Genomic Consideration in Chemotherapy-Induced Ovarian Damage and Fertility Preservation
Abstract
1. Introduction
2. Genes Involved in the Regulation of Ovarian Follicular reserve
2.1. Primordial Germ Cells Formation and Gonad Colonization
2.2. Germ Cell Survival and DNA Damage Repair
2.3. Follicular Assembly and Turnover
3. Mechanism of Chemotherapy-Induced Ovarian Damage
3.1. Chemotherapy-Induced DNA DSBs
3.2. Burnout Effect
3.3. Stromal and Microvascular Damage
3.4. Genes Related to Chemotherapy-Induced Ovarian Damage
3.4.1. DNA Damage Repair
3.4.2. Apoptosis
3.4.3. Follicular Activation and Development
4. Prevention Strategy for Ovarian Damage
4.1. Consideration for Protective Genetic Variants for Chemotherapy-Induced Ovarian Damage
4.2. Genetic Screening of Candidate Markers
4.3. Other Options for Prevention of Ovarian Damage
4.3.1. Gonadotropin-Releasing Hormone (GnRH)
4.3.2. AMH
4.3.3. AS101
4.3.4. Imatinib
4.3.5. Sphingosine-1-Phosphate
4.4. Cryopreservation
4.4.1. Embryo Cryopreservation
4.4.2. Oocyte Cryopreservation
4.4.3. Ovarian Tissue Cryopreservation and Transplantation
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults, 2020. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef]
- Sklar, C.A.; Mertens, A.C.; Mitby, P.; Whitton, J.; Stovall, M.; Kasper, C.; Mulder, J.; Green, D.; Nicholson, H.S.; Yasui, Y.; et al. Premature menopause in survivors of childhood cancer: A report from the childhood cancer survivor study. J. Natl. Cancer Inst. 2006, 98, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ozkavukcu, S.; Heytens, E.; Moy, F.; Oktay, K. Value of early referral to fertility preservation in young women with breast cancer. J. Clin. Oncol. 2010, 28, 4683–4686. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Heytens, E.; Moy, F.; Ozkavukcu, S.; Oktay, K. Determinants of access to fertility preservation in women with breast cancer. Fertil. Steril. 2011, 95, 1932–1936. [Google Scholar] [CrossRef] [PubMed]
- Letourneau, J.M.; Ebbel, E.E.; Katz, P.P.; Oktay, K.H.; McCulloch, C.E.; Ai, W.Z.; Chien, A.J.; Melisko, M.E.; Cedars, M.I.; Rosen, M.P. Acute ovarian failure underestimates age-specific reproductive impairment for young women undergoing chemotherapy for cancer. Cancer 2012, 118, 1933–1939. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.W.; Han, S.J.; Lee, S.; Park, H.T.; Song, J.Y.; Kim, T. Molecular Mechanism and Prevention Strategy of Chemotherapy- and Radiotherapy-Induced Ovarian Damage. Int. J. Mol. Sci. 2021, 22, 7484. [Google Scholar] [CrossRef]
- Bedoschi, G.; Navarro, P.A.; Oktay, K. Chemotherapy-induced damage to ovary: Mechanisms and clinical impact. Future Oncol. 2016, 12, 2333–2344. [Google Scholar] [CrossRef]
- Wallace, W.H.; Kelsey, T.W. Human ovarian reserve from conception to the menopause. PLoS ONE 2010, 5, e8772. [Google Scholar] [CrossRef]
- Hirshfield, A.N. Development of follicles in the mammalian ovary. Int. Rev. Cytol. 1991, 124, 43–101. [Google Scholar] [CrossRef]
- Pepling, M.E. Follicular assembly: Mechanisms of action. Reproduction 2012, 143, 139–149. [Google Scholar] [CrossRef]
- Skinner, M.K. Regulation of primordial follicle assembly and development. Hum. Reprod. Update 2005, 11, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Fortuno, C.; Labarta, E. Genetics of primary ovarian insufficiency: A review. J. Assist. Reprod. Genet. 2014, 31, 1573–1585. [Google Scholar] [CrossRef]
- Jiao, X.; Ke, H.; Qin, Y.; Chen, Z.J. Molecular Genetics of Premature Ovarian Insufficiency. Trends Endocrinol. Metab. 2018, 29, 795–807. [Google Scholar] [CrossRef]
- Pelosi, E.; Forabosco, A.; Schlessinger, D. Genetics of the ovarian reserve. Front. Genet. 2015, 6, 308. [Google Scholar] [CrossRef] [PubMed]
- Lawson, K.A.; Dunn, N.R.; Roelen, B.A.; Zeinstra, L.M.; Davis, A.M.; Wright, C.V.; Korving, J.P.; Hogan, B.L. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999, 13, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Liu, X.-M.; Marble, A.; Lawson, K.A.; Zhao, G.-Q. Requirement of Bmp8b for the generation of primordial germ cells in the mouse. Mol. Endocrinol. 2000, 14, 1053–1063. [Google Scholar] [CrossRef]
- Chang, H.; Matzuk, M.M. Smad5 is required for mouse primordial germ cell development. Mech. Dev. 2001, 104, 61–67. [Google Scholar] [CrossRef]
- Ying, Y.; Zhao, G.Q. Cooperation of endoderm-derived BMP2 and extraembryonic ectoderm-derived BMP4 in primordial germ cell generation in the mouse. Dev. Biol. 2001, 232, 484–492. [Google Scholar] [CrossRef]
- Tremblay, K.D.; Dunn, N.R.; Robertson, E.J. Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation. Development 2001, 128, 3609–3621. [Google Scholar] [CrossRef]
- Nichols, J.; Zevnik, B.; Anastassiadis, K.; Niwa, H.; Klewe-Nebenius, D.; Chambers, I.; Scholer, H.; Smith, A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998, 95, 379–391. [Google Scholar] [CrossRef]
