PCNA Loaders and Unloaders—One Ring That Rules Them All
Abstract
1. Introduction
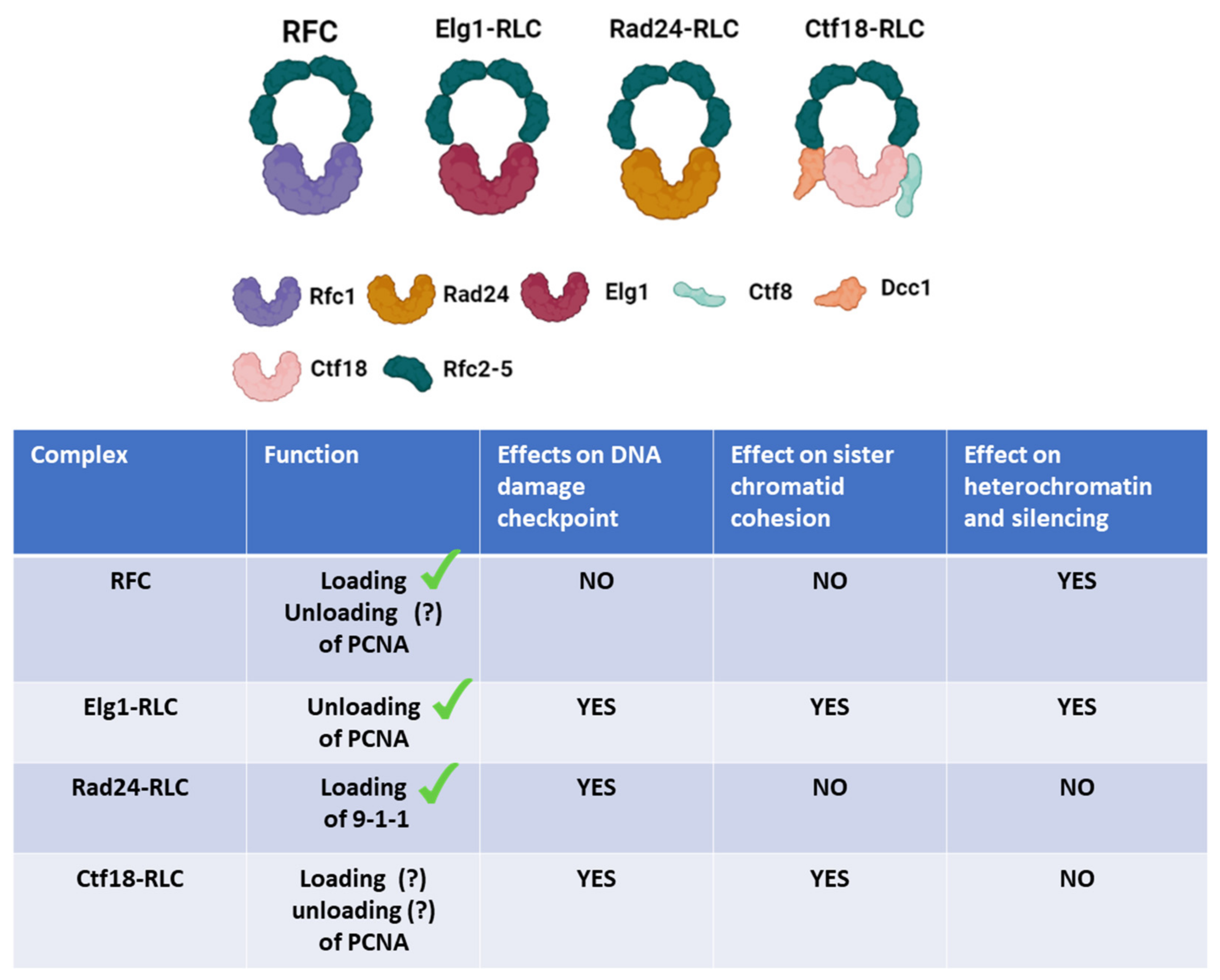
2. RFC and RFC-like Complexes (RLCs)
2.1. Rfc1
2.2. Elg1 (Mammalian ATAD5)
2.3. Ctf18
2.4. 9-1-1 and Rad24 (Rad17 in Humans)
3. DNA Replication
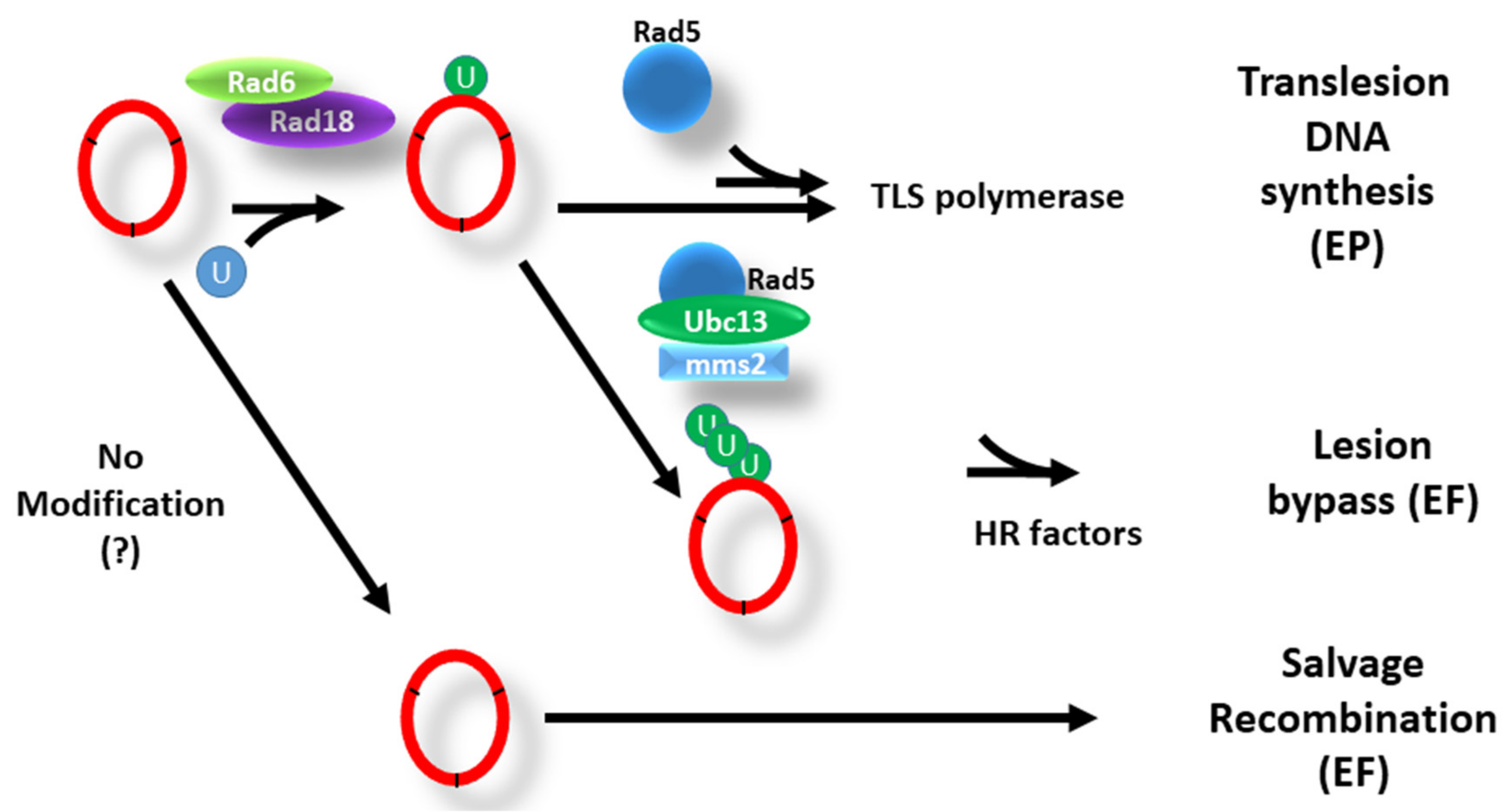
4. PCNA and the DNA Damage Tolerance Pathways
5. Heterochromatin and Silencing
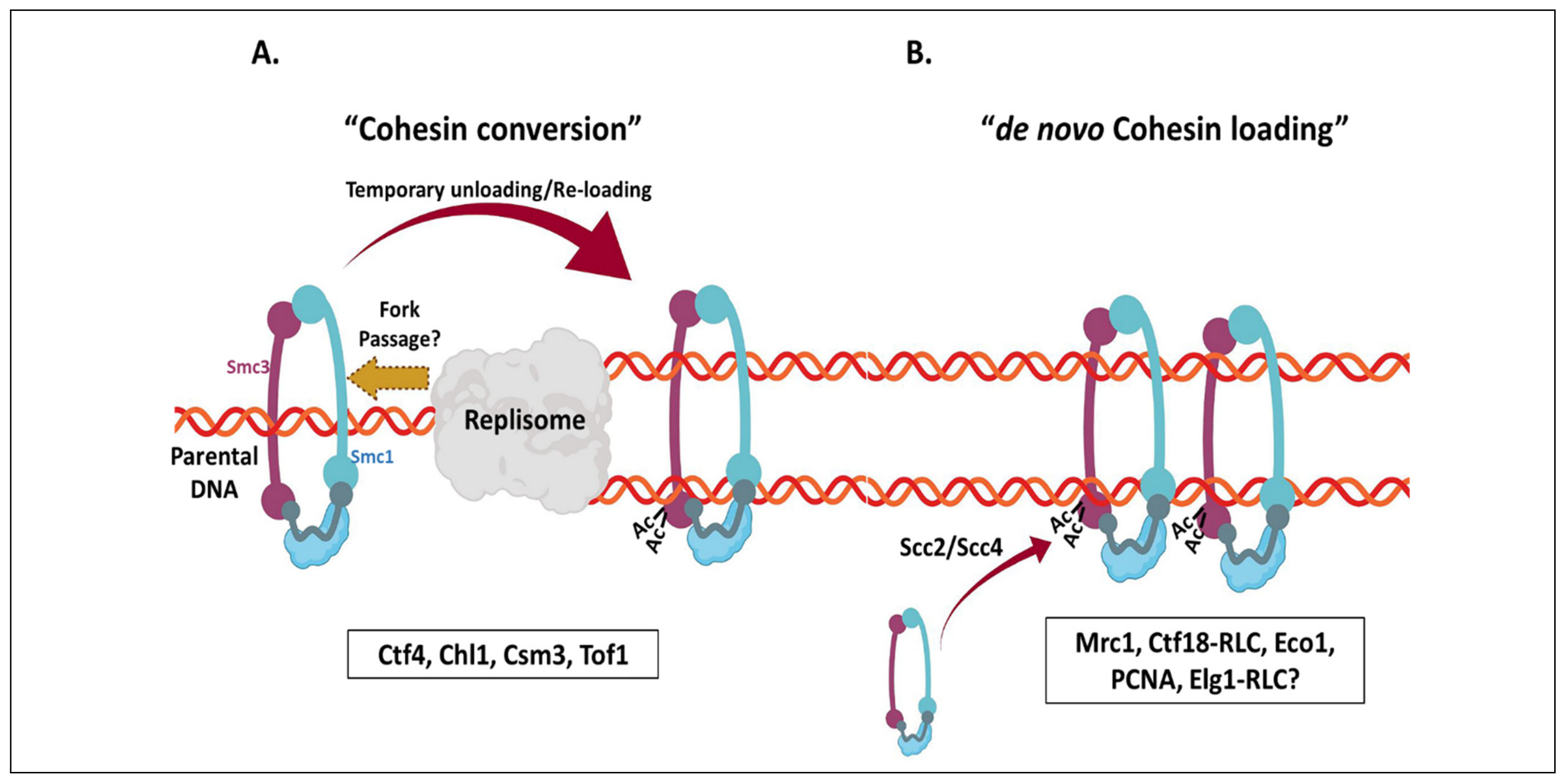
6. Sister Chromatid Cohesion
Coupling of SCC Establishment with DNA Replication and the Role of RLCs
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arbel, M.; Liefshitz, B.; Kupiec, M. DNA damage bypass pathways and their effect on mutagenesis in yeast. FEMS Microbiol. Rev. 2020, 45, fuaa038. [Google Scholar] [CrossRef]
- Boiteux, S.; Jinks-Robertson, S. DNA repair mechanisms and the bypass of DNA damage in Saccharomyces cerevisiae. Genetics 2013, 193, 1025–1064. [Google Scholar] [CrossRef] [PubMed]
- Kupiec, M. Alternative clamp loaders/unloaders. FEMS Yeast Res. 2016, 16, fow084. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Park, S.H. Eukaryotic clamp loaders and unloaders in the maintenance of genome stability. Exp. Mol. Med. 2020, 52, 1948–1958. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, E.; Tsurimoto, T. Functions of multiple clamp and clamp-loader complexes in eukaryotic DNA replication. Adv. Exp. Med. Biol. 2017, 1042, 135–162. [Google Scholar]
- Shiomi, Y.; Nishitani, H. Control of genome integrity by RFC complexes; conductors of PCNA loading onto and unloading from chromatin during DNA replication. Genes 2017, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Majka, J.; Burgers, P.M.J. The PCNA-RFC Families of DNA Clamps and Clamp Loaders. Prog. Nucleic Acid Res. Mol. Biol. 2004, 78, 227–260. [Google Scholar]
- Sakato, M.; Zhou, Y.; Hingorani, M.M. ATP binding and hydrolysis-driven rate-determining events in the RFC-catalyzed PCNA clamp loading reaction. J. Mol. Biol. 2012, 416, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Dionne, I.; Brown, N.J.; Woodgate, R.; Bell, S.D. On the mechanism of loading the PCNA sliding clamp by RFC. Mol. Microbiol. 2008, 68, 216–222. [Google Scholar] [CrossRef]
- Cullmann, G.; Fien, K.; Kobayashi, R.; Stillman, B. Characterization of the five replication factor C genes of Saccharomyces cerevisiae. Mol. Cell. Biol. 1995, 15, 4661–4671. [Google Scholar] [CrossRef] [PubMed]
- Mossi, R.; Hübscher, U. Clamping down on clamps and clamp loaders—The eukaryotic replication factor C. Eur. J. Biochem. 1998, 254, 209–216. [Google Scholar]
- Bunz, F.; Kobayashi, R.; Stillman, B. cDNAs encoding the large subunit of human replication factor C. Proc. Natl. Acad. Sci. USA 1993, 90, 11014–11018. [Google Scholar] [CrossRef] [PubMed]
- Uhlmann, F.; Cai, J.; Flores-Rozas, H.; Dean, F.B.; Finkelstein, J.; O’Donnell, M.; Hurwitz, J. In vitro reconstitution of human replication factor C from its five subunits. Proc. Natl. Acad. Sci. USA 1996, 93, 6521–6526. [Google Scholar] [CrossRef] [PubMed]
- Bowman, G.D.; O’Donnell, M.; Kuriyan, J. Structural analysis of a eukaryotic sliding DNA clamp-clamp loader complex. Nature 2004, 429, 724–730. [Google Scholar] [CrossRef]
- Gomes, X.V.; Gary, S.L.; Burgers, P.M.J. Overproduction in Escherichia coli and characterization of yeast replication factor C lacking the ligase homology domain. J. Biol. Chem. 2000, 275, 14541–14549. [Google Scholar] [CrossRef]
- Yao, N.Y.; O’Donnell, M. The RFC clamp loader: Structure and Function. Subcell. Biochem. 2012, 62, 259–279. [Google Scholar] [CrossRef]
- Gaubitz, C.; Liu, X.; Magrino, J.; Stone, N.P.; Landeck, J.; Hedglin, M.; Kelch, B.A. Structure of the human clamp loader reveals an autoinhibited conformation of a substrate-bound AAA+ switch. Proc. Natl. Acad. Sci. USA 2020, 117, 23571–23580. [Google Scholar] [CrossRef] [PubMed]
- Kelch, B.A. Review: The lord of the rings: Structure and mechanism of the sliding clamp loader. Biopolymers 2016, 105, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Ryu, E.; Lee, S.W.; Park, J.; Ha, N.Y.; Ra, J.S.; Kim, Y.J.; Kim, J.; Abdel-Rahman, M.; Park, S.H.; et al. Regulation of PCNA cycling on replicating DNA by RFC and RFC-like complexes. Nat. Commun. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Hedglin, M.; Aitha, M.; Benkovic, S.J. Monitoring the Retention of Human Proliferating Cell Nuclear Antigen at Primer/Template Junctions by Proteins That Bind Single-Stranded DNA. Biochemistry 2017, 56, 3415–3421. [Google Scholar] [CrossRef] [PubMed]
- Hedglin, M.; Perumal, S.K.; Hu, Z.; Benkovic, S. Stepwise assembly of the human replicative polymerase holoenzyme. Elife 2013, 2, e00278. [Google Scholar] [CrossRef] [PubMed]
- Ben-Aroya, S.; Koren, A.; Liefshitz, B.; Steinlauf, R.; Kupiec, M. ELG1, a yeast gene required for genome stability, forms a complex related to replication factor C. Proc. Natl. Acad. Sci. USA 2003, 100, 9906–9911. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Gan, H.; Han, J.; Zhou, Z.X.; Jia, S.; Chabes, A.; Farrugia, G.; Ordog, T.; Zhang, Z. Strand-Specific Analysis Shows Protein Binding at Replication Forks and PCNA Unloading from Lagging Strands when Forks Stall. Mol. Cell 2014, 56, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Bouchoux, C.; Panarotto, M.; Kakui, Y.; Patel, H.; Uhlmann, F. Division of Labor between PCNA Loaders in DNA Replication and Sister Chromatid Cohesion Establishment. Mol. Cell 2020, 78, 725–738.e4. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-G.; Roig, M.B.; Jansma, M.; Petela, N.; Metson, J.; Nasmyth, K.; Löwe, J.; Johnson, C.; Gali, V.K.V.K.; Takahashi, T.S.; et al. ELG1, a yeast gene required for genome stability, forms a complex related to replication factor C. Mol. Cell 2016, 10, 2420. [Google Scholar] [CrossRef]
- Johnson, C.; Gali, V.K.; Takahashi, T.S.; Kubota, T. PCNA Retention on DNA into G2/M Phase Causes Genome Instability in Cells Lacking Elg1. Cell Rep. 2016, 16, 684–695. [Google Scholar] [CrossRef]
- Kubota, T.; Nishimura, K.; Kanemaki, M.T.; Donaldson, A.D. The Elg1 Replication Factor C-like Complex Functions in PCNA Unloading during DNA Replication. Mol. Cell 2013, 50, 273–280. [Google Scholar] [CrossRef]
- Scholes, D.T.; Banerjee, M.; Bowen, B.; Curcio, M.J. Multiple regulators of Ty1 transposition in Saccharomyces cerevisiae have conserved roles in genome maintenance. Genetics 2001, 159, 1449–1465. [Google Scholar] [CrossRef]
