The Terpene Mini-Path, a New Promising Alternative for Terpenoids Bio-Production
Abstract
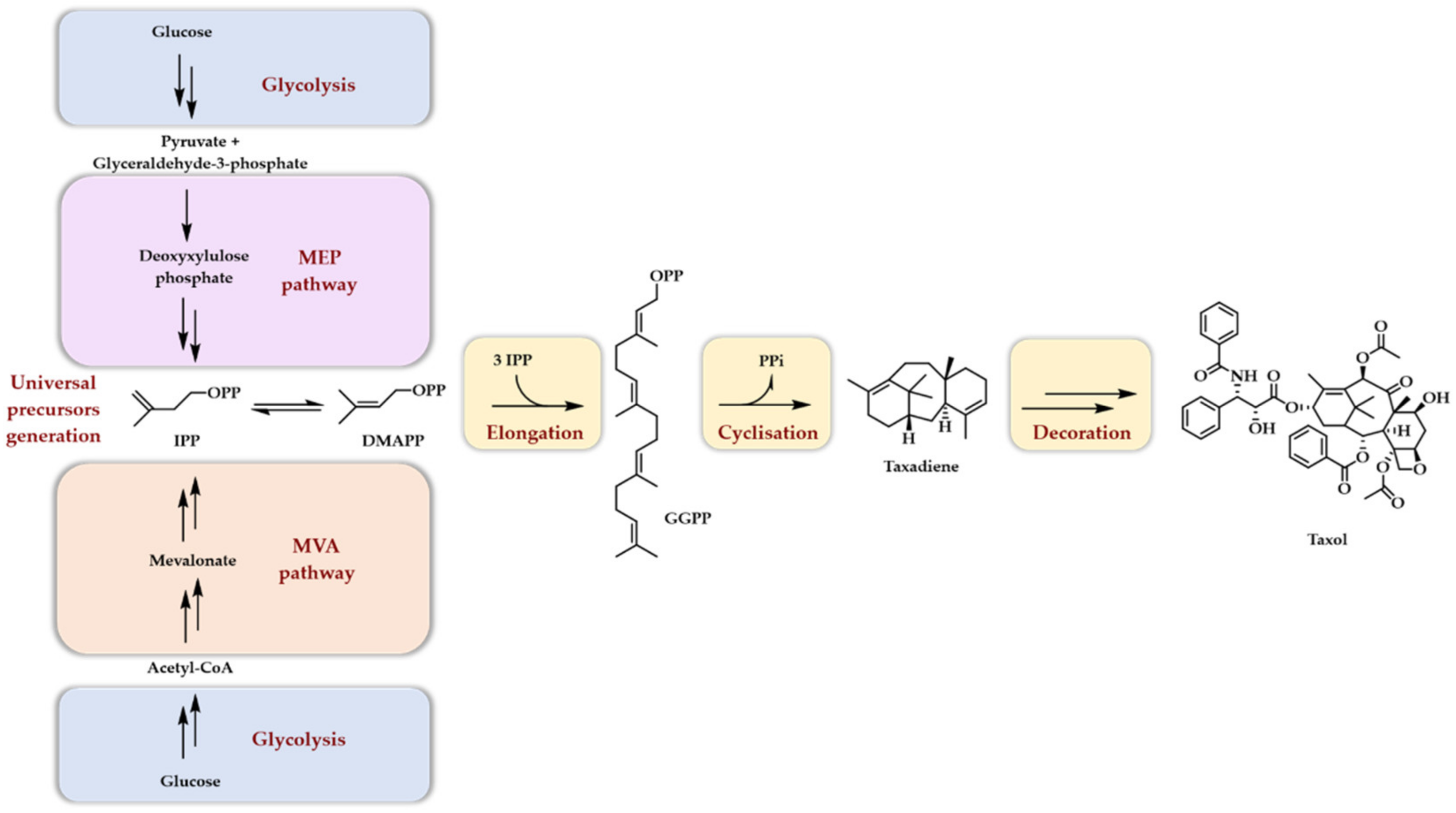
:1. Introduction
2. Premises
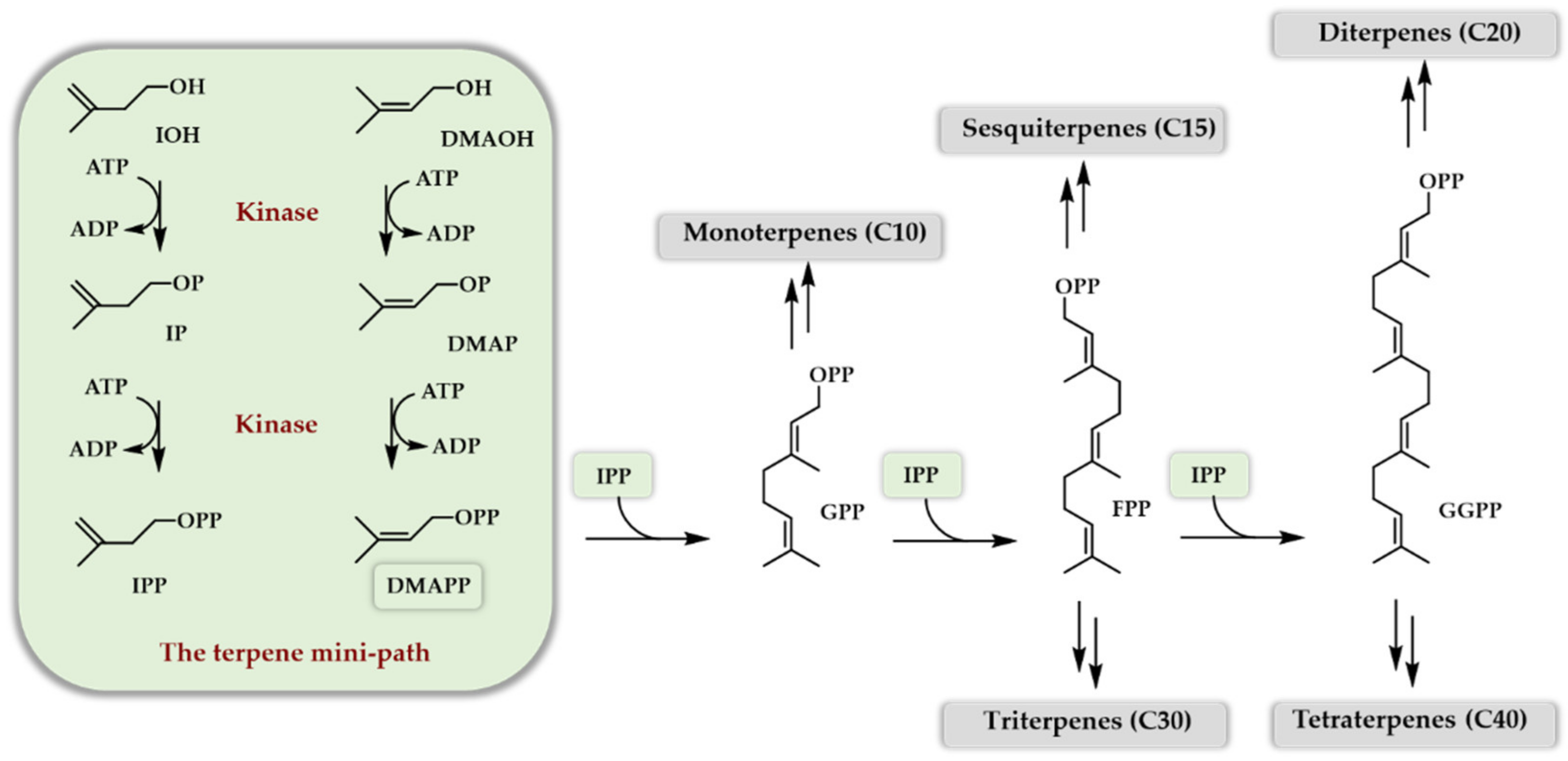
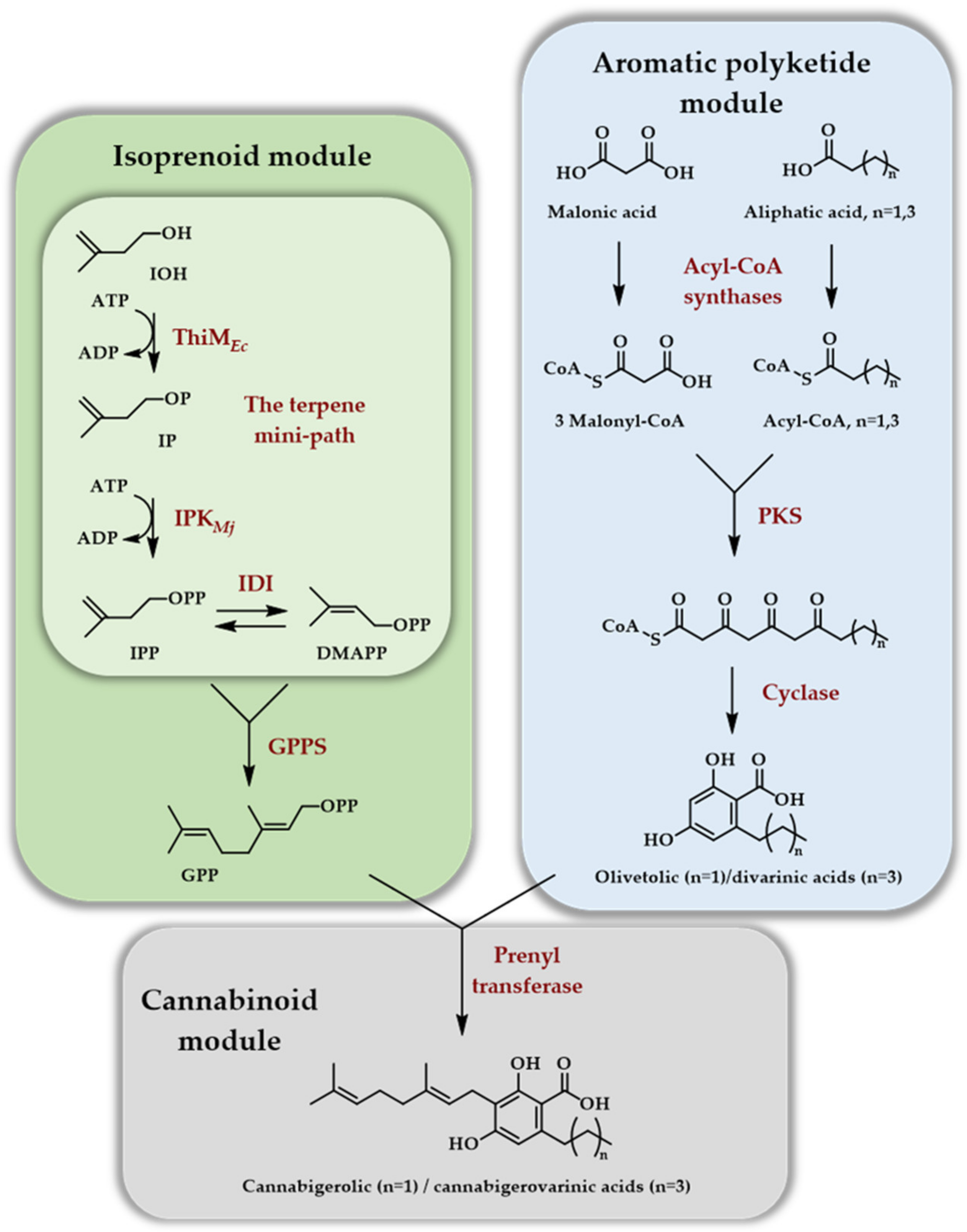
3. One Enzyme Access to DMAPP/IPP
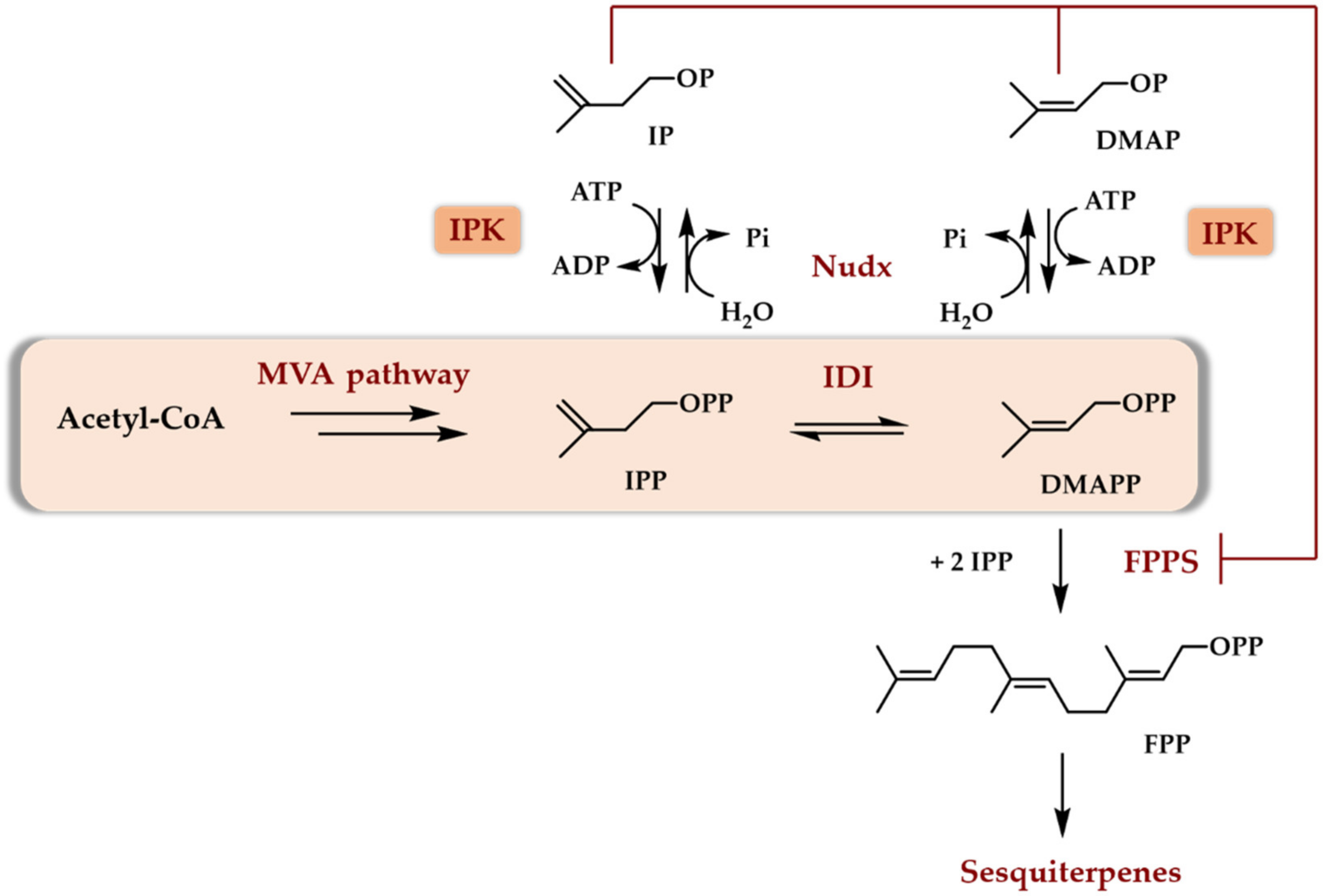
3.1. The Isopentenyl Phosphate Kinase Enzyme
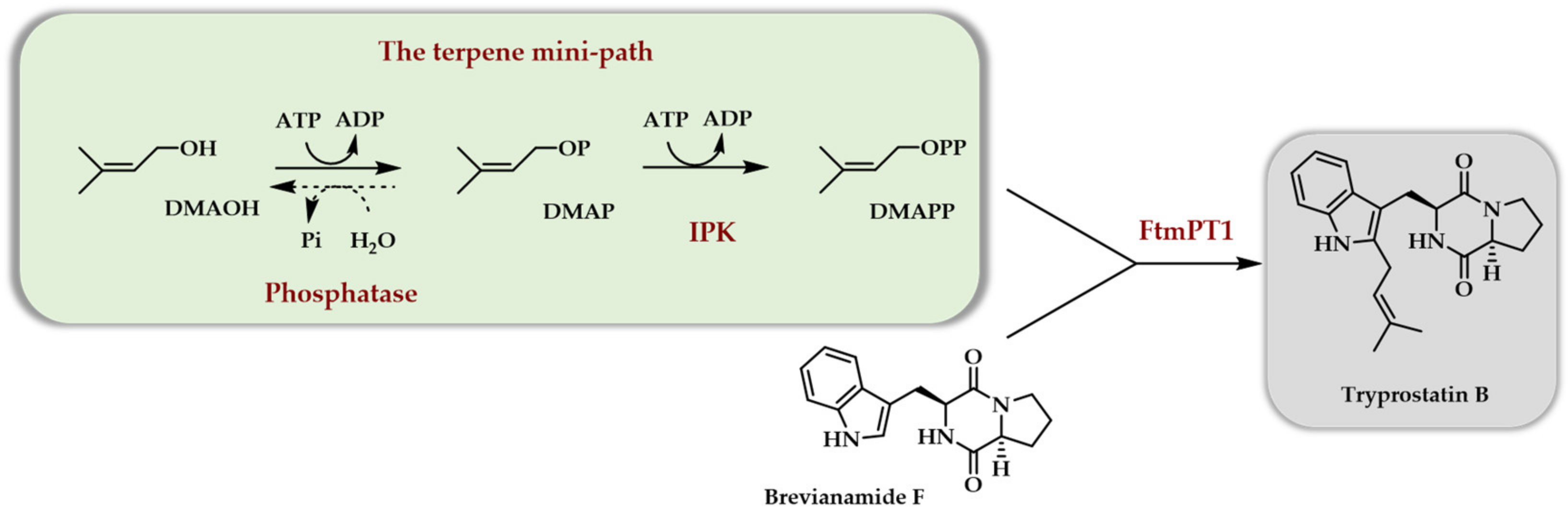
