Age and Serum AMH and FSH Levels as Predictors of the Number of Oocytes Retrieved from Chromosomal Translocation Carriers after Controlled Ovarian Hyperstimulation: Applicability and Limitations
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Karyotype Verification
2.3. Controlled Ovarian Hyperstimulation
2.4. Statistical Analysis
2.5. Ethical Issues
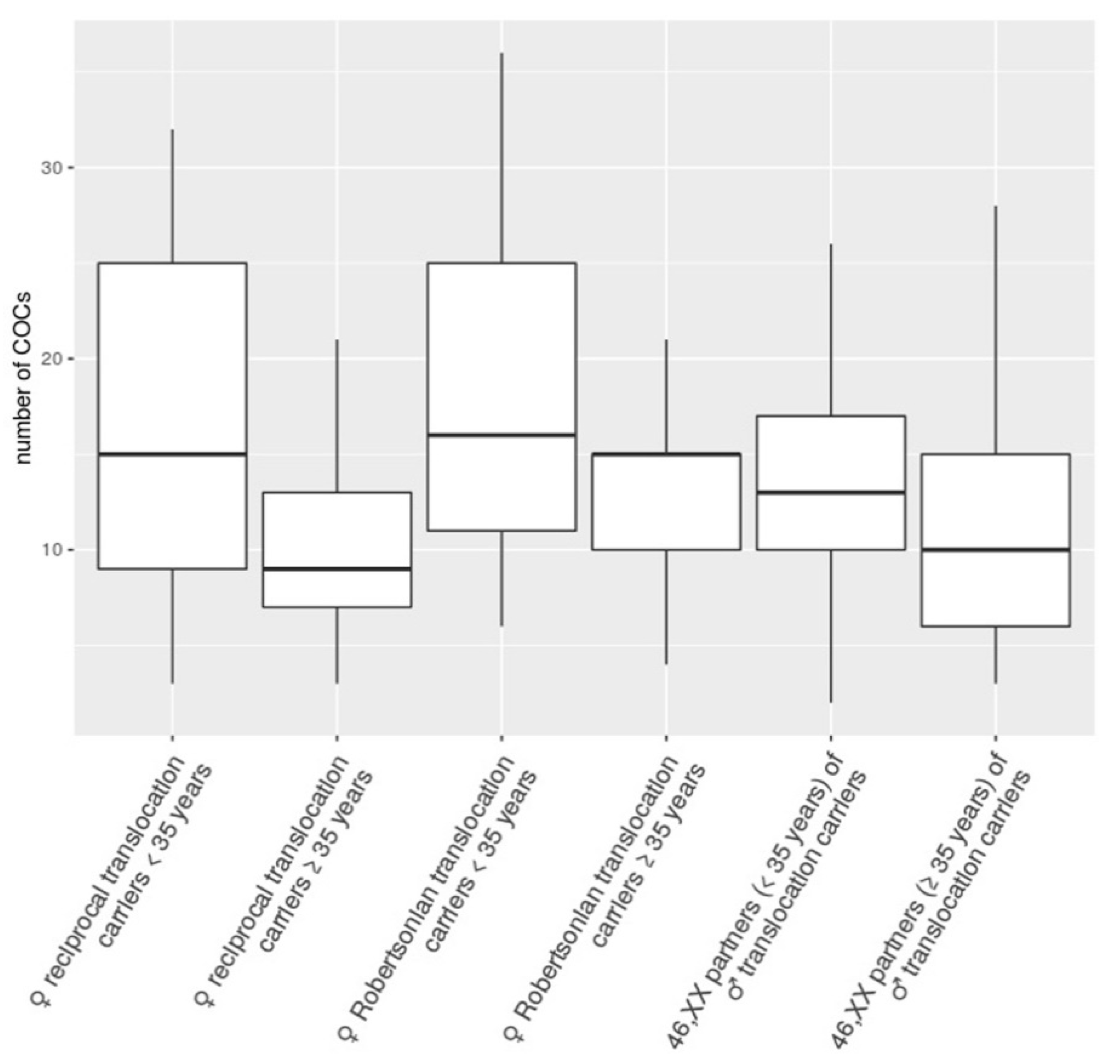
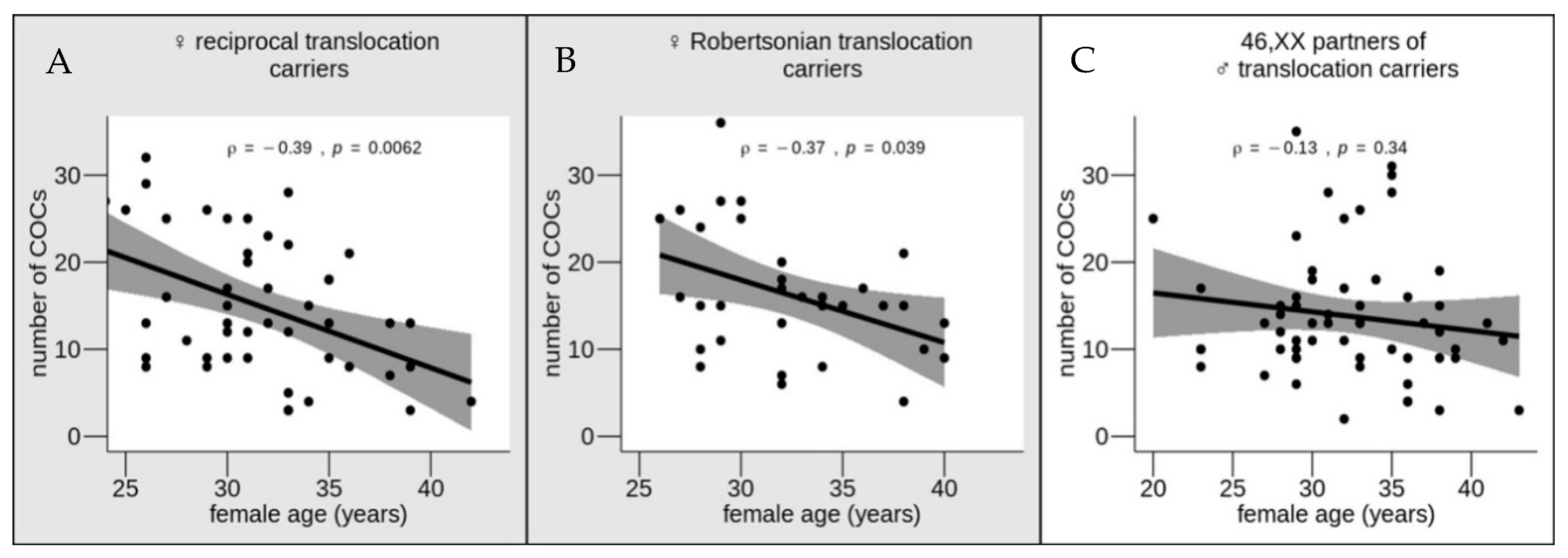
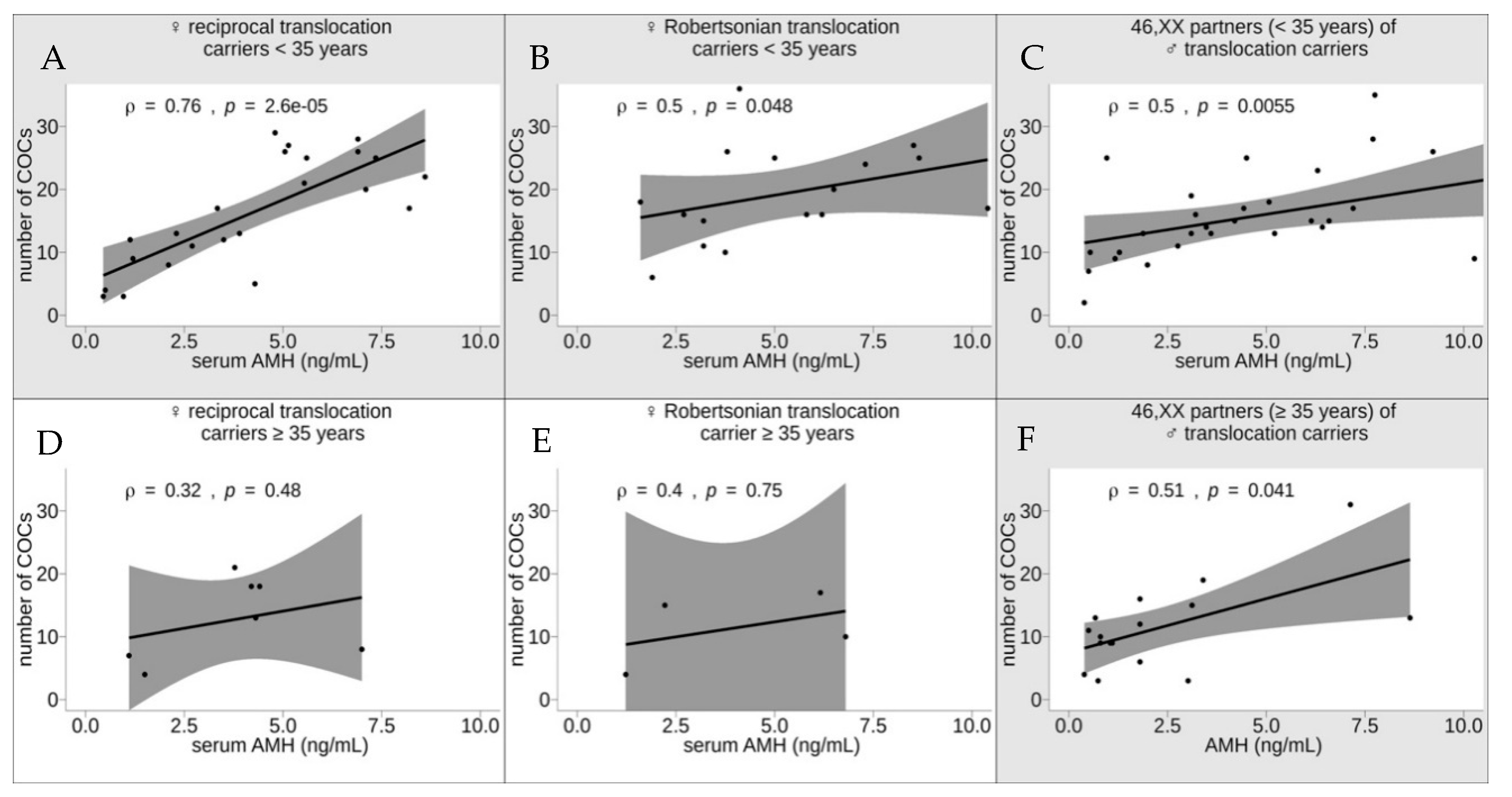
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Couple # | Carrier’s Karyotype | Partner’s Karyotype | Number of Cycles | Female Age, Years | Number of Cumulus–Oocyte Complexes (COCs) | Serum Anti-Müllerian Hormone (AMH), ng/mL | Serum Follicle-Stimulating Hormone (FSH), mIU/mL |
|---|---|---|---|---|---|---|---|
| 1 | 46,XX,t(1;16)(p31;q21) | 46,XY | 2 | 26 | 8 | 2.1 | 4.6 |
| 9 | |||||||
| 2 | 46,XX,t(2;17)(p21;p13) | 46,XY | 1 | 31 | 9 | 1.2 | 7.49 |
| 3 | 46,XX,t(10;15)(q22;q15) | 46,XY | 3 | 33 | 5 | 4.29 | 7.49 |
| 12 | |||||||
| 35 | 9 | ||||||
| 4 | 46,XX,t(10;18)(q21;q22) | 46,XY | 1 | 31 | 12 | 3.5 | 4.49 |
| 5 | 46,XX,t(2;11)(p13;q21) | 46,XY | 2 | 30 | 12 | 1.13 | 7.7 |
| 6 | 46,XX,t(4;12)(q32;q23) | 46,XY | 1 | 33 | 3 | 0.96 | 6.2 |
| 7 | 46,XX,t(2;16)(q33;q22) | 46,XY | 4 | 36 | 8 | 7 | 5.57 |
| 38 | 13 | ||||||
| 39 | 8 | ||||||
| 3 | |||||||
| 8 | 46,XX,t(3;14)(q25;q11.2) | 46,XY | 2 | 29 | 8 | 18.75 | 6.22 |
| 9 | |||||||
| 9 | 46,XX,t(4;11)(q22;q24) | 46,XY | 1 | 32 | 13 | 3.9 | 6.18 |
| 10 | 46,XX,t(1;2)(q41;p23) | 46,XY | 1 | 28 | 11 | 2.7 | 5.2 |
| 46,XX,t(3;16)(p21.2;p13.3) | 46,XY | 2 | 30 | 17 | 3.34 | 6.08 | |
| 25 | |||||||
| 12 | 46,XX,t(7;13)(p22;q32) | 46,XY | 5 | 26 | 29 | 4.8 | 5.83 |
| 32 | |||||||
| 30 | 15 | ||||||
| 13 | |||||||
| 32 | 23 | ||||||
| 13 | 46,XX,t(13;20)(q32;p12) | 46,XY | 1 | 31 | 21 | 5.54 | 5.3 |
| 14 | 46,XX,t(5;15)(q15;q25) | 46,XY | 2 | 24 | 27 | 5.14 | 4.48 |
| 27 | 16 | ||||||
| 15 | 46,XX,t(1;22)(q25;13) | 46,XY | 2 | 33 | 22 | 8.6 | 5.4 |
| 34 | 15 | ||||||
