Serpin Family E Member 1 Tag Single-Nucleotide Polymorphisms in Patients with Diabetic Nephropathy: An Association Study and Meta-Analysis Using a Genetic Model-Free Approach
Abstract
:1. Background
2. Materials and Methods
2.1. Association Study
2.1.1. Study Population
2.1.2. Genotyping
2.1.3. Data Analysis
2.2. Meta-Analysis
2.2.1. Identification and Eligibility of Relevant Studies
2.2.2. Data Extraction
2.2.3. Data Synthesis and Analysis
3. Results
3.1. Association Study
3.2. Meta-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DN | diabetic nephropathy; |
| GWASs | Genome-Wide Association Studies; |
| GWLS | Genome-Wide Linkage Studies; |
| HWE | Hardy–Weinberg equilibrium; |
| MAF | Minor Allele Frequency; |
| ORG | Generalized Odds Ratio; |
| PAI-1 | Plasminogen activator inhibitor-1; |
| serpin | serine proteinase inhibitor; |
| SERPINE1 | serpin family E member 1; |
| SNPs | Single-Nucleotide Polymorphisms; |
| T1DM | type 1 diabetes mellitus; |
| T2DM | type 2 diabetes mellitus; |
| tPA | tissue plasminogen activator; |
| uPA | urokinase. |
References
- Cowie, C.C.; Port, F.K.; Wolfe, R.A.; Savage, P.J.; Moll, P.P.; Hawthorne, V.M. Disparities in incidence of diabetic end-stage renal disease according to race and type of diabetes. N. Engl. J. Med. 1989, 321, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Dronavalli, S.; Duka, I.; Bakris, G. The pathogenesis of diabetic nephropathy. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 444–452. Available online: http://www.ncbi.nlm.nih.gov/pubmed/18607402 (accessed on 23 November 2021). [CrossRef] [PubMed]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.M.; Zoungas, S.; Rossing, P.; Groop, P.-H.; Cooper, M.E. Diabetic kidney disease. Nat. Rev. Dis. Primers 2015, 1, 15070. Available online: http://www.nature.com/articles/nrdp201570 (accessed on 23 November 2021). [CrossRef] [Green Version]
- Thomas, M.; Groop, P.-H.; Tryggvason, K. Towards understanding the inherited susceptibility for nephropathy in diabetes. Curr. Opin. Nephrol. Hypertens. 2012, 21, 195–202. [Google Scholar] [CrossRef]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef]
- Lander, E.; Kruglyak, L. Genetic dissection of complex traits: Guidelines for interpreting and reporting linkage results. Nat. Genet. 1995, 11, 241–247. [Google Scholar] [CrossRef]
- Cordell, H.J.; Clayton, D.G. Genetic association studies. Lancet 2005, 366, 1121–1131. Available online: http://www.sciencedirect.com/science/article/pii/S0140673605674247 (accessed on 23 November 2021). [CrossRef]
- Iyengar, S.K.; Abboud, H.E.; Goddard, K.A.B.; Saad, M.F.; Adler, S.G.; Arar, N.H.; Bowden, D.W.; Duggirala, R.; Elston, R.C.; Hanson, R.L.; et al. Genome-Wide Scans for Diabetic Nephropathy and Albuminuria in Multiethnic Populations. Diabetes 2007, 56, 1577–1585. [Google Scholar] [CrossRef] [Green Version]
