Non-Invasive Prenatal Testing: Current Perspectives and Future Challenges
Abstract
1. Introduction
2. Evolution of Non-Invasive Prenatal Screening for Fetal Aneuploidies
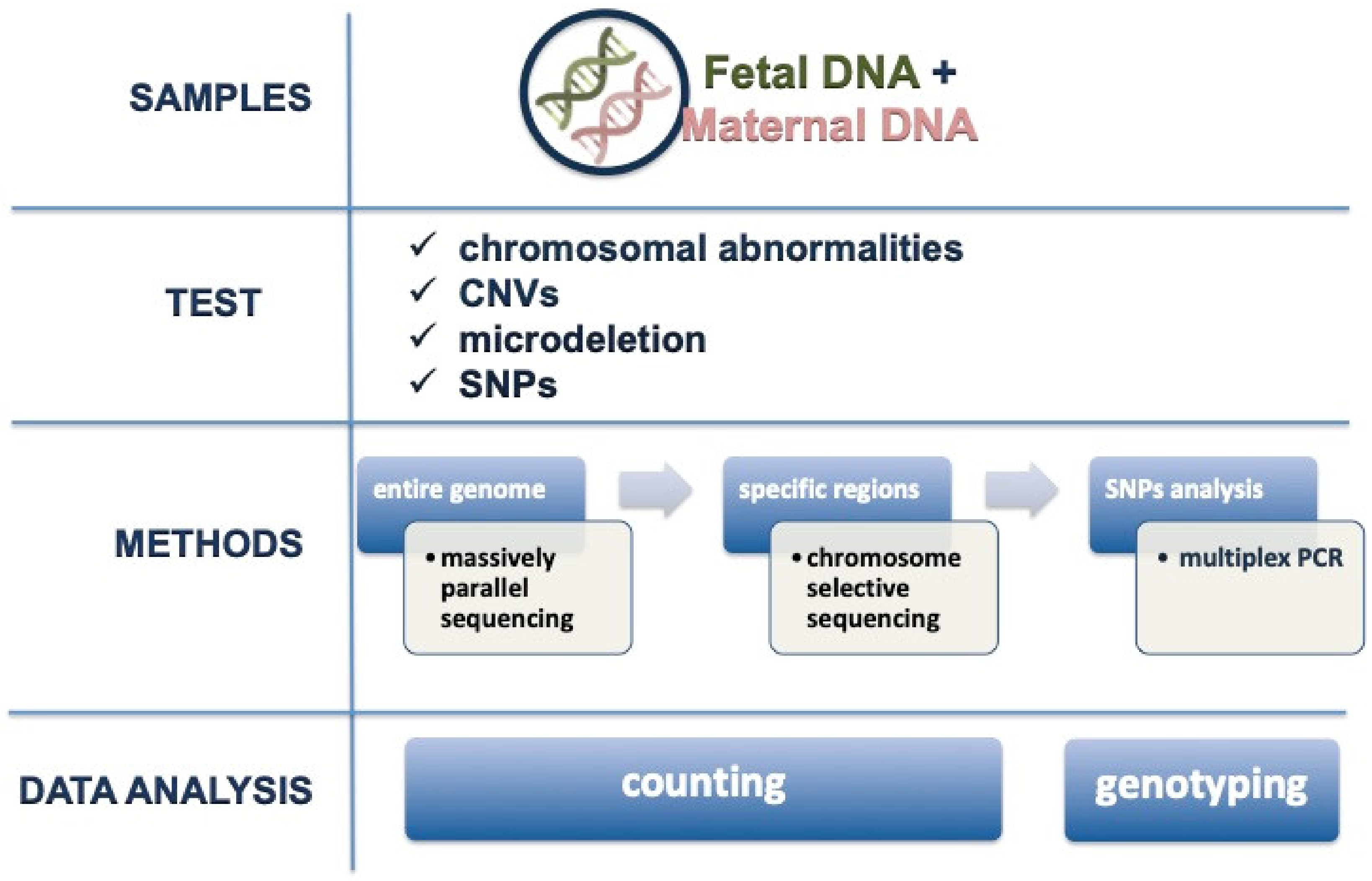
3. Available Techniques for Analysis
4. Role of Fetal Fraction and Failure Rate
5. Reliability of the Test
6. Implementation of NIPT into Clinical Practice
7. The Challenge of a Non-Invasive Prenatal Diagnosis for Monogenic Disease
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steele, M.W.; Breg, W.R., Jr. Chromosome analysis of human amniotic-fluid cells. Lancet 1966, 1, 383–385. [Google Scholar] [CrossRef]
- Nadler, H.L.; Gerbie, A.B. Role of amniocentesis in the intrauterine detection of genetic disorders. N. Engl. J. Med. 1970, 282, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Holzgreve, W.; Hogge, W.A.; Golbus, M.S. Chorion villi sampling (CVS) for prenatal diagnosis of genetic disorders: First results and future research. Eur. J. Obstet. Gynecol. Reprod. Biol. 1984, 17, 121–130. [Google Scholar] [CrossRef]
- Simoni, G.; Brambati, B.; Danesino, C.; Rossella, F.; Terzoli, G.L.; Ferrari, M.; Fraccaro, M. Efficient direct chromosome analyses and enzyme determinations from chorionic villi samples in the first trimester of pregnancy. Hum. Genet. 1983, 63, 349–357. [Google Scholar] [CrossRef]
- Beta, J.; Lesmes-Heredia, C.; Bedetti, C.; Akolekar, R. Risk of miscarriage following amniocentesis and chorionic villus sampling: A systematic review of the literature. Minerva Ginecol. 2018, 70, 215–219. [Google Scholar] [CrossRef]
- Alfirevic, Z.; Navaratnam, K.; Mujezinovic, F. Amniocentesis and chorionic villus sampling for prenatal diagnosis. Cochrane Database Syst. Rev. 2017, 9, CD003252. [Google Scholar] [CrossRef]
- Salomon, L.J.; Sotiriadis, A.; Wulff, C.B.; Odibo, A.; Akolekar, R. Risk of miscarriage following amniocentesis or chorionic villus sampling: Systematic review of the literature and updated meta-analysis. Ultrasound Obstet Gynecol. 2019, 54, 442–451. [Google Scholar] [CrossRef]
- Syngelaki, A.; Chelemen, T.; Dagklis, T.; Allan, L.; Nicolaides, K.H. Challenges in the diagnosis of fetal non-chromosomal abnormalities at 11–13 weeks. Prenat. Diagn. 2011, 31, 90–102. [Google Scholar] [CrossRef]
- Merkatz, I.R.; Nitowsky, H.M.; Macri, J.N.; Johnson, W.E. An association between low maternal serum alpha-fetoprotein and fetal chromosomal abnormalities. Am. J. Obstet. Gynecol. 1984, 148, 886–894. [Google Scholar] [CrossRef]
- Bogart, M.H.; Pandian, M.R.; Jones, O.W. Abnormal maternal serum chorionic gonadotrophin levels in pregnancies with fetal chromosome abnormalities. Prenat. Diagn. 1987, 7, 623–630. [Google Scholar] [CrossRef]
- Wald, N.J.; Cuckle, H.S.; Densem, J.W.; Nanchahal, K.; Canick, J.A.; Haddow, J.E.; Knight, G.J.; Palomaki, G.E. Maternal serum unconjugated oestriol as an antenatal screening test for Down’s syndrome. Br. J. Obstet. Gynaecol. 1988, 95, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Wald, N.J.; Cuckle, H.S.; Densem, J.W.; Nanchahal, K.; Royston, P.; Chard, T.; Haddow, J.E.; Knight, G.J.; Palomaki, G.E.; Canick, J.A. Maternal serum screening for Down’s syndrome in early pregnancy. BMJ 1988, 297, 883–887, Erratum in 1988, 297, 1029. [Google Scholar] [CrossRef] [PubMed]
- Wald, N.J.; Densem, J.W.; George, L.; Muttukrishna, S.; Knight, P.G. Prenatal screening for Down’s syndrome using inhibin-A as a serum marker. Prenat. Diagn. 1996, 16, 143–153. [Google Scholar] [CrossRef]
