Genetic Structure and Relationships among Wild and Cultivated Grapevines from Central Europe and Part of the Western Balkan Peninsula
Abstract
1. Introduction
2. Materials and Methods
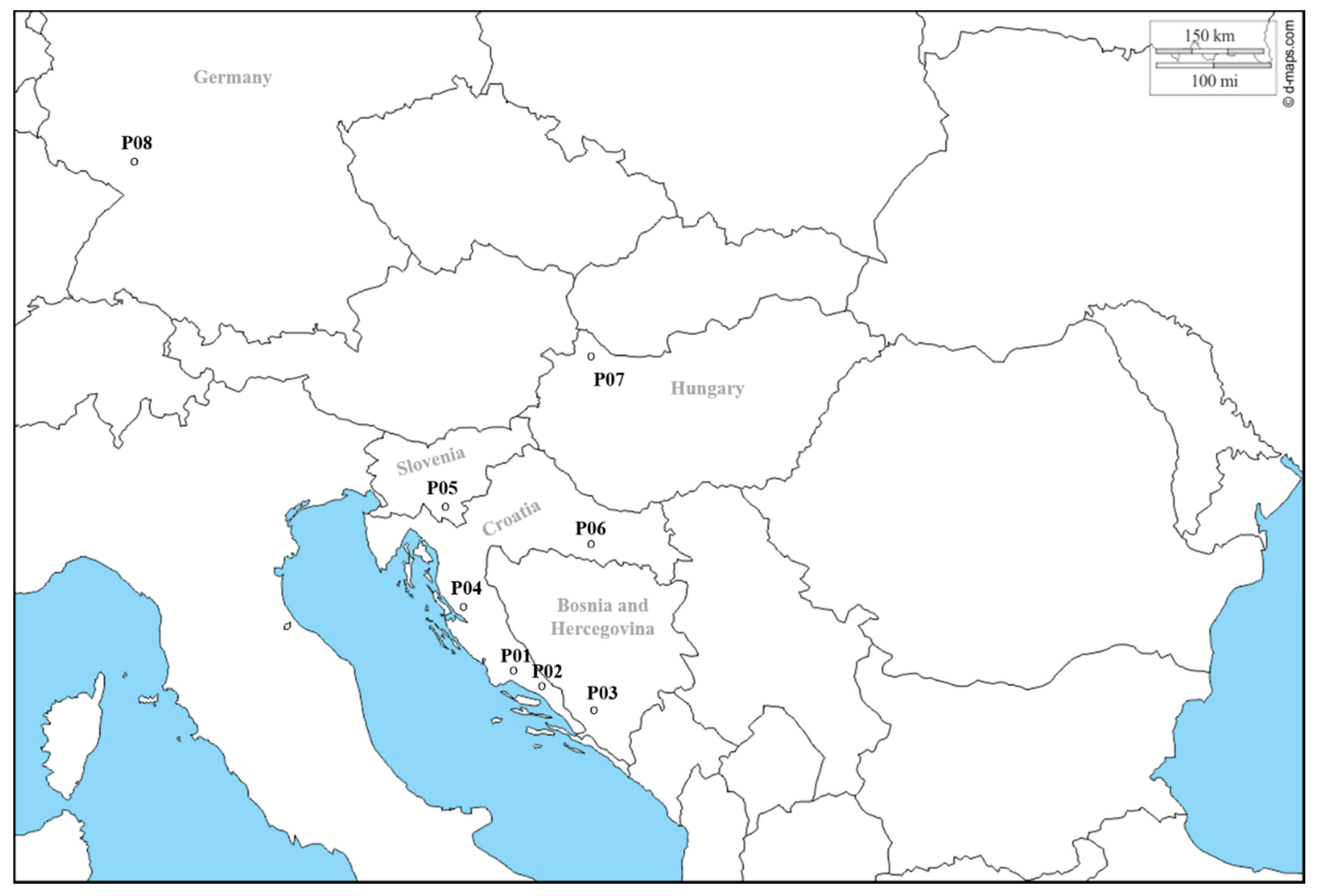
2.1. Plant Material
2.2. DNA Extraction and Microsatellite Analysis
2.3. Data Analysis
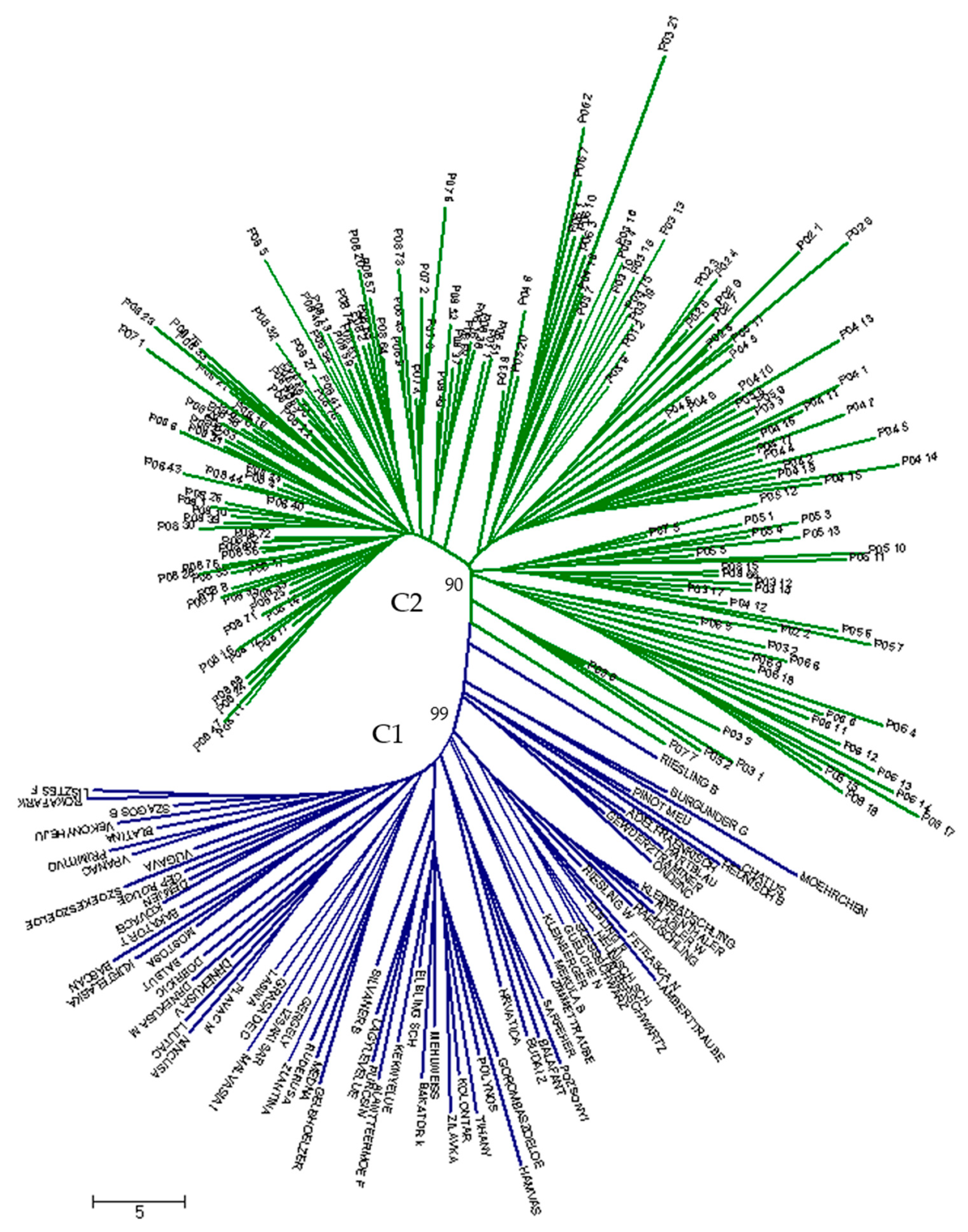
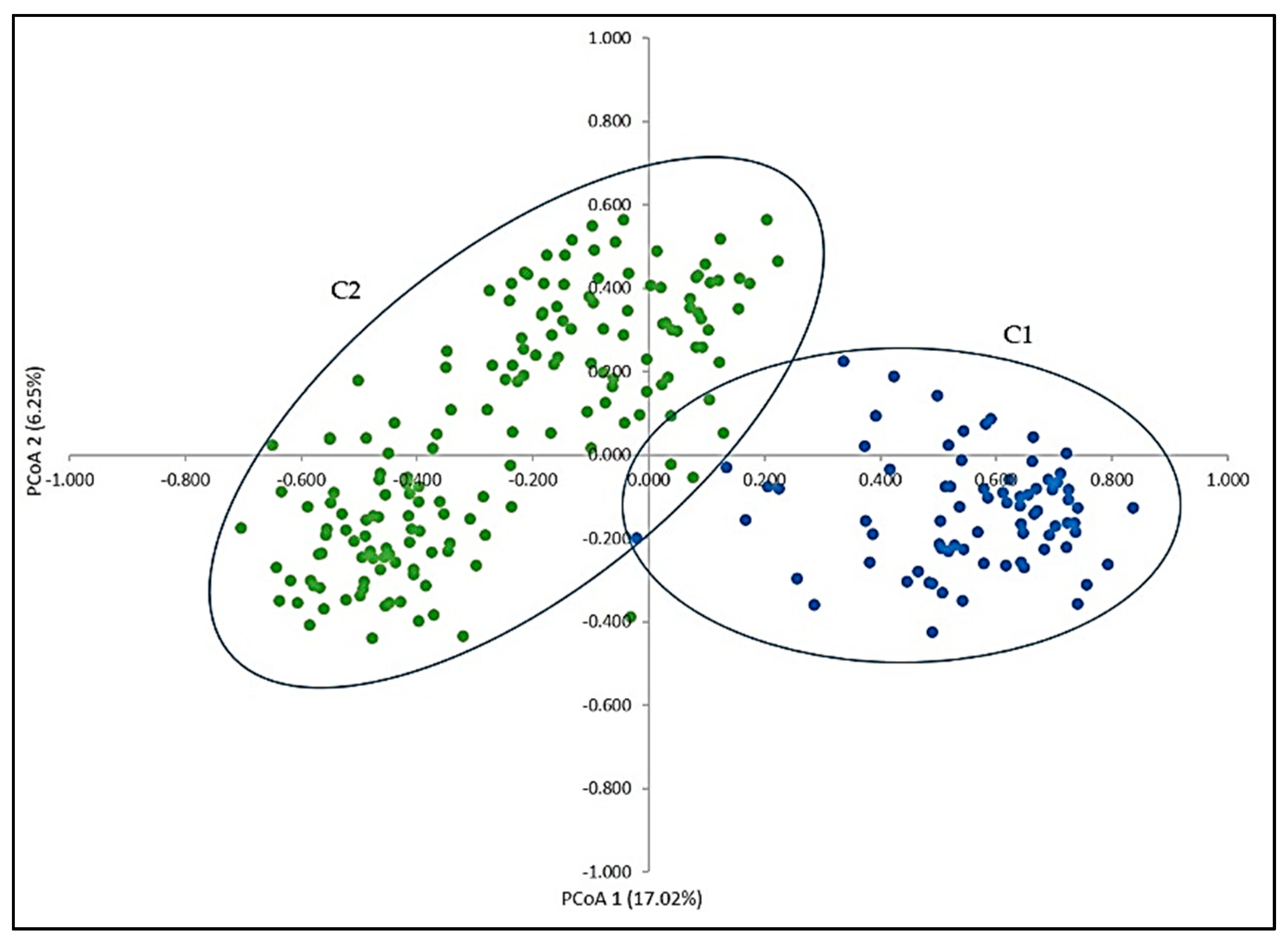
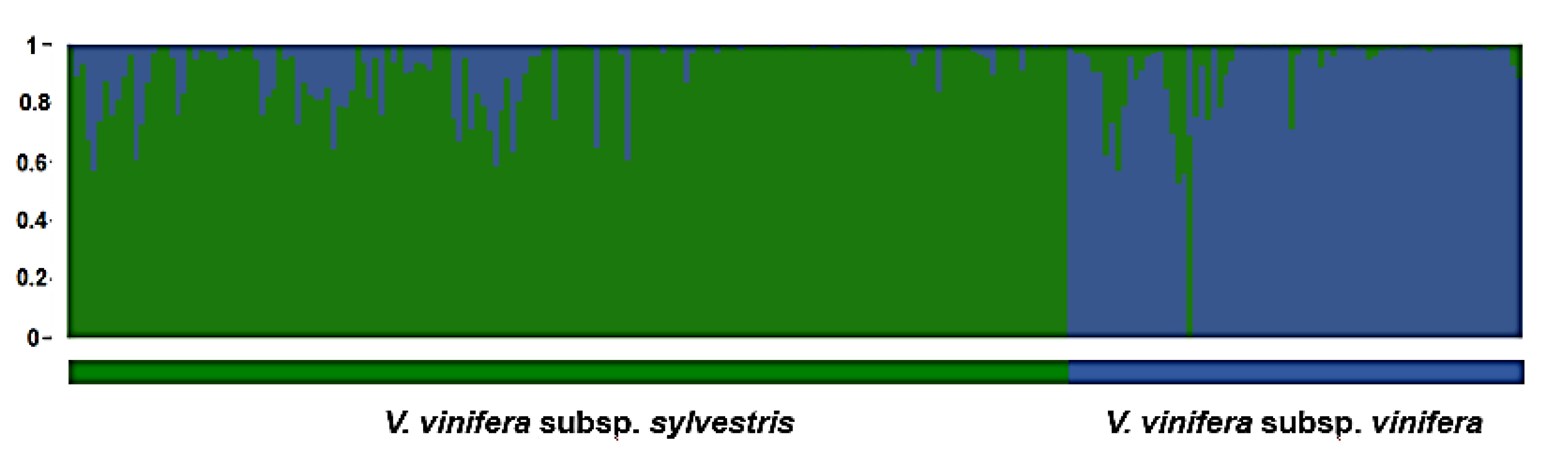
3. Results
4. Disscussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Andrés, M.T.; Benito, A.; Pérez-Rivera, G.; Ocete, R.; Lopez, M.A.; Gaforio, L.; Muñoz, G.; Cabello, F.; Martínez Zapater, J.M.; Arroyo-García, R. Genetic diversity of wild grapevine populations in Spain and their genetic relationships with cultivated grapevines: Genetic diversity of wild grapevine populations from Spain. Mol. Ecol. 2012, 21, 800–816. [Google Scholar] [CrossRef] [PubMed]
- Ocete, R.; Lopez, M.; Gallardo, A.; Arnold, C. Comparative analysis of wild and cultivated grapevine (Vitis vinifera) in the Basque Region of Spain and France. Agric. Ecosyst. Environ. 2008, 123, 95–98. [Google Scholar] [CrossRef][Green Version]
- Schröder, S.; Kortekamp, A.; Heene, E.; Daumann, J.; Valea, I.; Nick, P. Crop wild relatives as genetic resources—The case of the European wild grape. Can. J. Plant. Sci. 2015, 95, 905–912. [Google Scholar] [CrossRef]