- Krolewski, A.S.; Poznik, G.D.; Placha, G.; Canani, L.; Dunn, J.; Walker, W.; Smiles, A.; Krolewski, B.; Fogarty, D.G.; Moczulski, D.; et al. A genome-wide linkage scan for genes controlling variation in urinary albumin excretion in type II diabetes. Kidney Int. 2006, 69, 129–136. Available online: http://www.ncbi.nlm.nih.gov/pubmed/16374433 (accessed on 23 November 2021). [CrossRef] [Green Version]
- Osterholm, A.-M.; He, B.; Pitkaniemi, J.; Albinsson, L.; Berg, T.; Sarti, C.; Tuomilehto, J.; Tryggvason, K. Genome-wide scan for type 1 diabetic nephropathy in the Finnish population reveals suggestive linkage to a single locus on chromosome 3q. Kidney Int. 2007, 71, 140–145. Available online: http://www.ncbi.nlm.nih.gov/pubmed/17021601 (accessed on 23 November 2021). [CrossRef] [Green Version]
- Rogus, J.J.; Poznik, G.D.; Pezzolesi, M.G.; Smiles, A.M.; Dunn, J.; Walker, W.; Wanic, K.; Moczulski, D.; Canani, L.; Araki, S.; et al. High-Density Single Nucleotide Polymorphism Genome-Wide Linkage Scan for Susceptibility Genes for Diabetic Nephropathy in Type 1 Diabetes Discordant Sibpair Approach. Diabetes 2008, 57, 2519–2526. [Google Scholar] [CrossRef] [Green Version]
- Wessman, M.; Forsblom, C.; Kaunisto, M.A.; Söderlund, J.; Ilonen, J.; Sallinen, R.; Hiekkalinna, T.; Parkkonen, M.; Maxwell, A.P.; Tarnow, L.; et al. Novel susceptibility locus at 22q11 for diabetic nephropathy in type 1 diabetes. PLoS ONE 2011, 6, e24053. Available online: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3164698&tool=pmcentrez&rendertype=abstract (accessed on 23 November 2021). [CrossRef] [Green Version]
- Igo, R.P.; Iyengar, S.K.; Nicholas, S.B.; Goddard, K.a.B.; Langefeld, C.D.; Hanson, R.L.; Duggirala, R.; Divers, J.; Abboud, H.; Adler, S.G.; et al. Genomewide linkage scan for diabetic renal failure and albuminuria: The FIND study. Am. J. Nephrol. 2011, 33, 381–389. Available online: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3078269&tool=pmcentrez&rendertype=abstract (accessed on 23 November 2021). [CrossRef] [Green Version]
- Stefanidis, I.; Tziastoudi, M.; Tsironi, E.E.; Dardiotis, E.; Tachmitzi, S.V.; Fotiadou, A.; Pissas, G.; Kytoudis, K.; Sounidaki, M.; Ampatzis, G.; et al. The contribution of genetic variants of SLC2A1 gene in T2DM and T2DM-nephropathy: Association study and meta-analysis. Ren. Fail. 2018, 40, 561–576. Available online: https://www.tandfonline.com/doi/full/10.1080/0886022X.2018.1496931 (accessed on 23 November 2021). [CrossRef]
