Long-Term Impact of Suppressive Antibiotic Therapy on Intestinal Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Processing
2.3. Bioinformatics and Statistical Analysis
2.4. Bacterial Viability
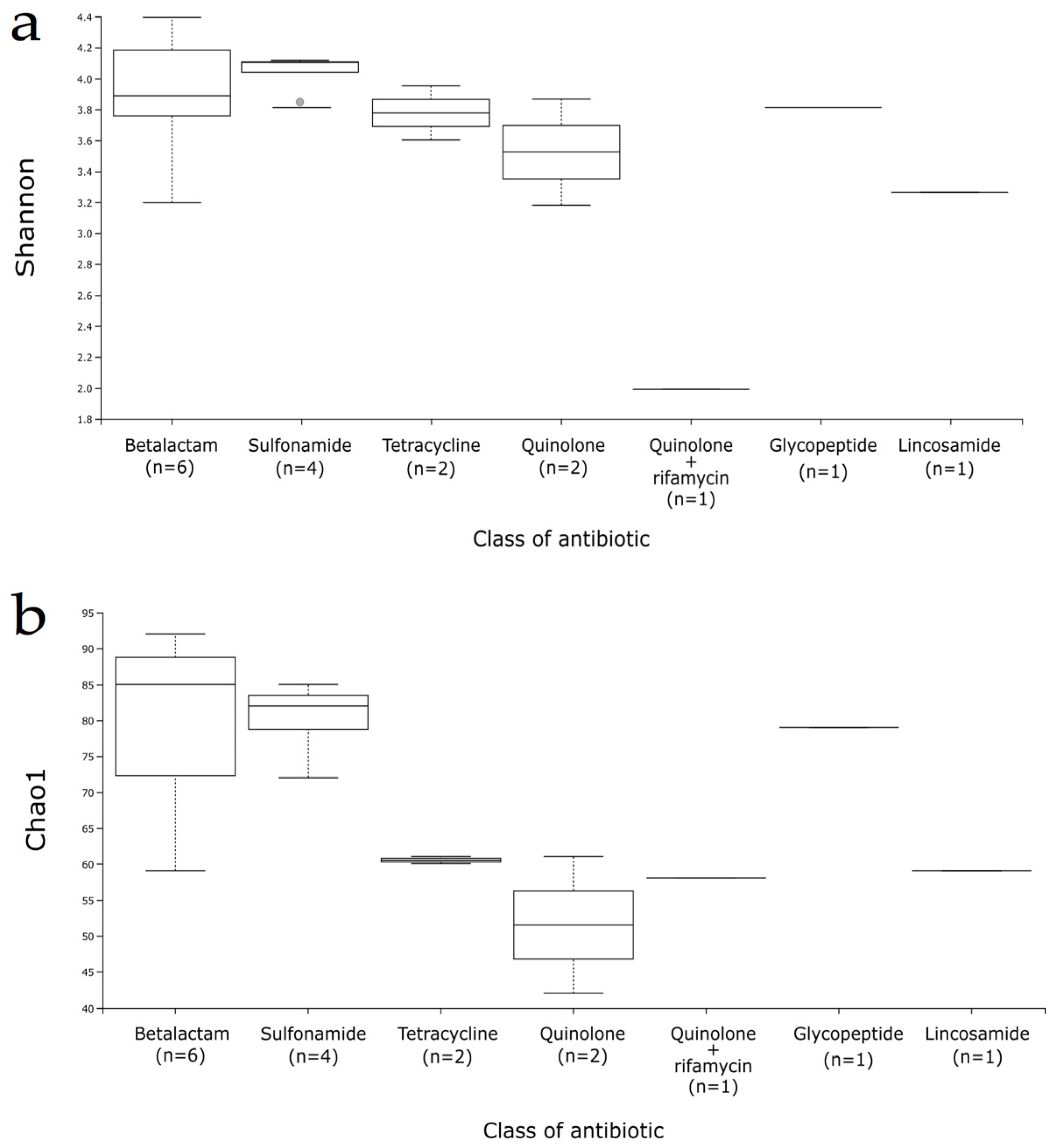
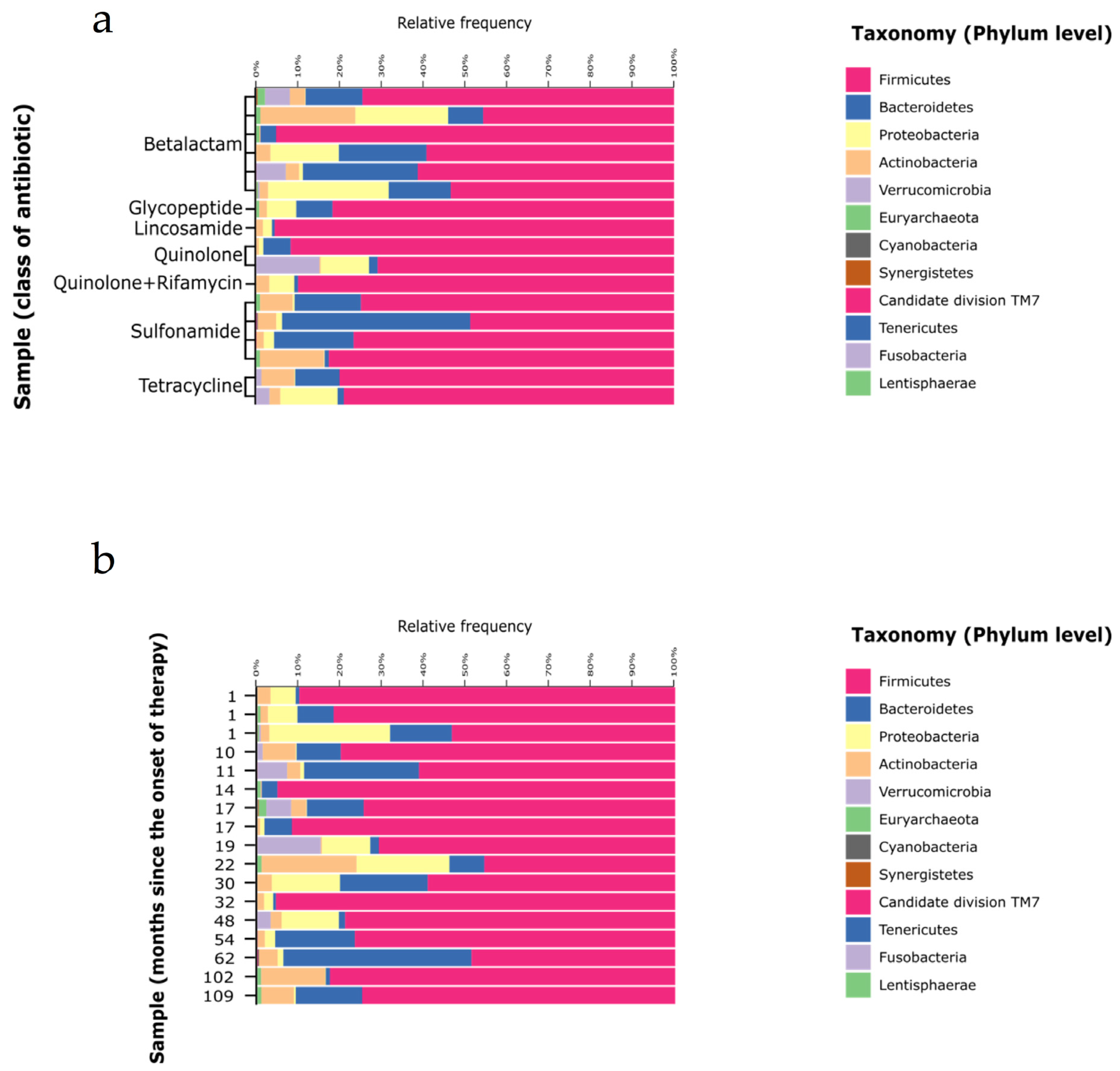
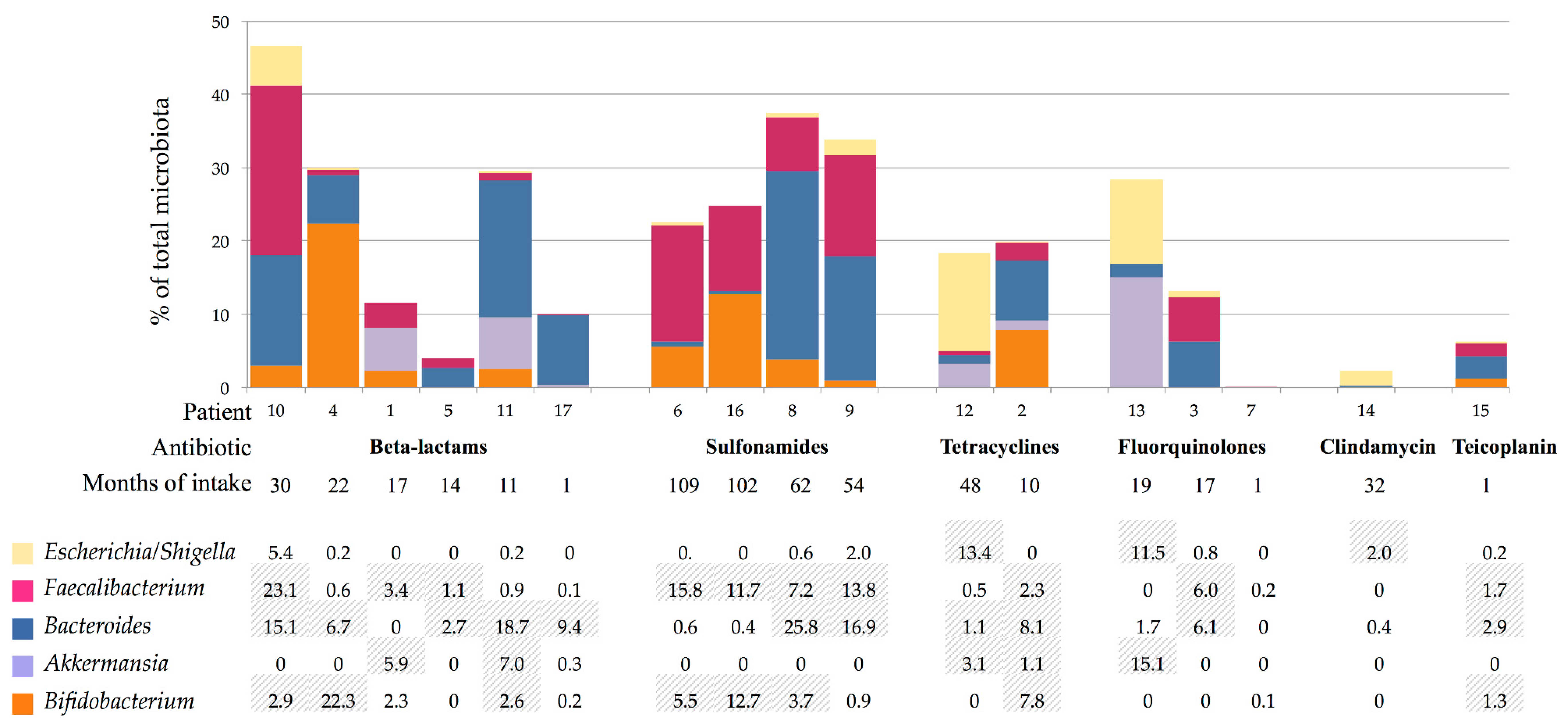
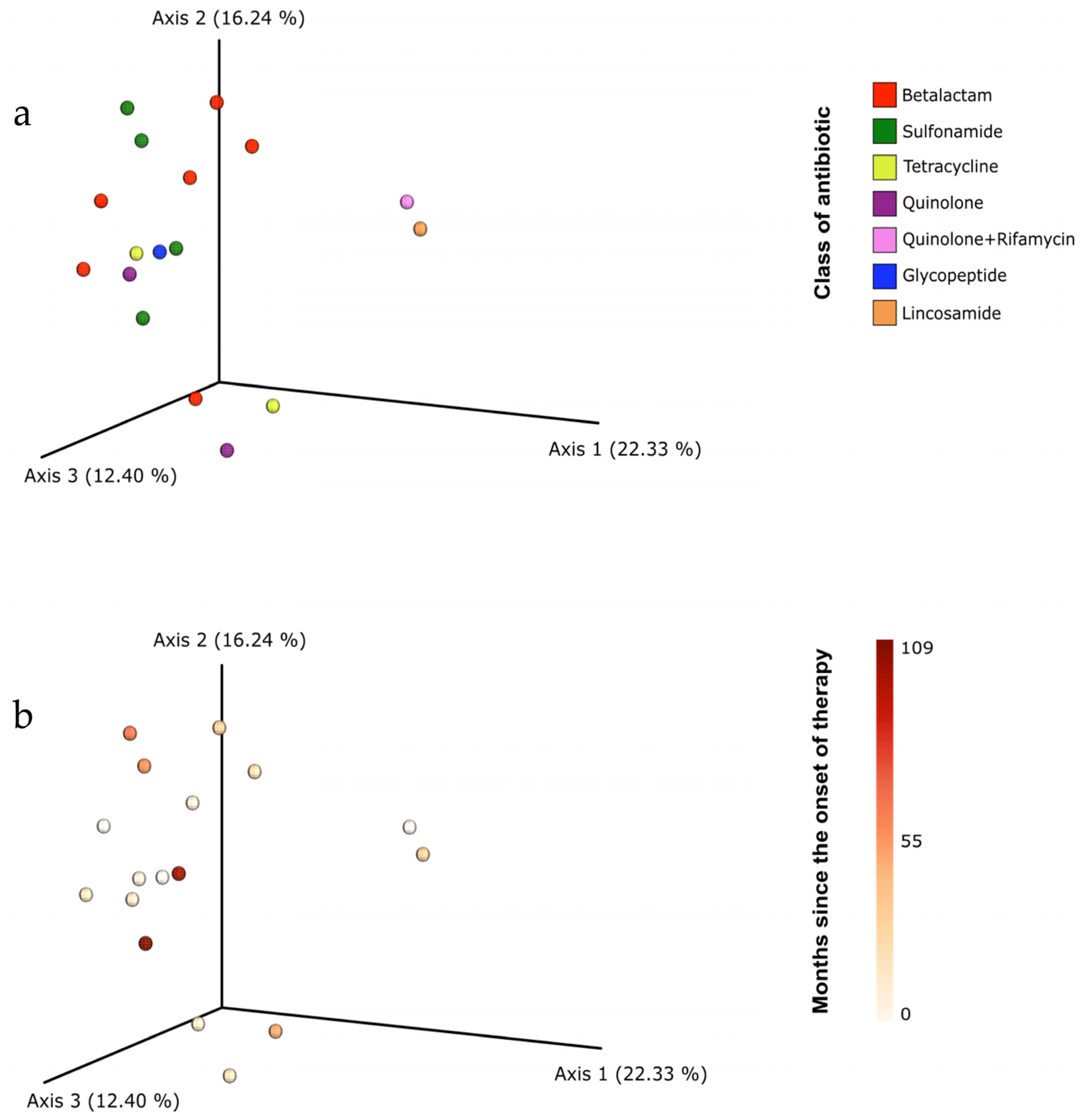
3. Results
