Hydrophilic Shell Matrix Proteins of Nautilus pompilius and the Identification of a Core Set of Conchiferan Domains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collections, Total RNA Extraction from Mantle Tissues and Total Shell Protein Extraction
2.2. Multiomics Analyses of the Shell Matrix Proteins of Nautilus pompilius
2.3. Characterizations of the Shell Matrix Proteins of Nautilus pompilius
2.4. Comparative Analysis of Conchiferan Shell Matrix Proteins
2.5. Phylogenetic Analyses of the Shell Matrix Proteins
3. Results
3.1. Transcriptomics and Proteomics of the Shell Matrix Proteins in Nautilus pompilius
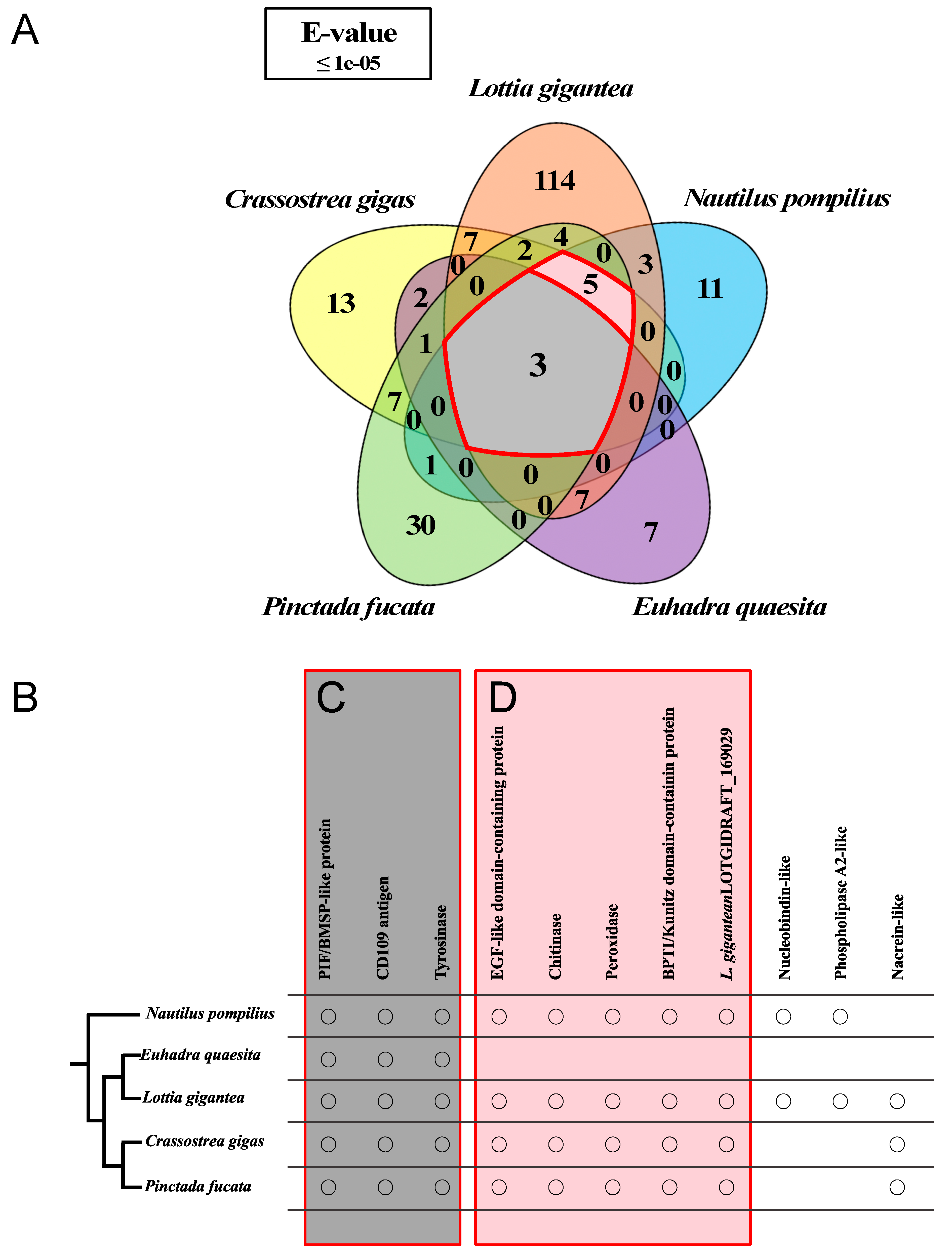
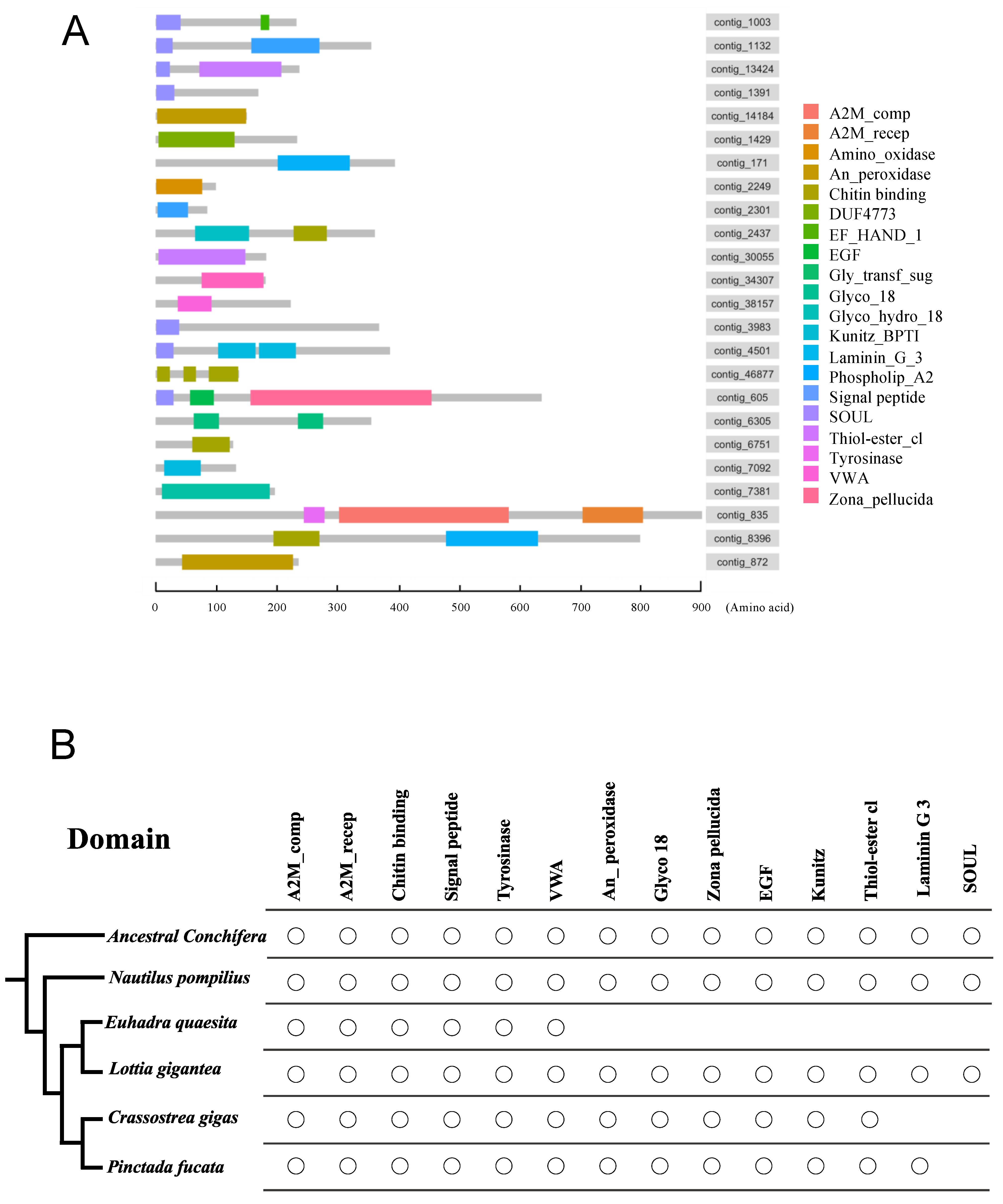
3.2. Homology Comparisons of the Shell Matrix Proteins and Their Domains among Several Conchiferan Mollusks
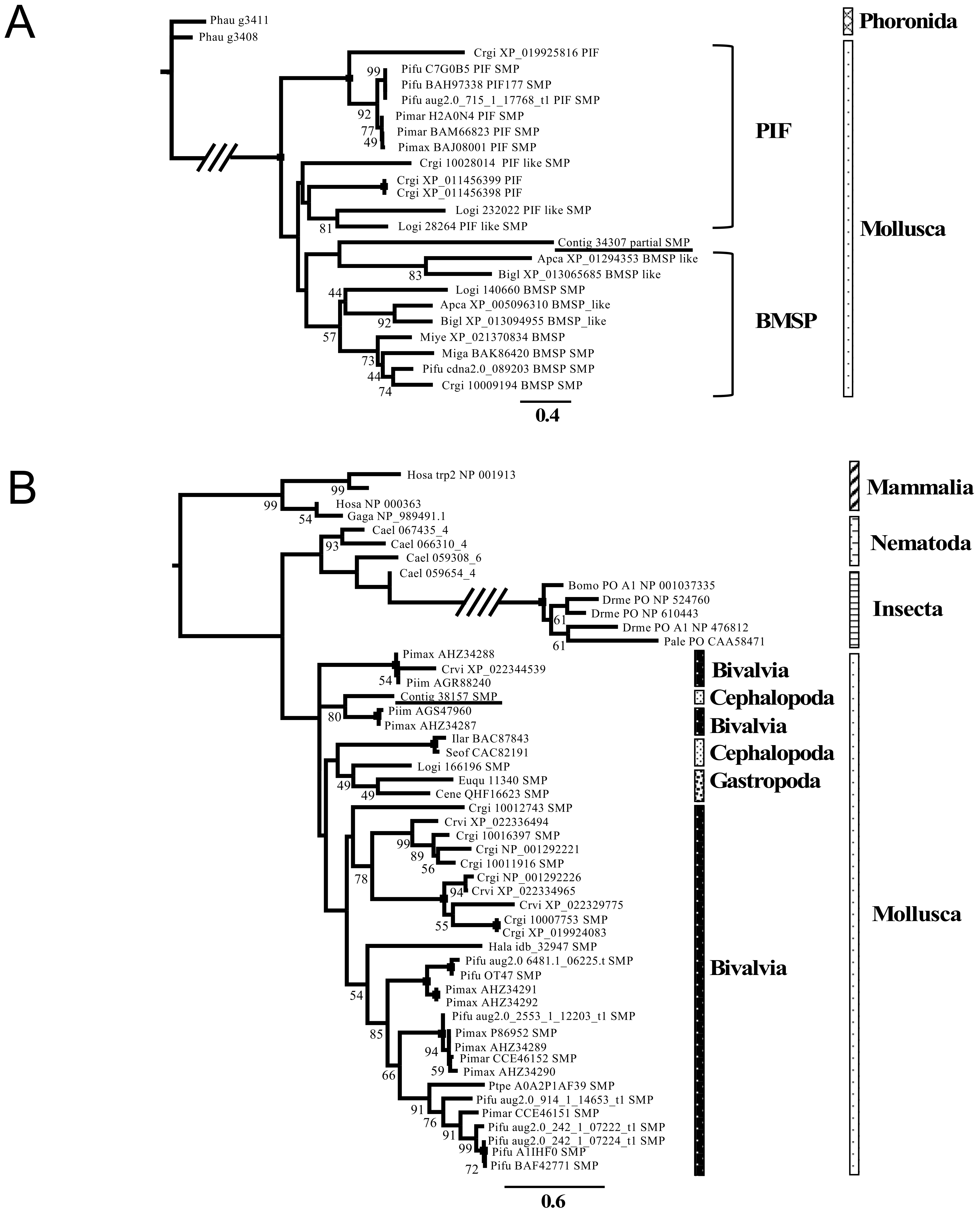
3.3. Phylogenetic Analysis of the Shell Matrix Proteins in Conchifera
4. Discussion
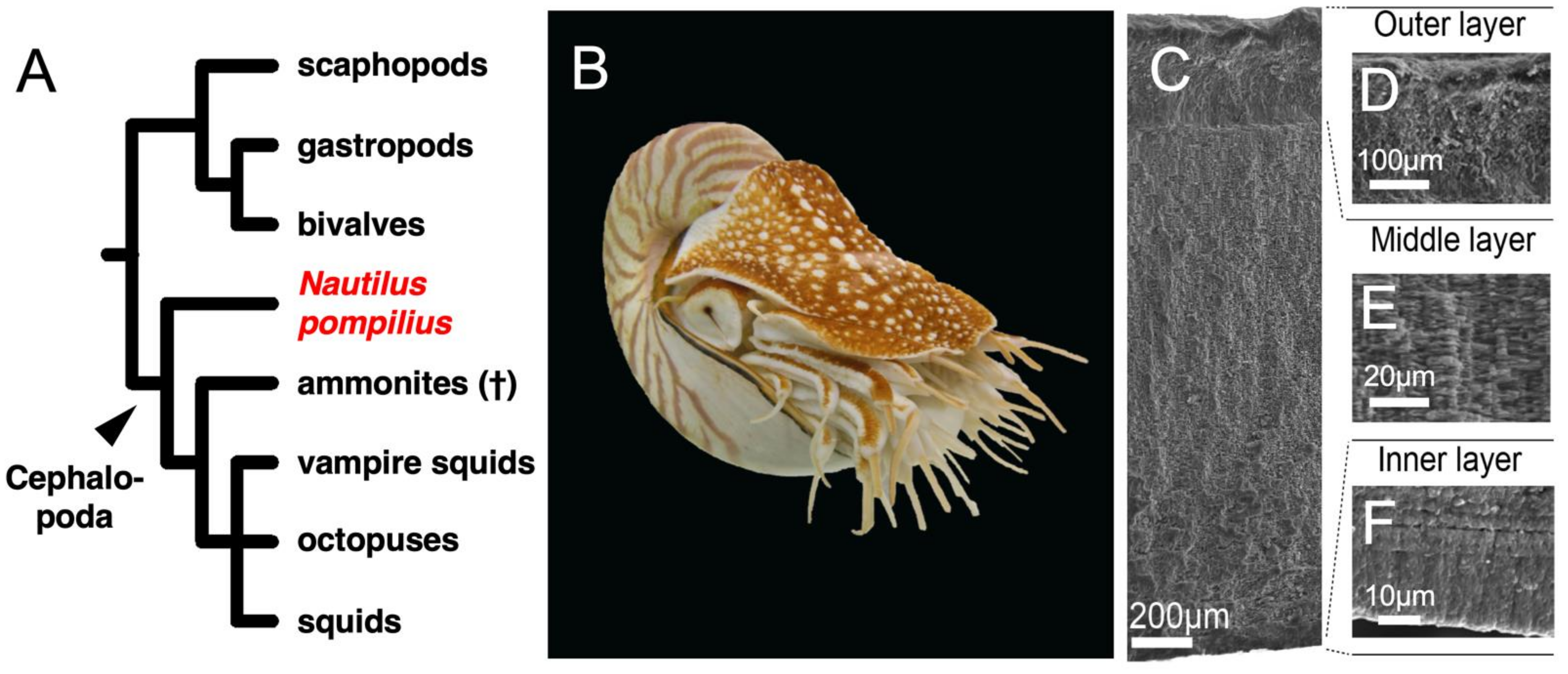
4.1. The Shell of Nautilus pompilius Is a Typical Conchiferan Shell
4.2. Homology Comparisons and the Evolution of the Shell Matrix Proteins and Their Domains among Several Conchiferan Mollusks
4.3. Transcriptomics of the Mantle Tissue in Nautilus pompilius Using ION Torrent PGM Is Arguably Enough to Reveal the Presence of Several Core Shell Matrix Proteins
4.4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cowen, R. History of Life, 4th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- Lowenstam, H.A.W.S. On Biomineralization; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Simkiss, K.; Wilbur, K.M. Biomineralization; Elsevier: New York, NY, USA, 2012. [Google Scholar]
- Shimizu, K.; Kimura, K.; Isowa, Y.; Oshima, K.; Ishikawa, M.; Kagi, H.; Kito, K.; Hattori, M.; Chiba, S.; Endo, L. Insights into the evolution of shells and love darts of land snails revealed from their matrix proteins. Genome Biol. Evol. 2019, 11, 380–397. [Google Scholar] [CrossRef]
- Kocot, K.M.; Cannon, J.T.; Todt, C.; Citarella, M.R.; Kohn, A.B.; Meyer, A.; Santos, S.R.; Schander, C.; Moroz, L.L.; Lieb, B.; et al. Phylogenomics reveals deep molluscan relationships. Nature 2011, 477, 452–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.A.; Wilson, N.G.; Goetz, F.E.; Feehery, C.; Andrade, S.C.S.; Rouse, G.W.; Giribet, G.; Dunn, C.W. Resolving the evolutionary relationships of molluscs with phylogenomic tools. Nature 2011, 480, 364–367. [Google Scholar] [CrossRef]
