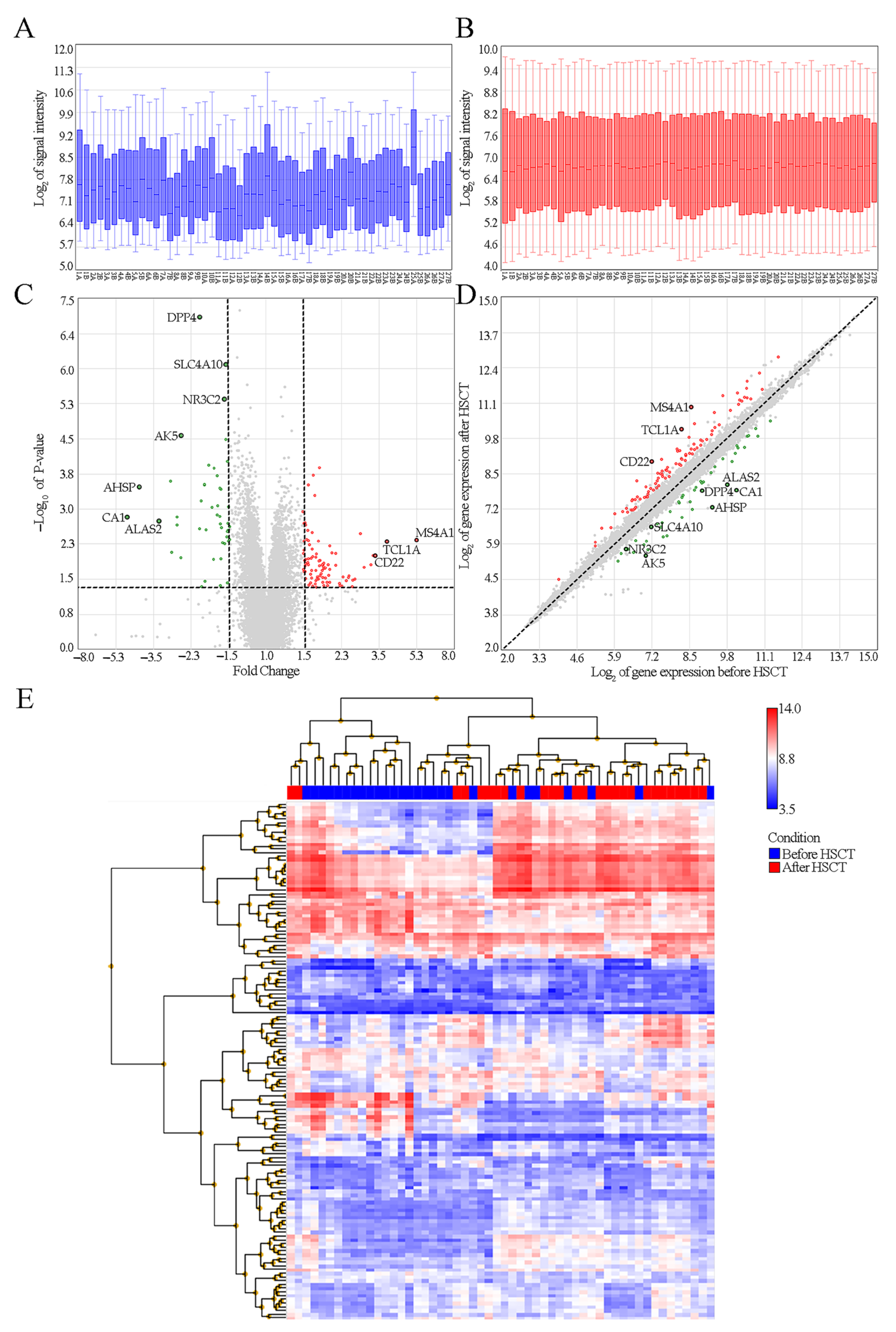
3.2. Identification of DEGs between Children before and after HSCT
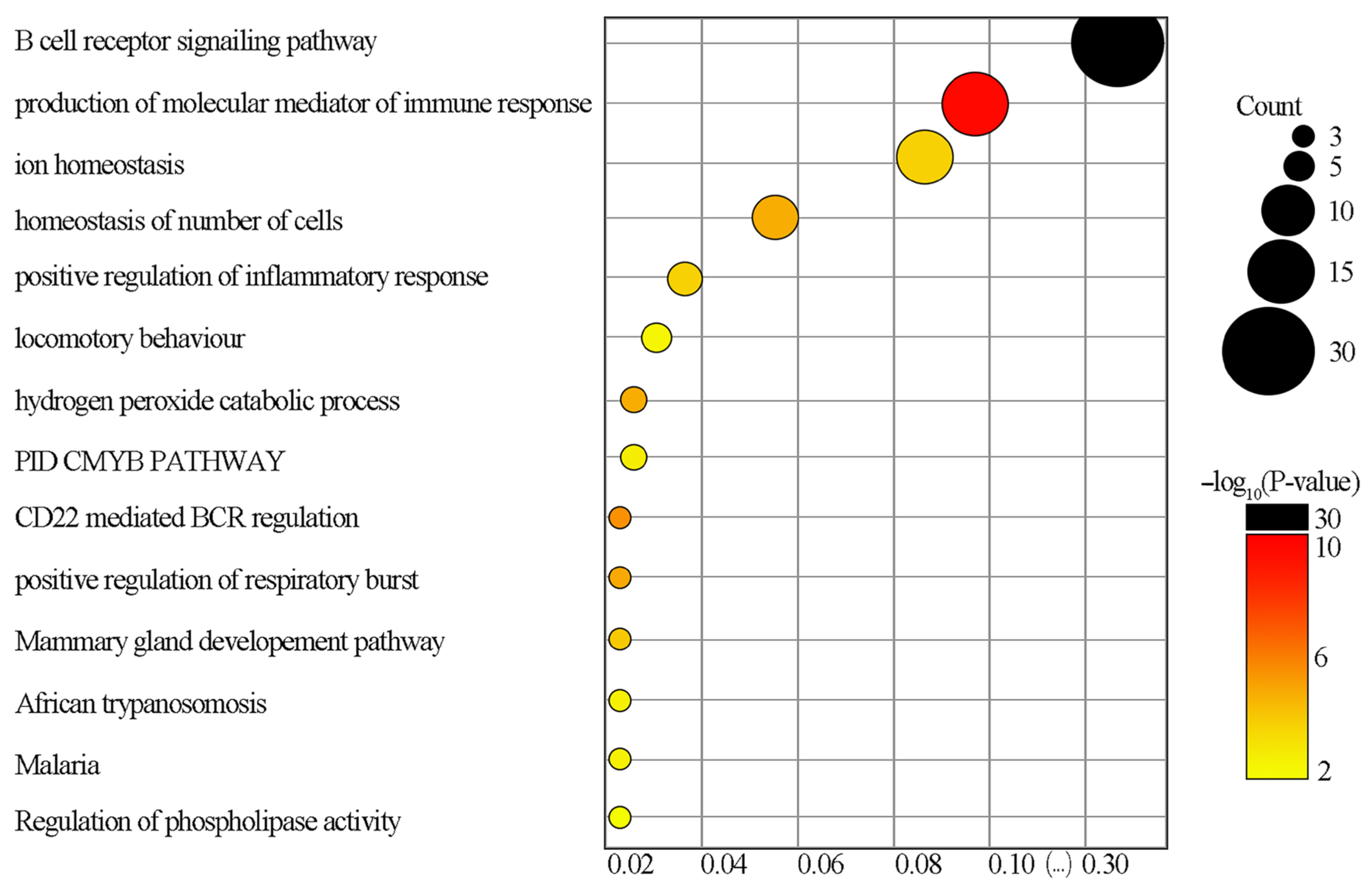
3.3. GO Functional Enrichment Analysis and KEGG Pathways Analysis
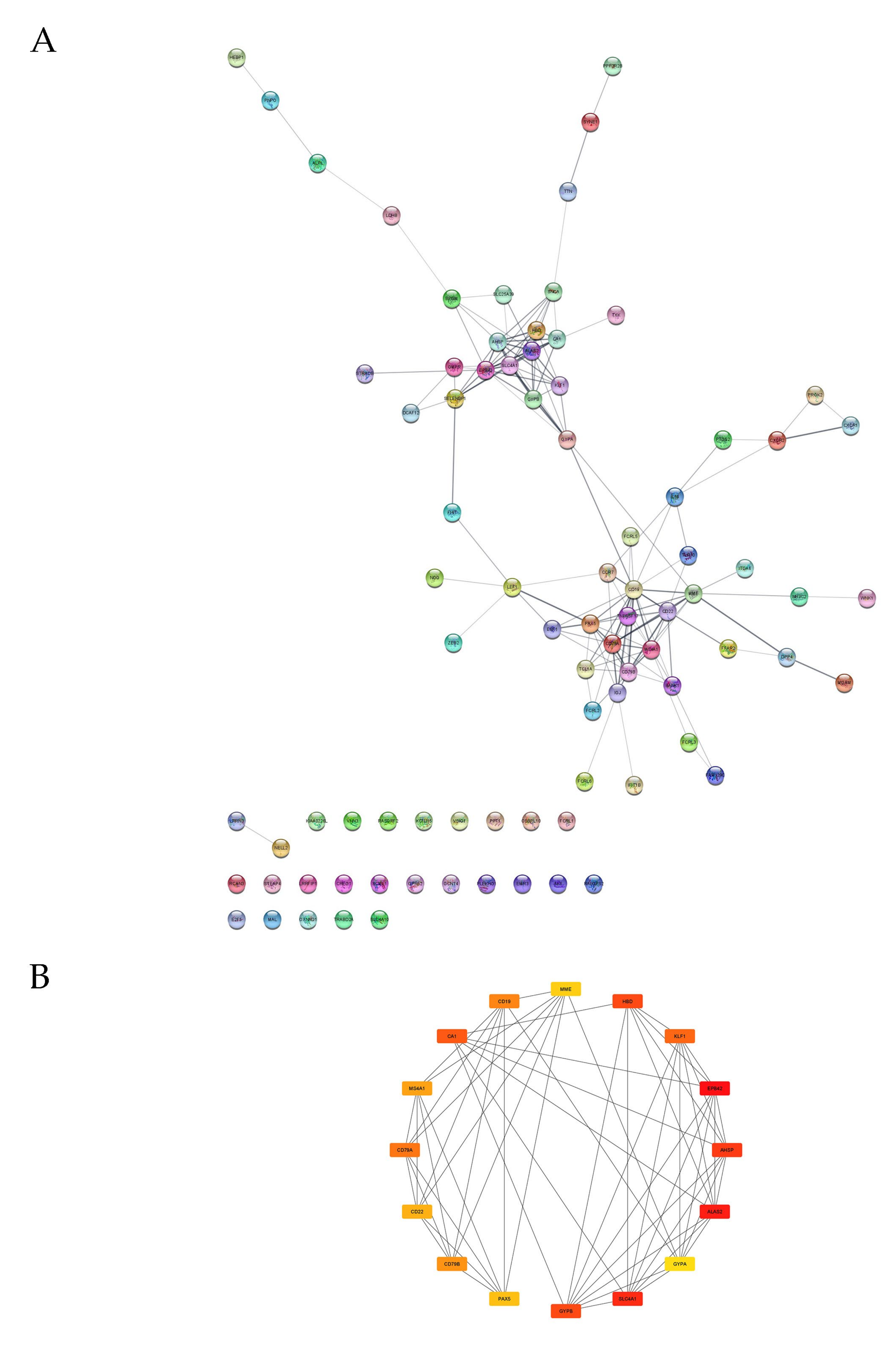
3.4. PPI Analysis
4. Discussion
4.1. GO and KEGG Enrichment Analyses
4.2. PPI Analysis and DEGs with the Most Significant Changes in Expressions
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khaddour, K.; Hana, C.K.; Mewawalla, P. Hematopoietic Stem Cell Transplantation; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Gratwohl, A.; Pasquini, M.C.; Aljurf, M.; Atsuta, Y.; Baldomero, H.; Foeken, L.; Gratwohl, M.; Bouzas, L.F.; Confer, D.; Frauendorfer, K.; et al. One Million Haemopoietic Stem-Cell Transplants: A Retrospective Observational Study. Lancet Haematol. 2015, 2, e91–e100. [Google Scholar] [CrossRef]
- Niederwieser, D.; Baldomero, H.; Szer, J.; Gratwohl, M.; Aljurf, M.; Atsuta, Y.; Bouzas, L.F.; Confer, D.; Greinix, H.; Horowitz, M.; et al. Hematopoietic Stem Cell Transplantation Activity Worldwide in 2012 and a SWOT Analysis of the Worldwide Network for Blood and Marrow Transplantation Group Including the Global Survey. Bone Marrow Transplant. 2016, 51, 778–785. [Google Scholar] [CrossRef]
- Gozdzik, J.; Czogala, W.; Skoczen, S.; Krasowska-Kwiecien, A.; Wiecha, O.; Mordel, A.; Lesko, E.; Majka, M.; Kowalczyk, D.; Zembala, M. Rapid Full Engraftment and Successful Immune Reconstitution after Allogeneic Hematopoietic Stem Cell Transplantation with Reduced Intensity Conditioning in Omenn Syndrome. Pediatr. Transplant. 2009, 13, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Czogała, M.; Balwierz, W.; Pawińska-Wąsikowska, K.; Książek, T.; Bukowska-Strakova, K.; Czogała, W.; Sikorska-Fic, B.; Matysiak, M.; Skalska-Sadowska, J.; Wachowiak, J.; et al. Advances in the First Line Treatment of Pediatric Acute Myeloid Leukemia in the Polish Pediatric Leukemia and Lymphoma Study Group from 1983 to 2019. Cancers 2021, 13, 4536. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, A.; Popradi, G. A General Practitioner’s Guide to Hematopoietic Stem-Cell Transplantation. Curr. Oncol. 2019, 26, 187–191. [Google Scholar] [CrossRef]
- Hierlmeier, S.; Eyrich, M.; Wölfl, M.; Schlegel, P.-G.; Wiegering, V. Early and Late Complications Following Hematopoietic Stem Cell Transplantation in Pediatric Patients—A Retrospective Analysis over 11 Years. PLoS ONE 2018, 13, e0204914. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, G.; Thacker, N.; Schechter, T.; Pole, J.D. Anxiety, Depression, and Mental Health-Related Quality of Life in Survivors of Pediatric Allogeneic Hematopoietic Stem Cell Transplantation: A Systematic Review. Bone Marrow Transplant. 2020, 55, 1240–1254. [Google Scholar] [CrossRef]