3.1. Clinical Data and Adverse Effects
3.2. Fecal Colonization by Antimicrobial Resistant Bacteria
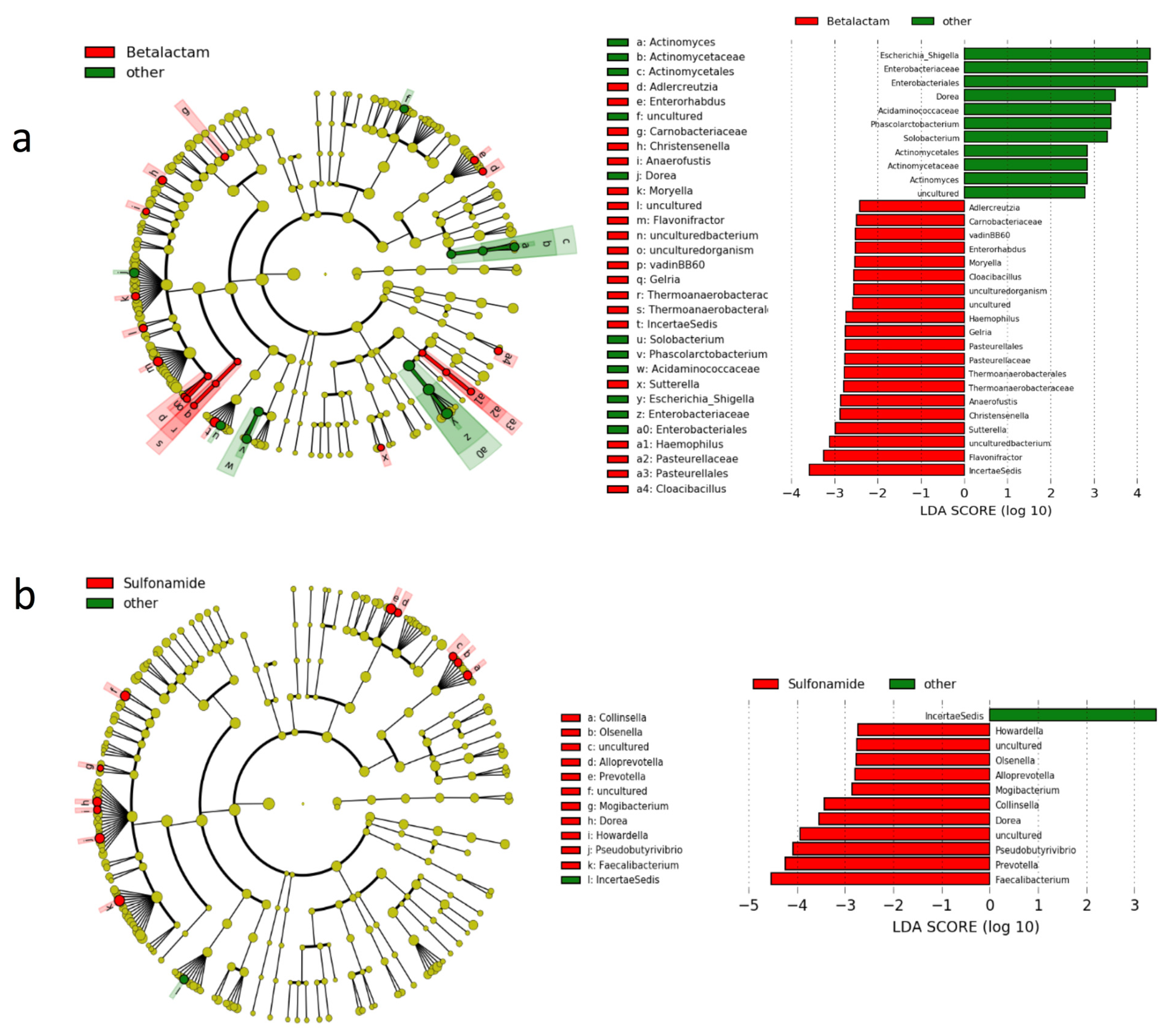
3.3. Gut Microbiota Composition
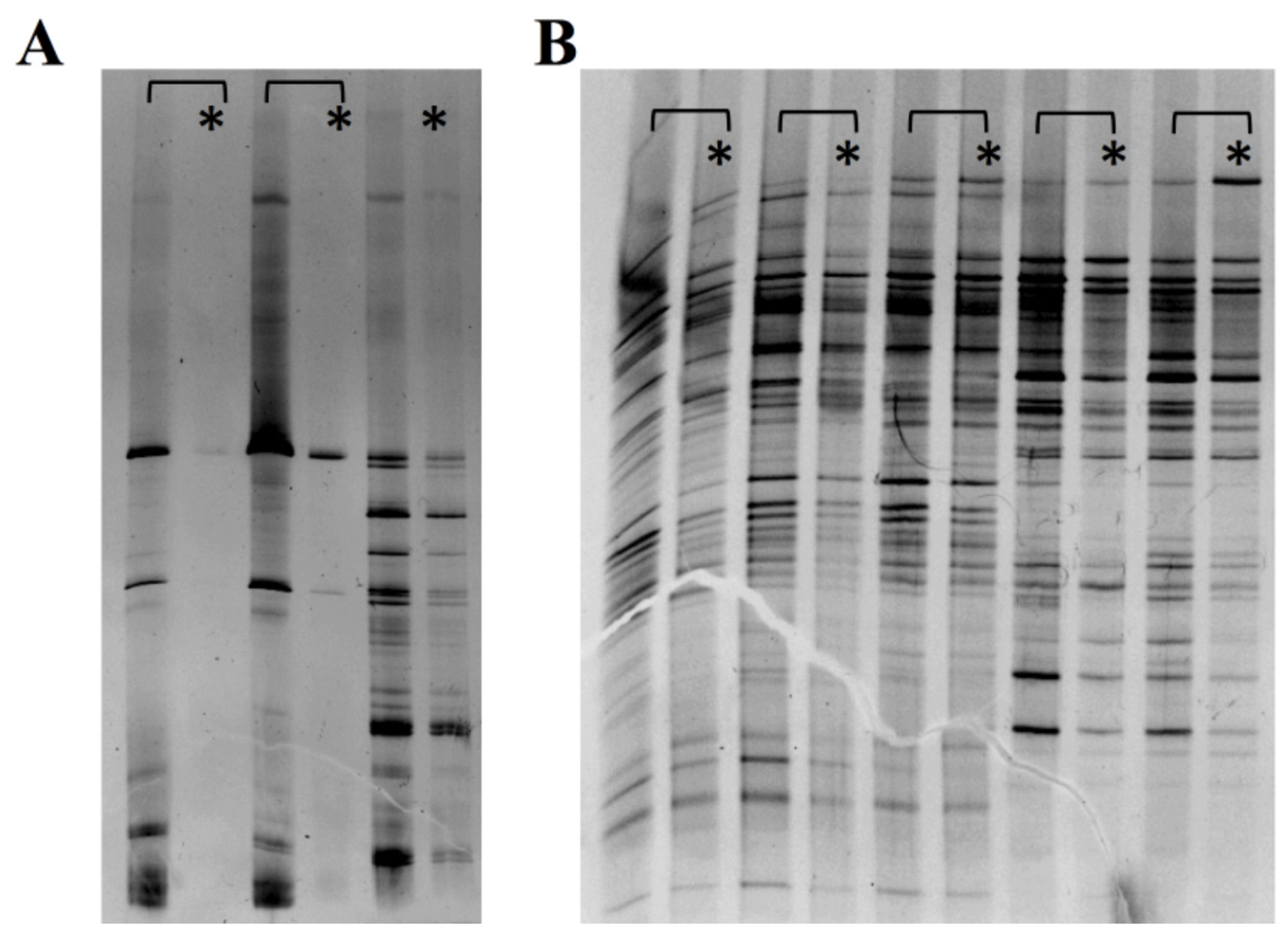
3.4. Bacterial Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministerio de Sanidad, Consumo y Bienestar Social. Subdirección General de Información Sanitaria e Innovación. Registro de Atención Especializada/Conjunto Mínimo Básico de Datos (RAE-CMBD) de los hospitales del Sistema Nacional de Salud. Available online: http://icmbd.es (accessed on 21 June 2020).