- Kocot, K.M. Recent advances and unanswered questions in deep molluscan phylogenetics. Am. Malacol. Bull. 2013, 31, 195–208. [Google Scholar] [CrossRef]
- Kocot, K.M.; Poustka, A.J.; Stöger, I.; Halanych, K.M.; Schrödl, M. New data from Monoplacophora and a carefully-curated dataset resolve molluscan relationships. Sci. Rep. 2020, 10, 101. [Google Scholar] [CrossRef]
- Jackson, D.J.; McDougall, C.; Woodcroft, B.; Moase, P.; Rose, R.; Kube, M.; Reinhardt, R.; Rokhsar, D.S.; Montagnani, C.; Joubert, C.; et al. Parallel evolution of nacre building gene sets in molluscs. Mol. Biol. Evol. 2010, 27, 591–608. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Yu, C.; Gu, Z.; Zhan, X.; Wang, Y.; Wang, A. Characterization of the pearl oyster (Pinctada martensii) mantle transcriptome unravels biomineralization genes. Mar. Biotechnol. 2013, 15, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Marin, F.; Le Roy, N.; Marie, B. The formation and mineralization of mollusk shell. Front. Biosci. 2012, 4, 1099–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, K.; Edsinger-Gonzales, E.; Mann, M. In-depth proteomic analysis of a mollusc shell: Acid-soluble and acid-insoluble matrix of the limpet Lottia gigantea. Proteome Sci. 2012, 10, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marie, B.; Joubert, C.; Tayalé, A.; Zanella-Cléon, I.; Belliard, C.; Piquemal, D.; Cochennec-Laureau, N.; Marin, F.; Gueguen, Y.; Montagnani, C. Different secretory repertoires control the processes of prism and nacre deposition of the pearl oyster shell. Proc. Natl. Acad. Sci. USA 2012, 109, 20986–20991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, H.; Endo, H.; Hashimoto, N.; Limura, K.; Isowa, Y.; Kinoshita, S.; Kotaki, T.; Masaoka, T.; Miki, T.; Nakayama, S.; et al. The diversity of Shell Matrix Proteins: Genome-wide investigation of the pearl oyster. Pinctada fucata. Zool. Sci. 2013, 30, 801–816. [Google Scholar] [CrossRef]
- Zhang, G.; Fang, X.; Guo, X.; Li, L.; Luo, R.; Xu, F.; Yang, P.; Zhang, L.; Wang, X.; Qi, H.; et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature 2012, 490, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, R.; Takeuchi, T.; Luo, Y.J.; Ishikawa, A.; Kobayashi, T.; Koyanagi, R.; Villar-Briones, A.; Yamada, L.; Sawada, H.; Iwanaga, S.; et al. Dual gene repertoires for larval and adult shells reveal molecules essential for molluscan shell formation. Mol. Biol. Evol. 2018, 35, 2751–2761. [Google Scholar] [CrossRef] [PubMed]