- Kobayashi, S.; Yamada, M.; Asaoka, M.; Kitamura, T. Essential role of the posterior morphogen nanos for germline development in Drosophila. Nature 1996, 380, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Sasaoka, Y.; Kiso, M.; Abe, K.; Haraguchi, S.; Kobayashi, S.; Saga, Y. Conserved role of nanos proteins in germ cell development. Science 2003, 301, 1239–1241. [Google Scholar] [CrossRef] [PubMed]
- Agoulnik, A.I.; Lu, B.; Zhu, Q.; Truong, C.; Ty, M.T.; Arango, N.; Chada, K.K.; Bishop, C.E. A novel gene, Pog, is necessary for primordial germ cell proliferation in the mouse and underlies the germ cell deficient mutation, gcd. Hum. Mol. Genet. 2002, 11, 3047–3053. [Google Scholar] [CrossRef] [PubMed]
- Atchison, F.W.; Capel, B.; Means, A.R. Pin1 regulates the timing of mammalian primordial germ cell proliferation. Development 2003, 130, 3579–3586. [Google Scholar] [CrossRef]
- Thomas, F.H.; Vanderhyden, B.C. Oocyte-granulosa cell interactions during mouse follicular development: Regulation of kit ligand expression and its role in oocyte growth. Reprod. Biol. Endocrinol. 2006, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Gawriluk, T.R.; Hale, A.N.; Flaws, J.A.; Dillon, C.P.; Green, D.R.; Rucker, E.B., 3rd. Autophagy is a cell survival program for female germ cells in the murine ovary. Reproduction 2011, 141, 759–765. [Google Scholar] [CrossRef]
- Juneja, S.C.; Barr, K.J.; Enders, G.C.; Kidder, G.M. Defects in the germ line and gonads of mice lacking connexin43. Biol. Reprod. 1999, 60, 1263–1270. [Google Scholar] [CrossRef]
- Romanienko, P.J.; Camerini-Otero, R.D. The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol. Cell 2000, 6, 975–987. [Google Scholar] [CrossRef]
- De Vries, S.S.; Baart, E.B.; Dekker, M.; Siezen, A.; de Rooij, D.G.; de Boer, P.; te Riele, H. Mouse MutS-like protein Msh5 is required for proper chromosome synapsis in male and female meiosis. Genes Dev. 1999, 13, 523–531. [Google Scholar] [CrossRef]
- Kneitz, B.; Cohen, P.E.; Avdievich, E.; Zhu, L.; Kane, M.F.; Hou, H.; Kolodner, R.D.; Kucherlapati, R.; Pollard, J.W.; Edelmann, W. MutS homolog 4 localization to meiotic chromosomes is required for chromosome pairing during meiosis in male and female mice. Genes Dev. 2000, 14, 1085–1097. [Google Scholar] [PubMed]
- Pittman, D.L.; Cobb, J.; Schimenti, K.J.; Wilson, L.A.; Cooper, D.M.; Brignull, E.; Handel, M.A.; Schimenti, J.C. Meiotic prophase arrest with failure of chromosome synapsis in mice deficient for Dmc1, a germline-specific RecA homolog. Mol. Cell 1998, 1, 697–705. [Google Scholar] [CrossRef]
- Barlow, C.; Liyanage, M.; Moens, P.B.; Tarsounas, M.; Nagashima, K.; Brown, K.; Rottinghaus, S.; Jackson, S.P.; Tagle, D.; Ried, T. Atm deficiency results in severe meiotic disruption as early as leptonema of prophase I. Development 1998, 125, 4007–4017. [Google Scholar] [CrossRef] [PubMed]
- Oktay, K.; Kim, J.Y.; Barad, D.; Babayev, S.N. Association of BRCA1 mutations with occult primary ovarian insufficiency: A possible explanation for the link between infertility and breast/ovarian cancer risks. J. Clin. Oncol. 2010, 28, 240. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.T.; Beattie, M.; Chen, L.M.; Oktay, K.; Crawford, S.L.; Gold, E.B.; Cedars, M.; Rosen, M. Comparison of age at natural menopause in BRCA1/2 mutation carriers with a non–clinic--based sample of women in northern California. Cancer 2013, 119, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Beasley, M.D.; Warren, W.D.; van der Horst, G.T.; McKay, M.J. Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev. Cell 2005, 8, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.; Richter, J.D. Germ cell differentiation and synaptonemal complex formation are disrupted in CPEB knockout mice. Dev. Cell 2001, 1, 201–213. [Google Scholar] [CrossRef]
- Ortega, S.; Prieto, I.; Odajima, J.; Martín, A.; Dubus, P.; Sotillo, R.; Barbero, J.L.; Malumbres, M.; Barbacid, M. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat. Genet. 2003, 35, 25–31. [Google Scholar] [CrossRef]
- Fu, C.; Begum, K.; Overbeek, P.A. Primary Ovarian Insufficiency Induced by Fanconi Anemia E Mutation in a Mouse Model. PLoS ONE 2016, 11, e0144285. [Google Scholar] [CrossRef]
- Koomen, M.; Cheng, N.C.; van de Vrugt, H.J.; Godthelp, B.C.; van der Valk, M.A.; Oostra, A.B.; Zdzienicka, M.Z.; Joenje, H.; Arwert, F. Reduced fertility and hypersensitivity to mitomycin C characterize Fancg/Xrcc9 null mice. Hum. Mol. Genet. 2002, 11, 273–281. [Google Scholar] [CrossRef]
- Whitney, M.A.; Royle, G.; Low, M.J.; Kelly, M.A.; Axthelm, M.K.; Reifsteck, C.; Olson, S.; Braun, R.E.; Heinrich, M.C.; Rathbun, R.K. Germ cell defects and hematopoietic hypersensitivity to gamma-interferon in mice with a targeted disruption of the Fanconi anemia C gene. Blood 1996, 88, 49–58. [Google Scholar] [CrossRef]
- Cheng, N.C.; van de Vrugt, H.J.; van der Valk, M.A.; Oostra, A.B.; Krimpenfort, P.; de Vries, Y.; Joenje, H.; Berns, A.; Arwert, F. Mice with a targeted disruption of the Fanconi anemia homolog Fanca. Hum. Mol. Genet. 2000, 9, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- Metchat, A.; Åkerfelt, M.; Bierkamp, C.; Delsinne, V.; Sistonen, L.; Alexandre, H.; Christians, E.S. Mammalian heat shock factor 1 is essential for oocyte meiosis and directly regulates Hsp90α expression. J. Biol. Chem. 2009, 284, 9521–9528. [Google Scholar] [CrossRef]