- Nicolaides, K.H.; Azar, G.; Byrne, D.; Mansur, C.; Marks, K. Fetal nuchal translucency: Ultrasound screening for chromosomal defects in first trimester of pregnancy. BMJ 1992, 304, 867–869. [Google Scholar] [CrossRef] [PubMed]
- De Biasio, P.; Siccardi, M.; Volpe, G.; Famularo, L.; Santi, F.; Canini, S. First-trimester screening for Down syndrome using nuchal translucency measurement with free beta-hCG and PAPP-A between 10 and 13 weeks of pregnancy—The combined test. Prenat Diagn. 1999, 19, 360–363. [Google Scholar] [CrossRef]
- Kagan, K.O.; Cicero, S.; Staboulidou, I.; Wright, D.; Nicolaides, K.H. Fetal nasal bone in screening for trisomies 21, 18 and 13 and Turner syndrome at 11–13 weeks of gestation. Ultrasound Obstet. Gynecol. 2009, 33, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Maiz, N.; Valencia, C.; Kagan, K.O.; Wright, D.; Nicolaides, K.H. Ductus venosus Doppler in screening for trisomies 21, 18 and 13 and Turner syndrome at 11–13 weeks of gestation. Ultrasound Obstet. Gynecol. 2009, 33, 512–517. [Google Scholar] [CrossRef]
- Kagan, K.O.; Valencia, C.; Livanos, P.; Wright, D.; Nicolaides, K.H. Tricuspid regurgitation in screening for trisomies 21, 18 and 13 and Turner syndrome at 11 + 0 to 13 + 6 weeks of gestation. Ultrasound Obstet. Gynecol. 2009, 33, 18–22. [Google Scholar] [CrossRef]
- Santorum, M.; Wright, D.; Syngelaki, A.; Karagioti, N.; Nicolaides, K.H. Accuracy of first-trimester combined test in screening for trisomies 21, 18 and 13. Obstet. Gynecol. 2017, 49, 714–720. [Google Scholar] [CrossRef]
- Walknowska, J.; Conte, F.A.; Grumbach, M.M. Practical and theoretical implications of fetal-maternal lymphocyte transfer. Lancet 1969, 1, 1119–1122. [Google Scholar] [CrossRef]
- Lo, Y.M.; Corbetta, N.; Chamberlain, P.F.; Rai, V.; Sargent, I.L.; Redman, C.W.; Wainscoat, J.S. Presence of fetal DNA in maternal plasma and serum. Lancet 1997, 350, 485–487. [Google Scholar] [CrossRef]
- Lo, Y.M.; Zhang, J.; Leung, T.N.; Lau, T.K.; Chang, A.M.; Hjelm, N.M. Rapid clearance of fetal DNA from maternal plasma. Am. J. Hum. Genet. 1999, 64, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.M.D.; Patel, P.; Wainscoat, J.S.; Sampietro, M.; Gillmer, M.D.G.; Fleming, K.A. Prenatal sex determination by DNA amplification from maternal peripheral blood. Lancet 1989, 2, 1363–1365. [Google Scholar] [CrossRef]
- Lo, Y.M.; Tein, M.S.; Lau, T.K.; Haines, C.J.; Leung, T.N.; Poon, P.M.; Wainscoat, J.S.; Johnson, P.J.; Chang, A.M.; Hjelm, N.M. Quantitative analysis of fetal DNA in maternal plasma and serum: Implications for noninvasive prenatal diagnosis. Am. J. Hum. Genet. 1998, 62, 768–775. [Google Scholar] [CrossRef]
- Fan, H.C.; Blumenfeld, Y.J.; Chitkara, U.; Hudgins, L.; Quake, S.R. Noninvasive diagnosis of fetal aneuploidy by shotgun sequencing DNA from maternal blood. Proc. Natl. Acad. Sci. USA 2008, 105, 16266–16271. [Google Scholar] [CrossRef]
- Chiu, R.W.K.; Allen Chan, K.C.; Gao, Y.; Lau, V.Y.M.; Zheng, W.; Leung, T.Y.; Foo, C.H.F.; Xie, B.; Tsui, N.B.; Lun, F.M.; et al. Noninvasive prenatal diagnosis of fetal chromosomal aneuploidy by massively parallel genomic sequencing of DNA in maternal plasma. Proc. Natl. Acad. Sci. USA 2008, 105, 20458–20463. [Google Scholar] [CrossRef]
- Cuckle, H.; Benn, P.; Pergament, E. Cell-free DNA screening for fetal aneuploidy as a clinical service. Clin. Biochem 2015, 48, 932–941. [Google Scholar] [CrossRef]
- Bianchi, D.W. Pregnancy: Prepare for unexpected prenatal test results. Nature 2015, 522, 29–30. [Google Scholar] [CrossRef]
