Transcription Factor 21 Promotes Chicken Adipocyte Differentiation at Least in Part via Activating MAPK/JNK Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Experimental Design
2.2. Oil Red O Staining and Extraction
2.3. Western Blotting
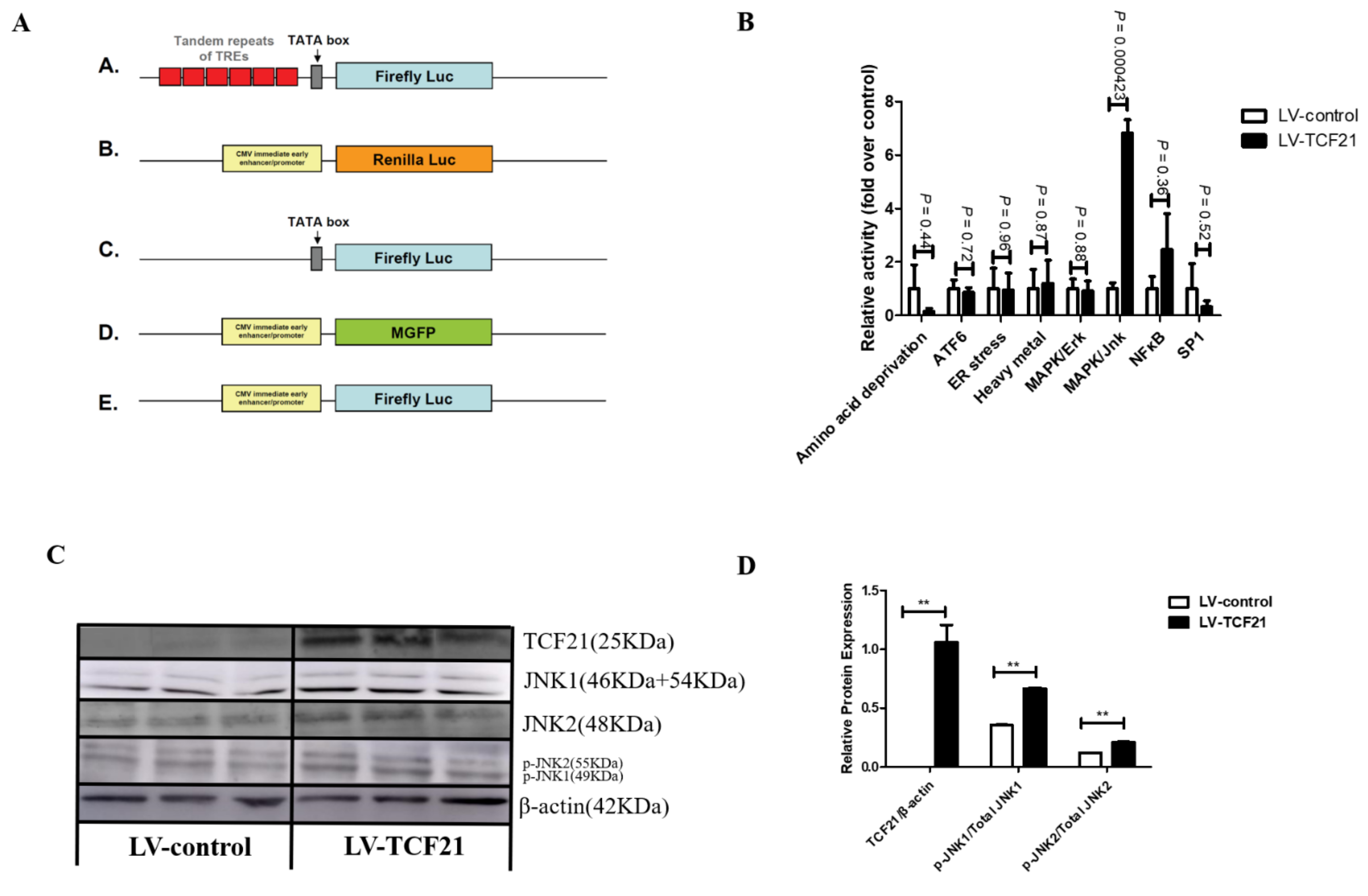
2.4. Luciferase Reporter Assay
2.5. RT-qPCR
2.6. Statistical Analysis
3. Results
3.1. Overexpression of TCF21 Leads to Enhanced Lipid Droplets Accumulation
3.2. MAPK/JNK Signaling Pathway Was Activated by TCF21 Overexpression
3.3. MAPK/JNK Signaling and Lipid Droplets Accumulation Are Inhibited by SP600125 in a Dose-Dependent Manner
3.4. Inhibition of MAPK/JNK Signaling Attenuates TCF21-Mediated Promotion of Preadipocyte Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TCF21 | transcription factor 21 |
| ICP | immortalized chicken preadipocytes cell line |
| LV-TCF21 | preadipocyte cell line stably overexpressing TCF21 |
| LV-control | control preadipocyte cell line |
| JNK | c-Jun N-terminal kinases |
| PPARγ | peroxisome proliferator-activated receptor γ |
| C/EBPα | CCAAT/enhancer binding protein α |
| A-FABP | adipocyte fatty acid binding protein |
| LPL | lipoprotein lipase |
| TBP | TATA-box binding protein |
| ERK | extracellular signal-regulated kinase |
| NLK | nemo-like kinase |
References
- Mallard, J.; Douaire, M. Strategies of selection for leanness in meat production. In Leanness in Domestic Birds: Genetic, Metabolic and Hormonal Aspects; Leclerq, B., Whitehead, C.C., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; pp. 3–23. [Google Scholar]
- Julibert, A.; Bibiloni, M.D.M.; Mateos, D.; Angullo, E.; Tur, J.A. Dietary fat intake and metabolic syndrome in older adults. Nutrients 2019, 11, 1901. [Google Scholar] [CrossRef] [Green Version]
- Vincent, M.J.; Allen, B.; Palacios, O.M.; Haber, L.T.; Maki, K.C. Meta-regression analysis of the effects of dietary cholesterol intake on LDL and HDL cholesterol. Am. J. Clin. Nutr. 2019, 109, 7–16. [Google Scholar] [CrossRef]
- Wu, G.Q.; Deng, X.M.; Li, J.Y.; Li, N.; Yang, N. A potential molecular marker for selection against abdominal fatness in chickens. Poult. Sci. 2006, 85, 1896–1899. [Google Scholar] [CrossRef] [PubMed]
- Sahraei, M. Feed restriction in broiler chickens production: A review. Glob. Vet. 2012, 8, 449–458. [Google Scholar] [CrossRef] [Green Version]