- Bierkamp, C.; Luxey, M.; Metchat, A.; Audouard, C.; Dumollard, R.; Christians, E. Lack of maternal Heat Shock Factor 1 results in multiple cellular and developmental defects, including mitochondrial damage and altered redox homeostasis, and leads to reduced survival of mammalian oocytes and embryos. Dev. Biol. 2010, 339, 338–353. [Google Scholar] [CrossRef] [PubMed]
- De Vries, L.; Behar, D.M.; Smirin-Yosef, P.; Lagovsky, I.; Tzur, S.; Basel-Vanagaite, L. Exome sequencing reveals SYCE1 mutation associated with autosomal recessive primary ovarian insufficiency. J. Clin. Endocrinol. Metab. 2014, 99, E2129–E2132. [Google Scholar] [CrossRef]
- Lacombe, A.; Lee, H.; Zahed, L.; Choucair, M.; Muller, J.-M.; Nelson, S.F.; Salameh, W.; Vilain, E. Disruption of POF1B binding to nonmuscle actin filaments is associated with premature ovarian failure. Am. J. Hum. Genet. 2006, 79, 113–119. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Jiang, H.; Wu, B.-L. Mutations in HFM1 in recessive primary ovarian insufficiency. N. Engl. J. Med. 2014, 370, 972–974. [Google Scholar] [CrossRef]
- Caburet, S.; Arboleda, V.A.; Llano, E.; Overbeek, P.A.; Barbero, J.L.; Oka, K.; Harrison, W.; Vaiman, D.; Ben-Neriah, Z.; García-Tuñón, I. Mutant cohesin in premature ovarian failure. N. Engl. J. Med. 2014, 370, 943–949. [Google Scholar] [CrossRef]
- Soyal, S.M.; Amleh, A.; Dean, J. FIGalpha, a germ cell-specific transcription factor required for ovarian follicle formation. Development 2000, 127, 4645–4654. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, Z.-J.; Qin, Y.; Shi, Y.; Wang, S.; Choi, Y.; Simpson, J.L.; Rajkovic, A. Transcription factor FIGLA is mutated in patients with premature ovarian failure. Am. J. Hum. Genet. 2008, 82, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Kerr, B.; Garcia-Rudaz, C.; Dorfman, M.; Paredes, A.; Ojeda, S.R. NTRK1 and NTRK2 receptors facilitate follicle assembly and early follicular development in the mouse ovary. Reproduction 2009, 138, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Spears, N.; Molinek, M.D.; Robinson, L.L.; Fulton, N.; Cameron, H.; Shimoda, K.; Telfer, E.E.; Anderson, R.A.; Price, D.J. The role of neurotrophin receptors in female germ-cell survival in mouse and human. Development 2003, 130, 5481–5491. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.; Dole, G.; Skinner, M.K. Neurotrophin NT3 promotes ovarian primordial to primary follicle transition. Reprod. Camb. Engl. 2009, 138, 697. [Google Scholar]
- Bergeron, L.; Perez, G.I.; Macdonald, G.; Shi, L.; Sun, Y.; Jurisicova, A.; Varmuza, S.; Latham, K.E.; Flaws, J.A.; Salter, J.C. Defects in regulation of apoptosis in caspase-2-deficient mice. Genes Dev. 1998, 12, 1304–1314. [Google Scholar] [CrossRef]
- Ratts, V.S.; Flaws, J.A.; Kolp, R.; Sorenson, C.M.; Tilly, J.L. Ablation of bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the post-natal female mouse gonad. Endocrinology 1995, 136, 3665–3668. [Google Scholar] [CrossRef]
- Greenfeld, C.R.; Pepling, M.E.; Babus, J.K.; Furth, P.A.; Flaws, J.A. BAX regulates follicular endowment in mice. Reproduction 2007, 133, 865–876. [Google Scholar] [CrossRef]
- Perez, G.I.; Robles, R.; Knudson, C.M.; Flaws, J.A.; Korsmeyer, S.J.; Tilly, J.L. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat. Genet. 1999, 21, 200–203. [Google Scholar] [CrossRef]
- Benedict, J.C.; Lin, T.-M.; Loeffler, I.; Peterson, R.E.; Flaws, J.A. Physiological role of the aryl hydrocarbon receptor in mouse ovary development. Toxicol. Sci. 2000, 56, 382–388. [Google Scholar] [CrossRef]
- Omari, S.; Waters, M.; Naranian, T.; Kim, K.; Perumalsamy, A.; Chi, M.; Greenblatt, E.; Moley, K.; Opferman, J.; Jurisicova, A. Mcl-1 is a key regulator of the ovarian reserve. Cell Death Dis. 2015, 6, e1755. [Google Scholar] [CrossRef]
- Winship, A.L.; Stringer, J.M.; Liew, S.H.; Hutt, K.J. The importance of DNA repair for maintaining oocyte quality in response to anti-cancer treatments, environmental toxins and maternal ageing. Hum. Reprod. Update 2018, 24, 119–134. [Google Scholar] [CrossRef]
- Amelio, I.; Grespi, F.; Annicchiarico-Petruzzelli, M.; Melino, G. p63 the guardian of human reproduction. Cell Cycle 2012, 11, 4545–4551. [Google Scholar] [CrossRef]
- Suh, E.K.; Yang, A.; Kettenbach, A.; Bamberger, C.; Michaelis, A.H.; Zhu, Z.; Elvin, J.A.; Bronson, R.T.; Crum, C.P.; McKeon, F. p63 protects the female germ line during meiotic arrest. Nature 2006, 444, 624–628. [Google Scholar] [CrossRef]
- Kerr, J.B.; Hutt, K.J.; Michalak, E.M.; Cook, M.; Vandenberg, C.J.; Liew, S.H.; Bouillet, P.; Mills, A.; Scott, C.L.; Findlay, J.K.; et al. DNA damage-induced primordial follicle oocyte apoptosis and loss of fertility require TAp63-mediated induction of Puma and Noxa. Mol. Cell 2012, 48, 343–352. [Google Scholar] [CrossRef]