- Esteves, S.C.; Yarali, H.; Ubaldi, F.M.; Carvalho, J.F.; Bento, F.C.; Vaiarelli, A.; Cimadomo, D.; Özbek, İ.Y.; Polat, M.; Bozdag, G.; et al. Validation of ART Calculator for Predicting the Number of Metaphase II Oocytes Required for Obtaining at Least One Euploid Blastocyst for Transfer in Couples Undergoing in vitro Fertilization/Intracytoplasmic Sperm Injection. Front. Endocrinol. 2020, 10, 917. [Google Scholar] [CrossRef]
- Ingerslev, H.J.; Kesmodel, U.S.; Jacobsson, B.; Vogel, I. Personalized medicine for the embryo and the fetus—Options in modern genetics influence preconception and prenatal choices. Acta Obstet. Gynecol. Scand. 2020, 99, 689–691. [Google Scholar] [CrossRef]
- Matar, A.; Höglund, A.T.; Segerdahl, P.; Kihlbom, U. Autonomous decisions by couples in reproductive care. BMC Med. Ethics 2020, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.; Sagi-Dain, L. Impact of a national genetic carrier-screening program for reproductive purposes. Acta Obstet. Gynecol. Scand. 2020, 99, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Mastantuoni, E.; Saccone, G.; Al-Kouatly, H.B.; Paternoster, M.; D’Alessandro, P.; Arduino, B.; Carbone, L.; Esposito, G.; Raffone, A.; De Vivo, V.; et al. Expanded carrier screening: A current perspective. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 230, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Casella, C.; Carbone, L.; Conforti, A.; Marrone, V.; Cioffi, G.; Buonfantino, C.; De Rosa, P.; Avino, L.; Capalbo, A.; Alviggi, C.; et al. Preimplantation genetic testing: Comparative analysis of jurisprudential regulations. It. J. Gynaecol. Obstet. 2020, 32, 237–247. [Google Scholar] [CrossRef]
- Cimadomo, D.; Rienzi, L.; Romanelli, V.; Alviggi, E.; Levi-Setti, P.E.; Albani, E.; Dusi, L.; Papini, L.; Livi, C.; Benini, F.; et al. Inconclusive chromosomal assessment after blastocyst biopsy: Prevalence, causative factors and outcomes after re-biopsy and re-vitrification. A multicenter experience. Hum. Reprod. 2018, 33, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Mazzilli, R.; Cimadomo, D.; Vaiarelli, A.; Capalbo, A.; Dovere, L.; Alviggi, E.; Dusi, L.; Foresta, C.; Lombardo, F.; Lenzi, A.; et al. Effect of the male factor on the clinical outcome of intracytoplasmic sperm injection combined with preimplantation aneuploidy testing: Observational longitudinal cohort study of 1,219 consecutive cycles. Fertil Steril. 2017, 108, 961–972.e3. [Google Scholar] [CrossRef] [PubMed]
- Coonen, E.; Van Montfoort, A.; Carvalho, F.; Kokkali, G.; Moutou, C.; Rubio, C.; De Rycke, M.; Goossens, V. ESHRE PGT Consortium data collection XVI-XVIII: Cycles from 2013 to 2015. Hum. Reprod. Open. 2020, 2020, hoaa043. [Google Scholar] [CrossRef]
- Kimelman, D.; Pavone, M.E. Non-invasive prenatal testing in the context of IVF and PGT-A. Best Pract Res. Clin. Obstet Gynaecol. 2020. [Google Scholar] [CrossRef]
- Cariati, F.; D’Argenio, V.; Tomaiuolo, R. Innovative technologies for diagnosis and screening of genetic diseases in antenatal age. J. Lab. Precis Med. 2020, 5, 6. [Google Scholar] [CrossRef]
- Chitty, L.S.; Lo, Y.M. Noninvasive Prenatal Screening for Genetic Diseases Using Massively Parallel Sequencing of Maternal Plasma DNA. Cold Spring Harb. Perspect Med. 2015, 5, a023085. [Google Scholar] [CrossRef]