- Snaman, J.M.; Talleur, A.C.; Lu, J.; Levine, D.R.; Kaye, E.C.; Sykes, A.; Lu, Z.; Triplett, B.M.; Baker, J.N. Treatment Intensity and Symptom Burden in Hospitalized Adolescent and Young Adult Hematopoietic Cell Transplant Recipients at the End of Life. Bone Marrow Transplant. 2018, 53, 84–90. [Google Scholar] [CrossRef] [PubMed]
- El-Jawahri, A.; LeBlanc, T.; VanDusen, H.; Traeger, L.; Greer, J.A.; Pirl, W.F.; Jackson, V.A.; Telles, J.; Rhodes, A.; Spitzer, T.R.; et al. Effect of Inpatient Palliative Care on Quality of Life 2 Weeks After Hematopoietic Stem Cell Transplantation. JAMA 2016, 316, 2094. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, C.K.; Lehmann, L.; London, W.B.; Guo, D.; Sridharan, M.; Koch, R.; Wolfe, J. End-of-Life Care Patterns Associated with Pediatric Palliative Care among Children Who Underwent Hematopoietic Stem Cell Transplant. Biol. Blood Marrow Transplant. 2016, 22, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Bis, G.; Szlasa, W.; Sondaj, K.; Zendran, I.; Mielcarek-Siedziuk, M.; Barg, E. Lipid Complications after Hematopoietic Stem Cell Transplantation (HSCT) in Pediatric Patients. Nutrients 2020, 12, 2500. [Google Scholar] [CrossRef] [PubMed]
- Ragbourne, S.C.; Crook, M.A. Metabolic Syndrome in Long-Term Survivors of Hematopoietic Stem-Cell Transplantation. Clin. Lymphoma Myeloma Leuk. 2017, 17, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Orio, F.; Muscogiuri, G.; Palomba, S.; Serio, B.; Sessa, M.; Giudice, V.; Ferrara, I.; Tauchmanovà, L.; Colao, A.; Selleri, C. Endocrinopathies after Allogeneic and Autologous Transplantation of Hematopoietic Stem Cells. Sci. World J. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.J.; Mueller, B.A.; Baker, K.S.; Cushing-Haugen, K.L.; Flowers, M.E.D.; Martin, P.J.; Friedman, D.L.; Lee, S.J. Cardiovascular Hospitalizations and Mortality Among Recipients of Hematopoietic Stem Cell Transplantation. Ann. Intern. Med. 2011, 155, 21. [Google Scholar] [CrossRef] [PubMed]
- Niederwieser, D.; Baldomero, H.; Bazuaye, N.; Bupp, C.; Chaudhri, N.; Corbacioglu, S.; Elhaddad, A.; Frutos, C.; Galeano, S.; Hamad, N.; et al. One and a Half Million Hematopoietic Stem Cell Transplants: Continuous and Differential Improvement in Worldwide Access with the Use of Non-Identical Family Donors. Haematologica 2021. [Google Scholar] [CrossRef] [PubMed]
- Passweg, J.R.; Baldomero, H.; Peters, C.; Gaspar, H.B.; Cesaro, S.; Dreger, P.; Duarte, R.F.; Falkenburg, J.H.F.; Farge-Bancel, D.; Gennery, A.; et al. Hematopoietic SCT in Europe: Data and Trends in 2012 with Special Consideration of Pediatric Transplantation. Bone Marrow Transplant. 2014, 49, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Morken, C.; Tevaarwerk, A.J.; Juckett, M.B.; Swiecichowski, A.K.; Haine, J.E.; Zhang, X.; Williams, Z.T.; Norslien, K.; Campbell, B.; Wassenaar, T.; et al. Barriers and Facilitators to the Use of Survivorship Care Plans by Hematopoietic Stem Cell Transplant Survivors and Clinicians. Support. Care Cancer 2021. [Google Scholar] [CrossRef]