- Tachmitzi, S.V.; Tsironi, E.E.; Kotoula, M.G.; Dardiotis, E.; Eleftheriadis, T.; Chatzoulis, D.Z.; Xanthopoulou, P.; Tziastoudi, M.; Koutsiaris, A.G.; Fotiadou, A.; et al. Association between Polymorphisms and Haplotypes in AKR1B1 and Diabetes Type 2 leading to Complications. Int. J. Med. Health Sci. 2015, 4, 430–436. [Google Scholar]
- Taira, M.; Imamura, M.; Takahashi, A.; Kamatani, Y.; Yamauchi, T.; Araki, S.-I.; Tanaka, N.; van Zuydam, N.R.; Ahlqvist, E.; Toyoda, M.; et al. A variant within the FTO confers susceptibility to diabetic nephropathy in Japanese patients with type 2 diabetes. PLoS ONE 2018, 13, e0208654. [Google Scholar] [CrossRef]
- Germain, M.; Pezzolesi, M.G.; Sandholm, N.; McKnight, A.J.; Susztak, K.; Lajer, M.; Forsblom, C.; Marre, M.; Parving, H.-H.; Rossing, P.; et al. SORBS1 gene, a new candidate for diabetic nephropathy: Results from a multi-stage genome-wide association study in patients with type 1 diabetes. Diabetologia 2015, 58, 543–548. [Google Scholar] [CrossRef] [Green Version]
- McDonough, C.W.; Palmer, N.D.; Hicks, P.J.; Roh, B.H.; An, S.S.; Cooke, J.N.; Hester, J.M.; Wing, M.R.; Bostrom, M.A.; Rudock, M.E.; et al. A genome-wide association study for diabetic nephropathy genes in African Americans. Kidney Int. 2011, 79, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Pezzolesi, M.G.; Poznik, G.D.; Mychaleckyj, J.C.; Paterson, A.D.; Barati, M.T.; Klein, J.B.; Ng, D.P.K.; Placha, G.; Canani, L.H.; Bochenski, J.; et al. Genome-Wide Association Scan for Diabetic Nephropathy Susceptibility Genes in Type 1 Diabetes. Diabetes 2009, 58, 1403–1410. [Google Scholar] [CrossRef] [Green Version]
- Maeda, S.; Osawa, N.; Hayashi, T.; Tsukada, S.; Kobayashi, M.; Kikkawa, R. Genetic variations associated with diabetic nephropathy and type II diabetes in a Japanese population. Kidney Int. Suppl. 2007, 72, S43–S48. [Google Scholar] [CrossRef] [Green Version]
- Tziastoudi, M.; Stefanidis, I.; Stravodimos, K.; Zintzaras, E. Identification of Chromosomal Regions Linked to Diabetic Nephropathy: A Meta-Analysis of Genome-Wide Linkage Scans. Genet. Test. Mol. Biomark. 2019, 23, 105–117. [Google Scholar] [CrossRef]
- Tziastoudi, M.; Stefanidis, I.; Zintzaras, E. The genetic map of diabetic nephropathy: Evidence from a systematic review and meta-analysis of genetic association studies. Clin. Kidney J. 2020, 13, 768–781. [Google Scholar] [CrossRef]
- Tziastoudi, M.; Stefanidis, I.; Hadjigeorgiou, G.M.; Stravodimos, K.; Zintzaras, E. A systematic review and meta-analysis of genetic association studies for the role of inflammation and the immune system in diabetic nephropathy. Clin. Kidney J. 2017, 10, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H.; Gejyo, F.; Suzuki, Y.; Suzuki, S.; Miyazaki, R.; Arakawa, M. Polymorphisms of angiotensin converting enzyme and plasminogen activator inhibitor-1 genes in diabetes and macroangiopathy1. Kidney Int. 1998, 54, 1659–1669. [Google Scholar] [CrossRef] [Green Version]