- Biagini, B.; Imazio, S.; Scienza, A.; Failla, O.; De Lorenzis, G. Renewal of wild grapevine (Vitis vinifera L. subsp. sylvestris (Gmelin) Hegi) populations through sexual pathway: Some Italian case studies. Flora 2016, 219, 85–93. [Google Scholar] [CrossRef]
- Meléndez, E.; Puras, P.; García, J.L.; Cantos, M.; Gómez-Rodríguez, J.A.; Íñiguez, M.; Rodríguez, Á.; Valle, J.M.; Arnold, C.; Ocete, C.A.; et al. Evolution of wild and feral vines from the Ega river gallery forest (Basque country and Navarra, Spain) from 1995 to 2015. OENO One 2016, 50. [Google Scholar] [CrossRef]
- Biagini, B.; De Lorenzis, G.; Imazio, S.; Failla, O.; Scienza, A. Italian wild grapevine (Vitis vinifera L. subsp. sylvestris) population: Insights into eco-geographical aspects and genetic structure. Tree Genet. Genomes 2014, 10, 1369–1385. [Google Scholar] [CrossRef]
- Arnold, C.; Gillet, F.; Gobat, J.M. Situation de la vigne sauvage Vitis vinifera ssp. Silvestris en Europe. Vitis 1998, 37, 159–170. [Google Scholar] [CrossRef]
- FCD-Flora Croatica Database. Available online: https://Hirc.Botanic.Hr/Fcd/ (accessed on 18 June 2020).
- De Michele, R.; La Bella, F.; Gristina, A.S.; Fontana, I.; Pacifico, D.; Garfi, G.; Motisi, A.; Crucitti, D.; Abbate, L.; Carimi, F. Phylogenetic Relationship Among Wild and Cultivated Grapevine in Sicily: A Hotspot in the Middle of the Mediterranean Basin. Front. Plant. Sci. 2019, 10, 1506. [Google Scholar] [CrossRef]