| 16 | 46,XX,t(10;13)(q11.2;q14) | 46,XY | 1 | 36 | 21 | 3.78 | 6.8 |
| 17 | 46,XX,t(3;8)(q13;p12) | 46,XY | 1 | 31 | 20 | 7.1 | 6.1 |
| 18 | 46,XX,t(1;4)(p22;p16) | 46,XY | 1 | 35 | 18 | 4.2 | 5.7 |
| 19 | 46,XX,t(11;22)(q23.3;q11.21) | 46,XY | 1 | 34 | 4 | 0.5 | 12 |
| 20 | 46,XX,t(5;7)(q35.2;q11.2) | 46,XY | 1 | 26 | 13 | 2.3 | 5.8 |
| 21 | 46,XX,t(11;22)(q23;q11.2) | 46,XY | 1 | 32 | 17 | 8.2 | 4.3 |
| 22 | 46,XX,t(2;8)(q23;p21) | 46,XY | 1 | 35 | 13 | 4.31 | 5.6 |
| 23 | 46,XX,t(1;8)(p22;q21) | 46,XY | 1 | 31 | 25 | 7.35 | 7.29 |
| 24 | 46,XX,t(2;17)(p11.2;q11.2) | 46,XY | 1 | 35 | 18 | 4.41 | 7.1 |
| 25 | 46,XX,t(4;13)(p16.3;q22.3) | 46,XY | 1 | 27 | 25 | 5.6 | 4.01 |
| 26 | 46,XX,t(1;19)(p13;q13.1) | 46,XY | 2 | 38 | 7 | 1.1 | 10.5 |
| 39 | 13 | ||||||
| 27 | 46,XX,t(9;11)(q13;q13) | 46,XY | 1 | 33 | 3 | 0.45 | 4.09 |
| 28 | 46,XX,t(6;8)(q12;q11.23) | 46,XY | 1 | 29 | 26 | 6.9 | 2.6 |
| 29 | 46,XX,t(5;14)(p15;q24) | 46,XY | 1 | 42 | 4 | 1.5 | 3.3 |
| 30 | 46,XX,t(9;10)(p24;q22) | 46,XY | 1 | 25 | 26 | 5.05 | 6.7 |
| 31 | 46,XX,t(1;5)(p34;q13) | 46,XY | 1 | 33 | 28 | 6.9 | 7.8 |
| 32 | 46,XY, t(1;2)(q41;q34) | 46,XX | 1 | 29 | 15 | 6.14 | 6.15 |
| 33 | 46,XY, t(2;9)(q11.2;q34) | 46,XX | 1 | 36 | 16 | 1.8 | 6.2 |
| 34 | 46,XY, t(8;18)(q12;p11.2) | 46,XX | 1 | 30 | 19 | 3.1 | 5.21 |
| 35 | 46,XY, t(7;11)(q32;q21) | 46,XX | 1 | 37 | 13 | 8.64 | 4.27 |
| 36 | 46,XY,t(10;21)(q11.2;q21) | 46,XX | 1 | 30 | 11 | 11.4 | 4.67 |
| 37 | 46,XY,t(16;19)(p11.2;p13.1) | 46,XX | 1 | 38 | 19 | 3.4 | 6.8 |
| 38 | 46,XY,t(7;21)(q32;q21) | 46,XX | 2 | 39 | 9 | 1.1 | 7.3 |
| 10 | - | ||||||
| 39 | 46,XY,t(5;11)(p15;q23) | 46,XX | 1 | 33 | 13 | 3.6 | 6.5 |
| 40 | 46,XY,t(16;19)(p11.2;p13.1) | 46,XX | 1 | 38 | 15 | 3.12 | 6.12 |
| 41 | 46,XY,t(1;7)(q42;p22) | 46,XX | 1 | 36 | 9 | 1.05 | 7.92 |
| 42 | 46,XY,t(3;6)(q26.2;q23) | 46,XX | 3 | 32 | 25 | 4.5 | 6.5 |
| 34 | 18 | ||||||
| 35 | 28 | ||||||
| 43 | 46,XY,t(11;20)(q21;q13) | 46,XX | 2 | 33 | 26 | 9.22 | 6.1 |
| 9 | |||||||
| 44 | 46,XY,t(9;15)(q12;q26) | 46,XX | 1 | 28 | 12 | 18.3 | 5.3 |