- Julian, R.J. Production and growth related disorders and other metabolic diseases of poultry—A review. Vet. J. 2015, 169, 350–369. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Huang, Y.F.; Ko, Y.J.; Liu, Y.J.; Chen, Y.H.; Walzem, R.L.; Chen, S.E. Obesity-associated cardiac pathogenesis in broiler breeder hens: Development of metabolic cardiomyopathy. Poult. Sci. 2017, 96, 2438–2446. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Lin, H.Y.; Chen, Y.W.; Ko, Y.J.; Liu, Y.J.; Chen, Y.H.; Walzem, R.L.; Chen, S.E. Obesity-associated cardiac pathogenesis in broiler breeder hens: Pathological adaption of cardiac hypertrophy. Poult. Sci. 2017, 96, 2428–2437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Wu, M.Q.; Wang, S.Z.; Zhang, H.; Du, Z.Q.; Li, Y.M.; Cao, Z.P.; Luan, P.; Leng, L.; Li, H. Genetic selection on abdominal fat content alters the reproductive performance of broilers. Animal 2018, 12, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Q.; Lane, M.D. Adipogenesis: From stem cell to adipocyte. Annu. Rev. Biochem. 2012, 81, 715–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwalie, P.C.; Dong, H.; Zachara, M.; Russeil, J.; Alpern, D.; Akchiche, N.; Caprara, C.; Sun, W.; Schlaudraff, K.; Soldati, G.; et al. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 2018, 559, 103–108. [Google Scholar] [CrossRef]
- Rosen, E.D.; Macdougald, O.A. Adipocyte differentiation from inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef]
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and adipogenesis. Eur. J. Cell Biol. 2013, 92, 229–236. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, B.; Liu, C.; Du, Z.; Zhang, H.; Wang, N.; Wu, M.; Li, Y.; Cao, Z.; Li, H. A novel regulator of preadipocyte differentiation, transcription factor TCF21, functions partially through promoting LPL expression. Front. Physiol. 2019, 10, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Zhang, T.; Wu, C.; Wang, S.; Wang, Y.; Li, H.; Wang, N. Immortalization of chicken preadipocytes by retroviral tranSEuction of chicken TERT and TR. PLoS ONE 2017, 12, e0177348. [Google Scholar]
- Han, J.; Li, L.; Wang, D.; Ma, H. (–)-Hydroxycitric acid reduced fat deposition via regulating lipid metabolism-related gene expression in broiler chickens. Lipids Health Dis. 2016, 15, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmoms, A.; Wennmalm, K.; Larsson, O.; Walden, T.B.; Lassmann, T.; Petrovic, N.; Hamilton, D.L.; Gimeno, R.E.; Wahlestedt, C.; Baar, K.; et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc. Natl. Acad. Sci. USA 2017, 104, 4401–4406. [Google Scholar] [CrossRef] [Green Version]
- Walden, T.B.; Hansen, I.R.; Timmons, J.A.; Cannon, B.; Nedergaard, J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am. J. Physiol. Endocrinol. Metab. 2012, 302, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, Z.; Ding, Y.; Xu, P.; Wang, T.; Xu, W.; Lu, H.; Li, J.; Wang, Y.; Li, S.; et al. Adipose tissues characteristics of normal, obesity, and type 2 diabetes in Uygurs population. J. Diabetes Res. 2015, 2015, 905042. [Google Scholar] [CrossRef] [Green Version]