- Dong, F.; Hao, S.; Zhang, S.; Zhu, C.; Cheng, H.; Yang, Z.; Hamey, F.K.; Wang, X.; Gao, A.; Wang, F.; et al. Differentiation of Transplanted Haematopoietic Stem Cells Tracked by Single-Cell Transcriptomic Analysis. Nat. Cell Biol. 2020, 22, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Cilloni, D.; Petiti, J.; Campia, V.; Podestà, M.; Squillario, M.; Montserrat, N.; Bertaina, A.; Sabatini, F.; Carturan, S.; Berger, M.; et al. Transplantation Induces Profound Changes in the Transcriptional Asset of Hematopoietic Stem Cells: Identification of Specific Signatures Using Machine Learning Techniques. J. Clin. Med. 2020, 9, 1670. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.-X.; Wen, J.-J.; Cheng, Y.-F.; Zhang, X.-H.; Xu, L.-P.; Wang, Y.; Yan, C.-H.; Chen, Y.-H.; Chen, H.; Han, W.; et al. Wilms’ Tumor Gene 1 Is an Independent Prognostic Factor for Pediatric Acute Myeloid Leukemia Following Allogeneic Hematopoietic Stem Cell Transplantation. BMC Cancer 2021, 21, 292. [Google Scholar] [CrossRef] [PubMed]
- Valkova, V.; Vydra, J.; Markova, M.; Cerovska, E.; Vrana, M.; Marinov, I.; Cechova, H.; Cetkovsky, P.; Vitek, A.; Salek, C. WT1 Gene Expression in Peripheral Blood Before and After Allogeneic Stem Cell Transplantation Is a Clinically Relevant Prognostic Marker in AML—A Single-Center 14-Year Experience. Clin. Lymphoma Myeloma Leuk. 2021, 21, e145–e151. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.-G.; Hong, Y.; Qin, Y.-Z.; Chang, Y.-J.; Wang, Y.; Zhang, X.-H.; Xu, L.-P.; Huang, X.-J.; Zhao, X.-S. Prognostic Significance of SET-NUP214 Fusion Gene in Acute Leukemia after Allogeneic Hematopoietic Stem Cell Transplantation. Medicine 2020, 99, e23569. [Google Scholar] [CrossRef] [PubMed]
- Seipel, K.; Messerli, C.; Wiedemann, G.; Bacher, U.; Pabst, T. MN1, FOXP1 and Hsa-MiR-181a-5p as Prognostic Markers in Acute Myeloid Leukemia Patients Treated with Intensive Induction Chemotherapy and Autologous Stem Cell Transplantation. Leuk. Res. 2020, 89, 106296. [Google Scholar] [CrossRef] [PubMed]
- Schenone, D.; Andolina, J.R.; Rademacher, B.; Fountaine, T.J.; Edwards, E.; Nunez, L.; Qiu, M.; Sharma, S.; Mullen, C.A. Gene Expression and Survival of Acute Lymphoblastic Leukemia Cells after Allogeneic Transplant. Anticancer Res. 2021, 41, 2781–2793. [Google Scholar] [CrossRef] [PubMed]
- Skoczen, S.; Bik-Multanowski, M.; Pietrzyk, J.J.; Grabowska, A.; Fijorek, K.; Strojny, W.; Klus-Kwiecinska, K.; Balwierz, W.; Siedlar, M. Genetic Background of Immune Complications after Allogeneic Hematopoietic Stem Cell Transplantation in Children. Stem Cells Int. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Czogała, W.; Czogała, M.; Kwiecińska, K.; Bik-Multanowski, M.; Tomasik, P.; Hałubiec, P.; Łazarczyk, A.; Miklusiak, K.; Skoczeń, S. The Expression of Genes Related to Lipid Metabolism and Metabolic Disorders in Children before and after Hematopoietic Stem Cell Transplantation—A Prospective Observational Study. Cancers 2021, 13, 3614. [Google Scholar] [CrossRef]