- Soleimani, R.; Heytens, E.; Darzynkiewicz, Z.; Oktay, K. Mechanisms of chemotherapy-induced human ovarian aging: Double strand DNA breaks and microvascular compromise. Aging 2011, 3, 782–793. [Google Scholar] [CrossRef]
- Roness, H.; Kashi, O.; Meirow, D. Prevention of chemotherapy-induced ovarian damage. Fertil. Steril. 2016, 105, 20–29. [Google Scholar] [CrossRef]
- Chen, X.Y.; Xia, H.X.; Guan, H.Y.; Li, B.; Zhang, W. Follicle Loss and Apoptosis in Cyclophosphamide-Treated Mice: What’s the Matter? Int. J. Mol. Sci. 2016, 17, 836. [Google Scholar] [CrossRef] [PubMed]
- Roness, H.; Gavish, Z.; Cohen, Y.; Meirow, D. Ovarian follicle burnout: A universal phenomenon? Cell Cycle 2013, 12, 3245–3246. [Google Scholar] [CrossRef] [PubMed]
- Marcello, M.F.; Nuciforo, G.; Romeo, R.; Di Dino, G.; Russo, I.; Russo, A.; Palumbo, G.; Schiliro, G. Structural and ultrastructural study of the ovary in childhood leukemia after successful treatment. Cancer 1990, 66, 2099–2104. [Google Scholar] [CrossRef]
- Nicosia, S.V.; Matus-Ridley, M.; Meadows, A.T. Gonadal effects of cancer therapy in girls. Cancer 1985, 55, 2364–2372. [Google Scholar] [CrossRef]
- Ben-Aharon, I.; Meizner, I.; Granot, T.; Uri, S.; Hasky, N.; Rizel, S.; Yerushalmi, R.; Sulkes, A.; Stemmer, S.M. Chemotherapy-induced ovarian failure as a prototype for acute vascular toxicity. Oncologist 2012, 17, 1386–1393. [Google Scholar] [CrossRef][Green Version]
- Meirow, D.; Dor, J.; Kaufman, B.; Shrim, A.; Rabinovici, J.; Schiff, E.; Raanani, H.; Levron, J.; Fridman, E. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum. Reprod. 2007, 22, 1626–1633. [Google Scholar] [CrossRef]
- Soleimani, R.; Heytens, E.; Oktay, K. Enhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplants. PLoS ONE 2011, 6, e19475. [Google Scholar] [CrossRef]
- De la Noval, B.D. Potential implications on female fertility and reproductive lifespan in BRCA germline mutation women. Arch. Gynecol. Obstet. 2016, 294, 1099–1103. [Google Scholar] [CrossRef]
- Titus, S.; Li, F.; Stobezki, R.; Akula, K.; Unsal, E.; Jeong, K.; Dickler, M.; Robson, M.; Moy, F.; Goswami, S. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci. Transl. Med. 2013, 5, ra121–ra172. [Google Scholar] [CrossRef]
- Michaelson-Cohen, R.; Mor, P.; Srebnik, N.; Beller, U.; Levy-Lahad, E.; Eldar-Geva, T. BRCA mutation carriers do not have compromised ovarian reserve. Int. J. Gynecol. Cancer 2014, 24, 233–237. [Google Scholar] [CrossRef]
- Shapira, M.; Raanani, H.; Feldman, B.; Srebnik, N.; Dereck-Haim, S.; Manela, D.; Brenghausen, M.; Geva-Lerner, L.; Friedman, E.; Levi-Lahad, E. BRCA mutation carriers show normal ovarian response in in vitro fertilization cycles. Fertil. Steril. 2015, 104, 1162–1167. [Google Scholar] [CrossRef]
- Lee, J.M.; Ledermann, J.A.; Kohn, E.C. PARP Inhibitors for BRCA1/2 mutation-associated and BRCA-like malignancies. Ann. Oncol. 2014, 25, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, H.R.; Kim, M.G.; Lee, J.S.; Jin, S.J.; Lee, H.T. The effect of poly(ADP-ribosyl)ation inhibition on the porcine cumulus-oocyte complex during in vitro maturation. Biochem. Biophys. Res. Commun. 2017, 483, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Takae, S.; Shiraishi, E.; Shinya, K.; Igualada, A.J.; Suzuki, N. Poly (ADP-ribose) polymerase inhibitor exposure reduces ovarian reserve followed by dysfunction in granulosa cells. Sci. Rep. 2020, 10, 17058. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Guo, T.; Li, G.; Zhou, L.; Qin, Y.; Chen, Z.J. Minichromosome maintenance complex component 8 mutations cause primary ovarian insufficiency. Fertil. Steril. 2016, 106, 1485–1489.e1482. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.J.; He, W.B.; Zhang, Y.X.; Meng, L.L.; Lu, G.X.; Lin, G.; Tan, Y.Q.; Du, J. In-Frame Variants in STAG3 Gene Cause Premature Ovarian Insufficiency. Front. Genet. 2019, 10, 1016. [Google Scholar] [CrossRef]
- Weinberg-Shukron, A.; Renbaum, P.; Kalifa, R.; Zeligson, S.; Ben-Neriah, Z.; Dreifuss, A.; Abu-Rayyan, A.; Maatuk, N.; Fardian, N.; Rekler, D.; et al. A mutation in the nucleoporin-107 gene causes XX gonadal dysgenesis. J. Clin. Investig. 2015, 125, 4295–4304. [Google Scholar] [CrossRef] [PubMed]
- Zhe, J.; Chen, S.; Chen, X.; Liu, Y.; Li, Y.; Zhou, X.; Zhang, J. A novel heterozygous splice-altering mutation in HFM1 may be a cause of premature ovarian insufficiency. J. Ovarian Res. 2019, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, B.; Dong, Z.; Zhou, S.; Liu, Z.; Shi, G.; Cao, Y.; Xu, Y. A NANOS3 mutation linked to protein degradation causes premature ovarian insufficiency. Cell Death Dis. 2013, 4, e825. [Google Scholar] [CrossRef]
- Franca, M.M.; Mendonca, B.B. Genetics of Primary Ovarian Insufficiency in the Next-Generation Sequencing Era. J. Endocr. Soc. 2020, 4, bvz037. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.R.; Schuster, J.; Badhai, J.; Stattin, E.L.; Losel, R.; Wehling, M.; Carlsson, B.; Hovatta, O.; Karlstrom, P.O.; Golovleva, I.; et al. Alterations in the expression, structure and function of progesterone receptor membrane component-1 (PGRMC1) in premature ovarian failure. Hum. Mol. Genet. 2008, 17, 3776–3783. [Google Scholar] [CrossRef]