- Bellaoui, M.; Chang, M.; Ou, J.; Xu, H.; Boone, C.; Brown, G.W. Elg1 forms an alternative RFC complex important for DNA replication and genome integrity. EMBO J. 2003, 22, 4304–4313. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-E.; Riot, A.-G.; Nicolas, A.; Kolodner, R.D. A genomewide screen in Saccharomyces cerevisiae for genes that suppress the accumulation of mutations. Proc. Natl. Acad. Sci. USA 2003, 100, 11529–11534. [Google Scholar] [CrossRef]
- Smith, S.; Hwang, J.-Y.; Banerjee, S.; Majeed, A.; Gupta, A.; Myung, K. Mutator genes for suppression of gross chromosomal rearrangements identified by a genome wide screening in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2004, 101, 9039–9044. [Google Scholar] [CrossRef]
- Smolikov, S.; Mazor, Y.; Krauskopf, A. ELG1, a regulator of genome stability, has a role in telomere length regulation and in silencing. Proc. Natl. Acad. Sci. USA 2004, 101, 1656–1661. [Google Scholar] [CrossRef]
- Ogiwara, H.; Ui, A.; Enomoto, T.; Seki, M. Role of Elg1 protein in double strand break repair. Nucleic Acids Res. 2007, 35, 353–362. [Google Scholar] [CrossRef]
- Askree, S.H.; Yehuda, T.; Smolikov, S.; Gurevich, R.; Hawk, J.; Coker, C.; Krauskopf, A.; Kupiec, M.; McEachern, M.J. A genome-wide screen for Saccharomyces cerevisiae deletion mutants that affect telomere length. Proc. Natl. Acad. Sci. USA 2004, 101, 8658–8663. [Google Scholar] [CrossRef] [PubMed]
- Gazy, I.; Liefshitz, B.; Bronstein, A.; Parnas, O.; Atias, N.; Sharan, R.; Kupiec, M. A genetic screen for high copy number suppressors of the synthetic lethality between elg1Δ and srs2Δ in yeast. G3 Genes Genomes Genet. 2013, 3, 917–926. [Google Scholar] [CrossRef]
- Gazy, I.; Liefshitz, B.; Parnas, O.; Kupiec, M. Elg1, a central player in genome stability. Mutat. Res. Rev. Mutat. Res. 2015, 763, 267–279. [Google Scholar] [CrossRef]
- Davidson, M.B.; Brown, G.W. The N- and C-termini of Elg1 contribute to the maintenance of genome stability. DNA Repair. (Amst). 2008, 7, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Alvaro, D.; Lisby, M.; Rothstein, R. Genome-wide analysis of Rad52 foci reveals diverse mechanisms impacting recombination. PLoS Genet. 2007, 3, e228. [Google Scholar] [CrossRef] [PubMed]
- Arbel, M.; Bronstein, A.; Sau, S.; Liefshitz, B.; Kupiec, M. Access to pcna by srs2 and elg1 controls the choice between alternative repair pathways in saccharomyces cerevisiae. MBio 2020, 11, e00705-20. [Google Scholar] [CrossRef]
- Bylund, G.O.; Majka, J.; Burgers, P.M.J. Overproduction and Purification of RFC-Related Clamp Loaders and PCNA-Related Clamps from Saccharomyces cerevisiae. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2006; Volume 409. [Google Scholar]
- Ho, B.; Baryshnikova, A.; Brown, G.W. Unification of Protein Abundance Datasets Yields a Quantitative Saccharomyces cerevisiae Proteome. Cell Syst. 2018, 6. [Google Scholar] [CrossRef]
- Kang, S.; Warner, M.D.; Bell, S.P. Multiple Functions for Mcm2-7 ATPase Motifs during Replication Initiation. Mol. Cell 2018, 6, 192–205.e3. [Google Scholar] [CrossRef]
- Kubota, T.; Myung, K.; Donaldson, A.D. Is PCNA unloading the central function of the Elg1/ ATAD5 replication factor C-like complex? Cell Cycle 2014, 55, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Parnas, O.; Zipin-Roitman, A.; Pfander, B.; Liefshitz, B.; Mazor, Y.; Ben-Aroya, S.; Jentsch, S.; Kupiec, M. Elg1, an alternative subunit of the RFC clamp loader, preferentially interacts with SUMOylated PCNA. EMBO J. 2010, 29, 2611–2622. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, K.; Sebesta, M.; Pacesa, M.; Sau, S.; Bronstein, A.; Parnas, O.; Liefshitz, B.; Venclovas, Č.; Krejci, L.; Kupiec, M. A structure-function analysis of the yeast Elg1 protein reveals the importance of PCNA unloading in genome stability maintenance. Nucleic Acids Res. 2017, 45, 3189–3203. [Google Scholar] [CrossRef]
- Pfander, B.; Moldovan, G.-L.; Sacher, M.; Hoege, C.; Jentsch, S. SUMO-modified PCNA recruits Srs2 to prevent recombination during S phase. Nature 2005, 436, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Burkovics, P.; Sebesta, M.; Sisakova, A.; Plault, N.; Szukacsov, V.; Robert, T.; Pinter, L.; Marini, V.; Kolesar, P.; Haracska, L.; et al. Srs2 mediates PCNA-SUMO-dependent inhibition of DNA repair synthesis. EMBO J. 2013, 32, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Veaute, X.; Jeusset, J.; Soustelle, C.; Kowalczykowski, S.C.; Le Cam, E.; Fahre, F. The Srs2 helicase prevents recombination by disrupting Rad51 nucleoprotein filaments. Nature 2003, 423, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Lanz, M.C.; Yugandhar, K.; Gupta, S.; Sanford, E.; Faça, V.; Vega, S.; Joiner, A.; Fromme, C.; Yu, H.; Smolka, M.B. In-depth and 3-dimensional exploration of the budding yeast phosphoproteome. bioRxiv 2019. [Google Scholar] [CrossRef] [PubMed]
- Shkedy, D.; Singh, N.; Shemesh, K.; Amir, A.; Geiger, T.; Liefshitz, B.; Harari, Y.; Kupiec, M. Regulation of Elg1 activity by phosphorylation. Cell Cycle 2015, 14, 3689–3697. [Google Scholar] [CrossRef]
- Pardo, B.; Crabbé, L.; Pasero, P. Signaling pathways of replication stress in yeast. FEMS Yeast Res. 2017, 17, fow101. [Google Scholar] [CrossRef] [PubMed]
- Toh, G.W.L.; Lowndes, N.F. Role of the Saccharomyces cerevisiae Rad9 protein in sensing and responding to DNA damage. Biochem. Soc. Trans. 2003, 31, 242–246. [Google Scholar] [CrossRef]
- Alcasabas, A.A.; Osborn, A.J.; Bachant, J.; Hu, F.; Werler, P.J.H.; Bousset, K.; Furuya, K.; Diffley, J.F.X.; Carr, A.M.; Elledge, S.J. Mrc1 transduces signals of DNA replication stress to activate Rad53. Nat. Cell Biol. 2001, 3, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, C.Y.; Melo, J.A.; Toczyski, D.P. Colocalization of Sensors Is Sufficient to Activate the DNA Damage Checkpoint in the Absence of Damage. Mol. Cell 2008, 30, 267–276. [Google Scholar] [CrossRef]
- Berens, T.J.; Toczyski, D.P. Colocalization of Mec1 and Mrc1 is sufficient for Rad53 phosphorylation in vivo. Mol. Biol. Cell 2012, 23, 1058–1067. [Google Scholar] [CrossRef]
- Sau, S.; Liefshitz, B.; Kupiec, M. The yeast pcna unloader Elg1 RFC-like complex plays a role in eliciting the DNA damage checkpoint. MBio 2019, 10, e01159-19. [Google Scholar] [CrossRef]