- Mersy, E.; Smits, L.J.; Van Winden, L.A.; De Die-Smulders, C.E.; Paulussen, A.D.; Macville, M.V.; Coumans, A.B.; Frints, S.G.; The South-East Netherlands NIPT Consortium. Noninvasive detection of fetal trisomy 21: Systematic review and report of quality and outcomes of diagnostic accuracy studies performed between 1997 and 2012. Hum. Reprod. Update. 2013, 19, 318–329. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, V.; Borrillo, F.; Cariati, F.; Di Maggio, F.; Tomaiuolo, R. Glossario di biologia molecolare e biologia molecolare clinica. Parte I: Termini generali. Biochim. Clin. 2019, 43, 90–105. [Google Scholar] [CrossRef]
- Cariati, F.; D’Argenio, V.; Tomaiuolo, R. The evolving role of genetic tests in reproductive medicine. J. Transl. Med. 2019, 17, 267. [Google Scholar] [CrossRef]
- Gil, M.M.; Galeva, S.; Jani, J.; Konstantinidou, L.; Akolekar, R.; Plana, M.N.; Nicolaides, K.H. Screening for trisomies by cfDNA testing of maternal blood in twin pregnancy: Update of The Fetal Medicine Foundation results and meta-analysis. Ultrasound Obstet. Gynecol. 2019, 53, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Stokowski, R.P.; Wang, E.; White, K.; Batey, A.; Jacobsson, B.; Brar, H.; Balanarasimha, M.; Hollemon, D.; Sparks, A.; Nicolaides, K.; et al. Clinical performance of non-invasive prenatal testing (NIPT) using targeted cell-free DNA analysis in maternal plasma with microarrays or next generation sequencing (NGS) is consistent across multiple controlled clinical studies. Prenat. Diagn. 2015, 35, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Benn, P.; Cuckle, H.; Pergament, E. Non-invasive prenatal testing for aneuploidy: Current status and future prospects. Ultrasound Obstet. Gynecol. 2013, 42, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Chen, X.; Wang, F.; Wang, D.; Cao, Z.; Zhu, X.; Lu, C.; Yang, W.; Gao, N.; Gao, H.; et al. A multiplex droplet digital PCR assay for non-invasive prenatal testing of fetal aneuploidies. Analyst 2019, 144, 2239–2247. [Google Scholar] [CrossRef]
- Hu, H.; Wang, L.; Wu, J.; Zhou, P.; Fu, J.; Sun, J.; Cai, W.; Liu, H.; Yang, Y. Noninvasive prenatal testing for chromosome aneuploidies and subchromosomal microdeletions/microduplications in a cohort of 8141 single pregnancies. Hum. Genom. 2019, 13, 14. [Google Scholar] [CrossRef]
- Lo, K.K.; Karampetsou, E.; Boustred, C.; McKay, F.; Mason, S.; Hill, M.; Plagnol, V.; Chitty, L.S. Limited Clinical Utility of Non-invasive Prenatal Testing for Subchromosomal Abnormalities. Am. J. Hum. Genet. 2016, 98, 34–44. [Google Scholar] [CrossRef]
- Hartwig, T.S.; Ambye, L.; Sørensen, S.; Jørgensen, F.S. Discordant non-invasive prenatal testing (NIPT)—A systematic review. Prenat. Diagn. 2017, 37, 527–539. [Google Scholar] [CrossRef]
- Wright, D.; Wright, A.; Nicolaides, K.H. A unified approach to risk assessment for fetal aneuploidies. Ultrasound Obstet. Gynecol. 2015, 45, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Canick, J.A.; Palomaki, G.E.; Kloza, E.M.; Lambert-Messerlian, G.M.; Haddow, J.E. The impact of maternal plasma DNA fetal fraction on next generation sequencing tests for common fetal aneuploidies. Prenat. Diagn. 2013, 33, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.M.; Quezada, M.S.; Bregant, B.; Ferraro, M.; Nicolaides, K.H. Implementation of maternal blood cell-free DNA testing in early screening for aneuploidies. Ultrasound Obstet. Gynecol. 2013, 42, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.F.; Burton, H. The use of cell-free fetal nucleic acids in maternal blood for non-invasive prenatal diagnosis. Hum. Reprod. Update 2009, 15, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Batey, A.; Struble, C.; Musci, T.; Song, K.; Oliphant, A. Gestational age and maternal weight effects on fetal cell-free DNA in maternal plasma. Prenat. Diagn. 2013, 33, 662–666. [Google Scholar] [CrossRef]