| 45 | 46,XY,t(7;11)(q22;q23) | 46,XX | 1 | 38 | 3 | 0.74 | 7.98 |
| 46 | 46,XY,t(3;8)(p25;q12) | 46,XX | 1 | 23 | 17 | 7.2 | 5.33 |
| 47 | 46,XY,t(3;13)(p21.2-p21.3;q32) | 46,XX | 1 | 38 | 12 | 1.8 | 9 |
| 48 | 46,XY,t(7;11)(q31.2;p14) | 46,XX | 2 | 27 | 13 | 5.21 | 5.9 |
| 29 | 10 | ||||||
| 49 | 46,XY,t(1;8)(q42.1;q24.2) | 46,XX | 2 | 29 | 9 | 10.27 | 7.27 |
| 31 | 13 | ||||||
| 50 | 46,XY,t(6;11)(p21.3;q13) | 46,XX | 1 | 35 | 31 | 7.13 | 5.1 |
| 51 | 46,XY,t(1;4)(q32;p16) | 46,XX | 1 | 36 | 6 | 1.8 | 5.6 |
| 52 | 46,XY,t(7;14)(q21.3;q31.2) | 46,XX | 1 | 29 | 35 | 7.75 | 5.37 |
| 53 | 46,XY, t(3;6)(q25;p21) | 46,XX | 1 | 29 | 11 | 2.76 | 8.61 |
| 54 | 46,XY,t(6;16)(q21;q24) | 46,XX | 1 | 43 | 3 | 3.02 | 4.4 |
| 55 | 46,XY,t(11;22)(q23.3;q11.2) | 46,XX | 1 | 30 | 18 | 5.07 | 6.9 |
| 56 | 46,XY,t(1;17)(p13;p11.2) | 46,XX | 1 | 28 | 10 | 1.28 | 7.2 |
| 57 | 46,XY,t(4;8)(q31.3;p21.3) | 46,XX | 1 | 33 | 15 | 6.59 | 7.4 |
| 58 | 46,XY,t(1;20)(p36.1;q13.3) | 46,XX | 1 | 31 | 28 | 7.7 | 7.6 |
| 59 | 46,XY,t(13;14)(q21.2;p11.2) | 46,XX | 3 | 23 | 10 | 0.54 | 5.6 |
| 8 | |||||||
| 10 | |||||||
| 60 | 45,XX,t(13;14)(q10;q10) | 46,XY | 1 | 33 | 16 | 6.2 | 0.27 |
| 61 | 45,XX, t(13;14)(q10;q10) | 46,XY | 1 | 27 | 26 | 3.8 | 4.5 |
| 62 | 45,XX, t(13;14)(q10;q10) | 46,XY | 1 | 32 | 18 | 1.6 | 5.3 |
| 63 | 45,XX,t(13;14)(q10;q10) | 46,XY | 1 | 32 | 17 | 10.4 | 5.58 |
| 64 | 45,XX,t(13;14)(q10;q10) | 46,XY | 2 | 32 | 20 | 6.5 | 5.8 |
| 13 | |||||||
| 65 | 45,XX,t(13;14)(q10;q10) | 46,XY | 2 | 34 | 16 | 2.7 | 5.3 |
| 8 | |||||||
| 66 | 45,XX,t(13;14)(q10;q10) | 46,XY | 1 | 35 | 15 | 17.5 | 6.39 |
| 67 | 45,XX,t(14;21)(q10;q10) | 46,XY | 1 | 28 | 24 | 7.3 | 4.91 |
| 68 | 45,XX,t(14;21)(q10;q10) | 46,XY | 2 | 32 | 6 | 1.9 | 7.7 |
| 7 | |||||||
| 69 | 45,XX,t(14;15)(q10;q10) | 46,XY | 2 | 29 | 11 | 3.2 | 6.6 |
| 34 | 15 | ||||||
| 70 | 45,XX,t(13;14)(q10;q10) | 46,XY | 3 | 29 | 27 | 8.52 | 6.21 |
| 15 | |||||||
| 30 | 27 | ||||||
| 71 | 45,XX,t(13;14)(q10;q10) | 46,XY | 1 | 28 | 15 | 3.2 | 7.36 |