- Bylund, G.O.; Burgers, P.M.J. Replication protein A-directed unloading of PCNA by the Ctf18 cohesion establishment complex. Mol. Cell. Biol. 2005, 25, 5445–5455. [Google Scholar] [CrossRef]
- Hanna, J.S.; Kroll, E.S.; Lundblad, V.; Spencer, F.A. Saccharomyces cerevisiae CTF18 and CTF4 are required for sister chromatid cohesion. Mol. Cell. Biol. 2001, 21, 3144–3158. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.L.; Gygi, S.P.; Aebersold, R.; Hieter, P. Identification of RFC(Ctf18p, Ctf8p, Dcc1p): An alternative RFC complex required for sister chromatid cohesion in S. cerevisiae. Mol. Cell 2001, 7, 959–970. [Google Scholar] [CrossRef]
- Spencer, F.; Gerring, S.L.; Connelly, C.; Hieter, P. Mitotic chromosome transmission fidelity mutants in Saccharomyces cerevisiae. Genetics 1990, 124, 237–249. [Google Scholar] [CrossRef]
- Kouprina, N.; Tsouladze, A.; Koryabin, M.; Hieter, P.; Spencer, F.; Larionov, V. Identification and genetic mapping of CHL genes controlling mitotic chromosome transmission in yeast. Yeast 1993, 9, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kouprina, N.; Kroll, E.; Kirillov, A.; Bannikov, V.; Zakharyev, V.; Larionov, V. CHL12, a gene essential for the fidelity of chromosome transmission in the yeast Saccharomyces cerevisiae. Genetics 1994, 138, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- Miles, J.; Formosa, T. Evidence that POB1, a Saccharomyces cerevisiae protein that binds to DNA polymerase α, acts in DNA metabolism in vivo. Mol. Cell. Biol. 1992, 12, 5724–5735. [Google Scholar] [CrossRef][Green Version]
- Pursell, Z.F.; Isoz, I.; Lundström, E.-B.; Johansson, E.; Kunkel, T.A. Yeast DNA polymerase ε participates in leading-strand DNA replication. Science 2007, 317, 127–130. [Google Scholar] [CrossRef]
- Stokes, K.; Winczura, A.; Song, B.; De Piccoli, G.; Grabarczyk, D.B. Ctf18-RFC and DNA Pol ϵ form a stable leading strand polymerase/clamp loader complex required for normal and perturbed DNA replication. Nucleic Acids Res. 2020, 48, 8128–8145. [Google Scholar] [CrossRef]
- Wade, B.O.; Liu, H.W.; Samora, C.P.; Uhlmann, F.; Singleton, M.R. Structural studies of RFC C tf18 reveal a novel chromatin recruitment role for Dcc1. EMBO Rep. 2017, 18, 558–568. [Google Scholar] [CrossRef]
- Crabbé, L.; Thomas, A.; Pantesco, V.; De Vos, J.; Pasero, P.; Lengronne, A. Analysis of replication profiles reveals key role of RFC-Ctf18 in yeast replication stress response. Nat. Struct. Mol. Biol. 2010, 17, 1391–1397. [Google Scholar] [CrossRef]
- Kubota, T.; Hiraga, S.-I.; Yamada, K.; Lamond, A.I.; Donaldson, A.D. Quantitative proteomic analysis of chromatin reveals that Ctf18 acts in the DNA replication checkpoint. Mol. Cell. Proteomics 2011, 10, m110.005561. [Google Scholar] [CrossRef]
- Terret, M.-E.; Sherwood, R.; Rahman, S.; Qin, J.; Jallepalli, P.V. Cohesin acetylation speeds the replication fork. Nature 2009, 462, 231–234. [Google Scholar] [CrossRef]
- Hiraga, S.-I.; Robertson, E.D.; Donaldson, A.D. The Ctf18 RFC-like complex positions yeast telomeres but does not specify their replication time. EMBO J. 2006, 25, 1505–1514. [Google Scholar] [CrossRef]
- García-Rodríguez, L.J.; De Piccoli, G.; Marchesi, V.; Jones, R.C.; Edmondson, R.D.; Labib, K. A conserved Polϵ binding module in Ctf18-RFC is required for S-phase checkpoint activation downstream of Mec1. Nucleic Acids Res. 2015, 43, 8830–8838. [Google Scholar] [CrossRef]
- Poli, J.; Tsaponina, O.; Crabbé, L.; Keszthelyi, A.; Pantesco, V.; Chabes, A.; Lengronne, A.; Pasero, P. dNTP pools determine fork progression and origin usage under replication stress. EMBO J. 2012, 31, 883–894. [Google Scholar] [CrossRef]
- Ogiwara, H.; Ohuchi, T.; Ui, A.; Tada, S.; Enomoto, T.; Seki, M. Ctf18 is required for homologous recombination-mediated double-strand break repair. Nucleic Acids Res. 2007, 35, 4989–5000. [Google Scholar] [CrossRef]
- Tercero, J.A.; Diffley, J.F.X. Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature 2001, 412, 553–557. [Google Scholar] [CrossRef]
- Santocanale, C.; Diffley, J.F.X. A Mec1-and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature 1998, 395, 615–618. [Google Scholar] [CrossRef]
- Bacal, J.; Moriel-Carretero, M.; Pardo, B.; Barthe, A.; Sharma, S.; Chabes, A.; Lengronne, A.; Pasero, P. Mrc1 and Rad9 cooperate to regulate initiation and elongation of DNA replication in response to DNA damage. EMBO J. 2018, 37, e99319. [Google Scholar] [CrossRef]
- Gershon, L.; Kupiec, M. A novel role for Dun1 in the regulation of origin firing upon hyper-acetylation of H3K56. PLoS Genet. 2021, 17, e1009391. [Google Scholar] [CrossRef]
- Topal, S.; Vasseur, P.; Radman-Livaja, M.; Peterson, C.L. Distinct transcriptional roles for Histone H3-K56 acetylation during the cell cycle in Yeast. Nat. Commun. 2019, 10, 4372. [Google Scholar] [CrossRef]
- Kaplan, T.; Liu, C.L.; Erkmann, J.A.; Holik, J.; Grunstein, M.; Kaufman, P.D.; Friedman, N.; Rando, O.J. Cell cycle- and chaperone-mediated regulation of H3K56ac incorporation in yeast. PLoS Genet. 2008, 4, e1000270. [Google Scholar] [CrossRef]
- Masumoto, H.; Hawke, D.; Kobayashi, R.; Verreault, A. A role for cell-cycle-regulated histone H3 lysine 56 acetylation in the DNA damage response. Nature 2005, 436, 294–298. [Google Scholar] [CrossRef]
- Celic, I.; Masumoto, H.; Griffith, W.P.; Meluh, P.; Cotter, R.J.; Boeke, J.D.; Verreault, A. The Sirtuins Hst3 and Hst4p Preserve Genome Integrity by Controlling Histone H3 Lysine 56 Deacetylation. Curr. Biol. 2006, 16, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Celic, I.; Verreault, A.; Boeke, J.D. Histone H3 K56 hyperacetylation perturbs replisomes and causes DNA damage. Genetics 2008, 179, 1769–1784. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Rothstein, R. The Dun1 checkpoint kinase phosphorylates and regulates the ribonucleotide reductase inhibitor Sml1. Proc. Natl. Acad. Sci. USA 2002, 99, 3746–3751. [Google Scholar] [CrossRef] [PubMed]
- Lanz, M.C.; Dibitetto, D.; Smolka, M.B. DNA damage kinase signaling: Checkpoint and repair at 30 years. EMBO J. 2019, 38, e101801. [Google Scholar] [CrossRef] [PubMed]
- Majka, J.; Binz, S.K.; Wold, M.S.; Burgers, P.M.J. Replication protein a directs loading of the DNA damage checkpoint clamp to 5′-DNA junctions. J. Biol. Chem. 2006, 281, 27855–27861. [Google Scholar] [CrossRef]
- Majka, J.; Burgers, P.M.J. Yeast Rad17/Mec3/Ddc1: A sliding clamp for the DNA damage checkpoint. Proc. Natl. Acad. Sci. USA 2003, 100, 2249–2254. [Google Scholar] [CrossRef]