- Stadion, M.; Schwerbel, K.; Graja, A.; Baumeier, C.; Rodiger, M.; Jonas, W.; Wolfrum, C.; Staiger, H.; Fritsche, A.; Haring, H.; et al. Increased Ifi202b/IFI16 expression stimulates adipogenesis in mice and human. Diabetologia 2018, 61, 1167–1179. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Duan, H.; Duan, C.; Zhu, H.; Zhou, R.; Pei, H.; Shen, L. TCF21 functions as a tumor suppressor in colorectal cancer through inactivation of PI3K/AKT signaling. OncoTargets Ther. 2017, 10, 1603–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, H.X.; Li, B.W.; Zhuang, X.; Wang, L.T.; Cao, Q.; Tan, L.H.; Qu, G.F.; Xiao, S. TCF21 inhibits tumor-associated angiogenesis and suppresses the growth of cholangiocarcinoma by targeting PI3K/Akt and ERK signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Li, S.; Xu, Z.; Yang, Y.; Chen, M.; Jiang, X.; Wu, H. Sumoylation of TCF21 downregulates the transcriptional activity of estrogen receptor-alpha. Oncotarget 2016, 7, 26220–26234. [Google Scholar] [CrossRef] [PubMed]
- Ide, S.; Finer, G.; Maezawa, Y.; Onay, T.; Souma, T.; Scott, R.; Ide, K.; Akimoto, Y.; Li, C.; Ye, M.; et al. Transcription factor 21 is required for branching morphogenesis and regulates Gdnf-axis in kidney development. J. Am. Soc. Nephrol. 2018, 29, 2795–2808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, J.; Lopez, J.M. Understanding MAPK signaling pathways in apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bost, F.; Aouadi, M.; Caron, L.; Binetruy, B. The role of MAPKs in adipocyte differentiation and obestity. Biochimie 2005, 87, 51–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, Y.; Sato, F.; Fukunaga, H.; Iwasaki, Y.; Chiba, Y.; Tebakari, M.; Daigo, Y.; Kawashima, J.; Kamei, J. Placental extract supresses differentiation of 3T3-L1 preadipocytes to mature adipocytes via accelerated activation of p38 MAPK during the early phase of adipogenesis. Nutr. Metab. 2019, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.S.; Nam, H.; Stephens, J.M.; Morrison, R.F. Mitogen-dependent regulation of DUSP1 governs ERK and p38 signaling during early 3T3-L1 adipocyte differentiation. J. Cell. Physiol. 2016, 231, 1562–1574. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Febbraio, M.A.; Lancaster, G.I. The roles of c-Jun NH2-terminal kinases (JNKs) in obesity and insulin resistance. J. Physiol. 