- Castrillon, D.H.; Miao, L.; Kollipara, R.; Horner, J.W.; DePinho, R.A. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 2003, 301, 215–218. [Google Scholar] [CrossRef]
- Watkins, W.J.; Umbers, A.J.; Woad, K.J.; Harris, S.E.; Winship, I.M.; Gersak, K.; Shelling, A.N. Mutational screening of FOXO3A and FOXO1A in women with premature ovarian failure. Fertil. Steril. 2006, 86, 1518–1521. [Google Scholar] [CrossRef]
- Wang, B.; Mu, Y.; Ni, F.; Zhou, S.; Wang, J.; Cao, Y.; Ma, X. Analysis of FOXO3 mutation in 114 Chinese women with premature ovarian failure. Reprod. Biomed. Online 2010, 20, 499–503. [Google Scholar] [CrossRef]
- Woad, K.J.; Pearson, S.M.; Harris, S.E.; Gersak, K.; Shelling, A.N. Investigating the association between inhibin alpha gene promoter polymorphisms and premature ovarian failure. Fertil. Steril. 2009, 91, 62–66. [Google Scholar] [CrossRef]
- Corre, T.; Schuettler, J.; Bione, S.; Marozzi, A.; Persani, L.; Rossetti, R.; Torricelli, F.; Giotti, I.; Vogt, P.; Toniolo, D. A large-scale association study to assess the impact of known variants of the human INHA gene on premature ovarian failure. Hum. Reprod. 2009, 24, 2023–2028. [Google Scholar] [CrossRef]
- Al-ajoury, R.; Kassem, E.; Al-halabi, B.; Moassess, F.; Al-achkar, W. Investigation of some genetic variations in BMP15 accompanied with premature ovarian failure (POF) in Syrian women. Middle East Fertil. Soc. J. 2015, 20, 91–96. [Google Scholar] [CrossRef]
- Cho, H.W.; Lee, S.; Min, K.J.; Hong, J.H.; Song, J.Y.; Lee, J.K.; Lee, N.W.; Kim, T. Advances in the Treatment and Prevention of Chemotherapy-Induced Ovarian Toxicity. Int. J. Mol. Sci. 2020, 21, 7792. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Z.; Cha, L.; Li, L.; Zhu, D.; Fang, Z.; He, Z.; Huang, J.; Pan, Z. Resveratrol Plays a Protective Role against Premature Ovarian Failure and Prompts Female Germline Stem Cell Survival. Int. J. Mol. Sci. 2019, 20, 3605. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Yeh, J. Onco-fertility and personalized testing for potential for loss of ovarian reserve in patients undergoing chemotherapy: Proposed next steps for development of genetic testing to predict changes in ovarian reserve. Fertil. Res. Pract. 2021, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Barzon, L.; Lavezzo, E.; Militello, V.; Toppo, S.; Palu, G. Applications of next-generation sequencing technologies to diagnostic virology. Int. J. Mol. Sci. 2011, 12, 7861–7884. [Google Scholar] [CrossRef]
- Blumenfeld, Z.; Dann, E. GnRH agonist for the prevention of chemotherapy-induced ovarian failure in lymphoma. J. Clin. Oncol. 2013, 31, 3721. [Google Scholar] [CrossRef]
- Blumenfeld, Z.; Avivi, I.; Linn, S.; Epelbaum, R.; Ben-Shahar, M.; Haim, N. Prevention of irreversible chemotherapy-induced ovarian damage in young women with lymphoma by a gonadotrophin-releasing hormone agonist in parallel to chemotherapy. Hum. Reprod. 1996, 11, 1620–1626. [Google Scholar] [CrossRef]
- Pereyra Pacheco, B.; Mendez Ribas, J.M.; Milone, G.; Fernandez, I.; Kvicala, R.; Mila, T.; Di Noto, A.; Contreras Ortiz, O.; Pavlovsky, S. Use of GnRH analogs for functional protection of the ovary and preservation of fertility during cancer treatment in adolescents: A preliminary report. Gynecol. Oncol. 2001, 81, 391–397. [Google Scholar] [CrossRef]
- Recchia, F.; Sica, G.; De Filippis, S.; Saggio, G.; Rosselli, M.; Rea, S. Goserelin as ovarian protection in the adjuvant treatment of premenopausal breast cancer: A phase II pilot study. Anticancer Drugs 2002, 13, 417–424. [Google Scholar] [CrossRef]
- Blumenfeld, Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist 2007, 12, 1044–1054. [Google Scholar] [CrossRef]
- Blumenfeld, Z.; Eckman, A. Preservation of fertility and ovarian function and minimization of chemotherapy-induced gonadotoxicity in young women by GnRH-a. J. Natl. Cancer Inst. Monogr. 2005, 34, 40–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lambertini, M.; Horicks, F.; Del Mastro, L.; Partridge, A.H.; Demeestere, I. Ovarian protection with gonadotropin-releasing hormone agonists during chemotherapy in cancer patients: From biological evidence to clinical application. Cancer Treat. Rev. 2019, 72, 65–77. [Google Scholar] [CrossRef] [PubMed]
- De Pedro, M.; Otero, B.; Martin, B. Fertility preservation and breast cancer: A review. Ecancermedicalscience 2015, 9, 503. [Google Scholar] [CrossRef]
- Kalechman, Y.; Albeck, M.; Oron, M.; Sobelman, D.; Gurwith, M.; Horwith, G.; Kirsch, T.; Maida, B.; Sehgal, S.N.; Sredni, B. Protective and restorative role of AS101 in combination with chemotherapy. Cancer Res. 1991, 51, 1499–1503. [Google Scholar]