- World Medical Association Declaration of Helsinki. JAMA 2013, 310, 2191. [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Bazzan, E.; Tinè, M.; Casara, A.; Biondini, D.; Semenzato, U.; Cocconcelli, E.; Balestro, E.; Damin, M.; Radu, C.M.; Turato, G.; et al. Critical Review of the Evolution of Extracellular Vesicles’ Knowledge: From 1946 to Today. Int. J. Mol. Sci. 2021, 22, 6417. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane Vesicles as Conveyors of Immune Responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Simak, J.; Gelderman, M.P. Cell Membrane Microparticles in Blood and Blood Products: Potentially Pathogenic Agents and Diagnostic Markers. Tansfus. Med. Rev. 2006, 20, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Vickers, K.C.; Remaley, A.T. Lipid-Based Carriers of MicroRNAs and Intercellular Communication. Curr. Opin. Lipidol. 2012, 23, 91–97. [Google Scholar] [CrossRef]
- Monleón, I.; Martínez-Lorenzo, M.J.; Monteagudo, L.; Lasierra, P.; Taulés, M.; Iturralde, M.; Piñeiro, A.; Larrad, L.; Alava, M.A.; Naval, J.; et al. Differential Secretion of Fas Ligand- or APO2 Ligand/TNF-Related Apoptosis-Inducing Ligand-Carrying Microvesicles During Activation-Induced Death of Human T Cells. J. Immunol. 2001, 167, 6736–6744. [Google Scholar] [CrossRef]
- Corraliza, A.M.; Ricart, E.; López-García, A.; Carme Masamunt, M.; Veny, M.; Esteller, M.; Mayorgas, A.; Le Bourhis, L.; Allez, M.; Planell, N.; et al. Differences in Peripheral and Tissue Immune Cell Populations Following Haematopoietic Stem Cell Transplantation in Crohn’s Disease Patients. J. Crohn’s Colitis 2019, 13, 634–647. [Google Scholar] [CrossRef]
- Lia, G.; Di Vito, C.; Cerrano, M.; Brunello, L.; Calcaterra, F.; Tapparo, M.; Giaccone, L.; Mavilio, D.; Bruno, B. Extracellular Vesicles After Allogeneic Hematopoietic Cell Transplantation: Emerging Role in Post-Transplant Complications. Front. Immunol. 2020, 11, 422. [Google Scholar] [CrossRef]
- Del Fattore, A.; Luciano, R.; Pascucci, L.; Goffredo, B.M.; Giorda, E.; Scapaticci, M.; Fierabracci, A.; Muraca, M. Immunoregulatory Effects of Mesenchymal Stem Cell-Derived Extracellular Vesicles on T Lymphocytes. Cell Transplant. 2015, 24, 2615–2627. [Google Scholar] [CrossRef] [PubMed]
- Mokarizadeh, A.; Delirezh, N.; Morshedi, A.; Mosayebi, G.; Farshid, A.-A.; Mardani, K. Microvesicles Derived from Mesenchymal Stem Cells: Potent Organelles for Induction of Tolerogenic Signaling. Immunol. Lett. 2012, 147, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gu, Z.; Zhao, X.; Yang, N.; Wang, F.; Deng, A.; Zhao, S.; Luo, L.; Wei, H.; Guan, L.; et al. Extracellular Vesicles Released from Human Umbilical Cord-Derived Mesenchymal Stromal Cells Prevent Life-Threatening Acute Graft-Versus-Host Disease in a Mouse Model of Allogeneic Hematopoietic Stem Cell Transplantation. Stem. Cells Dev. 2016, 25, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-L.; Li, J.