2016, 594, 267–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Jung, E.; Lee, J.; Huh, S.; Kim, Y.S.; Kim, Y.W.; Kim, Y.S.; Park, D. Anti-adipogenesis by 6-thioinosine is mediated by downregulation of PPAR gamma through JNK-dependent upregulation of iNOS. Cell. Mol. Life Sci. 2010, 67, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, R.; Gerin, I.; Cawthorn, W.P.; Macdougald, O.A.; Chen, X.W.; Saltiel, A.R.; Koenig, R.J.; Xu, B. SRA regulates adipogenesis by modulating p38/JNK phosphorylation and stimulating insulin receptor gene expression and downstream signaling. PLoS ONE 2014, 9, e95416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusuyama, J.; Ohnishi, T.; Bandow, K.; Amir, M.S.; Shima, K.; Semba, I.; Matsuguchi, T. Constitutive activation of p46JNK2 is indispensable for C/EBPδ induction in the initial stage of adipogenic differentiation. Biochem. J. 2017, 474, 3421–3437. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Dong, Y.G.; Li, H.L. PPARgamma phosphorylation mediated by JNK MAPK: A potential role in macrophage-derived foam cell formation. Acta Pharmocol. Sin. 2006, 27, 1146–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Sá, P.M.; Richard, A.J.; Hang, H.; Stephens, J.M. Transcriptional regulation of adipogenesis. Compr. Physiol. 2017, 7, 635–674. [Google Scholar]
- Bain, J.; Mclauchlan, H.; Elliott, M.; Cohen, P. The specificities of protein kinase inhibitors: An update. Biochem. J. 2003, 371, 199–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Gene | Accession Number | Primer Sequence (5’ to 3’) | Product Length (bp) |
|---|---|---|---|
| PPARγ | NM_001001460 | F:GTGCAATCAAAATGGAGCC | 170 |
| R:CTTACAACCTTCACATGCAT | |||
| C/EBPa | NM_001031459 | F:GCGACATCTGCGAGAACG | 266 |
| R:GTACAGCGGGTCGAGCTT | |||
| A-FABP | NM_204290 | F:ATGTGCGACCAGTTTGT R:TCACCATTGATGCTGATAG | 143 |
| LPL | NM_205282 | F:ATGTTCATTGATTGGATGGAGGAG | 159 |
| R:AAAGGTGGGACCAGCAGGAT | |||
| TBP | NM_205103 | F:GCGTTTTGCTGCTGTTATTATGAG | 122 |
| R:TCCTTGCTGCCAGTCTGGAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Cheng, B.; Jiang, H.; Liu, C.; Cao, Z.; Luan, P.; Wang, N.; Li, H. Transcription Factor 21 Promotes Chicken Adipocyte Differentiation at Least in Part via Activating MAPK/JNK Signaling. Genes 2021, 12, 1971. https://doi.org/10.3390/genes12121971
Zhang X, Cheng B, Jiang H, Liu C, Cao Z, Luan P, Wang N, Li H. Transcription Factor 21 Promotes Chicken Adipocyte Differentiation at Least in Part via Activating MAPK/JNK Signaling. Genes. 2021; 12(12):1971. https://doi.org/10.3390/genes12121971
Chicago/Turabian StyleZhang, Xinyang, Bohan Cheng, Haixu Jiang, Chang Liu, Zhiping Cao, Peng Luan, Ning Wang, and Hui Li. 2021. "Transcription Factor 21 Promotes Chicken Adipocyte Differentiation at Least in Part via Activating MAPK/JNK Signaling" Genes 12, no. 12: 1971. https://doi.org/10.3390/genes12121971
APA StyleZhang, X., Cheng, B., Jiang, H., Liu, C., Cao, Z., Luan, P., Wang, N., & Li, H. (2021). Transcription Factor 21 Promotes Chicken Adipocyte Differentiation at Least in Part via Activating MAPK/JNK Signaling. Genes, 12(12), 1971. https://doi.org/10.3390/genes12121971