- Kapadia, B.H.; Berg, R.A.; Daley, J.A.; Fritz, J.; Bhave, A.; Mont, M.A. Periprosthetic joint infection. Lancet 2016, 387, 386–394. [Google Scholar] [CrossRef]
- Escudero-Sanchez, R.; Senneville, E.; Digumber, M.; Soriano, A.; Del Toro, M.D.; Bahamonde, A.; Del Pozo, J.L.; Guio, L.; Murillo, O.; Rico, A.; et al. Suppressive antibiotic therapy in prosthetic joint infections: A multicentre cohort study. Clin. Microbiol. Infect. 2020, 26, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Leijtens, B.; Weerwag, L.; Schreurs, B.W.; Kullberg, B.J.; Rijnen, W. Clinical outcome of antibiotic suppressive therapy in patients with a prosthetic joint infection after hip replacement. J. Bone Jt. Infect. 2019, 4, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Prendki, V.; Ferry, T.; Sergent, P.; Oziol, E.; Forestier, E.; Fraisse, T.; Tounes, S.; Ansart, S.; Gaillat, J.; Bayle, S.; et al. Prolonged suppressive antibiotic therapy for prosthetic joint infection in the elderly: A national multicentre cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Wouthuyzen-Bakker, M.; Nijman, J.M.; Kampinga, G.A.; van Assen, S.; Jutte, P.C. Efficacy of antibiotic suppressive therapy in patients with a prosthetic joint infection. J. Bone Jt. Infect. 2017, 2, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Jernberg, C.; Löfmark, S.; Edlund, C.; Jansson, J.K. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007, 1, 56–66. [Google Scholar] [CrossRef]
- Jakobsson, H.E.; Jernberg, C.; Andersson, A.F.; Sjölund-Karlsson, M.; Jansson, J.K.; Engstrand, L. Short-term antibiotic treatment has differing long- term impacts on the human throat and gut microbiome. PLoS ONE 2010, 5, e9836. [Google Scholar] [CrossRef]
- Burdet, C.; Nguyen, T.T.; Duval, X.; Ferreira, S.; Andremont, A.; Guedj, J. Impact of antibiotic gut exposure on the temporal changes in microbiome diversity. Antimicrob. Agents Chemother. 2019, 63, e00820-19. [Google Scholar] [CrossRef]
- Becattini, S.; Taur, Y.; Pamer, E.G. Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol. Med. 2016, 22, 458–478. [Google Scholar] [CrossRef]
- Lange, K.; Buerger, M.; Stallmach, A.; Bruns, T. Effects of antibiotics on gut microbiota. Dig. Dis. 2016, 34, 260–268. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucl. Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Vázquez-Baeza, Y.; Pirrung, M.; Gonzalez, A.; Knight, R. EMPeror: A tool for visualizing high-throughput microbial community data. Gigascience 2013, 2, 16. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Jernberg, C.; Lofmark, S.; Edlund, C.; Jansson, J.K. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 2010, 156, 3216–3223. [Google Scholar] [CrossRef]
- Palleja, A.; Mikkelsen, K.H.; Forslund, S.K.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Liang, S.; Feng, Q.; Zhang, C.; et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat. Microbiol. 2018, 3, 1255–1265. [Google Scholar] [CrossRef]
- Fouhy, F.; Guinane, C.M.; Hussey, S.; Wall, R.; Ryan, C.A.; Dempsey, E.M.; Murphy, B.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C.; et al. High-throughput sequencing reveals the incomplete, short-term recovery of infant gut microbiota following parenteral antibiotic treatment with ampicillin and gentamicin. Antimicrob. Agents Chemother. 2012, 56, 5811–5820. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Relman, D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4554–4561. [Google Scholar] [CrossRef]
- Pérez-Cobas, A.E.; Gosalbes, M.J.; Friedrichs, A.; Knecht, H.; Artacho, A.; Eismann, K.; Otto, W.; Rojo, D.; Bargiela, R.; von Bergen, M.; et al. Gut microbiota disturbance during antibiotic therapy: A multi-omic approach. Gut 2013, 62, 1591–1601. [Google Scholar] [CrossRef]