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, F. Mollusk shell formation: A source of new concepts for understanding biomineralization processes. Chem. A Eur. J. 2006, 12, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Marin, F.; Marie, B.; Hamada, S.; Romos-Silva, P. ‘Shellome’: Proteins involved in mollusk shell biomineralization-diversity, functions. In Recent Advances in Pearl Research; Watabe, S., Maeyama, K., Nagasawa, H., Eds.; Terrapub: Tokyo, Japan, 2013; pp. 149–166. [Google Scholar]
- Kröger, B.; Vinther, J.; Fuchs, D. Cephalopod origin and evolution: A congruent picture emerging from fossils, development and molecules. BioEssays 2011, 33, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.R.; Phakey, P.P. Notes on the microstructure of the Nautilus shell. Scanning Microsc. 1995, 9, 16. [Google Scholar]
- Grégoire, C. Ultrastructure of the Nautilus shell. In Nautilus: The Biology and Paleobiology of a Living Fossil; Saunders, W.B., Landman, N.H., Eds.; Plenum Press: New York, NY, USA, 1987; pp. 463–486. [Google Scholar]
- Naef, A. Cephalopoda. In Fauna und Flora des Golfes von Naples; Monografia; Progress for Scientific Translations: Jerusalem, Israel, 1923; p. 863. [Google Scholar]
- Wolfe, K.; Smith, A.M.; Trimby, P.; Byrne, M. Vulnerability of the Paper Nautilus (Argonauta nodosa) Shell to a Climate-Change Ocean: Potential for Extinction by Dissolution. Biol. Bull. 2012, 223, 236–244. [Google Scholar] [CrossRef]
- Hirota, K.; Yoshida, M.-A.; Itoh, T.; Toyoda, A.; Setiamarga, D.H.E. The full mitochondrial genome sequence of the greater argonaut Argonauta argo (Cephalopoda, Argonautoidea) and its phylogenetic position in Octopodiformes. Mitochondrial DNA B Resour. 2021, 6, 1451–1453. [Google Scholar] [CrossRef]
- Butler-Struben, H.M.; Brophy, S.M.; Johnson, N.A.; Crook, R.J. In vivo recording of neural and behavioral correlates of anesthesia induction, reversal, and euthanasia in cephalopod molluscs. Front. Physiol. 2018, 9, 109. [Google Scholar] [CrossRef]
- Kinjo, S.; Monma, N.; Misu, S.; Kitamura, N.; Imoto, J.; Yoshitake, K.; Gojobori, T.; Ikeo, K. Maser: One-stop platform for NGS big data from analysis to visualization. Database 2018, 2018, bay027. [Google Scholar] [CrossRef] [Green Version]