3.2. One Enzyme Double Phosphorylation of DMAOH and IOH
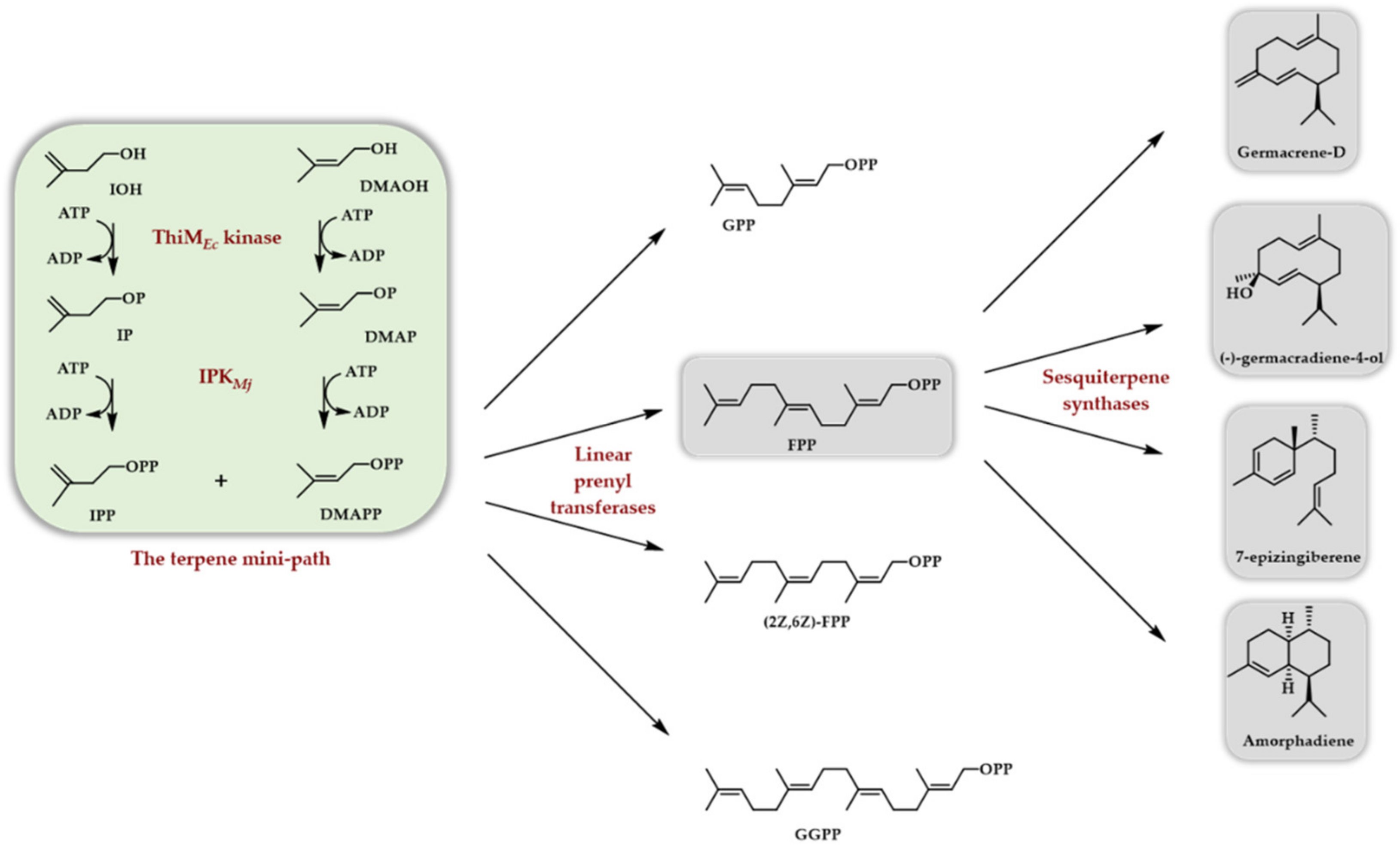
4. Two Enzyme Access to DMAPP/IPP
4.1. What Enzyme to Be Coupled with an IPK
4.2. The TMP In Vitro—Purified Enzymes
4.3. The TMP In Vivo
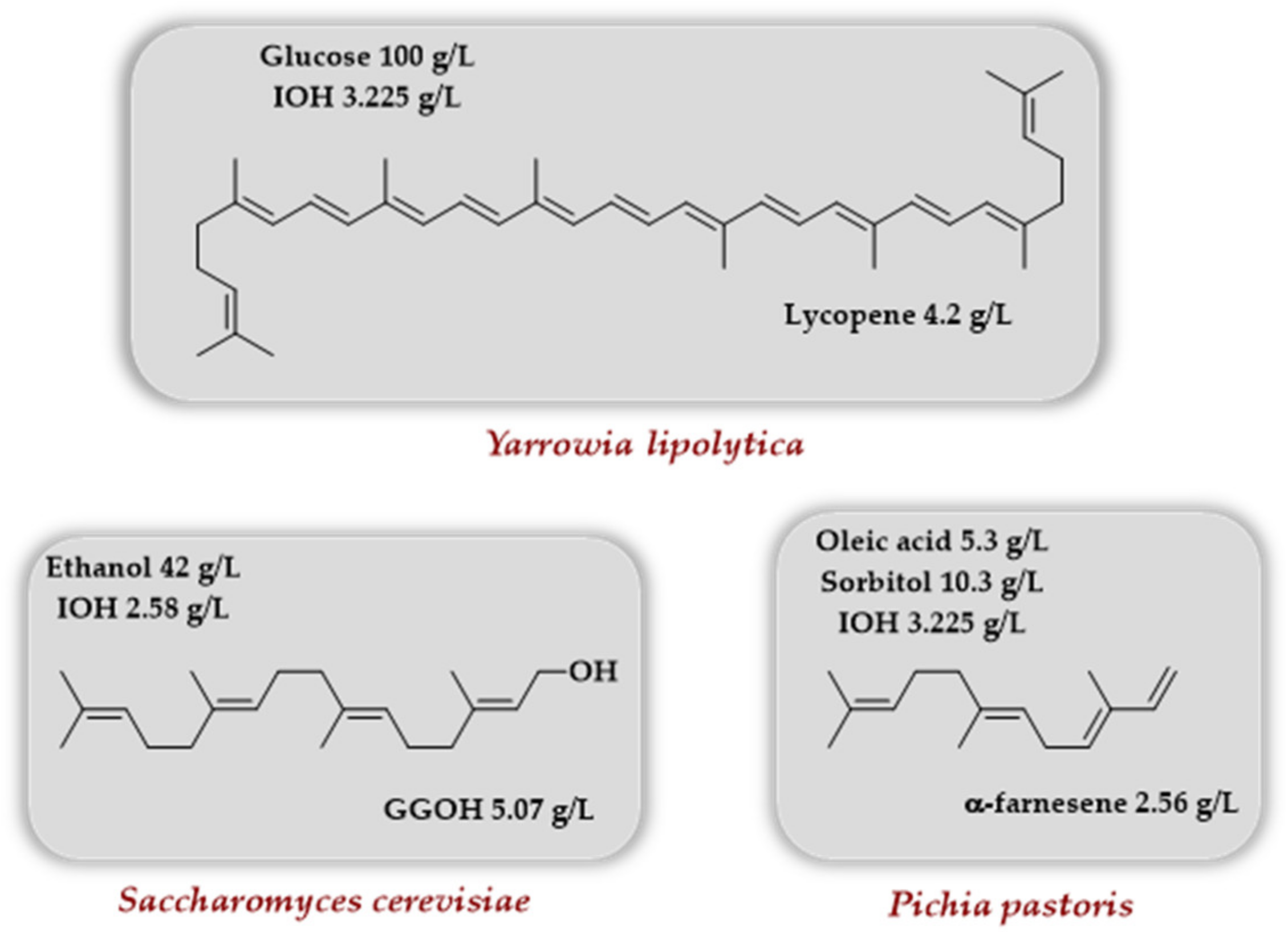
4.3.1. Escherichia coli as Host
4.3.2. Yeasts as Host