| 72 | 45,XX,t(13;14)(q10;q10) | 46,XY | 1 | 27 | 16 | 5.81 | 6.74 |
| 73 | 45,XX,t(13;14)(q10;q10) | 46,XY | 1 | 26 | 25 | 5 | 4.9 |
| 74 | 45,XX,t(13;14)(q10;q10) | 46,XY | 1 | 29 | 36 | 4.11 | 9.32 |
| 75 | 45,XX,t(14;22)(q10;q10) | 46,XY | 2 | 28 | 10 | 3.75 | 8.2 |
| 46,XY | 8 | ||||||
| 76 | 45,XX,t(13;14)(q10;q10) | 46,XY | 3 | 38 | 15 | 2.22 | 7.5 |
| 40 | 13 | ||||||
| 9 | |||||||
| 77 | 45,XX,t(13;14)(q10;q10) | 46,XY | 3 | 36 | 17 | 6.16 | 5.3 |
| 37 | 15 | ||||||
| 38 | 21 | ||||||
| 78 | 45,XX,t(13;14)(q10;q10) | 46,XY | 1 | 38 | 4 | 1.23 | 8.3 |
| 79 | 45,XX,t(14;21)(q10;q10) | 46,XY | 1 | 39 | 10 | 6.8 | 5.8 |
| 80 | 45,XX,t(13;14)(q10;q10) | 46,XY | 1 | 30 | 25 | 8.66 | 6.74 |
| 81 | 45,XY,t(14;21)(q10;q10) | 46,XX | 1 | 32 | 17 | 4.43 | 7.27 |
| 82 | 45,XY,t(13,14)(q10;q10) | 46,XX | 1 | 28 | 14 | 6.42 | 5.38 |
| 83 | 45,XY,t(13;14)(p11.2;p11.2) | 46,XX | 2 | 29 | 9 | 1.17 | 7.33 |
| 6 | |||||||
| 84 | 45,XY,t(13;14)(q10;q10) | 46,XX | 1 | 33 | 13 | 3.1 | 6.1 |
| 85 | 45,XY,t(14;15)(q10;q10) | 46,XX | 1 | 29 | 16 | 3.21 | 6.05 |
| 86 | 45,XY,t(13;14)(q10;q10) | 46,XX | 1 | 28 | 15 | 4.2 | 9.8 |
| 87 | 45,XY,t(14;22)(q10;q10) | 46,XX | 2 | 31 | 14 | 3.48 | 7.1 |
| 32 | 11 | ||||||
| 88 | 45,XY,t(14;21)(q10;q10) | 46,XX | 1 | 30 | 13 | 1.88 | 8.11 |
| 89 | 45,XY,t(13;14)(q10;q10) | 46,XX | 1 | 29 | 23 | 6.3 | 5.36 |
| 90 | 45,XY,t(14;21)(q10;q10) | 46,XX | 1 | 38 | 9 | 0.8 | 10.9 |
| 91 | 45,XY,t(14;21)(q10;q10) | 46,XX | 1 | 35 | 10 | 0.8 | 10.1 |
| 92 | 45,XY,(15;21)(q10;q10) | 46,XX | 1 | 41 | 13 | 0.67 | 10.1 |
| 93 | 45,XY,t(13;14)(q10;q10) | 46,XX | 1 | 42 | 11 | 0.5 | 11 |
| 94 | 45,XY,t(13;14)(q10;q10) | 46,XX | 1 | 20 | 25 | 0.96 | 6.67 |
| 95 | 45,XY,t(13;14)(q10;q10) | 46,XX | 1 | 36 | 4 | 0.39 | 11.5 |
| 96 | 45,XY,t(13;14)(q10;q10) | 46,XX | 1 | 33 | 8 | 1.99 | 5.9 |
| 97 | 45,XY,t(13;14)(q10;q10) | 46,XX | 1 | 36 | 4 | 17.33 | 4.32 |
| 98 | 45,XY,t(13;14)(q10;q10) | 46,XX | 1 | 27 | 7 | 0.5 | 11 |
| 99 | 45,XY(13;14)(q10;q10) | 46,XX | 1 | 32 | 2 | 0.39 | 11 |