- De Cosmo, S.; Margaglione, M.; Tassi, V.; Garrubba, M.; Thomas, S.; Olivetti, C.; Piras, G.P.; Trevisan, R.; Vedovato, M.; Cavallo Perin, P.; et al. ACE, PAI-1, decorin and Werner helicase genes are not associated with the development of renal disease in European patients with type 1 diabetes. Diabetes Metab. Res. Rev. 1999, 15, 247–253. [Google Scholar] [CrossRef]
- Tarnow, L.; Stehouwer, C.D.; Emeis, J.J.; Poirier, O.; Cambien, F.; Hansen, B.V.; Parving, H.-H. Plasminogen activator inhibitor-1 and apolipoprotein E gene polymorphisms and diabetic angiopathy. Nephrol. Dial. Transplant. 2000, 15, 625–630. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.Y.; Poon, P.; Szeto, C.C.; Chan, J.C.; Li, P.K. Association of plasminogen activator inhibitor-1 4G/4G genotype and type 2 diabetic nephropathy in Chinese patients. Kidney Int. 2000, 57, 632–638. [Google Scholar] [CrossRef]
- Liu, S.; Xue, Y.; Yang, G.; He, F.; Zhao, X. Relationship between plasminogen activator inhibitor-1 gene 4G/5G polymorphism and type 2 diabetic nephropathy in Chinese Han patients in Guangdong Province. Di 1 Jun Yi Da Xue Xue Bao Acad. J. First Med. Coll. PLA 2004, 24, 904–907. [Google Scholar]
- Martin, R.J.L.; Savage, D.A.; Patterson, C.C.; Brady, H.R.; Maxwell, A.P. Common polymorphisms of the PAI1 gene do not play a major role in the development of diabetic nephropathy in Type 1 diabetes. Diabet. Med. J. Br. Diabet. Assoc. 2007, 24, 259–265. Available online: http://www.ncbi.nlm.nih.gov/pubmed/17263760 (accessed on 23 November 2021). [CrossRef]
- Prasad, P.; Tiwari, A.K.; Kumar, K.M.P.; Ammini, A.C.; Gupta, A.; Gupta, R.; Thelma, B.K. Association analysis of ADPRT1, AKR1B1, RAGE, GFPT2 and PAI-1 gene polymorphisms with chronic renal insufficiency among Asian Indians with type-2 diabetes. BMC Med. Genet. 2010, 11, 52. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Liu, H.; Sun, Y. Association of plasminogen activator inhibitor-1 gene polymorphism and type 2 diabetic nephropathy. Ren. Fail. 2016, 38, 157–162. Available online: http://www.tandfonline.com/doi/full/10.3109/0886022X.2015.1089464 (accessed on 23 November 2021). [CrossRef] [PubMed] [Green Version]
- Xue, C.; Nie, W.; Zhou, C.; Yu, F.; Wang, D.-M.; Dai, B.; Mei, C.-L. 4 G/4 G polymorphism of plasminogen activator inhibitor-1 gene increases the risk of diabetic nephropathy. Ren. Fail. 2014, 36, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, X.; Yang, F.; Cui, D.; Shi, Y.; Shen, C.; Tang, W.; Yang, T. PAI-1-675 4G/5G polymorphism in association with diabetes and diabetic complications susceptibility: A meta-analysis study. PLoS ONE 2013, 8, e79150. [Google Scholar]