- Ocete, C.A.; Arroyo, R.; Lovicu, G.; Rodríguez-Miranda, Á.; Valle, J.M.; Cantos, M.; Garcia, J.L.; Lara, M.; González De Canales, F.; Llompart, J.; et al. An inventory of the relic Eurasian wild grapevine populational nuclei in Huelva province (Andalusia, Spain). Vitis 2019, 58, 53–57. [Google Scholar] [CrossRef]
- Riaz, S.; Boursiquot, J.-M.; Dangl, G.S.; Lacombe, T.; Laucou, V.; Tenscher, A.C.; Walker, M. Identification of mildew resistance in wild and cultivated Central Asian grape germplasm. BMC Plant. Biol. 2013, 13, 149. [Google Scholar] [CrossRef]
- Ramos, M.J.N.; Coito, J.L.; Fino, J.; Cunha, J.; Silva, H.; De Almeida, P.G.; Costa, M.M.R.; Amâncio, S.; Paulo, O.S.; Rocheta, M. Deep analysis of wild Vitis flower transcriptome reveals unexplored genome regions associated with sex specification. Plant. Mol. Biol. 2017, 93, 151–170. [Google Scholar] [CrossRef] [PubMed]
- This, P.; Lacombe, T.; Thomas, M. Historical origins and genetic diversity of wine grapes. Trends Genet. 2006, 22, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.; De Lorenzis, G.; Velasco, D.; Koehmstedt, A.; Maghradze, D.; Bobokashvili, Z.; Musayev, M.; Zdunic, G.; Laucou, V.; Walker, A.M.; et al. Genetic diversity analysis of cultivated and wild grapevine (Vitis vinifera L.) accessions around the Mediterranean basin and Central Asia. BMC Plant. Biol. 2018, 18, 137. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.; Bachmann, O.; Schnitzler, A. Insights into the Vitis complex in the Danube floodplain (Austria). Ecol. Evol. 2017, 7, 7796–7806. [Google Scholar] [CrossRef]