-Y.; Xie, G.-L.; Ma, X.-Y. Exosomes Released From Human Bone Marrow–Derived Mesenchymal Stem Cell Attenuate Acute Graft-Versus-Host Disease After Allogeneic Hematopoietic Stem Cell Transplantation in Mice. Front. Cell Dev. Biol. 2021, 9, 617589. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, S.; Yang, P.; Cao, H.; Li, L. The Role of Mesenchymal Stem Cells in Hematopoietic Stem Cell Transplantation: Prevention and Treatment of Graft-versus-Host Disease. Stem. Cell Res. Ther. 2019, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Kordelas, L.; Rebmann, V.; Ludwig, A.-K.; Radtke, S.; Ruesing, J.; Doeppner, T.R.; Epple, M.; Horn, P.A.; Beelen, D.W.; Giebel, B. MSC-Derived Exosomes: A Novel Tool to Treat Therapy-Refractory Graft-versus-Host Disease. Leukemia 2014, 28, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Blazar, B.R.; MacDonald, K.P.A.; Hill, G.R. Immune Regulatory Cell Infusion for Graft-versus-Host Disease Prevention and Therapy. Blood 2018, 131, 2651–2660. [Google Scholar] [CrossRef]
- Ranganathan, P.; Heaphy, C.E.A.; Costinean, S.; Stauffer, N.; Na, C.; Hamadani, M.; Santhanam, R.; Mao, C.; Taylor, P.A.; Sandhu, S.; et al. Regulation of Acute Graft-versus-Host Disease by MicroRNA-155. Blood 2012, 119, 4786–4797. [Google Scholar] [CrossRef]
- Pomatto, M.A.C.; Bussolati, B.; D’Antico, S.; Ghiotto, S.; Tetta, C.; Brizzi, M.F.; Camussi, G. Improved Loading of Plasma-Derived Extracellular Vesicles to Encapsulate Antitumor MiRNAs. Mol. Ther. Methods Clin. Dev. 2019, 13, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Brunello, L.; Lia, G.; Bruno, S.; Tapparo, M.; Omede, P.; Festuccia, M.; Maffini, E.; Ciccone, G.; Comba, L.; Tosti, L.; et al. Biomarkers of Acute Graft-Versus-Host Disease: Surface Antigens and Micro Rnas in Extracellular Vesicles. Biol. Blood Marrow Transplant. 2019, 25, S232. [Google Scholar] [CrossRef]
- Lia, G.; Brunello, L.; Bruno, S.; Carpanetto, A.; Omedè, P.; Festuccia, M.; Tosti, L.; Maffini, E.; Giaccone, L.; Arpinati, M.; et al. Extracellular Vesicles as Potential Biomarkers of Acute Graft-vs-Host Disease. Leukemia 2018, 32, 765–773. [Google Scholar] [CrossRef]
- Crossland, R.E.; Norden, J.; Kralj Juric, M.; Pearce, K.F.; Lendrem, C.; Bibby, L.A.; Collin, M.; Greinix, H.T.; Dickinson, A.M. Serum and Extracellular Vesicle MicroRNAs MiR-423, MiR-199, and MiR-93* As Biomarkers for Acute Graft-versus-Host Disease. Front. Immunol. 2017, 8, 1446. [Google Scholar] [CrossRef]