- Elias, J.E.; Gygi, S.P. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods 2007, 4, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Isowa, Y.; Sarashina, I.; Oshima, K.; Kito, K.; Hattori, M.; Endo, K. Proteome analysis of shell matrix proteins in the brachiopod Laqueus rubellus. Proteome Sci. 2015, 13, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Feng, D.; Li, Q.; Yu, H.; Kong, L.; Du, S. Identification of conserved proteins from diverse shell matrix proteome in Crassostrea gigas: Characterization of genetic bases regulating shell formation. Sci. Rep. 2017, 7, 45754. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, S.; Shimizu, K.; Negishi, L.; Suzuki, N.; Nagata, K.; Suzuki, M. Characterization of the chalky layer-derived EGF-like domain-containing protein (CgELC) in the pacific oyster, Crassostrea gigas. J. Struct. Biol. 2020, 212, 107594. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef]
- Hulo, N.; Bairoch, A.; Bulliard, V.; Cerutti, L.; De Carstro, E.; Langendijk-Genevaux, P.S.; Pagni, M.; Sigrist, C.J.A. The PROSITE database. Nucleic Acids Res. 2006, 34, D227–D230. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Eddy, S.R. Profile Hidden Markov Models. Bioinformatics 1998, 14, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.N.; Brunak, S.; Von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.I.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Kuma, K.I.; Toh, H.; Miyata, T. MAFFT version 5: Improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005, 33, 511–518. [Google Scholar] [CrossRef]
- Castresana, J. Genes on human chromosome 19 show extreme divergence from the mouse orthologs and a high GC content. Nucleic Acids Res. 2002, 30, 1751–1756. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvestro, D.; Michalak, I. raxmlGUI: A graphical front-end for RAxML. Org. Divers. Evol. 2012, 12, 335–337. [Google Scholar] [CrossRef]
- Albertin, C.; Simakov, O.; Mitros, T.; Wang, Z.Y.; Pungor, J.R.; Edsinger-Gonzales, E.; Brenner, S.; Ragsdale, C.W.; Rokhsar, D.S. The octopus genome and the evolution of cephalopod neural and morphological novelties. Nature 2015, 524, 220–224. [Google Scholar] [CrossRef] [Green Version]
- Tanabe, K. Record of trapping experiment. Kagoshima Univ. Res. Center South Pac. Occas. Pap. 1988, 15, 5–15. [Google Scholar]
- Takeda, Y.; Tanabe, K. Low durophagous predation on Toarcian (Early Jurassic) ammonoids in the northwestern Panthalassa shelf basin. Acta Palaeontol. Pol. 2015, 60, 781–794. [Google Scholar] [CrossRef] [Green Version]
- Takeda, Y.; Tanabe, K.; Sasaki, T.; Landman, N.H. Durophagous predation on scaphitid ammonoids in the Late Cretaceous Western Interior Seaway of North America. Lethaia 2016, 49, 28–42. [Google Scholar] [CrossRef]