- Panda, S.; El khader, I.; Casellas, F.; López Vivancos, J.; García Cors, M.; Santiago, A.; Cuenca, S.; Guarner, F.; Manichanh, C. Short-term effect of antibiotics on human gut microbiota. PLoS ONE 2014, 9, e95476. [Google Scholar] [CrossRef]
- Raymond, F.; Ouameur, A.A.; Déraspe, M.; Iqbal, N.; Gingras, H.; Dridi, B.; Leprohon, P.; Plante, P.L.; Giroux, R.; Bérubé, È.; et al. The initial state of the human gut microbiome determines its reshaping by antibiotics. ISME J. 2016, 10, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Schubert, A.M.; Sinani, H.; Schloss, P.D. Antibiotic-induced alterations of the murine gut microbiota and subsequent effects on colonization resistance against Clostridium difficile. mBio 2015, 6, e00974. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, H.; Shi, Q.; Wang, N.; Zhang, Z.; Xiong, C.; Liu, J.; Chen, Y.; Jiang, L.; Jiang, Q. Effects of oral florfenicol and azithromycin on gut microbiota and adipogenesis in mice. PLoS ONE 2017, 12, e0181690. [Google Scholar] [CrossRef] [PubMed]
- Thuny, F.; Richet, H.; Casalta, J.P.; Angelakis, E.; Habib, G.; Raoult, D. Vancomycin treatment of infective endocarditis is linked with recently acquired obesity. PLoS ONE 2010, 5, e9074. [Google Scholar] [CrossRef]
- Angelakis, E.; Million, M.; Kankoe, S.; Lagier, J.C.; Armougom, F.; Giorgi, R.; Raoult, D. Abnormal weight gain and gut microbiota modifications are side effects of long-term doxycycline and hydroxychloroquin. Antimicrob. Agents Chemother. 2014, 58, 3342–3347. [Google Scholar] [CrossRef]
- Ianiro, G.; Tilg, H.; Gasbarrini, A. Antibiotics as deep modulators of gut microbiota: Between good and evil. Gut 2016, 65, 1906–1915. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.; Zhang, Y.; Zheng, K.; Xiang, Q.; Chen, N.; Chen, Z.; Zhang, N.; Zhu, J.; He, Q. Antibiotic-Induced disruption of gut microbiota alters local metabolomes and immune responses. Front. Cell Infect. Microbiol. 2019, 9, 99. [Google Scholar] [CrossRef]
- Mu, C.; Zhu, W. Antibiotic effects on gut microbiota, metabolism, and beyond. Appl. Microbiol. Biotechnol. 2019, 103, 9277–9285. [Google Scholar] [CrossRef]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef]
- Ng, K.M.; Aranda-Díaz, A.; Tropini, C.; Frankel, M.R.; Van Treuren, W.; O’Laughlin, C.T.; Merrill, B.D.; Yu, F.B.; Pruss, K.M.; Oliveira, R.A.; et al. Recovery of the gut microbiota after antibiotics depends on host diet, community context, and environmental reservoirs. Cell Host Microbe. 2019, 26, 650–665.e4. [Google Scholar] [CrossRef] [PubMed]
- Morjaria, S.; Schluter, J.; Taylor, B.P.; Littmann, E.R.; Carter, R.A.; Fontana, E.; Peled, J.U.; van den Brink, M.R.M.; Xavier, J.B.; Taur, Y. Antibiotic-induced shifts in fecal microbiota density and composition during hematopoietic stem cell transplantation. Infect. Immun. 2019, 87, e00206-19. [Google Scholar] [CrossRef] [PubMed]
- Burdet, C.; Sayah-Jeanne, S.; Nguyen, T.T.; Miossec, C.; Saint-Lu, N.; Pulse, M.; Weiss, W.; Andremont, A.; Mentré, F.; de Gunzburg, J. Protection of hamsters from mortality by reducing fecal moxifloxacin concentration with dav131a in a model of moxifloxacin-induced Clostridium difficile colitis. Antimicrob. Agents Chemother. 2017, 61, e00543-17. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.S.W.; Derraik, J.G.B.; Hofman, P.L.; Cutfield, W.S. Antibiotics, gut microbiome and obesity. Clin. Endocrinol. 2018, 88, 185–200. [Google Scholar] [CrossRef]
- Yi, H.; Kim, H.S. Antibiotic scars left on the gut microbiota from the stringent response. Trends Microbiol. 2018, 26, 735–737. [Google Scholar] [CrossRef]