- Dastgheib, S.A.; Najafi, F.; Shajari, A.; Bahrami, R.; Asadian, F.; Sadeghizadeh-Yazdi, J.; Akbarian, E.; Emarati, S.A.; Neamatzadeh, H. Association of plasminogen activator inhibitor-1 4G5G Polymorphism with risk of diabetic nephropathy and retinopathy: A systematic review and meta-analysis. J. Diabetes Metab. Disord. 2020, 19, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Festa, A.; D’Agostino, R.J.; Tracy, R.P.; Haffner, S.M. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: The insulin resistance atherosclerosis study. Diabetes 2002, 51, 1131–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gori, A.M.; Marcucci, R.; Fatini, C.; Gensini, F.; Sticchi, E.; Sodi, A.; Cappelli, S.; Menchini, U.; Gensini, G.F.; Abbate, R.; et al. Impaired fibrinolysis in retinal vein occlusion: A role for genetic determinants of PAI-1 levels. Thromb. Haemost. 2004, 92, 54–60. [Google Scholar] [PubMed]
- Steinberger, J.; Daniels, S.R. Obesity, insulin resistance, diabetes, and cardiovascular risk in children: An American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism). Circulation 2003, 107, 1448–1453. [Google Scholar]
- Siokas, V.; Dardiotis, E.; Sokolakis, T.; Kotoula, M.; Tachmitzi, S.V.; Chatzoulis, D.Z.; Almpanidou, P.; Stefanidis, I.; Hadjigeorgiou, G.M.; Tsironi, E.E. Plasminogen Activator Inhibitor Type-1 Tag Single-Nucleotide Polymorphisms in Patients with Diabetes Mellitus Type 2 and Diabetic Retinopathy. Curr. Eye Res. 2017, 42, 1048–1053. [Google Scholar] [CrossRef]
- Zintzaras, E. The generalized odds ratio as a measure of genetic risk effect in the analysis and meta-analysis of association studies. Stat. Appl. Genet. Mol. Biol. 2010, 9, 21. Available online: http://www.ncbi.nlm.nih.gov/pubmed/20597847 (accessed on 23 November 2021). [CrossRef]
- Zintzaras, E. The power of generalized odds ratio in assessing association in genetic studies. J. Appl. Stat. 2012, 39, 2569–2581. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986, 7, 177–188. Available online: http://www.ncbi.nlm.nih.gov/pubmed/3802833 (accessed on 23 November 2021). [CrossRef]
- Cochran, W. The combination of estimates from different experiments. Biometrics 1954, 10, 101–129. Available online: http://www.jstor.org/stable/3001666 (accessed on 23 November 2021). [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12111919 (accessed on 23 November 2021). [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997, 315, 629–634. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9310563 (accessed on 23 November 2021). [CrossRef] [Green Version]
- Liu, Y.; Cheng, J.; Guo, X.; Mo, J.; Gao, B.; Zhou, H.; Wu, Y.; Li, Z. The roles of PAI-1 gene polymorphisms in atherosclerotic diseases: A systematic review and meta-analysis involving 149,908 subjects. Gene 2018, 673, 167–173. [Google Scholar] [CrossRef]