- Makarovsky, D.; Kalechman, Y.; Sonino, T.; Freidkin, I.; Teitz, S.; Albeck, M.; Weil, M.; Geffen-Aricha, R.; Yadid, G.; Sredni, B. Tellurium compound AS101 induces PC12 differentiation and rescues the neurons from apoptotic death. Ann. N. Y. Acad. Sci. 2003, 1010, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Kalich-Philosoph, L.; Roness, H.; Carmely, A.; Fishel-Bartal, M.; Ligumsky, H.; Paglin, S.; Wolf, I.; Kanety, H.; Sredni, B.; Meirow, D. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci. Transl. Med. 2013, 5, 185ra162. [Google Scholar] [CrossRef]
- Druker, B.J.; Tamura, S.; Buchdunger, E.; Ohno, S.; Segal, G.M.; Fanning, S.; Zimmermann, J.; Lydon, N.B. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat. Med. 1996, 2, 561–566. [Google Scholar] [CrossRef]
- Gonfloni, S.; Di Tella, L.; Caldarola, S.; Cannata, S.M.; Klinger, F.G.; Di Bartolomeo, C.; Mattei, M.; Candi, E.; De Felici, M.; Melino, G.; et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat. Med. 2009, 15, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.B.; Hutt, K.J.; Cook, M.; Speed, T.P.; Strasser, A.; Findlay, J.K.; Scott, C.L. Cisplatin-induced primordial follicle oocyte killing and loss of fertility are not prevented by imatinib. Nat. Med. 2012, 18, 1172–1174. [Google Scholar] [CrossRef]
- Kim, S.Y.; Cordeiro, M.H.; Serna, V.A.; Ebbert, K.; Butler, L.M.; Sinha, S.; Mills, A.A.; Woodruff, T.K.; Kurita, T. Rescue of platinum-damaged oocytes from programmed cell death through inactivation of the p53 family signaling network. Cell Death Differ. 2013, 20, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.; Lopes, F.; Gourley, C.; Anderson, R.A.; Spears, N. Cisplatin and doxorubicin induce distinct mechanisms of ovarian follicle loss; imatinib provides selective protection only against cisplatin. PLoS ONE 2013, 8, e70117. [Google Scholar] [CrossRef]
- Morita, Y.; Perez, G.I.; Paris, F.; Miranda, S.R.; Ehleiter, D.; Haimovitz-Friedman, A.; Fuks, Z.; Xie, Z.; Reed, J.C.; Schuchman, E.H.; et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat. Med. 2000, 6, 1109–1114. [Google Scholar] [CrossRef]
- Li, F.; Turan, V.; Lierman, S.; Cuvelier, C.; De Sutter, P.; Oktay, K. Sphingosine-1-phosphate prevents chemotherapy-induced human primordial follicle death. Hum. Reprod. 2014, 29, 107–113. [Google Scholar] [CrossRef]
- Hancke, K.; Strauch, O.; Kissel, C.; Gobel, H.; Schafer, W.; Denschlag, D. Sphingosine 1-phosphate protects ovaries from chemotherapy-induced damage in vivo. Fertil. Steril. 2007, 87, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.; Desdicioglu, R.; Sezik, M.; Ulukaya, E.; Ozkaya, O.; Yilmaztepe, A.; Demirci, M. Does sphingosine-1-phosphate have a protective effect on cyclophosphamide- and irradiation-induced ovarian damage in the rat model? Fertil. Steril. 2008, 89, 732–735. [Google Scholar] [CrossRef]
- Gook, D.A.; Edgar, D.H. Human oocyte cryopreservation. Hum. Reprod. Update 2007, 13, 591–605. [Google Scholar] [CrossRef]
- Lee, S.; Song, J.Y.; Ku, S.Y.; Kim, S.H.; Kim, T. Fertility preservation in women with cancer. Clin. Exp. Reprod. Med. 2012, 39, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Rienzi, L.; Gracia, C.; Maggiulli, R.; LaBarbera, A.R.; Kaser, D.J.; Ubaldi, F.M.; Vanderpoel, S.; Racowsky, C. Oocyte, embryo and blastocyst cryopreservation in ART: Systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum. Reprod. Update 2017, 23, 139–155. [Google Scholar] [CrossRef] [PubMed]
- AbdelHafez, F.F.; Desai, N.; Abou-Setta, A.M.; Falcone, T.; Goldfarb, J. Slow freezing, vitrification and ultra-rapid freezing of human embryos: A systematic review and meta-analysis. Reprod. Biomed. Online 2010, 20, 209–222. [Google Scholar] [CrossRef]
- Debrock, S.; Peeraer, K.; Fernandez Gallardo, E.; De Neubourg, D.; Spiessens, C.; D’Hooghe, T.M. Vitrification of cleavage stage day 3 embryos results in higher live birth rates than conventional slow freezing: A RCT. Hum. Reprod. 2015, 30, 1820–1830. [Google Scholar] [CrossRef]
- Oktay, K.; Cil, A.P.; Bang, H. Efficiency of oocyte cryopreservation: A meta-analysis. Fertil. Steril. 2006, 86, 70–80. [Google Scholar] [CrossRef]
- Dolmans, M.M.; Hollanders de Ouderaen, S.; Demylle, D.; Pirard, C. Utilization rates and results of long-term embryo cryopreservation before gonadotoxic treatment. J. Assist. Reprod. Genet. 2015, 32, 1233–1237. [Google Scholar] [CrossRef]
- Courbiere, B.; Decanter, C.; Bringer-Deutsch, S.; Rives, N.; Mirallie, S.; Pech, J.C.; De Ziegler, D.; Carre-Pigeon, F.; May-Panloup, P.; Sifer, C.; et al. Emergency IVF for embryo freezing to preserve female fertility: A French multicentre cohort study. Hum. Reprod. 2013, 28, 2381–2388. [Google Scholar] [CrossRef]