| 100 | 45,XY(13;14)(q10;q10) | 46,XX | 1 | 35 | 30 | 15 | 4.44 |
References
- Shah, K.; Sivapalan, G.; Gibbons, N.; Tempest, H.; Griffin, D.K. The genetic basis of infertility. Reproduction 2003, 126, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Wilch, E.S.; Morton, C.C. Historical and clinical perspectives on chromosomal translocations. In Advances in Experimental Medicine and Biology; Zhang, Y., Ed.; Springer: New York, NY, USA, 2018; Volume 1044, pp. 1–14. [Google Scholar] [CrossRef]
- Gardner, R.J.M.; Sutherland, G.; Shaffer, L. Chromosome Abnormalities and Genetic Counseling, 4th ed.; Oxford University Press: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Imudia, A.N.; Plosker, S. The Past, Present, and Future of Preimplantation Genetic Testing. Clin. Lab. Med. 2016, 36, 385–399. [Google Scholar] [CrossRef]
- Kuliev, A.; Rechitsky, S. Preimplantation genetic testing: Current challenges and future prospects. Expert Rev. Mol. Diagn. 2017, 17, 1071–1088. [Google Scholar] [CrossRef] [PubMed]
- Pendina, A.A.; Efimova, O.A.; Tikhonov, A.V.; Chiryaeva, O.G.; Fedorova, I.D.; Koltsova, A.S.; Krapivin, M.I.; Parfenyev, S.E.; Kuznetzova, T.V.; Baranov, V.S. Immunofluorescence Staining for Cytosine Modifications Like 5-Methylcytosine and Its Oxidative Derivatives and FISH. In Springer Protocols Handbooks; Liehr, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 337–346. [Google Scholar] [CrossRef]
- Grigorian, A.S.; Kruglyakov, P.V.; Taminkina, U.A.; Efimova, O.A.; Pendina, A.A.; Voskresenskaya, A.V.; Kuznetsova, T.V.; Polyntsev, D.G. Alterations of cytological and karyological profile of human mesenchymal stem cells during in vitro culturing. Bull. Exp. Biol. Med. 2010, 150, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Pendina, A.A.; Shilenkova, Y.V.; Talantova, O.E.; Efimova, O.A.; Chiryaeva, O.G.; Malysheva, O.V.; Dudkina, V.S.; Petrova, L.I.; Serebryakova, E.A.; Shabanova, E.S.; et al. Reproductive History of a Woman with 8p and 18p Genetic Imbalance and Minor Phenotypic Abnormalities. Front. Genet. 2019, 10, 1164. [Google Scholar] [CrossRef] [PubMed]