- Cunha, J.; Ibáñez, J.; Teixeira-Santos, M.; Brazão, J.; Fevereiro, P.; Martínez-Zapater, J.M.; Eiras-Dias, J.E. Genetic Relationships Among Portuguese Cultivated and Wild Vitis vinifera L. Germplasm. Front. Plant. Sci. 2020, 11, 127. [Google Scholar] [CrossRef]
- Di Vecchi-Staraz, M.; Laucou, V.; Bruno, G.; Lacombe, T.; Gerber, S.; Bourse, T.; Boselli, M.; This, P. Low Level of Pollen-Mediated Gene Flow from Cultivated to Wild Grapevine: Consequences for the Evolution of the Endangered Subspecies Vitis vinifera L. subsp. silvestris. J. Hered. 2009, 100, 66–75. [Google Scholar] [CrossRef]
- Ghaffari, S.; Hasnaoui, N.; Zinelabidine, L.H.; Ferchichi, A.; Martínez-Zapater, J.M.; Ibáñez, J. Genetic diversity and parentage of Tunisian wild and cultivated grapevines (Vitis vinifera L.) as revealed by single nucleotide polymorphism (SNP) markers. Tree Genet. Genomes 2014, 10, 1103–1112. [Google Scholar] [CrossRef]
- Lewter, J.; Worthington, M.L.; Clark, J.R.; Varanasi, A.V.; Nelson, L.; Owens, C.L.; Conner, P.; Gunawan, G. High-density linkage maps and loci for berry color and flower sex in muscadine grape (Vitis rotundifolia). Theor. Appl. Genet. 2019, 132, 1571–1585. [Google Scholar] [CrossRef]
- D’Onofrio, C. Introgression Among Cultivated and Wild Grapevine in Tuscany. Front. Plant. Sci. 2020, 11, 202. [Google Scholar] [CrossRef]