- Denton, E.J.; Gilpin-Brown, J.B. On the buoyancy of the pearly Nautilus. J. Mar. Biol. Assoc. United Kingd. 1966, 46, 723–759. [Google Scholar] [CrossRef]
- Carter, J.G. Skeletal biomineralization: Patterns, processes and evolutionary trends. Geol. Mag. 1990, 128, 411–413. [Google Scholar]
- Suzuki, M.; Saruwatari, K.; Kogure, T.; Yamamoto, Y.; Nishimura, T.; Kato, T.; Nagasawa, H. An acidic matrix protein, Pif, is a key macromolecule for nacre formation. Science 2009, 325, 1388–1390. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Iwashima, A.; Tsutsui, N.; Ohira, T.; Kogure, T.; Nagasawa, H. Identification and characterisation of a calcium carbonate-binding protein, blue mussel shell protein (BMSP), from the nacreous layer. Chembiochem 2011, 12, 2478–2487. [Google Scholar] [CrossRef] [PubMed]
- Blobel, G.; Dobberstein, B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J. Cell Biol. 1975, 67, 835–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, K.; Yano, M.; Morimoto, K.; Miyamoto, H. Tyrosinase localization in mollusc shells. Comp. Biochem. Physiol. Part. B Biochem. Mol. Biol. 2007, 146, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Cui, B.; Li, X.; Lin, Z.; Dong, Y. Characteristics of a novel tyrosinase gene involved in the formation of shell color in hard clam Meretrix meretrix. J. Ocean. Univ. China 2020, 19, 183–190. [Google Scholar] [CrossRef]
- Wang, Y.; Inger, M.; Jiang, H.; Tenenbaum, H.; Glogauer, M. CD109 plays a role in osteoclastogenesis. PLoS ONE 2013, 8, e61213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finnson, K.W.; Tam, B.Y.; Liu, K.; Marcoux, A.; Lepage, P.; Roy, S.; Bizet, A.A.; Philip, A. Identification of CD109 as part of the TGF-β receptor system in human keratinocytes. FASEB J. 2006, 20, 1525–1527. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, I.V.; Bizet, A.A.; Binamer, Y.; Jones, D.A.; Sasseville, D.; Philip, A. CD109 release from the cell surface in human keratinocytes regulates TGF-β receptor expression, TGF-β signalling and STAT3 activation: Relevance to psoriasis. Exp. Dermatol. 2011, 20, 627–632. [Google Scholar] [CrossRef]
- Kintsu, H.; Okumura, T.; Negishi, L.; Ifuku, S.; Kogure, T.; Sakuda, S.; Suzuki, M. Crystal defects induced by chitin and chitinolytic enzymes in the prismatic layer of Pinctada fucata. Biochem. Biophys. Res. Commun. 2017, 489, 89–95. [Google Scholar] [CrossRef]