- Mayeur, A.; Puy, V.; Windal, V.; Hesters, L.; Gallot, V.; Benoit, A.; Grynberg, M.; Sonigo, C.; Frydman, N. Live birth rate after use of cryopreserved oocytes or embryos at the time of cancer diagnosis in female survivors: A retrospective study of ten years of experience. J. Assist. Reprod. Genet. 2021, 38, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, Y.; Lee, S.; Kim, T. Ovarian tissue cryopreservation and transplantation in patients with cancer. Obstet. Gynecol. Sci. 2018, 61, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Cobo, A.; Garcia-Velasco, J.A.; Domingo, J.; Remohí, J.; Pellicer, A. Is vitrification of oocytes useful for fertility preservation for age-related fertility decline and in cancer patients? Fertil. Steril. 2013, 99, 1485–1495. [Google Scholar] [CrossRef]
- Cobo, A.; Diaz, C. Clinical application of oocyte vitrification: A systematic review and meta-analysis of randomized controlled trials. Fertil. Steril. 2011, 96, 277–285. [Google Scholar] [CrossRef]
- Ubaldi, F.; Anniballo, R.; Romano, S.; Baroni, E.; Albricci, L.; Colamaria, S.; Capalbo, A.; Sapienza, F.; Vajta, G.; Rienzi, L. Cumulative ongoing pregnancy rate achieved with oocyte vitrification and cleavage stage transfer without embryo selection in a standard infertility program. Hum. Reprod. 2010, 25, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Rienzi, L.; Romano, S.; Albricci, L.; Maggiulli, R.; Capalbo, A.; Baroni, E.; Colamaria, S.; Sapienza, F.; Ubaldi, F. Embryo development of fresh ‘versus’ vitrified metaphase II oocytes after ICSI: A prospective randomized sibling-oocyte study. Hum. Reprod. 2010, 25, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, R.; Lotz, L.; Mueller, A.; Hoffmann, I.; Wachter, D.L.; Amann, K.U.; Beckmann, M.W.; Hildebrandt, T. Oncofertility: Combination of ovarian stimulation with subsequent ovarian tissue extraction on the day of oocyte retrieval. Reprod. Biol. Endocrinol. 2013, 11, 19. [Google Scholar] [CrossRef]
- Suzuki, N. Ovarian tissue cryopreservation in young cancer patients for fertility preservation. Reprod. Med. Biol. 2015, 14, 1–4. [Google Scholar] [CrossRef]
- Lee, S.; Ryu, K.J.; Kim, B.; Kang, D.; Kim, Y.Y.; Kim, T. Comparison between Slow Freezing and Vitrification for Human Ovarian Tissue Cryopreservation and Xenotransplantation. Int. J. Mol. Sci. 2019, 20, 3346. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M.; Pellicer, A.; Diaz-Garcia, C.; Sanchez Serrano, M.; Schmidt, K.T.; Ernst, E.; Luyckx, V.; Andersen, C.Y. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: A review of 60 cases of reimplantation. Fertil. Steril. 2013, 99, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.M.; Luyckx, V.; Donnez, J.; Andersen, C.Y.; Greve, T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil. Steril. 2013, 99, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Loren, A.W.; Senapati, S. Fertility preservation in patients with hematologic malignancies and recipients of hematopoietic cell transplants. Blood 2019, 134, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Bedaiwy, M.A.; Falcone, T. Ovarian tissue banking for cancer patients: Reduction of post-transplantation ischaemic injury: Intact ovary freezing and transplantation. Hum. Reprod. 2004, 19, 1242–1244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martinez-Madrid, B.; Dolmans, M.M.; Van Langendonckt, A.; Defrere, S.; Donnez, J. Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil. Steril. 2004, 82, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, X.; Kim, S.S.; Chen, H.; Tan, S.L.; Gosden, R.G. Transplantation of intact rat gonads using vascular anastomosis: Effects of cryopreservation, ischaemia and genotype. Hum. Reprod. 2003, 18, 1165–1172. [Google Scholar] [CrossRef]
- Zhang, J.M.; Sheng, Y.; Cao, Y.Z.; Wang, H.Y.; Chen, Z.J. Cryopreservation of whole ovaries with vascular pedicles: Vitrification or conventional freezing? J. Assist. Reprod. Genet. 2011, 28, 445–452. [Google Scholar] [CrossRef]
- Zhang, S.; Yao, H.; Liu, Y.; Ren, L.; Xiang, D.; Wang, Y. Hypothermic machine perfusion after static cold storage improves ovarian function in rat ovarian tissue transplantation. J. Assist. Reprod. Genet. 2020, 37, 1745–1753. [Google Scholar] [CrossRef]
- Hossay, C.; Donnez, J.; Dolmans, M.M. Whole Ovary Cryopreservation and Transplantation: A Systematic Review of Challenges and Research Developments in Animal Experiments and Humans. J. Clin. Med. 2020, 9, 3196. [Google Scholar] [CrossRef]
- Lee, J.A.; Barritt, J.; Moschini, R.M.; Slifkin, R.E.; Copperman, A.B. Optimizing human oocyte cryopreservation for fertility preservation patients: Should we mature then freeze or freeze then mature? Fertil. Steril. 2013, 99, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Son, W.Y.; Henderson, S.; Cohen, Y.; Dahan, M.; Buckett, W. Immature Oocyte for Fertility Preservation. Front. Endocrinol. 2019, 10, 464. [Google Scholar] [CrossRef] [PubMed]
- Prasath, E.B.; Chan, M.L.; Wong, W.H.; Lim, C.J.; Tharmalingam, M.D.; Hendricks, M.; Loh, S.F.; Chia, Y.N. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum. Reprod. 2014, 29, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Uzelac, P.S.; Delaney, A.A.; Christensen, G.L.; Bohler, H.C.; Nakajima, S.T. Live birth following in vitro maturation of oocytes retrieved from extracorporeal ovarian tissue aspiration and embryo cryopreservation for 5 years. Fertil. Steril. 2015, 104, 1258–1260. [Google Scholar] [CrossRef]