5. Discussion and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cane, D.E. Enzymatic Formation of Sesquiterpenes. Chem. Rev. 1990, 90, 1089–1103. [Google Scholar] [CrossRef]
- Christianson, D.W. Structural Biology and Chemistry of the Terpenoid Cyclases. Chem. Rev. 2006, 106, 3412–3442. [Google Scholar] [CrossRef]
- Dickschat, J.S. Isoprenoids in Three-Dimensional Space: The Stereochemistry of Terpene Biosynthesis. Nat. Prod. Rep. 2011, 28, 1917–1936. [Google Scholar] [CrossRef]
- Christianson, D.W. Structural and Chemical Biology of Terpenoid Cyclases. Chem. Rev. 2017, 117, 11570–11648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tantillo, D.J. Biosynthesis via Carbocations: Theoretical Studies on Terpene Formation. Nat. Prod. Rep. 2011, 28, 1035–1053. [Google Scholar] [CrossRef] [PubMed]
- Tantillo, D.J. Importance of Inherent Substrate Reactivity in Enzyme-Promoted Carbocation Cyclization/Rearrangements. Angew. Chem. Int. Ed. 2017, 56, 10040–10045. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Gou, J.; Liao, Q.; Li, Y.; Zhou, Q.; Bi, G.; Li, C.; Du, R.; Wang, X.; Sun, T.; et al. The Taxus Genome Provides Insights into Paclitaxel Biosynthesis. Nat. Plants 2021, 7, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Paddon, C.J.; Keasling, J.D. Semi-Synthetic Artemisinin: A Model for the Use of Synthetic Biology in Pharmaceutical Development. Nat. Rev. Microbiol. 2014, 12, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Sparks of Creation. Available online: https://www.rsc.org/images/Synth_tcm18-126654.pdf (accessed on 9 December 2021).