- Eckardt-Schupp, F.; Siede, W.; Game, J.C. The RAD24 (= R(S1)) gene product of Saccharomyces cerevisiae participates in two different pathways of DNA repair. Genetics 1987, 115, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Aylon, Y.; Kupiec, M. The Checkpoint Protein Rad24 of Saccharomyces cerevisiae Is Involved in Processing Double-Strand Break Ends and in Recombination Partner Choice. Mol. Cell. Biol. 2003, 23, 6585–6596. [Google Scholar] [CrossRef]
- De la Torre-Ruiz, M.A.; Green, C.M.; Lowndes, N.F. RAD9 and RAD24 define two additive, interacting branches of the DNA damage checkpoint pathway in budding yeast normally required for Rad53 modification and activation. EMBO J. 1998, 17, 2687–2698. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.X.; Lujan, S.A.; Burkholder, A.B.; Garbacz, M.A.; Kunkel, T.A. Roles for DNA polymerase δ in initiating and terminating leading strand DNA replication. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.E.; Klassen, R.; Prakash, L.; Prakash, S. A Major Role of DNA Polymerase δ in Replication of Both the Leading and Lagging DNA Strands. Mol. Cell 2015, 59, 163–175. [Google Scholar] [CrossRef]
- Georgescu, R.E.; Schauer, G.D.; Yao, N.Y.; Langston, L.D.; Yurieva, O.; Zhang, D.; Finkelstein, J.; O’Donnell, M.E. Reconstitution of a eukaryotic replisome reveals suppression mechanisms that define leading/lagging strand operation. Elife 2015, 4, e04988. [Google Scholar] [CrossRef] [PubMed]
- Paunesku, T.; Mittal, S.; Protić, M.; Oryhon, J.; Korolev, S.V.; Joachimiak, A.; Woloschak, G.E. Proliferating cell nuclear antigen (PCNA): Ringmaster of the genome. Int. J. Radiat. Biol. 2001, 77, 1007–1021. [Google Scholar] [CrossRef]
- Moldovan, G.-L.; Pfander, B.; Jentsch, S. PCNA, the Maestro of the Replication Fork. Cell 2007, 129, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Maga, G.; Hübscher, U. Proliferating cell nuclear antigen (PCNA): A dancer with many partners. J. Cell Sci. 2003, 116, 3051–3060. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Kamimura, Y.; Okawa, M.; Muramatsu, S.; Sugino, A.; Araki, H. GINS, a novel multiprotein complex required for chromosomal DNA replication in budding yeast. Genes Dev. 2003, 17, 1153–1165. [Google Scholar] [CrossRef]
- Sengupta, S.; Van Deursen, F.; De Piccoli, G.; Labib, K. Dpb2 Integrates the Leading-Strand DNA Polymerase into the Eukaryotic Replisome. Curr. Biol. 2013, 23, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Langston, L.D.; Zhang, D.; Yurieva, O.; Georgescu, R.E.; Finkelstein, J.; Yao, N.Y.; Indiani, C.; O’Donnell, M.E. CMG helicase and DNA polymerase ε form a functional 15-subunit holoenzyme for eukaryotic leading-strand DNA replication. Proc. Natl. Acad. Sci. USA 2014, 111, 15390–15395. [Google Scholar] [CrossRef]
- Stepchenkova, E.I.; Zhuk, A.S.; Cui, J.; Tarakhovskaya, E.R.; Barbari, S.R.; Shcherbakova, P.V.; Polev, D.E.; Fedorov, R.; Poliakov, E.; Rogozin, I.B.; et al. Compensation for the absence of the catalytically active half of DNA polymerase ε in yeast by positively selected mutations in CDC28. Genetics 2021, 218, iyab060. [Google Scholar] [CrossRef]
- Johansson, E.; Garg, P.; Burgers, P.M.J. The Pol32 Subunit of DNA Polymerase δ Contains Separable Domains for Processive Replication and Proliferating Cell Nuclear Antigen (PCNA) Binding. J. Biol. Chem. 2004, 279, 1907–1915. [Google Scholar] [CrossRef]
- Krogan, N.J.; Cagney, G.; Yu, H.; Zhong, G.; Guo, X.; Ignatchenko, A.; Li, J.; Pu, S.; Datta, N.; Tikuisis, A.P.; et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 2006, 440, 637–643. [Google Scholar] [CrossRef]
- Burgers, P.M.J. Saccharomyces cerevisiae replication factor C. II. Formation and activity of complexes with the proliferating cell nuclear antigen and with DNA polymerases delta and epsilon. J. Biol. Chem. 1991, 266, 22698–22706. [Google Scholar] [CrossRef]
- Yuan, Z.; Georgescu, R.; Schauer, G.D.; O’Donnell, M.E.; Li, H. Structure of the polymerase ε holoenzyme and atomic model of the leading strand replisome. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, R.; Okazaki, T.; Sakabe, K.; Sugimoto, K.; Sugino, A. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proc. Natl. Acad. Sci. USA 1968, 59, 598–605. [Google Scholar] [CrossRef]
- Sugino, A. Yeast DNA polymerases and their role at the replication fork. Trends Biochem. Sci. 1995, 20, 319–323. [Google Scholar] [CrossRef]
- Perera, R.L.; Torella, R.; Klinge, S.; Kilkenny, M.L.; Maman, J.D.; Pellegrini, L. Mechanism for priming DNA synthesis by yeast DNA Polymerase α. Elife 2013, 2, e00482. [Google Scholar] [CrossRef] [PubMed]
- Bebenek, K.; Kunkel, T.A. Functions of DNA polymerases. Adv. Protein Chem. 2004, 69, 137–165. [Google Scholar] [CrossRef] [PubMed]
- Maga, G.; Stucki, M.; Spadari, S.; Hübscher, U. DNA polymerase switching: I. Replication factor C displaces DNA polymerase α prior to PCNA loading. J. Mol. Biol. 2000, 295, 791–801. [Google Scholar] [CrossRef]
- Bauer, G.A.; Burgers, P.M.J. The yeast analog of mammalian cyclin/proliferating-cell nuclear antigen interacts with mammalian DNA polymerase δ. Proc. Natl. Acad. Sci. USA 1988, 85, 7506–7510. [Google Scholar] [CrossRef]
- Sporbert, A.; Domaing, P.; Leonhardt, H.; Cardoso, M.C. PCNA acts as a stationary loading platform for transiently interacting Okazaki fragment maturation proteins. Nucleic Acids Res. 2005, 33, 3521–3528. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pavlov, Y.I.; Frahm, C.; McElhinny, S.A.N.; Niimi, A.; Suzuki, M.; Kunkel, T.A. Evidence that errors made by DNA polymerase α are corrected by DNA polymerase δ. Curr. Biol. 2006, 16, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Stodola, J.L.; Burgers, P.M. Resolving individual steps of Okazaki-fragment maturation at a millisecond timescale. Nat. Struct. Mol. Biol. 2016, 23, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.H. The cdc9 ligase joins completed replicons in Baker’s yeast. MGG Mol. Gen. Genet. 1983, 190, 315–317. [Google Scholar] [CrossRef]
- Kubota, T.; Katou, Y.; Nakato, R.; Shirahige, K.; Donaldson, A.D. Replication-Coupled PCNA Unloading by the Elg1 Complex Occurs Genome-wide and Requires Okazaki Fragment Ligation. Cell Rep. 2015, 12, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Guilliam, T.A.; Yeeles, J.T.P. Reconstitution of translesion synthesis reveals a mechanism of eukaryotic DNA replication restart. Nat. Struct. Mol. Biol. 2020, 27, 450–460. [Google Scholar] [CrossRef]