- Zhang, Q.; Jin, Y.; Li, X.; Peng, X.; Peng, N.; Song, J.; Xu, M. Plasminogen activator inhibitor-1 (PAI-1) 4G/5G promoter polymorphisms and risk of venous thromboembolism—A meta-analysis and systematic review. VASA Z. Gefasskrankh. 2020, 49, 141–146. [Google Scholar] [CrossRef]
- Hu, X.; Zan, X.; Xie, Z.; Li, Y.; Lin, S.; Li, H.; You, C. Association Between Plasminogen Activator Inhibitor-1 Genetic Polymorphisms and Stroke Susceptibility. Mol. Neurobiol. 2017, 54, 328–341. [Google Scholar] [CrossRef]
- Gao, W.-F.; Guo, Y.-B.; Bai, Y.; Ding, X.-Y.; Yan, Y.-J.; Wu, Z.-Q. Association between PAI-1 4G/5G polymorphism and diabetic nephropathy: A meta-analysis in the Chinese population. Int. Urol. Nephrol. 2016, 48, 1483–1489. [Google Scholar] [CrossRef]
- Chen, J.; Zhai, C.; Wang, Z.; Li, R.; Wu, W.; Hou, K.; Alzogool, M.; Wang, Y.; Cong, H. The susceptibility of SERPINE1 rs1799889 SNP in diabetic vascular complications: A meta-analysis of fifty-one case-control studies. BMC Endocr. Disord. 2021, 21, 195. [Google Scholar] [CrossRef]



| Parameters | Case-Control Study Population Groups (n = 578) | |||||
|---|---|---|---|---|---|---|
| HC | DM | p Value | DM-DN | DM + DN | p Value | |
| N | 238 | 340 | n.a. | 150 | 190 | n.a. |
| Gender [m; n (%)] | 136 (42.9) | 181 (57.1) | 0.361 | 74 (47.7) | 107 (54.3) | 0.305 |
| Age (years) | 71 ± 9.2 | 68 ± 8.9 | <.001 | 68 ± 9.1 | 69 ± 8.8 | 0.380 |
| DM duration (years) | n.a. | 16.3 ± 8.0 | n.a. | 15.7 ± 8.3 | 16.8 ± 7.8 | 0.203 |
| HbA1c | n.d. | 7.36 ± 1.32 | n.a. | 7.20 ± 1.34 | 7.47 ± 1.29 | 0.064 |
| Insulin treatment (%) | n.d. | 105 (32.3) | n.a. | 50 (32.3) | 55 (27.9) | 0.412 |
| Hypertension (%) | 0 | 222 (63.4) | <.001 | 97 (63.0) | 125 (63.8) | 0.912 |
| Cardiovascular disease (%) | 0 | 110 (31.3) | <.001 | 41 (26.5) | 69 (35.0) | 0.105 |
| Creatinine (mg/dL) | 0.77 ± 0.15 | 1.46 ± 1.37 | <.001 | 0.90 ± 0.18 | 1.85 ± 1.67 | <0.001 |
| Urea (mg/dL) | 30 ± 7.9 | 59 ± 34 | <.001 | 42 ± 13.6 | 71 ± 38.3 | <0.001 |
| UACR | 36.7 ± 63.5 | 470 ± 856 | 0.382 | 43.9 ± 53.4 | 783 ± 1020 | <0.001 |
| Proteinuria (mg/dL) | 136.6 ± 118.5 | 788 ± 1468 | 0.444 | 105 ± 80.0 | 1311 ± 1784 | <0.001 |
| Variant | Genotype | DN | ORG (95% CI) | ||
|---|---|---|---|---|---|
| Healthy | Diseased Controls | Cases | |||
| rs2227667 | AA | 110 | 72 | 100 | 0.85 (0.66, 1.08) |
| GA | 106 | 63 | 69 | ||
| GG | 18 | 14 | 14 | ||
| rs2070682 | TT | 81 | 51 | 74 | 0.97 (0.77, 1.23) |
| TC | 126 | 71 | 83 | ||
| CC | 30 | 25 | 32 | ||
| rs1050813 | GG | 158 | 113 | 129 | 0.92 (0.69, 1.23) |
| AG | 67 | 31 | 50 | ||
| AA | 8 | 5 | 6 | ||
| rs2227690 | AA | 153 | 93 | 132 | 0.87 (0.66, 1.15) |