- Puppo, I.L.; Saifitdinova, A.F.; Loginova, Y.A.; Kinunen, A.A.; Tonyan, Z.N.; Pastukhova, Y.R.; Leontyeva, O.A.; Kuznetsova, R.A.; Chiryaeva, O.G.; Pendina, A.A.; et al. Y/15 and Y/22 Derivative Chromosomes in Couples with Reproductive Failures: Algorithm of Preimplantation Genetic Testing and Specificity of Inheritance. Russ. J. Genet. 2020, 56, 488–495. [Google Scholar] [CrossRef]
- Efimova, O.A.; Pendina, A.A.; Tikhonov, A.V.; Parfenyev, S.E.; Mekina, I.D.; Komarova, E.M.; Mazilina, M.A.; Daev, E.V.; Chiryaeva, O.G.; Galembo, I.A.; et al. Genome-wide 5-hydroxymethylcytosine patterns in human spermatogenesis are associated with semen quality. Oncotarget 2017, 8, 88294–88307. [Google Scholar] [CrossRef] [PubMed]
- Morin, S.J.; Eccles, J.; Iturriaga, A.; Zimmerman, R.S. Translocations, inversions and other chromosome rearrangements. Fertil. Steril. 2017, 107, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Escudero, T.; Cekleniak, N.A.; Sable, D.B.; Garrisi, G.; Munne, S. Patterns of ovarian response to gonadotropin stimulation in female carriers of balanced translocation. Fertil. Steril. 2005, 83, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Dechanet, C.; Castelli, C.; Reyftmann, L.; Hamamah, S.; Hedon, B.; Dechaud, H.; Anahory, T. Do female translocations influence the ovarian response pattern to controlled ovarian stimulation in preimplantation genetic diagnosis? Hum. Reprod. 2011, 26, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Mayeur, A.; Ahdad, N.; Hesters, L.; Grynberg, M.; Romana, S.; Sonigo, C.; Frydman, N. Does the prognosis after PGT for structural rearrangement differ between female and male translocation carriers? Reprod. Biomed. Online 2020, 40, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Franasiak, J.M.; Forman, E.J.; Hong, K.H.; Werner, M.D.; Upham, K.M.; Treff, N.R.; Scott, R.T., Jr. The nature of aneuploidy with increasing age of the female partner: A review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil. Steril. 2014, 101, 656–663.e1. [Google Scholar] [CrossRef] [PubMed]