- Bailly, V.; Lauder, S.; Prakash, S.; Prakash, L. Yeast DNA repair proteins Rad6 and Rad18 form a heterodimer that has ubiquitin conjugating, DNA binding, and ATP hydrolytic activities. J. Biol. Chem. 1997, 272, 23360–23365. [Google Scholar] [CrossRef]
- Hoege, C.; Pfander, B.; Moldovan, G.-L.; Pyrowolakis, G.; Jentsch, S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 2002, 419, 135–141. [Google Scholar] [CrossRef]
- Bienko, M.; Green, C.M.; Crosetto, N.; Rudolf, F.; Zapart, G.; Coull, B.; Kannouche, P.; Wider, G.; Peter, M.; Lehmann, A.R.; et al. Biochemistry: Ubiquitin-binding domains in Y-family polymerases regulate translesion synthesis. Science 2005, 310, 1821–1824. [Google Scholar] [CrossRef]
- Brocas, C.; Charbonnier, J.B.; Dhérin, C.; Gangloff, S.; Maloisel, L. Stable interactions between DNA polymerase δ catalytic and structural subunits are essential for efficient DNA repair. DNA Repair 2010, 9, 1098–1111. [Google Scholar] [CrossRef]
- Tellier-Lebegue, C.; Dizet, E.; Ma, E.; Veaute, X.; Coïc, E.; Charbonnier, J.B.; Maloisel, L. The translesion DNA polymerases Pol ζ and Rev1 are activated independently of PCNA ubiquitination upon UV radiation in mutants of DNA polymerase δ. PLoS Genet. 2017, 13, e1007119. [Google Scholar] [CrossRef]
- Ulrich, H.D.; Jentsch, S. Two RING finger proteins mediate cooperation between ubiquitin-conjugating enzymes in DNA repair. EMBO J. 2000, 19, 3388–3397. [Google Scholar] [CrossRef]
- Branzei, D.; Vanoli, F.; Foiani, M. SUMOylation regulates Rad18-mediated template switch. Nature 2008, 456, 915–920. [Google Scholar] [CrossRef]
- Branzei, D.; Psakhye, I. DNA damage tolerance. Curr. Opin. Cell Biol. 2016, 40, 137–144. [Google Scholar] [CrossRef]
- Giannattasio, M.; Zwicky, K.; Follonier, C.; Foiani, M.; Lopes, M.; Branzei, D. Visualization of recombination-mediated damage bypass by template switching. Nat. Struct. Mol. Biol. 2014, 21, 884–892. [Google Scholar] [CrossRef]
- Blastyák, A.; Pintér, L.; Unk, I.; Prakash, L.; Prakash, S.; Haracska, L. Yeast Rad5 Protein Required for Postreplication Repair Has a DNA Helicase Activity Specific for Replication Fork Regression. Mol. Cell 2007, 28, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Vujanovic, M.; Krietsch, J.; Raso, M.C.; Terraneo, N.; Zellweger, R.; Schmid, J.A.; Taglialatela, A.; Huang, J.W.; Holland, C.L.; Zwicky, K.; et al. Replication Fork Slowing and Reversal upon DNA Damage Require PCNA Polyubiquitination and ZRANB3 DNA Translocase Activity. Mol. Cell 2017, 67, 882–890.e5. [Google Scholar] [CrossRef]
- Kolinjivadi, A.M.; Sannino, V.; De Antoni, A.; Zadorozhny, K.; Kilkenny, M.; Técher, H.; Baldi, G.; Shen, R.; Ciccia, A.; Pellegrini, L.; et al. Smarcal1-Mediated Fork Reversal Triggers Mre11-Dependent Degradation of Nascent DNA in the Absence of Brca2 and Stable Rad51 Nucleofilaments. Mol. Cell 2017, 67, 867–881.e7. [Google Scholar] [CrossRef] [PubMed]
- Vaisman, A.; Tissier, A.; Frank, E.G.; Goodman, M.F.; Woodgate, R. Human DNA Polymerase ι Promiscuous Mismatch Extension. J. Biol. Chem. 2001, 276, 30615–30622. [Google Scholar] [CrossRef] [PubMed]
- Quinet, A.; Lemaçon, D.; Vindigni, A. Replication Fork Reversal: Players and Guardians. Mol. Cell 2017, 68, 830–833. [Google Scholar] [CrossRef]
- Joseph, S.A.; Taglialatela, A.; Leuzzi, G.; Huang, J.W.; Cuella-Martin, R.; Ciccia, A. Time for remodeling: SNF2-family DNA translocases in replication fork metabolism and human disease. DNA Repair 2020, 95, 102943. [Google Scholar] [CrossRef] [PubMed]
- Fabre, F.; Chan, A.; Heyer, W.D.; Gangloff, S. Alternate pathways involving Sgs1/Top3, Mus81/ Mms4, and Srs2 prevent formation of toxic recombination intermediates from single-stranded gaps created by DNA replication. Proc. Natl. Acad. Sci. USA 2002, 99, 16887–16892. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, A.; Ogawa, H.; Ogawa, T. Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell 1992, 69, 457–470. [Google Scholar] [CrossRef]
- Sung, P. Catalysis of ATP-dependent homologous DNA pairing and strand exchange by yeast RAD51 protein. Science 1994, 265, 1241–1243. [Google Scholar] [CrossRef]
- Ulrich, H.D. The srs2 suppressor of UV sensitivity acts specifically on the RAD5- and MMS2-dependent branch of the RAD6 pathway. Nucleic Acids Res. 2001, 29, 3487–3494. [Google Scholar] [CrossRef]
- Aboussekhra, A.; Chanet, R.; Zgaga, Z.; Cassier-Chauvat, C.; Heude, M.; Fabre, F. RADH, a gene of Saccharomyces cerevisiae encoding a putative DNA helicase involved in DNA repair. Characteristics of radH mutants and sequence of the gene. Nucleic Acids Res. 1989, 17, 7211–7219. [Google Scholar] [CrossRef]
- Rong, L.; Palladino, F.; Aguilera, A.; Klein, H.L. The hyper-gene conversion hpr5-1 mutation of Saccharomyces cerevisiae is an allele of the SRS2/RADH gene. Genetics 1991, 127, 75–85. [Google Scholar] [CrossRef]
- Yuan, G.C.; Liu, Y.J.; Dion, M.F.; Slack, M.D.; Wu, L.F.; Altschuler, S.J.; Rando, O.J. Molecular biology: Genome-scale identification of nucleosome positions in S. cerevisiae. Science 2005, 309, 626–630. [Google Scholar] [CrossRef]
- Anderson, J.D.; Widom, J. Sequence and position-dependence of the equilibrium accessibility of nucleosomal DNA target sites. J. Mol. Biol. 2000, 296, 979–987. [Google Scholar] [CrossRef]
- Schäfer, G.; Smith, E.M.; Patterton, H.G. The Saccharomyces cerevisiae linker histone Hho1p, with two globular domains, can simultaneously bind to two four-way junction DNA molecules. Biochemistry 2005, 44, 16766–16775. [Google Scholar] [CrossRef]
- Richmond, T.J.; Davey, C.A. The structure of DNA in the nucleosome core. Nature 2003, 423, 145–150. [Google Scholar] [CrossRef]
- Ushinsky, S.C.; Bussey, H.; Ahmed, A.A.; Wang, Y.; Friesen, J.; Williams, B.A.; Storms, R.K. Histone H1 in Saccharomyces cerevisiae. Yeast 1997, 13, 151–161. [Google Scholar] [CrossRef]
- Lacal, I.; Ventura, R. Epigenetic Inheritance: Concepts, Mechanisms and Perspectives. Front. Mol. Neurosci. 2018, 11, 292. [Google Scholar] [CrossRef]
- Kayne, P.S.; Kim, U.J.; Han, M.; Mullen, J.R.; Yoshizaki, F.; Grunstein, M. Extremely conserved histone H4 N terminus is dispensable for growth but essential for repressing the silent mating loci in yeast. Cell 1988, 55, 27–39. [Google Scholar] [CrossRef]