- Yoshizawa, S.; Umezu, T.; Saitoh, Y.; Gotoh, M.; Akahane, D.; Kobayashi, C.; Ohyashiki, J.; Ohyashiki, K. Exosomal MiRNA Signatures for Late-Onset Acute Graft-Versus-Host Disease in Allogenic Hematopoietic Stem Cell Transplantation. Int. J. Mol. Sci. 2018, 19, 2493. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, M.; Mitsuiki, N.; Yanagimachi, M.; Yamamoto, M.; Fukuda, T.; Miura, O.; Oba, R.; Igarashi, A.; Nagata, K.; Morio, T. Utility of Novel T-Cell-Specific Extracellular Vesicles in Monitoring and Evaluation of Acute GVHD. Int. J. Hematol. 2021, 113, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro-Vornhagen, A.; Hallek, M.J.; Storb, R.F.; von Bergwelt-Baildon, M.S. The Role of B Cells in the Pathogenesis of Graft-versus-Host Disease. Blood 2009, 114, 4919–4927. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, J.L.; Levine, J.E.; Reddy, P.; Holler, E. Graft-versus-Host Disease. Lancet 2009, 373, 1550–1561. [Google Scholar] [CrossRef]
- Koyama, M.; Hill, G.R. Alloantigen Presentation and Graft-versus-Host Disease: Fuel for the Fire. Blood 2016, 127, 2963–2970. [Google Scholar] [CrossRef]
- Neefjes, J.; Jongsma, M.L.M.; Paul, P.; Bakke, O. Towards a Systems Understanding of MHC Class I and MHC Class II Antigen Presentation. Nat. Rev. Immunol. 2011, 11, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Jodele, S.; Medvedovic, M.; Luebbering, N.; Chen, J.; Dandoy, C.E.; Laskin, B.L.; Davies, S.M. Interferon-Complement Loop in Transplant-Associated Thrombotic Microangiopathy. Blood Adv. 2020, 4, 1166–1177. [Google Scholar] [CrossRef]
- Raanani, P.; Levi, I.; Holzman, F.; Grotto, I.; Brok-Simoni, F.; Avigdor, A.; Davidson, J.; Shpilberg, O.; Ben-Bassat, I. Engraftment-Associated Hypophosphatemia–the Role of Cytokine Release and Steep Leukocyte Rise Post Stem Cell Transplantation. Bone Marrow Transplant. 2001, 27, 311–317. [Google Scholar] [CrossRef][Green Version]
- Faghihi, T.; Iravani, M.; Shamshiri, A.R.; Hadjibabaie, M.; Mousavi, S.A.; Alimoghaddam, K.; Ghavamzadeh, A. Serum Electrolyte Changes at Engraftment Time in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Ann. Transplant. 2009, 14, 51–57. [Google Scholar]
- Uçkan, D.; Çetin, M.; Dida, A.; Batu, A.; Tuncer, M.; Tezcan, İ. Hypophosphatemia and Hypouricemia in Pediatric Allogeneic Bone Marrow Transplant Recipients. Pediatr. Transplant. 2003, 7, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Feske, S.; Skolnik, E.Y.; Prakriya, M. Ion Channels and Transporters in Lymphocyte Function and Immunity. Nat. Rev. Immunol. 2012, 12, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.; Martin, M.-J.; Orchard, S.; Magrane, M.; Agivetova, R.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; Bursteinas, B.; et al. UniProt: The Universal Protein Knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- ALAS2–5-Aminolevulinate Synthase, Erythroid-Specific, Mitochondrial Precursor–Homo Sapiens (Human)–ALAS2 Gene & Protein. Available online: https://www.uniprot.org/uniprot/P22557 (accessed on 22 October 2021).
- EPB42–Protein 4.2–Homo Sapiens (Human)–EPB42 Gene & Protein. Available online: https://www.uniprot.org/uniprot/P16452 (accessed on 22 October 2021).