- Pendina, A.A.; Efimova, O.A.; Chiryaeva, O.G.; Tikhonov, A.V.; Petrova, L.I.; Dudkina, V.S.; Sadik, N.A.; Fedorova, I.D.; Galembo, I.A.; Kuznetzova, T.V.; et al. A comparative cytogenetic study of miscarriages after IVF and natural conception in women aged under and over 35 years. J. Assist. Reprod. Genet. 2014, 31, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Sauer, M.V. Reproduction at an advanced maternal age and maternal health. Fertil. Steril. 2015, 103, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Kuliev, A.; Zlatopolsky, Z.; Kirillova, I.; Spivakova, J.; Janzen, J.C. Meiosis errors in over 20,000 oocytes studied in the practice of preimplantation aneuploidy testing. Reprod. Biomed. Online 2011, 22, 2–8. [Google Scholar] [CrossRef] [PubMed]
- The ESHRE Guideline Group on Ovarian Stimulation; Bosch, E.; Broer, S.; Griesinger, G.; Grynberg, M.; Humaidan, P.; Kolibianakis, E.; Kunicki, M.; La Marca, A.; Lainas, G.; et al. ESHRE guideline: Ovarian stimulation for IVF/ICSI. Hum. Reprod. Open. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- DIppolito, G.; Tirelli, A.; Giulini, S.; Volpe, A.; La Marca, A. Hormonal and ultrasound markers of ovarian function in a woman with a balanced 1;11 translocation. Fertil. Steril. 2011, 95, 803.e7–803.e8. [Google Scholar] [CrossRef]
- Keymolen, K.; Staessen, C.; Verpoest, W.; Michiels, A.; Bonduelle, M.; Haentjens, P.; Vanderelst, J.; Liebaers, I. A proposal for reproductive counselling in carriers of Robertsonian translocations: 10 years of experience with preimplantation genetic diagnosis. Hum. Reprod. 2009, 24, 2365–2371. [Google Scholar] [CrossRef] [PubMed]