- Laurenson, P.; Rine, J. Silencers, silencing, and heritable transcriptional states. Microbiol. Rev. 1992, 56, 543–560. [Google Scholar] [CrossRef] [PubMed]
- Brownell, J.E.; Zhou, J.; Ranalli, T.; Kobayashi, R.; Edmondson, D.G.; Roth, S.Y.; Allis, C.D. Tetrahymena histone acetyltransferase A: A homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell 1996, 84, 843–851. [Google Scholar] [CrossRef]
- Nakajima, H. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Tanpakushitsu Kakusan Koso 2007, 52, 1790–1791. [Google Scholar]
- Shogren-Knaak, M.; Ishii, H.; Sun, J.M.; Pazin, M.J.; Davie, J.R.; Peterson, C.L. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science 2006, 311, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.-I.; Armstrong, C.M.; Kaeberlein, M.; Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 2000, 403, 795–800. [Google Scholar] [CrossRef]
- Hoppe, G.J.; Tanny, J.C.; Rudner, A.D.; Gerber, S.A.; Danaie, S.; Gygi, S.P.; Moazed, D. Steps in Assembly of Silent Chromatin in Yeast: Sir3-Independent Binding of a Sir2/Sir4 Complex to Silencers and Role for Sir2-Dependent Deacetylation. Mol. Cell. Biol. 2002, 22, 4167–4180. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Vega-Palas, M.A.; Grunstein, M. Rap1-Sir4 binding independent of other Sir, yKu, or histone interactions initiates the assembly of telomeric heterochromatin in yeast. Genes Dev. 2002, 16, 1528–1539. [Google Scholar] [CrossRef]
- Rusche, L.N.; Kirchmaier, A.L.; Rine, J. Ordered Nucleation and Spreading of Silenced Chromatin in Saccharomyces cerevisiae. Mol. Biol. Cell 2002, 13, 2207–2222. [Google Scholar] [CrossRef]
- Pillus, L.; Rine, J. Epigenetic inheritance of transcriptional states in S. cerevisiae. Cell 1989, 59, 637–647. [Google Scholar] [CrossRef]
- Morse, R.H. RAP, RAP, open up! New wrinkles for RAP1 in yeast. Trends Genet. 2000, 16, 51–53. [Google Scholar] [CrossRef]
- Aparicio, O.M.; Billington, B.L.; Gottschling, D.E. Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell 1991, 66. [Google Scholar] [CrossRef]
- Gardner, K.A.; Rine, J.; Fox, C.A. A Region of the Sir1 Protein Dedicated to Recognition of a Silencer and Required for Interaction with the Orc1 Protein in Saccharomyces cerevisiae. Genetics 1999, 151, 31–44. [Google Scholar] [CrossRef]
- Pillus, L.; Rine, J. SIR1 and the origin of epigenetic states in Saccharomyces cerevisiae. Cold Spring Harb. Symp. Quant. Biol. 2004, 69, 259–266. [Google Scholar] [CrossRef][Green Version]
- Jackson, V. Deposition of newly synthesized histones: Hybrid nucleosomes are not tandemly arranged on daughter DNA strands. Biochemistry 1988, 27, 2109–2120. [Google Scholar] [CrossRef] [PubMed]
- Gershon, L.; Kupiec, M. The Amazing Acrobat: Yeast’s Histone H3K56 Juggles Several Important Roles while Maintaining Perfect Balance. Genes 2021, 12, 342. [Google Scholar] [CrossRef] [PubMed]
- English, C.M.; Adkins, M.W.; Carson, J.J.; Churchill, M.E.A.; Tyler, J.K. Structural Basis for the Histone Chaperone Activity of Asf1. Cell 2006, 127, 495–508. [Google Scholar] [CrossRef]
- Campos, E.I.; Fillingham, J.; Li, G.; Zheng, H.; Voigt, P.; Kuo, W.-H.W.; Seepany, H.; Gao, Z.; Day, L.A.; Greenblatt, J.F.; et al. The program for processing newly synthesized histones H3.1 and H4. Nat. Struct. Mol. Biol. 2010, 17, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Schwabish, M.A.; Struhl, K. Asf1 Mediates Histone Eviction and Deposition during Elongation by RNA Polymerase II. Mol. Cell 2006, 22, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, R.; Hudson, A.; Jackson, S.P. Yeast Rtt109 Promotes Genome Stability by Acetylating Histone H3 on Lysine 56. Science 2007, 315, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.A.; Lam, W.M.; Burgers, P.M.; Kaufman, P.D. Histone deposition protein Asf1 maintains DNA replisome integrity and interacts with replication factor C. Genes Dev. 2005, 19, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Hu, Q.; Li, Q.; Thompson, J.R.; Cui, G.; Fazly, A.; Davies, B.A.; Botuyan, M.V.; Zhang, Z.; Mer, G. Structural basis for recognition of H3K56-acetylated histone H3–H4 by the chaperone Rtt106. Nature 2012, 483, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shahar, T.R.; Castillo, A.G.; Osborne, M.J.; Borden, K.L.B.; Kornblatt, J.; Verreault, A. Two Fundamentally Distinct PCNA Interaction Peptides Contribute to Chromatin Assembly Factor 1 Function. Mol. Cell. Biol. 2009, 29, 6353–6365. [Google Scholar] [CrossRef]
- Kondratick, C.M.; Litman, J.M.; Shaffer, K.V.; Washington, M.T.; Dieckman, L.M. Crystal structures of PCNA mutant proteins defective in gene silencing suggest a novel interaction site on the front face of the PCNA ring. PLoS ONE 2018, 13, e0193333. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, H.; Wurtele, H.; Davies, B.; Horazdovsky, B.; Verreault, A.; Zhang, Z. Acetylation of Histone H3 Lysine 56 Regulates Replication-Coupled Nucleosome Assembly. Cell 2008, 134, 244–255. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Feng, J.; Leng, H.; Li, S.; Xiao, J.; Liu, S.; Xu, Z.; Xu, J.; Li, D.; et al. The Histone Chaperone FACT Contributes to DNA Replication-Coupled Nucleosome Assembly. Cell Rep. 2016, 14, 1128–1141. [Google Scholar] [CrossRef]
- Gali, V.K.; Dickerson, D.; Katou, Y.; Fujiki, K.; Shirahige, K.; Owen-Hughes, T.; Kubota, T.; Donaldson, A.D. Identification of Elg1 interaction partners and effects on post-replication chromatin re-formation. PLoS Genet. 2018, 14, e1007783. [Google Scholar] [CrossRef]
- Hartman, T.; Stead, K.; Koshland, D.; Guacci, V. Pds5p is an essential chromosomal protein required for both sister chromatid cohesion and condensation in Saccharomyces cerevisiae. J. Cell Biol. 2000, 151, 613–626. [Google Scholar] [CrossRef]
- Kirchmaier, A.L.; Rine, J. DNA Replication-Independent Silencing in S. cerevisiae. Science 2001, 291, 646–650. [Google Scholar] [CrossRef]
- Li, Y.-C.; Cheng, T.-H.; Gartenberg, M.R. Establishment of Transcriptional Silencing in the Absence of DNA Replication. Science 2001, 291, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Dodson, A.E.; Rine, J. Heritable capture of heterochromatin dynamics in Saccharomyces cerevisiae. eLife 2015, 4, e05007. [Google Scholar] [CrossRef] [PubMed]