- Chiu, R.W.; Lo, Y.M. Non-invasive prenatal diagnosis by fetal nucleic acid analysis in maternal plasma: The coming of age. Semin Fetal Neonatal. Med. 2011, 16, 88–93. [Google Scholar] [CrossRef]
- Poon, L.C.; Musci, T.; Song, K.; Syngelaki, A.; Nicolaides, K.H. Maternal plasma cell-free fetal and maternal DNA at 11–13 weeks’ gestation: Relation to fetal and maternal characteristics and pregnancy outcomes. Fetal Diagn. Ther. 2013, 33, 215–223. [Google Scholar] [CrossRef]
- Sarno, L.; Revello, R.; Hanson, E.; Akolekar, R.; Nicolaides, K.H. Prospective first-trimester screening for trisomies by cell-free DNA testing of maternal blood in twin pregnancy. Ultrasound Obstet. Gynecol. 2016, 47, 705–711. [Google Scholar] [CrossRef]
- Galeva, S.; Gil, M.M.; Konstantinidou, L.; Akolekar, R.; Nicolaides, K.H. First-trimester screening for trisomies by cfDNA testing of maternal blood in singleton and twin pregnancies: Factors affecting test failure. Ultrasound Obstet. Gynecol. 2019, 53, 804–809. [Google Scholar] [CrossRef]
- Gil, M.M.; Akolekar, R.; Quezada, M.S.; Bregant, B.; Nicolaides, K.H. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: Meta-analysis. Fetal Diagn Ther. 2014, 35, 156–173. [Google Scholar] [CrossRef]
- Leung, T.Y.; Qu, J.Z.; Liao, G.J.; Jiang, P.; Cheng, Y.K.; Chan, K.C.; Chiu, R.W.; Lo, Y.M. Noninvasive twin zygosity assessment and aneuploidy detection by maternal plasma DNA sequencing. Prenat. Diagn. 2013, 33, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Struble, C.A.; Syngelaki, A.; Oliphant, A.; Song, K.; Nicolaides, K.H. Fetal fraction estimate in twin pregnancies using directed cell-free DNA analysis. Fetal Diagn. Ther. 2014, 35, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Palomaki, G.E.; Chiu, R.W.K.; Pertile, M.D.; Sistermans, E.A.; Yaron, Y.; Vermeesch, J.R.; Vora, N.L.; Best, R.G.; Wilkins-Haug, L. International Society for Prenatal Diagnosis Position Statement: Cell free (cf)DNA screening for Down syndrome in multiple pregnancies. Prenat. Diagn. 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Rava, R.P.; Srinivasan, A.; Sehnert, A.J.; Bianchi, D.W. Circulating fetal cell-free DNA fractions differ in autosomal aneuploidies and monosomy X. Clin. Chem. 2014, 60, 243–250. [Google Scholar] [CrossRef]
- Revello, R.; Sarno, L.; Ispas, A.; Akolekar, R.; Nicolaides, K.H. Screening for trisomies by cell-free DNA testing of maternal blood: Consequences of a failed result. Ultrasound Obstet. Gynecol. 2016, 47, 698–704. [Google Scholar] [CrossRef]
- Palomaki, G.E.; Kloza, E.M.; Lambert-Messerlian, G.M.; Boom, D.V.D.; Ehrich, M.; Deciu, C.; Bombard, A.T.; Haddow, J.E. Circulating cell free DNA testing: Are some test failures informative? Prenat. Diagn. 2015, 35, 289–293. [Google Scholar] [CrossRef]
- Gregg, A.R.; Skotko, B.G.; Benkendorf, J.L.; Monaghan, K.G.; Bajaj, K.; Best, R.G.; Klugman, S.; Watson, M.S. Noninvasive prenatal screening for fetal aneuploidy, 2016 update: A position statement of the American College of Medical Genetics and Genomics. Genet. Med. 2016, 18, 1056–1065. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Committee Opinion No. 640: Cell-Free DNA Screening For Fetal Aneuploidy. Obstet. Gynecol. 2015, 126, e31–e37. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Screening for Fetal Chromosomal Abnormalities: ACOG Practice Bulletin Summary, Number 226. Obstet. Gynecol. 2020, 136, 859–867. [Google Scholar] [CrossRef]