- Liao, Z.; Jiang, Y.T.; Sun, Q.; Fan, M.H.; Wang, J.X.; Liang, H.Y. Microstructure and in-depth proteomic analysis of Perna viridis shell. PLoS ONE 2019, 14, e0219699. [Google Scholar] [CrossRef] [Green Version]
- Hohagen, J.; Jackson, D.J. An ancient process in a modern mollusc: Early development of the shell in Lymnaea stagnalis. BMC Dev. Biol. 2013, 13, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaudet, P.; Livstone, M.S.; Lewis, S.E.; Thomas, P.D. Phylogenetic-based propagation of functional annotations within the Gene Ontology consortium. Brief. Bioinform. 2011, 12, 449–462. [Google Scholar] [CrossRef] [Green Version]
- Dennis, E.A. Diversity of group types, regulation, and function of phospholipase A2. J. Biol. Chem. 1994, 269, 13057–13060. [Google Scholar] [CrossRef]
- Yochelson, E.L.; Flower, R.H.; Webers, G.F. The bearing of the new Late Cambrian monoplacophoran genus Knightoconus upon the origin of the Cephalopoda. Lethaia 1973, 6, 275–309. [Google Scholar] [CrossRef]
- Salvini-Plawen, L.; Steiner, G. Synapomorphies and plesiomorphies in higher classification of Mollusca. In Origin and Evolutionary Radiation of the Mollusca; Taylor, J., Ed.; Oxford University Press: New York, NY, USA, 1996. [Google Scholar]
- Arivalagan, J.; Marie, B.; Sleight, V.A.; Clark, M.S.; Berland, S.; Marie, A. Shell matrix proteins of the clam, Mya truncata: Roles beyond shell formation through proteomic study. Mar. Genom. 2016, 27, 69–74. [Google Scholar] [CrossRef]
- Arivalagan, J.; Yarra, T.; Marie, B.; Sleight, V.; Duvernois-Berthet, E.; Clark, M.S.; Marie, A.; Berland, S. Insights from the shell proteome: Biomineralization to adaptation. Mol. Biol. Evol. 2017, 34, 66–77. [Google Scholar] [CrossRef] [Green Version]
- Liao, Z.; Bao, L.F.; Fan, M.H.; Gao, P.; Wang, X.X.; Qin, C.L.; Li, X.M. In-depth proteomic analysis of nacre, prism, and myostracum of Mytilus shell. J. Proteom. 2015, 122, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, S.; Kong, J.; Liu, Y.; Wang, T.; Xie, L.; Zhang, R. In-depth proteomic analysis of Shell Matrix Proteins of Pinctada fucata. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, K.; Jackson, D.J. Characterization of the pigmented shell-forming proteome of the common grove snail Cepaea nemoralis. BMC Genom. 2014, 15, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marie, B.; Zanella-Cléon, I.; Corneillat, M.; Becchi, M.; Alcaraz, G.; Plasseraud, L.; Luquet, G.; Marin, F. Nautilin-63, a novel acidic glycoprotein from the shell nacre of Nautilus macromphalus. FEBS J. 2011, 278, 2117–2130. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.; Miyashita, T.; Okushima, M.; Nakano, S.; Morita, T.; Matsushiro, A. A carbonic anhydrase from the nacreous layer in oyster pearls. Proc. Natl. Acad. Sci. USA 1996, 93, 9657–9660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Contig ID | FPKM | BLASTn Searches Result | e-Value |
|---|---|---|---|
| contig_130 | 73.5 | None | |
| contig_145 | 140,864.70 | None | |
| contig_171 | 2072.40 | Sushi-like protein [Mytilus coruscus] | 3.00 × 10−21 |
| contig_175 | 2671.90 | None | |
| contig_218 | 29,476.20 | None | |
| contig_605 | 321.6 | EGF-like domain-containing protein 2 isoform X3 [Octopus bimaculoides] | 2.00 × 10−107 |
| contig_737 | 41,106.30 | None | |
| contig_749 | 175,497.30 | None | |
| contig_790 | 97350.2 | None | |
| contig_835 | 862.5 | CD109 Antigen-like isoform X1 [Crassostrea gigas] | 0 |
| contig_872 | 88.1 | Chorion Peroxidase-like [Octopus vulgaris] | 3.00 × 10−45 |
| contig_1003 | 2305.10 | protein PFC0760c-like [Octopus vulgaris] | 1.00 × 10−3 |
| contig_1132 | 1428.80 | Phospholipase A2-like [Centruroides sculpturatus] | 1.00 × 10−39 |
| contig_1391 | 239 | hypothetical protein KP79_PYT17609 [Mizuhopecten yessoensis] | 6.00 × 10−10 |
| contig_1429 | 1.9 | None | |
| contig_2249 | 6547 | Aplysianin-A-like [Crassostrea virginica] | 9.00 × 10−6 |
| contig_2301 | 77,909.50 | hypothetical protein LOTGIDRAFT_176428 [Lottia gigantea] | 3.00 × 10−8 |
| contig_2437 | 224.1 | Chitinase [Sepia esculenta] | 2.00 × 10−42 |
| contig_3214 | 1694.2 | hypothetical protein LOTGIDRAFT_236297 [Lottia gigantea] | 1.00 × 10−4 |
| contig_3983 | 1112.5 | None | |
| contig_4501 | 663.3 | Papilin-like [Lingula anatina] | 2.00 × 10−37 |
| contig_6305 | 420.8 | uncharacterized protein LOC112560033 isoform X3 [Pomacea canaliculata] | 2.00 × 10−24 |
| contig_6751 | 2281.00 | BMSP [Mytilus galloprovincialis] | 3.00 × 10−19 |
| contig_7092 | 93.4 | Collagen Alpha-3(VI) chain isoform X2 [Cricetulus griseus] | 6.00 × 10−8 |
| contig_7381 | 440 | hypothetical protein OCBIM_22014960mg [Octopus bimaculoides] | 3.00 × 10−51 |
| contig_8396 | 288.3 | Sushi-like protein [Mytilus coruscus] | 6.00 × 10−56 |
| contig_8398 | 6029.2 | None | |
| contig_11910 | 1079.4 | PREDICTED: nucleobindin-1-like, partial [Paralichthys olivaceus] | 2.00 × 10−7 |
| contig_13424 | 197.6 | Heme-binding protein 2-like [Limulus polyphemus] | 3.00 × 10−8 |