- Janke, R.; King, G.A.; Kupiec, M.; Rine, J. Pivotal roles of PCNA loading and unloading in heterochromatin function. Proc. Natl. Acad. Sci. USA 2018, 115, E2030–E2039. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.-M.; Nishiyama, T. Sister Chromatid Cohesion. Cold Spring Harb. Perspect. Biol. 2012, 4, a011130. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.-M.; Tedeschi, A.; Schmitz, J. The cohesin complex and its roles in chromosome biology. Genes Dev. 2008, 22, 3089–3114. [Google Scholar] [CrossRef] [PubMed]
- Nasmyth, K. Disseminating the Genome: Joining, Resolving, and Separating Sister Chromatids during Mitosis and Meiosis. Annu. Rev. Genet. 2001, 35, 673–745. [Google Scholar] [CrossRef]
- Nicklas, R.B. The Forces that Move Chromosomes in Mitosis. Annu. Rev. Biophys. Biophys. Chem. 1988, 17, 431–449. [Google Scholar] [CrossRef]
- Joglekar, A.P.; Hunt, A.J. A Simple, Mechanistic Model for Directional Instability during Mitotic Chromosome Movements. Biophys. J. 2002, 83, 42–58. [Google Scholar] [CrossRef]
- Hirano, T. At the heart of the chromosome: SMC proteins in action. Nat. Rev. Mol. Cell Biol. 2006, 7, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Gruber, S.; Haering, C.H.; Nasmyth, K. Chromosomal Cohesin Forms a Ring. Cell 2003, 112, 765–777. [Google Scholar] [CrossRef]
- Haering, C.H.; Löwe, J.; Hochwagen, A.; Nasmyth, K. Molecular Architecture of SMC Proteins and the Yeast Cohesin Complex. Mol. Cell 2002, 9, 773–788. [Google Scholar] [CrossRef]
- Guacci, V.; Koshland, D.; Strunnikov, A. A Direct Link between Sister Chromatid Cohesion and Chromosome Condensation Revealed through the Analysis of MCD1 in S. cerevisiae. Cell 1997, 91, 47–57. [Google Scholar] [CrossRef]
- Michaelis, C.; Ciosk, R.; Nasmyth, K. Cohesins: Chromosomal Proteins that Prevent Premature Separation of Sister Chromatids. Cell 1997, 91, 35–45. [Google Scholar] [CrossRef]
- Haering, C.H.; Schoffnegger, D.; Nishino, T.; Helmhart, W.; Nasmyth, K.; Löwe, J. Structure and Stability of Cohesin’s Smc1-Kleisin Interaction. Mol. Cell 2004, 15, 951–964. [Google Scholar] [CrossRef]
- Arumugam, P.; Gruber, S.; Tanaka, K.; Haering, C.H.; Mechtler, K.; Nasmyth, K. ATP Hydrolysis Is Required for Cohesin’s Association with Chromosomes. Curr. Biol. 2003, 13, 1941–1953. [Google Scholar] [CrossRef]
- Tanaka, K.; Hao, Z.; Kai, M.; Okayama, H. Establishment and maintenance of sister chromatid cohesion in fission yeast by a unique mechanism. EMBO J. 2001, 20, 5779–5790. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.-L.; Gligoris, T.; Upcher, W.; Kato, Y.; Shirahige, K.; Nasmyth, K.; Beckouët, F. Pds5 promotes and protects cohesin acetylation. Proc. Natl. Acad. Sci. USA 2013, 110, 13020–13025. [Google Scholar] [CrossRef] [PubMed]
- Sutani, T.; Kawaguchi, T.; Kanno, R.; Itoh, T.; Shirahige, K. Budding Yeast Wpl1(Rad61)-Pds5 Complex Counteracts Sister Chromatid Cohesion-Establishing Reaction. Curr. Biol. 2009, 19, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Henrikus, S.S.; Costa, A. Towards a Structural Mechanism for Sister Chromatid Cohesion Establishment at the Eukaryotic Replication Fork. Biology 2021, 10, 466. [Google Scholar] [CrossRef] [PubMed]
- Nasmyth, K.; Haering, C.H. Cohesin: Its Roles and Mechanisms. Annu. Rev. Genet. 2009, 43, 525–558. [Google Scholar] [CrossRef]
- Milutinovich, M.; Koshland, D.E. Molecular biology: SMC Complexes—Wrapped Up in Controversy. Science 2003, 300, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Eng, T.; Guacci, V.; Koshland, D. Interallelic complementation provides functional evidence for cohesin–cohesin interactions on DNA. Mol. Biol. Cell 2015, 26, 4224–4235. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Scheinost, J.C.; Petela, N.J.; Gligoris, T.G.; Wissler, M.; Ogushi, S.; Collier, J.E.; Voulgaris, M.; Kurze, A.; Chan, K.-L.; et al. The Cohesin Ring Uses Its Hinge to Organize DNA Using Non-topological as well as Topological Mechanisms. Cell 2018, 173, 1508–1519.e18. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Koshland, D. Cohesin architecture and clustering in vivo. eLife 2021, 10, e62243. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Zhao, S.; Zuo, M.-Q.; Zhang, J.; Hou, W.; Dong, M.-Q.; Cao, Q.; Lou, H. The acetyltransferase Eco1 elicits cohesin dimerization during S phase. J. Biol. Chem. 2020, 295, 7554–7565. [Google Scholar] [CrossRef] [PubMed]
- Uhlmann, F.; Lottspeich, F.; Nasmyth, K. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 1999, 400, 37–42. [Google Scholar] [CrossRef]
- Uhlmann, F.; Wernic, D.; Poupart, M.-A.; Koonin, E.V.; Nasmyth, K. Cleavage of Cohesin by the CD Clan Protease Separin Triggers Anaphase in Yeast. Cell 2000, 103, 375–386. [Google Scholar] [CrossRef]
- Ciosk, R.; Shirayama, M.; Shevchenko, A.; Tanaka, T.; Toth, A.; Shevchenko, A.; Nasmyth, K. Cohesin’s Binding to Chromosomes Depends on a Separate Complex Consisting of Scc2 and Scc4 Proteins. Mol. Cell 2000, 5, 243–254. [Google Scholar] [CrossRef]
- Lengronne, A.; McIntyre, J.; Katou, Y.; Kanoh, Y.; Hopfner, K.-P.; Shirahige, K.; Uhlmann, F. Establishment of Sister Chromatid Cohesion at the S. cerevisiae Replication Fork. Mol. Cell 2006, 23, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Uhlmann, F.; Nasmyth, K. Cohesion between sister chromatids must be established during DNA replication. Curr. Biol. 1998, 8, 1095–1102. [Google Scholar] [CrossRef]
- Ivanov, D.; Schleiffer, A.; Eisenhaber, F.; Mechtler, K.; Haering, C.H.; Nasmyth, K. Eco1 Is a Novel Acetyltransferase that Can Acetylate Proteins Involved in Cohesion. Curr. Biol. 2002, 12, 323–328. [Google Scholar] [CrossRef]
- Hou, F.; Zou, H. Two Human Orthologues of Eco1/Ctf7 Acetyltransferases Are Both Required for Proper Sister-Chromatid Cohesion. Mol. Biol. Cell 2005, 16, 3908–3918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, X.; Li, Y.; Kim, B.-J.; Jia, J.; Huang, Z.; Yang, T.; Fu, X.; Jung, S.Y.; Wang, Y.; et al. Acetylation of Smc3 by Eco1 Is Required for S Phase Sister Chromatid Cohesion in Both Human and Yeast. Mol. Cell 2008, 31, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.; Gillespie, P.J.; Hirano, T. Human Wapl Is a Cohesin-Binding Protein that Promotes Sister-Chromatid Resolution in Mitotic Prophase. Curr. Biol. 2006, 16, 2406–2417. [Google Scholar] [CrossRef] [PubMed]
- Unal, E.; Heidinger-Pauli, J.M.; Kim, W.; Guacci, V.; Onn, I.; Gygi, S.P.; Koshland, D.E. A Molecular Determinant for the Establishment of Sister Chromatid Cohesion. Science 2008, 321, 566–569. [Google Scholar] [CrossRef]