- Shook, L.L.; Clapp, M.A.; Roberts, P.S.; Bernstein, S.N.; Goldfarb, I.T. High Fetal Fraction on First Trimester Cell-Free DNA Aneuploidy Screening and Adverse Pregnancy Outcomes. Am. J. Perinatol. 2020, 37, 8–13. [Google Scholar] [CrossRef]
- Yuan, X.; Zhou, L.; Zhang, B.; Wang, H.; Yu, B.; Xu, J. Association between low fetal fraction of cell free DNA at the early second-trimester and adverse pregnancy outcomes. Pregnancy Hypertens. 2020, 22, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Carbone, I.F.; Conforti, A.; Picarelli, S.; Morano, D.; Alviggi, C.; Farina, A. Circulating Nucleic Acids in Maternal Plasma and Serum in Pregnancy Complications: Are They Really Useful in Clinical Practice? A Systematic Review. Mol. Diagn. Ther. 2020, 24, 409–431. [Google Scholar] [CrossRef] [PubMed]
- Flori, E.; Doray, B.; Gautier, E.; Kohler, M.; Ernault, P.; Flori, J.; Costa, J.M. Circulating cell-free fetal DNA in maternal serum appears to originate from cyto- and syncytio-trophoblastic cells. Case report. Hum. Reprod. 2004, 19, 723–724. [Google Scholar] [CrossRef] [PubMed]
- Srebniak, M.I.; Diderich, K.E.; Noomen, P.; Dijkman, A.; de Vries, F.A.; van Opstal, D. Abnormal non-invasive prenatal test results concordant with karyotype of cytotrophoblast but not reflecting abnormal fetal karyotype. Ultrasound Obstet. Gynecol. 2014, 44, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Grati, F.R.; Malvestiti, F.; Ferreira, J.C.; Bajaj, K.; Gaetani, E.; Agrati, C.; Grimi, B.; Dulcetti, F.; Ruggeri, A.M.; De Toffol, S.; et al. Fetoplacental mosaicism: Potential implications for false-positive and false-negative noninvasive prenatal screening results. Genet. Med. 2014, 16, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.L.; Drendel, H.M.; Verbrugge, J.L.; Reese, A.M.; Schumacher, K.L.; Griffith, C.B.; Weaver, D.D.; Abernathy, M.P.; Litton, C.G.; Vance, G.H. Positive cell-free fetal DNA testing for trisomy 13 reveals confined placental mosaicism. Genet. Med. 2013, 15, 729–732. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, L.; Zhang, H.; Jiang, F.; Hu, H.; Chen, F.; Jiang, H.; Mu, F.; Zhao, L.; Liang, Z.; et al. Noninvasive prenatal genetic testing for fetal aneuploidy detects maternal trisomy X. Prenat. Diagn. 2012, 32, 1114–1116. [Google Scholar] [CrossRef]
- Bianchi, D.W.; Wilkins-Haug, L.E.; Enders, A.C.; Hay, E.D. Origin of extraembryonic mesoderm in experimental animals: Relevance to chorionic mosaicism in humans. Am. J. Med. Genet. 1993, 46, 542–550. [Google Scholar] [CrossRef]
- Grömminger, S.; Yagmur, E.; Erkan, S.; Nagy, S.; Schöck, U.; Bonnet, J.; Smerdka, P.; Ehrich, M.; Wegner, R.D.; Hofmann, W.; et al. Fetal Aneuploidy Detection by Cell-Free DNA Sequencing for Multiple Pregnancies and Quality Issues with Vanishing Twins. J. Clin. Med. 2014, 3, 679–692. [Google Scholar] [CrossRef]
- Curnow, K.J.; Wilkins-Haug, L.; Ryan, A.; Kırkızlar, E.; Stosic, M.; Hall, M.P.; Sigurjonsson, S.; Demko, Z.; Rabinowitz, M.; Gross, S.J. Detection of triploid, molar, and vanishing twin pregnancies by a single-nucleotide polymorphism-based noninvasive prenatal test. Am. J. Obstet Gynecol. 2015, 212, 79.e1-9. [Google Scholar] [CrossRef]