| contig_14184 | 431.7 | Peroxidase-like protein [Mizuhopecten yessoensis] | 9.00 × 10−42 |
| contig_14880 | 772.8 | None | |
| contig_16223 | 267.3 | None | |
| contig_17506 | 164.2 | Protein PIF [Mizuhopecten yessoensis] | 1.00 × 10−2 |
| contig_21095 | 770.1 | None | |
| contig_21964 | 195.5 | None | |
| contig_23085 | 71.3 | None | |
| contig_25822 | 83.8 | hypothetical protein KP79_PYT14004 [Mizuhopecten yessoensis] | 9.00 × 10−8 |
| contig_30055 | 123.8 | uncharacterized protein LOC106876168 [Octopus bimaculoides] | 3.00 × 10−18 |
| contig_30170 | 134.5 | Mucin-5AC-like isoform X2 [Pomacea canaliculata] | 4.00 × 10−15 |
| contig_30322 | 109.8 | None | |
| contig_33774 | 152.2 | None | |
| contig_34307 | 12.1 | Collagen-like protein-1, partial [Mytilus coruscus] | 3.00 × 10−13 |
| contig_35294 | 13.7 | None | |
| contig_38157 | 3.4 | Tyrosinase-like protein [Octopus vulgaris] | 3.00 × 10−77 |
| contig_38801 | 167 | None | |
| contig_46079 | 0 | None | |
| contig_46877 | 59.3 | hypothetical protein LOTGIDRAFT_169029 [Lottia gigantea] | 3.00 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setiamarga, D.H.E.; Hirota, K.; Yoshida, M.-a.; Takeda, Y.; Kito, K.; Ishikawa, M.; Shimizu, K.; Isowa, Y.; Ikeo, K.; Sasaki, T.; et al. Hydrophilic Shell Matrix Proteins of Nautilus pompilius and the Identification of a Core Set of Conchiferan Domains. Genes 2021, 12, 1925. https://doi.org/10.3390/genes12121925
Setiamarga DHE, Hirota K, Yoshida M-a, Takeda Y, Kito K, Ishikawa M, Shimizu K, Isowa Y, Ikeo K, Sasaki T, et al. Hydrophilic Shell Matrix Proteins of Nautilus pompilius and the Identification of a Core Set of Conchiferan Domains. Genes. 2021; 12(12):1925. https://doi.org/10.3390/genes12121925
Chicago/Turabian StyleSetiamarga, Davin H. E., Kazuki Hirota, Masa-aki Yoshida, Yusuke Takeda, Keiji Kito, Makiko Ishikawa, Keisuke Shimizu, Yukinobu Isowa, Kazuho Ikeo, Takenori Sasaki, and et al. 2021. "Hydrophilic Shell Matrix Proteins of Nautilus pompilius and the Identification of a Core Set of Conchiferan Domains" Genes 12, no. 12: 1925. https://doi.org/10.3390/genes12121925
APA StyleSetiamarga, D. H. E., Hirota, K., Yoshida, M.-a., Takeda, Y., Kito, K., Ishikawa, M., Shimizu, K., Isowa, Y., Ikeo, K., Sasaki, T., & Endo, K. (2021). Hydrophilic Shell Matrix Proteins of Nautilus pompilius and the Identification of a Core Set of Conchiferan Domains. Genes, 12(12), 1925. https://doi.org/10.3390/genes12121925