- Weenen, C.; Laven, J.S.; Von Bergh, A.R.; Cranfield, M.; Groome, N.P.; Visser, J.A.; Kramer, P.; Fauser, B.C.; Themmen, A.P. Anti-Müllerian hormone expression pattern in the human ovary: Potential implications for initial and cyclic follicle recruitment. Mol. Hum. Reprod. 2004, 10, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.A.; Themmen, A.P.N. Anti-Müllerian hormone and folliculogenesis. Mol. Cell. Endocrinol. 2005, 234, 81–86. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shilenkova, Y.V.; Pendina, A.A.; Mekina, I.D.; Efimova, O.A.; Komarova, E.M.; Lesik, E.A.; Ishchuk, M.A.; Fedorova, E.M.; Chiryaeva, O.G.; Petrova, L.I.; et al. Age and Serum AMH and FSH Levels as Predictors of the Number of Oocytes Retrieved from Chromosomal Translocation Carriers after Controlled Ovarian Hyperstimulation: Applicability and Limitations. Genes 2021, 12, 18. https://doi.org/10.3390/genes12010018
Shilenkova YV, Pendina AA, Mekina ID, Efimova OA, Komarova EM, Lesik EA, Ishchuk MA, Fedorova EM, Chiryaeva OG, Petrova LI, et al. Age and Serum AMH and FSH Levels as Predictors of the Number of Oocytes Retrieved from Chromosomal Translocation Carriers after Controlled Ovarian Hyperstimulation: Applicability and Limitations. Genes. 2021; 12(1):18. https://doi.org/10.3390/genes12010018
Chicago/Turabian StyleShilenkova, Yulia V., Anna A. Pendina, Irina D. Mekina, Olga A. Efimova, Evgeniia M. Komarova, Elena A. Lesik, Mariia A. Ishchuk, Elena M. Fedorova, Olga G. Chiryaeva, Lubov’ I. Petrova, and et al. 2021. "Age and Serum AMH and FSH Levels as Predictors of the Number of Oocytes Retrieved from Chromosomal Translocation Carriers after Controlled Ovarian Hyperstimulation: Applicability and Limitations" Genes 12, no. 1: 18. https://doi.org/10.3390/genes12010018
APA StyleShilenkova, Y. V., Pendina, A. A., Mekina, I. D., Efimova, O. A., Komarova, E. M., Lesik, E. A., Ishchuk, M. A., Fedorova, E. M., Chiryaeva, O. G., Petrova, L. I., Dudkina, V. S., Talantova, O. E., Gzgzyan, A. M., & Kogan, I. Y. (2021). Age and Serum AMH and FSH Levels as Predictors of the Number of Oocytes Retrieved from Chromosomal Translocation Carriers after Controlled Ovarian Hyperstimulation: Applicability and Limitations. Genes, 12(1), 18. https://doi.org/10.3390/genes12010018