- Kelley, J.F.; Henning, G.; Ambrose, A.; Adelman, A. Vanished Twins and Misdiagnosed Sex: A Case Report with Implications in Prenatal Counseling Using Noninvasive Cell-Free DNA Screening. J. Am. Board Fam. Med. 2016, 29, 411–413. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Osborne, C.M.; Hardisty, E.; Devers, P.; Kaiser-Rogers, K.; Hayden, M.A.; Goodnight, W.; Vora, N.L. Discordant noninvasive prenatal testing results in a patient subsequently diagnosed with metastatic disease. Prenat. Diagn. 2013, 33, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.M.; Accurti, V.; Santacruz, B.; Plana, M.N.; Nicolaides, K.H. Analysis of Cell-Free DNA in Maternal Blood in Screening for Aneuploidies: Updated Meta-Analysis. Ultrasound Obstet. Gynecol. 2017, 50, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, K.H.; Musci, T.J.; Struble, C.A.; Syngelaki, A.; Gil, M.M. Assessment of fetal sex chromosome aneuploidy using directed cell-free DNA analysis. Fetal Diagn. Ther. 2014, 35, 1–6. [Google Scholar] [CrossRef]
- Syngelaki, A.; Pergament, E.; Homfray, T.; Akolekar, R.; Nicolaides, K.H. Replacing the combined test by cell-free DNA testing in screening for trisomies 21, 18 and 13: Impact on the diagnosis of other chromosomal abnormalities. Fetal Diagn. Ther. 2014, 35, 174–184. [Google Scholar] [CrossRef]
- Bianchi, D.W.; Platt, L.D.; Goldberg, J.D.; Abuhamad, A.Z.; Sehnert, A.J.; Rava, R.P.; MatErnal BLood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet. Gynecol. 2012, 119, 890–901, Erratum in 2012, 120, 957. [Google Scholar] [CrossRef]
- Neocleous, A.C.; Syngelaki, A.; Nicolaides, K.H.; Schizas, C.N. Two-stage approach for risk estimation of fetal trisomy 21 and other aneuploidies using computational intelligence systems. Ultrasound Obstet. Gynecol. 2018, 51, 503–508. [Google Scholar] [CrossRef]
- Gil, M.M.; Quezada, M.S.; Revello, R.; Akolekar, R.; Nicolaides, K.H. Analysis of cell-free DNA in maternal blood in screening for fetal aneuploidies: Updated meta-analysis. Ultrasound Obstet. Gynecol. 2015, 45, 249–266. [Google Scholar] [CrossRef]
- Palomaki, G.E.; Kloza, E.M.; Lambert-Messerlian, G.M.; Haddow, J.E.; Neveux, L.M.; Ehrich, M.; Boom, D.V.D.; Bombard, A.T.; Deciu, C.; Grody, W.W.; et al. DNA sequencing of maternal plasma to detect Down syndrome: An international clinical validation study. Genet. Med. 2011, 13, 913–920. [Google Scholar] [CrossRef]
- Bevilacqua, E.; Gil, M.M.; Nicolaides, K.H.; Ordoñez, E.; Cirigliano, V.; Dierickx, H.; Willems, P.J.; Jani, J.C. Performance of screening for aneuploidies by cell-free DNA analysis of maternal blood in twin pregnancies. Ultrasound Obstet Gynecol. 2015, 45, 61–66. [Google Scholar] [CrossRef]
- Gratacós, E.; Nicolaides, K. Clinical perspective of cell-free DNA testing for fetal aneuploidies. Fetal Diagn. Ther. 2014, 35, 151–155, Erratum in 2014, 36, 68 and 2014, 36, 262. [Google Scholar] [CrossRef] [PubMed]
- Vora, N.L.; Powell, B.; Brandt, A.; Strande, N.; Hardisty, E.; Gilmore, K.; Foreman, A.K.M.; Wilhelmsen, K.; Bizon, C.; Reilly, J.; et al. Prenatal exome sequencing in anomalous fetuses: New opportunities and challenges. Genet. Med. 2017, 19, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Sarno, L.; Maruotti, G.M.; Izzo, A.; Mazzaccara, C.; Carbone, L.; Esposito, G.; Di Cresce, M.; Saccone, G.; Sirico, A.; Genesio, R.; et al. First trimester ultrasound features of X-linked Opitz syndrome and early molecular diagnosis: Case report and review of the literature. J. Matern. Fetal Neonatal. Med. 2019, 21, 1–5. [Google Scholar] [CrossRef]