- SLC4A1–Band 3 Anion Transport Protein–Homo Sapiens (Human)–SLC4A1 Gene & Protein. Available online: https://www.uniprot.org/uniprot/P02730 (accessed on 22 October 2021).
- AHSP–Alpha-Hemoglobin-Stabilizing Protein–Homo Sapiens (Human)–AHSP Gene & Protein. Available online: https://www.uniprot.org/uniprot/Q9NZD4 (accessed on 22 October 2021).
- GYPB–Glycophorin-B Precursor–Homo Sapiens (Human)–GYPB Gene & Protein. Available online: https://www.uniprot.org/uniprot/P06028 (accessed on 22 October 2021).
- HBD–Hemoglobin Subunit Delta–Homo Sapiens (Human)–HBD Gene & Protein. Available online: https://www.uniprot.org/uniprot/P02042 (accessed on 22 October 2021).
- CA1–Carbonic Anhydrase 1–Homo Sapiens (Human)–CA1 Gene & Protein. Available online: https://www.uniprot.org/uniprot/P00915 (accessed on 22 October 2021).
- KLF1–Krueppel-like Factor 1–Homo Sapiens (Human)–KLF1 Gene & Protein. Available online: https://www.uniprot.org/uniprot/Q13351 (accessed on 22 October 2021).
- CD79A–B-Cell Antigen Receptor Complex-Associated Protein Alpha Chain Precursor–Homo Sapiens (Human)–CD79A Gene & Protein. Available online: https://www.uniprot.org/uniprot/P11912 (accessed on 22 October 2021).
- CD79B–B-Cell Antigen Receptor Complex-Associated Protein Beta Chain Precursor–Homo Sapiens (Human)–CD79B Gene & Protein. Available online: https://www.uniprot.org/uniprot/P40259 (accessed on 22 October 2021).
- CD19–B-Lymphocyte Antigen CD19 Precursor–Homo Sapiens (Human)–CD19 Gene & Protein. Available online: https://www.uniprot.org/uniprot/P15391 (accessed on 22 October 2021).
- MS4A1–B-Lymphocyte Antigen CD20–Homo Sapiens (Human)–MS4A1 Gene & Protein. Available online: https://www.uniprot.org/uniprot/P11836 (accessed on 22 October 2021).
- CD22–B-Cell Receptor CD22 Precursor–Homo Sapiens (Human)–CD22 Gene & Protein. Available online: https://www.uniprot.org/uniprot/P20273 (accessed on 22 October 2021).
- PAX5–Paired Box Protein Pax-5–Homo Sapiens (Human)–PAX5 Gene & Protein. Available online: https://www.uniprot.org/uniprot/Q02548 (accessed on 22 October 2021).
- TCL1A–T-Cell Leukemia/Lymphoma Protein 1A–Homo Sapiens (Human)–TCL1A Gene & Protein. Available online: https://www.uniprot.org/uniprot/P56279 (accessed on 25 October 2021).
- DPP4–Dipeptidyl Peptidase 4–Homo Sapiens (Human)–DPP4 Gene & Protein. Available online: https://www.uniprot.org/uniprot/P27487 (accessed on 25 October 2021).
- SLC4A10–Sodium-Driven Chloride Bicarbonate Exchanger–Homo Sapiens (Human)–SLC4A10 Gene & Protein. Available online: https://www.uniprot.org/uniprot/Q6U841 (accessed on 25 October 2021).
- NR3C2–Mineralocorticoid Receptor–Homo Sapiens (Human)–NR3C2 Gene & Protein. Available online: https://www.uniprot.org/uniprot/P08235 (accessed on 25 October 2021).
- AK5–Adenylate Kinase Isoenzyme 5–Homo Sapiens (Human)–AK5 Gene & Protein. Available online: https://www.uniprot.org/uniprot/Q9Y6K8 (accessed on 25 October 2021).
- Zhao, M.Y.; Yu, Y.; Xie, M.; Yang, M.H.; Zhu, S.; Yang, L.C.; Kang, R.; Tang, D.L.; Zhao, L.L.; Cao, L.Z. Digital Gene Expression Profiling Analysis of Childhood Acute Lymphoblastic Leukemia. Mol. Med. Rep. 2016, 13, 4321–4328. [Google Scholar] [CrossRef] [PubMed]

| Diagnosis | Number (%), n = 27 |
|---|---|
| Neoplastic diseases | 18 (67) |
| Acute lymphoblastic leukemia | 11 (41) |
| Acute myeloblastic leukemia | 4 (15) |
| Juvenile myelomonocytic leukemia and acute myeloblastic leukemia | 1 (4) |
| Myelodysplastic syndrome | 1 (4) |
| Chronic myelocytic leukemia | 1 (4) |
| Non-Neoplastic diseases | 9 (33) |
| Hyper IgM syndrome | 1 (4) |
| Chronic granulomatous disease | 3 (11) |
| Autoimmune lymphoproliferative syndrome | 1 (4) |
| Severe aplastic anemia | 4 (15) |
| Treatment | Number of Patients, n = 27 | |
|---|---|---|
| Time since diagnosis (years) | Neoplastic diseases | median: 1.0, mean: 2.0, range: 0.1–7.0 |
| Non-neoplastic diseases | median: 1.5, mean: 3.8, range: 0.1–13.0 | |
| Local radiotherapy (n, %) | 5 (19): CNS-4 (15), testes-1 (4) | |
| Total body irradiation-12 Gy/6 fractions (n, %) | 7 (27) | |