| GA | 77 | 44 | 52 | ||
| GG | 6 | 7 | 5 | ||
| rs2227692 | CC | 184 | 119 | 153 | 0.88 (0.64, 1.23) |
| CT | 52 | 30 | 33 | ||
| TT | 2 | 1 | 4 | ||
| Clinical Features | p-Value | ||||
|---|---|---|---|---|---|
| rs2227667 | rs2070682 | rs1050813 | rs2227690 | rs2227692 | |
| DM duration | 0.806 | 0.178 | 0.806 | 0.299 | 0.619 |
| HbA1c | 0.357 | 0.720 | 0.751 | 0.264 | 0.704 |
| Insulin | 0.522 | 0.223 | 0.927 | 0.871 | 0.712 |
| Hypertension | 0.644 | 0.144 | 0.849 | 0.392 | 0.826 |
| CVD | 0.644 | 0.334 | 0.945 | 0.464 | 0.788 |
| Creatinine | 0.500 | 0.199 | 0.491 | 0.480 | 0.037 |
| Urea | 0.498 | 0.674 | 0.138 | 0.290 | 0.687 |
| UACR | 0.306 | 0.688 | 0.328 | 0.833 | 0.609 |
| Proteinuria | 0.574 | 0.905 | 0.217 | 0.592 | 0.859 |
| eGFR | 0.195 | 0.970 | 0.088 | 0.310 | 0.609 |
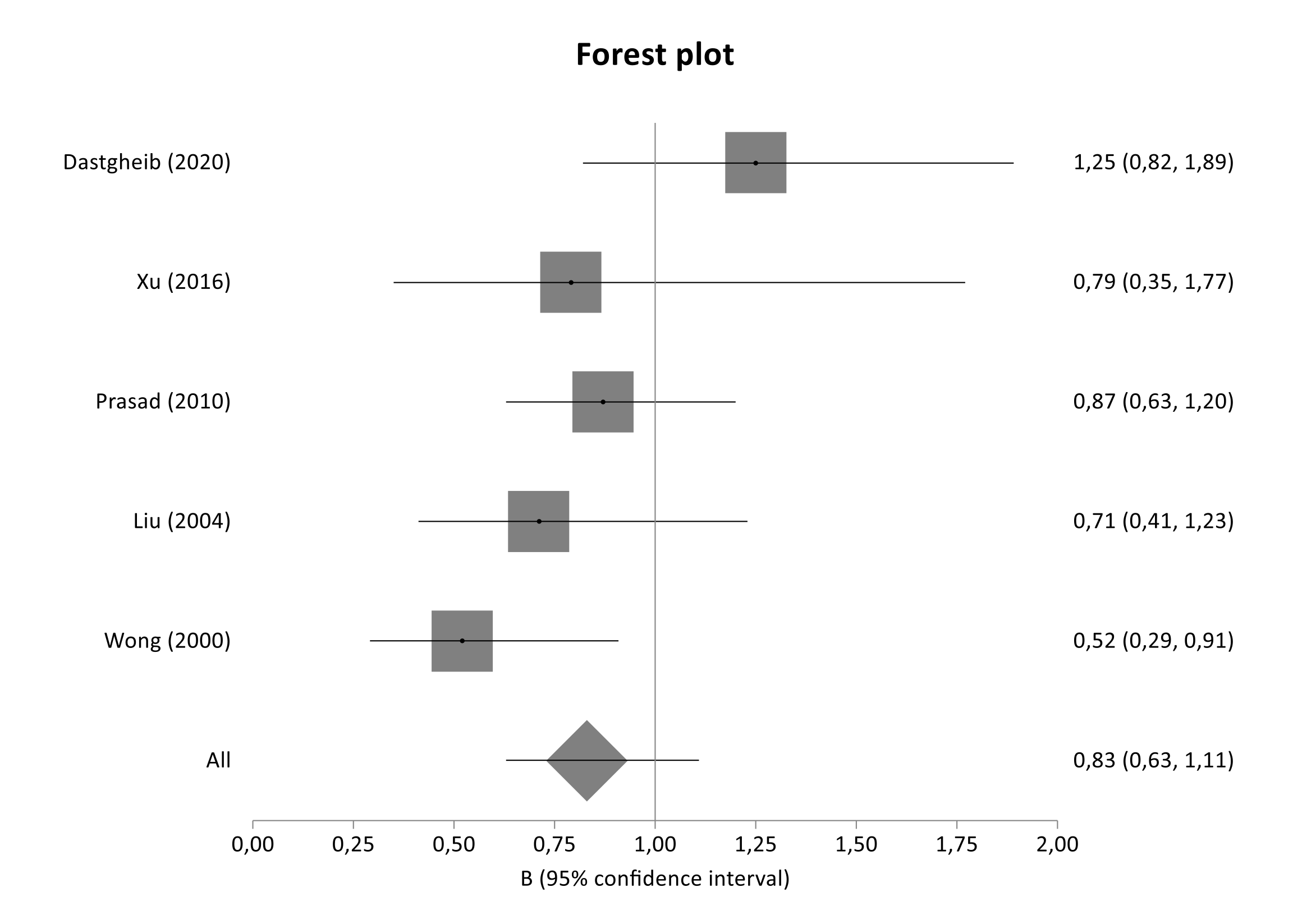
| SERPINE1 rs1799768 | Dastgheib (2020) [34] | E. Asians | 33520873 | T2DM | DN | 118 | macr/ria | 120 | norm/ria without diabetic retinopathy | DC-C | |||
| Xu (2016) [31] | E. Asians | 26616527 | T2DM | DN | 33 | macr/ria | 44 | norm/ria | DC-C, HT-DC-C, HT-C | ||||
| Prasad (2010) [30] | Asian Indians | 20353610 | T2DM | DN | 196 | CRI, serum Cr. ≥ 3.0 mg/dL | 225 | normal renal function and norm/ria, DM duration ≥ 10 yrs matched for age, ethnicity | DC-C | ||||
| Martin (2007) [29] | Caucasians | 17263760 | T1DM | DN | 222 | DM ≥ 10 yrs, pers. proteinuria, DR, no evidence of non-diabetic renal disease | 361 | DM > 15 yrs, pers. norm/ria, no anti-HT meds, background DR | 86 | non-diabetics | DC-C, HT-DC-C, HT-C | ||
| Tarnow (2000) [26] | Caucasians | 10809802 | T1DM | DN | 198 | diabetic glomerulosclerosis, pers. macr/ria, retinopathy | 192 | pers. norm/ria age, gender, DM duration | No | DC-C | |||
| Wong (2000) [27] | E. Asians | 10652041 | T2DM | DN | 95 | pers. micro/macroalbuminuriaor dialysis | 46 | pers. norm/ria, DM duration > 12 yrs matched for age, gender | No | DC-C, HT-DC-C, HT-C | |||
| De Cosmo (1999) [25] | Caucasians | 10495473 | T1DM | DN | 175 | micro/macroalbuminuria, retinopathy | 136 | norm/ria, DM > 15 yrs | 200 | non-diabetics | DC-C, HT-DC-C, HT-C | ||
| Kimura (1998) [24] | E. Asians | 9844142 | T2DM | DN | 98 | overt proteinuria, impaired renal function, DR or ESRD requiring dialysis | - | 177 | non-diabetics | HT-C |
| Diseased Controls versus Cases | ||||||||||
| Gene | Polymorphism | Rs number | N | Cases/Controls | RE ORG | LL ORG | UL ORG | I2 | PQ | PE |
| SERPINE1 | c.-821_-820insG (4G>5G) | rs1799768 | 7 | 1035/1121 | 0.91 | 0.78 | 1.06 | 6.76 | 0.34 | 0.13 |
| SERPINE1 | All in HWE | 4 | 626/764 | 0.88 | 0.74 | 1.06 | 0 | 0.98 | 0.17 | |
| T1DM/Caucasians | ||||||||||