- Chiu, E.K.L.; Hui, W.W.I.; Chiu, R.W.K. cfDNA screening and diagnosis of monogenic disorders—Where are we heading? Prenat. Diagn. 2018, 38, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Di Maggio, F.; Borrillo, F.; Cariati, F.; Tomaiuolo, R.; D’Argenio, V. Glossary of molecular biology and clinical molecular biology. Part III: Molecular diagnostics. Biochim. Clin. 2020, 44, 174–178. [Google Scholar]
- D’argenio, V.; Nunziato, M.; D’Uonno, N.D.; Borrillo, F.; Vallone, R.; Conforti, A.; De Rosa, P.; Tomaiuolo, R.; Cariati, F. Indications and limitations for preimplantation genetic diagnosis. Biochim. Clin. 2017, 41, 314–321. [Google Scholar] [CrossRef]
- Cariati, F.; Savarese, M.; D’Argenio, V.; Salvatore, F.; Tomaiuolo, R. The SEeMORE strategy: Single-tube electrophoresis analysis-based genotyping to detect monogenic diseases rapidly and effectively from conception until birth. Clin. Chem. Lab. Med. 2017, 56, 40–50. [Google Scholar] [CrossRef]
- Guissart, C.; Debant, V.; Desgeorges, M.; Bareil, C.; Raynal, C.; Toga, C.; Pritchard, V.; Koenig, M.; Claustres, M.; Vincent, M.C. Non-invasive prenatal diagnosis of monogenic disorders: An optimized protocol using MEMO qPCR with miniSTR as internal control. Clin. Chem. Lab. Med. 2015, 53, 205–215. [Google Scholar] [CrossRef]
- Jenkins, L.A.; Deans, Z.C.; Lewis, C.; Allen, S. Delivering an accredited non-invasive prenatal diagnosis service for monogenic disorders and recommendations for best practice. Prenat. Diagn. 2018, 38, 44–51. [Google Scholar] [CrossRef]
- Scotchman, E.; Shaw, J.; Paternoster, B.; Chandler, N.; Chitty, L.S. Non-invasive prenatal diagnosis and screening for monogenic disorders. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 320–327. [Google Scholar] [CrossRef]
- Wong, F.C.K.; Lo, D.Y.M. Prenatal Diagnosis Innovation: Genome Sequencing of Maternal Plasma. Annu. Rev. Med. 2016, 67, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.K.; Cheung, S.W.; Smith, J.L.; Bi, W.; Ward, P.A.; Peacock, S.; Braxton, A.; Veyver, I.B.V.D.; Breman, A.M. Positive predictive value estimates for cell-free noninvasive prenatal screening from data of a large referral genetic diagnostic laboratory. Am. J. Obstet Gynecol. 2017, 217, 691.e1–691.e6. [Google Scholar] [CrossRef] [PubMed]
- Wapner, R.J.; Babiarz, J.E.; Levy, B.; Stosic, M.; Zimmermann, B.; Sigurjonsson, S.; Wayham, N.; Ryan, A.; Banjevic, M.; Lacroute, P.; et al. Expanding the scope of noninvasive prenatal testing: Detection of fetal microdeletion syndromes. Am. J. Obstet Gynecol. 2015, 212, 332.e1–9. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbone, L.; Cariati, F.; Sarno, L.; Conforti, A.; Bagnulo, F.; Strina, I.; Pastore, L.; Maruotti, G.M.; Alviggi, C. Non-Invasive Prenatal Testing: Current Perspectives and Future Challenges. Genes 2021, 12, 15. https://doi.org/10.3390/genes12010015
Carbone L, Cariati F, Sarno L, Conforti A, Bagnulo F, Strina I, Pastore L, Maruotti GM, Alviggi C. Non-Invasive Prenatal Testing: Current Perspectives and Future Challenges. Genes. 2021; 12(1):15. https://doi.org/10.3390/genes12010015
Chicago/Turabian StyleCarbone, Luigi, Federica Cariati, Laura Sarno, Alessandro Conforti, Francesca Bagnulo, Ida Strina, Lucio Pastore, Giuseppe Maria Maruotti, and Carlo Alviggi. 2021. "Non-Invasive Prenatal Testing: Current Perspectives and Future Challenges" Genes 12, no. 1: 15. https://doi.org/10.3390/genes12010015
APA StyleCarbone, L., Cariati, F., Sarno, L., Conforti, A., Bagnulo, F., Strina, I., Pastore, L., Maruotti, G. M., & Alviggi, C. (2021). Non-Invasive Prenatal Testing: Current Perspectives and Future Challenges. Genes, 12(1), 15. https://doi.org/10.3390/genes12010015