- Cho, E.; Kim, Y.Y.; Noh, K.; Ku, S.Y. A new possibility in fertility preservation: The artificial ovary. J. Tissue Eng. Regen. Med. 2019, 13, 1294–1315. [Google Scholar] [CrossRef]
- Luyckx, V.; Dolmans, M.M.; Vanacker, J.; Legat, C.; Fortuno Moya, C.; Donnez, J.; Amorim, C.A. A new step toward the artificial ovary: Survival and proliferation of isolated murine follicles after autologous transplantation in a fibrin scaffold. Fertil. Steril. 2014, 101, 1149–1156. [Google Scholar] [CrossRef]
- Vanacker, J.; Luyckx, V.; Dolmans, M.M.; Des Rieux, A.; Jaeger, J.; Van Langendonckt, A.; Donnez, J.; Amorim, C.A. Transplantation of an alginate-matrigel matrix containing isolated ovarian cells: First step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials 2012, 33, 6079–6085. [Google Scholar] [CrossRef]
- Johnson, J.; Canning, J.; Kaneko, T.; Pru, J.K.; Tilly, J.L. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 2004, 428, 145–150. [Google Scholar] [CrossRef]
- Tilly, J.L.; Telfer, E.E. Purification of germline stem cells from adult mammalian ovaries: A step closer towards control of the female biological clock? Mol. Hum. Reprod. 2009, 15, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Hutt, K.J.; Albertini, D.F. Clinical applications and limitations of current ovarian stem cell research: A review. J. Exp. Clin. Assist. Reprod 2006, 3, 6. [Google Scholar] [CrossRef] [PubMed][Green Version]
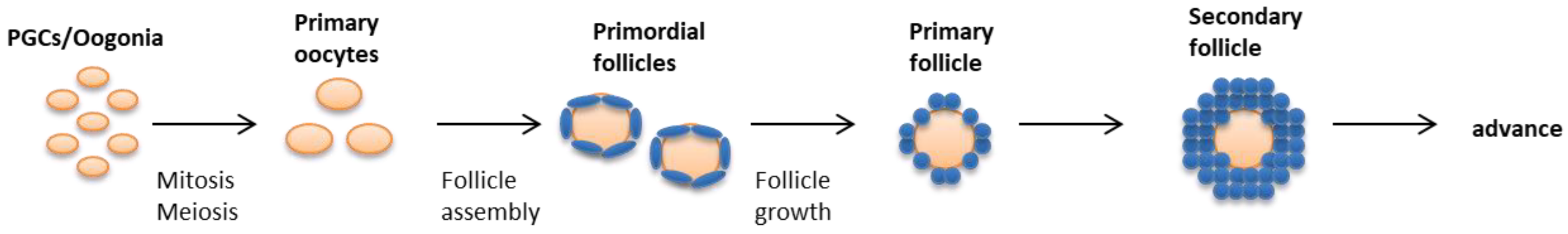
| Morphogenesis Process | Genes |
|---|---|
| From ovarian stem cell to ovigerous cords | Wnt4, Rspo1, Bmp2, Bmp4, Bmp8a, Smads, KltL, Oct4, Nanos1, Nanos3, Kit, Dazl, Bcl-x, Pin1, Pog, Gja1, Bax |
| Follicular assembly | Msx1, Msx2, Dicer, Notch, Follistain, Stra8, Stag3, Syce1, Sycp2l, Spo11, Msh4, Msh5, Dmc1, Rec8, Atm, Lsh, Cdk2, Cbep, Hsf1, Fanc-a, Fanc-c, Fanc-g, Bcl2, Brca1, Mcl1, Gdf9, Bmp15 |
| Primordial follicles formation and growth | Foxl2, Ccn2, Cyp19a1, KitL, p27kip1, Figla, Pdk1, Mcm8, Foxo3, Pten, Sohlh1, Sohlh2, Lhx8, Nobox8, Ybx2, Tsc1/2, Diaph2/3, Ngf, Nt3, Nt4, Bdnf, Ntrk1, Ntrk2, Ntrk3, Birc1, Becn1, Atg7 |
| Secondary follicle formation | Gdf9, Bmp15, Zp1, Zp2, Zp3, Cx43, Rspo2, Inha, Taf4b |
| Advance to latter stages | Esr1, Fshr, Nppc, Nprb, Kras, Erk1/2, Egfr, Bax, Ahr, Clast4, Polg |
| Type of Chemotherapy | Agents | Target Disease | Mechanisms of Action |
|---|---|---|---|
| Alkylating agents | Cyclophosphamide Ifosfamide Nitrosoureas Chlorambucil Melphalan Busulphan Mechlorethamine | Leukemia, breast cancer, lung cancer, ovarian cancer, prostate cancer, lymphoma, Hodgkin’s disease | Interference with cell division via cross-linking of DNA; Mitochondrial transmembrane potential reduction; Inhibition of the accumulation of cytochrome c in the cytosol; Induction of DSBs in oocytes |
| Vinka alkaloids | Vinblastine Vincristine | Testicular cancer, lymphoma, Hodgkin’s disease, breast cancer, germ cell tumors, lung cancer, | Inhibition of tubulin forming into microtubules; Low gonadotoxic risk |
| Antimetabolites | Cytarabine Methotrexate 5-fluorouracil | Leukemia, breast cancer, ovarian cancer, gastrointestinal cancer | Inhibition of purine, pyrimidine becoming incorporated into DNA; Inhibition of RNA synthesis; Low gonadotoxic risk |
| Platinum agents | Cisplatin Carboplatin Oxaliplatin | Bladder cancer, colorectal cancer, head and neck cancer, lung cancer, ovarian cancer, testicular cancer | DNA damage by the formation of DNA adducts, which interfere with cellular transcription and replication, leading to oocyte death. |
| Anthracycline antibiotics | Daunorubicin Bleomycin Doxorubicin | Lymphoma, leukemia, breast cancer, sarcoma | Intercalation with DNA and prevention of its replication and transcription via the inhibition of topoisomerase II; Upregulation of P53 protein which induces apoptosis; DNA DSBs leading to activation of ATM, which initiates apoptosis |
| Others | Procarbazine | Hodgkin’s disease, brain tumor | Inhibition of DNA methylation and RNA and protein synthesis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Lee, S.; Park, H.-T.; Song, J.-Y.; Kim, T. Genomic Consideration in Chemotherapy-Induced Ovarian Damage and Fertility Preservation. Genes 2021, 12, 1525. https://doi.org/10.3390/genes12101525
Kim S, Lee S, Park H-T, Song J-Y, Kim T. Genomic Consideration in Chemotherapy-Induced Ovarian Damage and Fertility Preservation. Genes. 2021; 12(10):1525. https://doi.org/10.3390/genes12101525
Chicago/Turabian StyleKim, Seongmin, Sanghoon Lee, Hyun-Tae Park, Jae-Yun Song, and Tak Kim. 2021. "Genomic Consideration in Chemotherapy-Induced Ovarian Damage and Fertility Preservation" Genes 12, no. 10: 1525. https://doi.org/10.3390/genes12101525
APA StyleKim, S., Lee, S., Park, H.-T., Song, J.-Y., & Kim, T. (2021). Genomic Consideration in Chemotherapy-Induced Ovarian Damage and Fertility Preservation. Genes, 12(10), 1525. https://doi.org/10.3390/genes12101525