| SERPINE1 | 4G>5G | rs1799768 | 3 | 594/688 | 0.93 | 0.77 | 1.13 | 0 | 0.78 | 0.08 |
| T2DM/Asians | ||||||||||
| SERPINE1 | 4G>5G | rs1799768 | 4 | 518/503 | 0.85 | 0.60 | 1.21 | 51.08 | 0.11 | 0.15 |
| SERPINE1 | 4G>5G | rs1799768 | 2 | 400/383 | 0.86 | 0.64 | 1.16 | 0 | 0.83 | 0.12 |
| Healthy Controls versus Diseased Controls versus Cases | ||||||||||
| SERPINE1 | c.-821_-820insG (4G>5G) | rs1799768 | 4 | 0.9 | 0.76 | 1.05 | 14.57 | 0.32 | 0 | |
| T1DM/Caucasians | ||||||||||
| SERPINE1 | 2 | 0.96 | 0.81 | 1.14 | 0 | 0.83 | - | |||
| T2DM/Asians | ||||||||||
| SERPINE1 | 2 | 0.74 | 0.53 | 1.04 | - | |||||
| Healthy Controls versus Cases | ||||||||||
| SERPINE1 | c.-821_-820insG (4G>5G) | rs1799768 | 5 | 622/659 | 0.92 | 0.74 | 1.13 | 9.02 | 0.36 | 0.07 |
| T1DM/Caucasians | ||||||||||
| SERPINE1 | c.-821_-820insG (4G>5G) | rs1799768 | 2 | 0.96 | 0.73 | 1.25 | 0 | 0.70 | - | |
| T2DM/Asians | ||||||||||
| SERPINE1 | c.-821_-820insG (4G>5G) | rs1799768 | 3 | 0.83 | 0.55 | 1.27 | 50.87 | 0.13 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tziastoudi, M.; Dardiotis, E.; Pissas, G.; Filippidis, G.; Golfinopoulos, S.; Siokas, V.; Tachmitzi, S.V.; Eleftheriadis, T.; Hadjigeorgiou, G.M.; Tsironi, E.; et al. Serpin Family E Member 1 Tag Single-Nucleotide Polymorphisms in Patients with Diabetic Nephropathy: An Association Study and Meta-Analysis Using a Genetic Model-Free Approach. Genes 2021, 12, 1887. https://doi.org/10.3390/genes12121887
Tziastoudi M, Dardiotis E, Pissas G, Filippidis G, Golfinopoulos S, Siokas V, Tachmitzi SV, Eleftheriadis T, Hadjigeorgiou GM, Tsironi E, et al. Serpin Family E Member 1 Tag Single-Nucleotide Polymorphisms in Patients with Diabetic Nephropathy: An Association Study and Meta-Analysis Using a Genetic Model-Free Approach. Genes. 2021; 12(12):1887. https://doi.org/10.3390/genes12121887
Chicago/Turabian StyleTziastoudi, Maria, Efthimios Dardiotis, Georgios Pissas, Georgios Filippidis, Spyridon Golfinopoulos, Vasileios Siokas, Sophia V. Tachmitzi, Theodoros Eleftheriadis, Georgios M. Hadjigeorgiou, Evangelia Tsironi, and et al. 2021. "Serpin Family E Member 1 Tag Single-Nucleotide Polymorphisms in Patients with Diabetic Nephropathy: An Association Study and Meta-Analysis Using a Genetic Model-Free Approach" Genes 12, no. 12: 1887. https://doi.org/10.3390/genes12121887
APA StyleTziastoudi, M., Dardiotis, E., Pissas, G., Filippidis, G., Golfinopoulos, S., Siokas, V., Tachmitzi, S. V., Eleftheriadis, T., Hadjigeorgiou, G. M., Tsironi, E., & Stefanidis, I. (2021). Serpin Family E Member 1 Tag Single-Nucleotide Polymorphisms in Patients with Diabetic Nephropathy: An Association Study and Meta-Analysis Using a Genetic Model-Free Approach. Genes, 12(12), 1887. https://doi.org/10.3390/genes12121887