| Chemotherapy before HSCT (n, %) | 17 (63) | |
| Conditioning regimen based on busulfan or treosulfan (n, %) | 16 (59) | |
| GvHD prophylaxis (n, %) | ATG | 20 (74) |
| CsA | 4 (15) | |
| Mtx + CsA | 23 (85) | |
| Mucositis (n, %) | 22 (81) | |
| Grade (n) | I-7, II-8, III-6, IV-1 | |
| Intravenous alimentation due to mucositis (%) | 13 (48) | |
| aGvHD (n, %) | 11 (41) | |
| Localization (%) | Gut-9, liver-27, skin-91 | |
| Grade (n) | IA-1, IB-4, IIB-1, IIC-3, IIIC-2 | |
| Systemic glucocorticoid treatment | n, % | 19 (70) |
| days | median: 3.5, mean: 3.6, range: 0.1–11.0 | |
| Time from HSCT to the second assessment (months) | median: 6.3, range: 5.9–19.1 | |
| Time from discontinuation of immunosuppressive treatment to the second assessment (months) | median: 1.6, range: 0.0–9.0 | |
| Time from discontinuation of systemic glucocorticoids to the second assessment (months) | median: 3.6, mean: 4.5, range: 0.5–14.0 | |
| Hematopoietic stem cells donor (n, %) | MUD: 16 (59), MSD: 9 (33), MFD: 2 (7) | |
| Conditioning Type | Regimen | Number (%), n = 27 |
|---|---|---|
| Non-myeloablative | CyATG Bu or Bux-based | 14 (52) |
| FluCyATG | 1 (4) | |
| Myeloablative | CyATG | 3 (11) |
| TBI-VP | 7 (26) | |
| Treo-based | 2 (7) |
| Characteristic | Pre-HSCT n = 27 | Post-HSCT n = 27 |
|---|---|---|
| Boys/girls (n, %) | 20(74)/7(26) | |
| Age (years) | 9.7 ± 5.2 | 10.4 ± 5.0 |
| Body mass (kg) | 37.4 ± 18.5 | 37.2 ± 17.4 |
| Height (cm) | 134.7 ± 29.8 | 137.7 ± 27.2 |
| Gene Symbol | Locus and Affimetrix Code | Pre-HSCT n = 27 | Post-HSCT n = 27 | Pre-HSCT vs. Post-HSCT | |
|---|---|---|---|---|---|
| FC | p/pBH-Value | ||||
| The Most Statistically Significantly Changed Genes (FDR < 0.05) | |||||
| DPP4 | 2q24.2 8056222 | 8.94 | 7.88 | −2.09 | 8.0 × 10−8/0.0012 |
| SLC4A10 | 2q24.2 8045974 | 7.18 | 6.54 | −1.56 | 8.1 × 10−7/0.0059 |
| NR3C2 | 4q31 8103094 | 6.38 | 5.71 | −1.59 | 4.5 × 10−6/0.0165 |
| AK5 | 1p31.1 7902452 | 7.06 | 5.69 | −2.58 | 2.7 × 10−5/0.0493 |
| The genes whose expressions were most decreased after HSCT | |||||
| AHSP | 16p11.2 7995237 | 9.28 | 7.25 | −4.09 | 0.0003/0.095 |
| CA1 | 8q21.2 8151592 | 10.11 | 7.89 | −4.67 | 0.0015/0.15 |
| ALAS2 | Xp11.21 8173135 | 9.81 | 8.09 | −3.29 | 0.0018/0.16 |
| The genes whose expressions were most increased after HSCT | |||||
| MS4A1 | 11q12.2 7940287 | 8.56 | 10.96 | 5.27 | 0.0046/0.20 |
| TCL1A | 14q32.13 7981183 | 8.22 | 10.14 | 3.78 | 0.005/0.21 |
| CD22 | 19q13.12 8027837 | 7.20 | 8.94 | 3.34 | 0.01/0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strojny, W.; Kwiecińska, K.; Hałubiec, P.; Kowalczyk, W.; Miklusiak, K.; Łazarczyk, A.; Skoczeń, S. Analysis of Peripheral Blood Mononuclear Cells Gene Expression Highlights the Role of Extracellular Vesicles in the Immune Response following Hematopoietic Stem Cell Transplantation in Children. Genes 2021, 12, 2008. https://doi.org/10.3390/genes12122008
Strojny W, Kwiecińska K, Hałubiec P, Kowalczyk W, Miklusiak K, Łazarczyk A, Skoczeń S. Analysis of Peripheral Blood Mononuclear Cells Gene Expression Highlights the Role of Extracellular Vesicles in the Immune Response following Hematopoietic Stem Cell Transplantation in Children. Genes. 2021; 12(12):2008. https://doi.org/10.3390/genes12122008
Chicago/Turabian StyleStrojny, Wojciech, Kinga Kwiecińska, Przemysław Hałubiec, Wojciech Kowalczyk, Karol Miklusiak, Agnieszka Łazarczyk, and Szymon Skoczeń. 2021. "Analysis of Peripheral Blood Mononuclear Cells Gene Expression Highlights the Role of Extracellular Vesicles in the Immune Response following Hematopoietic Stem Cell Transplantation in Children" Genes 12, no. 12: 2008. https://doi.org/10.3390/genes12122008
APA StyleStrojny, W., Kwiecińska, K., Hałubiec, P., Kowalczyk, W., Miklusiak, K., Łazarczyk, A., & Skoczeń, S. (2021). Analysis of Peripheral Blood Mononuclear Cells Gene Expression Highlights the Role of Extracellular Vesicles in the Immune Response following Hematopoietic Stem Cell Transplantation in Children. Genes, 12(12), 2008. https://doi.org/10.3390/genes12122008