- Ben-Shahar, T.R.; Heeger, S.; Lehane, C.; East, P.; Flynn, H.; Skehel, M.; Uhlmann, F. Eco1-Dependent Cohesin Acetylation During Establishment of Sister Chromatid Cohesion. Science 2008, 321, 563–566. [Google Scholar] [CrossRef]
- Rowland, B.D.; Roig, M.B.; Nishino, T.; Kurze, A.; Uluocak, P.; Mishra, A.; Beckouet, F.; Underwood, P.; Metson, J.; Imre, R.; et al. Building Sister Chromatid Cohesion: Smc3 Acetylation Counteracts an Antiestablishment Activity. Mol. Cell 2009, 33, 763–774. [Google Scholar] [CrossRef]
- Guacci, V.; Chatterjee, F.; Robison, B.; Koshland, D.E. Communication between distinct subunit interfaces of the cohesin complex promotes its topological entrapment of DNA. eLife 2019, 8, e46347. [Google Scholar] [CrossRef]
- Moldovan, G.-L.; Pfander, B.; Jentsch, S. PCNA Controls Establishment of Sister Chromatid Cohesion during S Phase. Mol. Cell 2006, 23, 723–732. [Google Scholar] [CrossRef]
- Bender, D.; Da Silva, E.M.L.; Chen, J.; Poss, A.; Gawey, L.; Rulon, Z.; Rankin, S. Multivalent interaction of ESCO2 with the replication machinery is required for sister chromatid cohesion in vertebrates. Proc. Natl. Acad. Sci. USA 2020, 117, 1081–1089. [Google Scholar] [CrossRef]
- Feytout, A.; Vaur, S.; Genier, S.; Vazquez, S.; Javerzat, J.-P. Psm3 Acetylation on Conserved Lysine Residues Is Dispensable for Viability in Fission Yeast but Contributes to Eso1-Mediated Sister Chromatid Cohesion by Antagonizing Wpl1. Mol. Cell. Biol. 2011, 31, 1771–1786. [Google Scholar] [CrossRef]
- Borges, V.; Smith, D.J.; Whitehouse, I.; Uhlmann, F. An Eco1-independent sister chromatid cohesion establishment pathway in S. cerevisiae. Chromosoma 2013, 122, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.L.; Pot, I.; Chang, M.; Xu, H.; Aneliunas, V.; Kwok, T.; Newitt, R.; Aebersold, R.; Boone, C.; Brown, G.W.; et al. Identification of Protein Complexes Required for Efficient Sister Chromatid Cohesion. Mol. Biol. Cell 2004, 15, 1736–1745. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.D.; Eckley, D.M.; Lee, M.S.; Hanna, J.S.; Hughes, A.; Peyser, B.; Jie, C.; Irizarry, R.; Spencer, F.A. S-Phase Checkpoint Genes Safeguard High-Fidelity Sister Chromatid Cohesion. Mol. Biol. Cell 2004, 15, 1724–1735. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Boone, C.; Klein, H.L. Mrc1 Is Required for Sister Chromatid Cohesion To Aid in Recombination Repair of Spontaneous Damage. Mol. Cell. Biol. 2004, 24, 7082–7090. [Google Scholar] [CrossRef][Green Version]
- Xu, H.; Boone, C.; Brown, G.W. Genetic Dissection of Parallel Sister-Chromatid Cohesion Pathways. Genetics 2007, 176, 1417–1429. [Google Scholar] [CrossRef]
- Uzunova, S.D.; Zarkov, A.S.; Ivanova, A.M.; Stoynov, S.S.; Nedelcheva-Veleva, M.N. The subunits of the S-phase checkpoint complex Mrc1/Tof1/Csm3: Dynamics and interdependence. Cell Div. 2014, 9, 4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mohanty, B.K.; Bairwa, N.K.; Bastia, D. The Tof1p-Csm3p protein complex counteracts the Rrm3p helicase to control replication termination of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2006, 103, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Shemesh, K.; Liefshitz, B.; Kupiec, M. Genetic and physical interactions between the yeastELG1gene and orthologs of the Fanconi anemia pathway. Cell Cycle 2013, 12, 1625–1636. [Google Scholar] [CrossRef]
- Katheeja, M.N.; Das, S.P.; Laha, S. The budding yeast protein Chl1p is required for delaying progression through G1/S phase after DNA damage. Cell Div. 2021, 16, 1–16. [Google Scholar] [CrossRef]
- Porcella, S.Y.; Koussa, N.C.; Tang, C.P.; Kramer, D.N.; Srivastava, P.; Smith, D.J. Separable, Ctf4-mediated recruitment of DNA Polymerase α for initiation of DNA synthesis at replication origins and lagging-strand priming during replication elongation. PLoS Genet. 2020, 16, e1008755. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Georgescu, R.; Santos, R.D.L.A.; Zhang, D.; Bai, L.; Yao, N.Y.; Zhao, G.; O’Donnell, M.E.; Li, H. Ctf4 organizes sister replisomes and Pol α into a replication factory. eLife 2019, 8, e47405. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Fumasoni, M.; Petela, N.J.; Murray, A.; Nasmyth, K.A. Cohesion is established during DNA replication utilising chromosome associated cohesin rings as well as those loaded de novo onto nascent DNAs. eLife 2020, 9, e56611. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Petela, N.J.; Scheinost, J.C.; Collier, J.; Voulgaris, M.; Roig, M.B.; Beckouet, F.; Hu, B.; Nasmyth, K.A. Scc2 counteracts a Wapl-independent mechanism that releases cohesin from chromosomes during G1. eLife 2019, 8, e44736. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Kubota, Y.; Tsujimura, T.; Kumano, M.; Masai, H.; Takisawa, H. Replisome progression complex links DNA replication to sister chromatid cohesion in Xenopusegg extracts. Genes Cells 2009, 14, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Parnas, O.; Zipin-Roitman, A.; Mazor, Y.; Liefshitz, B.; Ben-Aroya, S.; Kupiec, M. The Elg1 Clamp Loader Plays a Role in Sister Chromatid Cohesion. PLoS ONE 2009, 4, e5497. [Google Scholar] [CrossRef] [PubMed]
- Maradeo, M.E.; Skibbens, R.V. The Elg1-RFC Clamp-Loading Complex Performs a Role in Sister Chromatid Cohesion. PLoS ONE 2009, 4, e4707. [Google Scholar] [CrossRef]
- Tong, K.; Skibbens, R.V. Pds5 regulators segregate cohesion and condensation pathways in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2015, 112, 7021–7026. [Google Scholar] [CrossRef]
- Maradeo, M.E.; Skibbens, R.V. Replication Factor C Complexes Play Unique Pro- and Anti-Establishment Roles in Sister Chromatid Cohesion. PLoS ONE 2010, 5, e15381. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arbel, M.; Choudhary, K.; Tfilin, O.; Kupiec, M. PCNA Loaders and Unloaders—One Ring That Rules Them All. Genes 2021, 12, 1812. https://doi.org/10.3390/genes12111812
Arbel M, Choudhary K, Tfilin O, Kupiec M. PCNA Loaders and Unloaders—One Ring That Rules Them All. Genes. 2021; 12(11):1812. https://doi.org/10.3390/genes12111812
Chicago/Turabian StyleArbel, Matan, Karan Choudhary, Ofri Tfilin, and Martin Kupiec. 2021. "PCNA Loaders and Unloaders—One Ring That Rules Them All" Genes 12, no. 11: 1812. https://doi.org/10.3390/genes12111812
APA StyleArbel, M., Choudhary, K., Tfilin, O., & Kupiec, M. (2021). PCNA Loaders and Unloaders—One Ring That Rules Them All. Genes, 12(11), 1812. https://doi.org/10.3390/genes12111812