- Chatzivasileiou, A.O.; Ward, V.; McBride, E.S.; Stephanopoulos, G. Two-Step Pathway for Isoprenoid Synthesis. Proc. Natl. Acad. Sci. USA 2019, 116, 506–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lund, S.; Hall, R.; Williams, G.J. An Artificial Pathway for Isoprenoid Biosynthesis Decoupled from Native Hemiterpene Metabolism. ACS Synth. Biol. 2019, 8, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Couillaud, J.; Rico, J.; Rubini, A.; Hamrouni, T.; Courvoisier-Dezord, E.; Petit, J.-L.; Mariage, A.; Darii, E.; Duquesne, K.; de Berardinis, V.; et al. Simplified in Vitro and in Vivo Bioaccess to Prenylated Compounds. ACS Omega 2019, 4, 7838–7849. [Google Scholar] [CrossRef] [Green Version]
- Clomburg, J.M.; Qian, S.; Tan, Z.; Cheong, S.; Gonzalez, R. The Isoprenoid Alcohol Pathway, a Synthetic Route for Isoprenoid Biosynthesis. Proc. Natl. Acad. Sci. USA 2019, 116, 12810–12815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Yan, Z.; Lu, X.; Xiao, D.; Jiang, H. Improving the Catalytic Activity of Isopentenyl Phosphate Kinase through Protein Coevolution Analysis. Sci. Rep. 2016, 6, 24117. [Google Scholar] [CrossRef] [PubMed]
- Rico, J.; Duquesne, K.; Petit, J.-L.; Mariage, A.; Darii, E.; Peruch, F.; de Berardinis, V.; Iacazio, G. Exploring Natural Biodiversity to Expand Access to Microbial Terpene Synthesis. Microbial. Cell Fact. 2019, 18, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawada, I.; Uchida, K.; Aida, K. Effects of Isopentenyl Alcohol and Its Homologues on the Ubiquinone Production by Various Microorganisms. Agric. Biol. Chem. 1980, 44, 407–411. [Google Scholar]
- Myung-Ji, S.; Kook, M.-C.; Kim, S.-O. Association of Colony Morphology with Coenzyme Q10 Production and Its Enhancement from Rhizobium radiobacter T6102W by Addition of Isopentenyl Alcohol as a Precursor. J. Microbiol. Biotechnol. 2012, 22, 230–233. [Google Scholar]
- Cunningham, F.X., Jr.; Lafond, T.P.; Gantt, E. Evidence of a Role for LytB in the Nonmevalonate Pathway of Isoprenoid Biosynthesis. J. Bacteriol. 2000, 182, 5841–5848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohdich, F.; Hecht, S.; Gärtner, K.; Adam, P.; Krieger, C.; Amslinger, S.; Arigoni, D.; Bacher, A.; Eisenreich, W. Studies on the Nonmevalonate Terpene Biosynthetic Pathway: Metabolic Role of IspH (LytB) Protein. Proc. Natl. Acad. Sci. USA 2002, 99, 1158–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dellas, N.; Thomas, S.T.; Manning, G.; Noel, J.P. Discovery of a Metabolic Alternative to the Classical Mevalonate Pathway. eLife 2013, 2, e00672. [Google Scholar] [CrossRef]
- Lange, B.M.; Croteau, R. Isopentenyl Diphosphate Biosynthesis via a Mevalonate-Independent Pathway: Isopentenylmonophosphate Kinase Catalyzes the Terminal Enzymatic Step. Proc. Natl. Acad. Sci. USA 1999, 96, 13714–13719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VanNice, J.C.; Skaff, D.A.; Keightley, A.; Addo, J.K.; Wyckoff, G.J.; Miziorko, H.M. Identification in Haloferax volcanii of Phosphomevalonate Decarboxylase and Isopentenyl Phosphate Kinase as Catalysts of the Terminal Enzyme Reactions in an Archaeal Alternate Mevalonate Pathway. J. Bacteriol. 2014, 196, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Grochowski, L.L.; Xu, H.; White, R.H. Methanocaldococcus jannaschii Uses a Modified Mevalonate Pathway for Biosynthesis of Isopentenyl Diphosphate. J. Bacteriol. 2006, 188, 3192–3198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, F.; Pichersky, E. More is Better: The Diversity of Terpene Metabolism in Plants. Curr. Opin. Plant Biol. 2020, 55, 1–10. [Google Scholar] [CrossRef]