- Wei, S.; Mortensen, M.S.; Stokholm, J.; Brejnrod, A.D.; Thorsen, J.; Rasmussen, M.A.; Trivedi, U.; Bisgaard, H.; Sørensen, S.J. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: A double-blind, randomized, placebo-controlled trial. EBioMedicine 2018, 38, 265–272. [Google Scholar] [CrossRef]
- Kim, S.; Covington, A.; Pamer, E.G. The intestinal microbiota: Antibiotics, colonization resistance, and enteric pathogens. Immunol. Rev. 2017, 279, 90–105. [Google Scholar] [CrossRef]
- Fujisaka, S.; Ussar, S.; Clish, C.; Devkota, S.; Dreyfuss, J.M.; Sakaguchi, M.; Soto, M.; Konishi, M.; Softic, S.; Altindis, E.; et al. Antibiotic effects on gut microbiota and metabolism are host dependent. J. Clin. Investig. 2016, 126, 4430–4443. [Google Scholar] [CrossRef]

| Patient | Sex (Age in Years) | Charlson Index | Location of Infection | Microorganism | Antibiotic (Family) [Months of Antibiotic Intake] | Symptomatology Attributable to SAT |
|---|---|---|---|---|---|---|
| 1 | male (79) | 1 | Abdominal (mesh) | MSSA | Cefadroxil (betalactam) (17) | Flatulence |
| 2 | male (73) | 1 | PJI (Knee) | MSSA | Doxycycline (glycopeptide) (10) | Urinary infection |
| 3 | female (83) | 0 | PJI (Hip) | Enterobacter cloacae | Ciprofloxacin (fluorquinolone) (17) | |
| 4 | female (83) | 0 | PJI (Hip) | MSSA | Cephalexin (betalactam) (18) | Asymptomatic bacteriuria |
| 5 | male (89) | 1 | PJI (Hip) | MSSA | Cephalexin (betalactam) (14) | |
| 6 | female (79) | 0 | PJI (Knee) | MRSE | Cotrimoxazole (109) | Diarrhoea and Asymptomatic bacteriuria |
| 7 | female (80) | 1 | PJI (Knee) | MSSA | Levofloxacin (fluorquinolone) /Rifamycin (1) | |
| 8 | female (88) | 0 | PJI (Knee) | Escherichia coli | Cotrimoxazole (62) | Asymptomatic bacteriuria |
| 9 | male (73) | 1 | Vertebral Instrumentation | Unidentified | Cotrimoxazole (54) | Constipation |
| 10 | male (84) | 3 | PJI (Hip) | MSSA | Cephalexin (betalactam) (19) | |
| 11 | female (70) | 1 | PJI (Shoulder) | Enterococcus faecalis | Amoxicillin (betalactam) (11) | Weight gain and Candidiasis |
| 12 | male (66) | 2 | PJI (Hip) | MSSE | Doxycycline (tetracycline) (48) | Diarrhoea |
| 13 | female (82) | 1 | PJI (Shoulder) | Cutibacterium granulosum | Moxifloxacin (fluorquinolone) (20) | Intestinal distension |
| 14 | female (74) | 0 | PJI (Knee) | MSSA | Clindamycin (macrolide) (21) | |
| 15 | male (72) | 6 | Osteosynthesis material (Humerus) | MRSE | Teicoplanin (glycopeptide) (1) | |
| 16 | male (34) | 1 | Vertebral Instrumentation | Polymicrobial | Cotrimoxazole (102) | |
| 17 | male (81) | 2 | Osteosynthesis material (Tibia) | MSSA | Cefadroxil (1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escudero-Sánchez, R.; Ponce-Alonso, M.; Barragán-Prada, H.; Morosini, M.I.; Cantón, R.; Cobo, J.; del Campo, R. Long-Term Impact of Suppressive Antibiotic Therapy on Intestinal Microbiota. Genes 2021, 12, 41. https://doi.org/10.3390/genes12010041
Escudero-Sánchez R, Ponce-Alonso M, Barragán-Prada H, Morosini MI, Cantón R, Cobo J, del Campo R. Long-Term Impact of Suppressive Antibiotic Therapy on Intestinal Microbiota. Genes. 2021; 12(1):41. https://doi.org/10.3390/genes12010041
Chicago/Turabian StyleEscudero-Sánchez, Rosa, Manuel Ponce-Alonso, Hugo Barragán-Prada, María Isabel Morosini, Rafael Cantón, Javier Cobo, and Rosa del Campo. 2021. "Long-Term Impact of Suppressive Antibiotic Therapy on Intestinal Microbiota" Genes 12, no. 1: 41. https://doi.org/10.3390/genes12010041
APA StyleEscudero-Sánchez, R., Ponce-Alonso, M., Barragán-Prada, H., Morosini, M. I., Cantón, R., Cobo, J., & del Campo, R. (2021). Long-Term Impact of Suppressive Antibiotic Therapy on Intestinal Microbiota. Genes, 12(1), 41. https://doi.org/10.3390/genes12010041