- Henry, L.K.; Gutensohn, M.; Thomas, S.T.; Noel, J.P.; Dudareva, N. Orthologs of the Archaeal Isopentenyl Phosphate Kinase Regulate Terpenoid Production in Plants. Proc. Natl. Acad. Sci. USA 2015, 112, 10050–10055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Poulter, C.D. Characterization of Thermophilic Archaeal Isopentenyl Phosphate Kinases. Biochemistry 2010, 49, 207–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Herk, T.; Hartog, A.F.; van der Burg, A.M.; Wever, R. Regioselective Phosphorylation of Carbohydrates and Various Alcohols by Bacterial Acid Phosphatases; Probing the Substrate Specificity of the Enzyme from Shigella flexneri. Adv. Synth. Catal. 2005, 347, 1155–1162. [Google Scholar] [CrossRef]
- Wang, P.-H.; Khusnutdinova, A.N.; Luo, F.; Xiao, J.; Nemr, K.; Flick, R.; Brown, G.; Mahadevan, R.; Edwards, E.A.; Yakunin, A.F. Biosynthesis and Activity of Prenylated FMN Cofactors. Cell Chem. Biol. 2018, 25, 560–570. [Google Scholar] [CrossRef] [Green Version]
- Valliere, M.A.; Korman, T.P.; Arbing, M.A.; Bowie, J.U. A Bio-Inspired Cell-Free System for Cannabinoid Production from Inexpensive Inputs. Nat. Chem. Biol. 2020, 16, 1427–1433. [Google Scholar] [CrossRef]
- Johnson, L.A.; Dunbabin, A.; Benton, J.C.R.; Mart, R.J.; Allemann, R.K. Modular Chemoenzymatic Synthesis of Terpenes and their Analogues. Angew. Chem. Int. Ed. 2020, 59, 8486–8490. [Google Scholar] [CrossRef] [PubMed]
- Ward, V.C.A.; Chatzivasileiou, A.O.; Stephanopoulos, G. Cell Free Biosynthesis of Isoprenoids from Isopentenol. Biotechnol. Bioeng. 2019, 116, 3269–3281. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, C.A.; Leferink, N.G.H.; Kosov, I.; Scrutton, N.S. Isopentenol Utilization Pathway for the Production of Linalool in Escherichia coli Using an Improved Bacterial Linalool/Nerolidol Synthase. Chem. Biol. Chem. 2021, 22, 2325–2334. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Liu, N.; Lazar, Z.; Chatzivasileiou, A.; Ward, V.; Chen, J.; Zhou, J.; Stephanopoulos, G. Enhancing Isoprenoid Synthesis in Yarrowia lipolytica by Expressing the Isopentenol Utilization Pathway and Modulating Intracellular Hydrophobicity. Metab. Eng. 2020, 61, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, L.; Li, Y.; Xu, S.; Jiang, W.; Liang, C.; Fang, Y.; Chu, A.; Zhang, L.; Ding, Z.; et al. Enhancing Geranylgeraniol Production by Metabolic Engineering and Utilization of Isoprenol as a Substrate in Saccharomyces cerevisiae. J. Agric. Food Chem. 2021, 69, 4480–4489. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, S.-L.; Xu, J.-Z.; Zhang, W.-G. Dual Regulation of Cytoplasm and Peroxisomes for Improved α-Farnesene Production in Recombinant Pichia pastoris. ACS Synth. Biol. 2021, 10, 1563–1573. [Google Scholar] [CrossRef]
- Global Bioenergies. Available online: https://www.global-bioenergies.com (accessed on 9 December 2021).



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couillaud, J.; Leydet, L.; Duquesne, K.; Iacazio, G. The Terpene Mini-Path, a New Promising Alternative for Terpenoids Bio-Production. Genes 2021, 12, 1974. https://doi.org/10.3390/genes12121974
Couillaud J, Leydet L, Duquesne K, Iacazio G. The Terpene Mini-Path, a New Promising Alternative for Terpenoids Bio-Production. Genes. 2021; 12(12):1974. https://doi.org/10.3390/genes12121974
Chicago/Turabian StyleCouillaud, Julie, Létitia Leydet, Katia Duquesne, and Gilles Iacazio. 2021. "The Terpene Mini-Path, a New Promising Alternative for Terpenoids Bio-Production" Genes 12, no. 12: 1974. https://doi.org/10.3390/genes12121974