- Negrul, A. Ampelography of USSR. Orig. Cultiv. Vine Classif. 1946, 159–206. [Google Scholar]
- Arroyo-García, R.; Ruiz-García, L.; Bolling, L.; Ocete, R.; López, M.A.; Arnold, C.; Ergul, A.; Söylemezo˝Lu, G.; Uzun, H.I.; Cabello, F.; et al. Multiple origins of cultivated grapevine (Vitis vinifera L. ssp. sativa) based on chloroplast DNA polymorphisms: Multiple origins of cultivated grapevine. Mol. Ecol. 2006, 15, 3707–3714. [Google Scholar] [CrossRef] [PubMed]
- Bacilieri, R.; Lacombe, T.; Le Cunff, L.; Di Vecchi-Staraz, M.; Laucou, V.; Genna, B.; Péros, J.-P.; This, P.; Boursiquot, J.-M. Genetic structure in cultivated grapevines is linked to geography and human selection. BMC Plant. Biol. 2013, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Emanuelli, F.; Lorenzi, S.; Grzeskowiak, L.; Catalano, V.; Stefanini, M.; Troggio, M.; Myles, S.; Martinez-Zapater, J.M.; Zyprian, E.; Moreira, F.M.; et al. Genetic diversity and population structure assessed by SSR and SNP markers in a large germplasm collection of grape. BMC Plant. Biol. 2013, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Zdunić, G.; Maul, E.; Hančević, K.; Leko, M.; Butorac, L.; Mucalo, A.; Radić, T.; Šimon, S.; Budić Leto, I.; Žulj Mihaljević, M.; et al. Genetic Diversity of Wild Grapevine [Vitis vinifera L. subsp. sylvestris (Gmel.) Hegi] in the Eastern Adriatic Region. Am. J. Enol. Vitic. 2017, 68, 252–257. [Google Scholar] [CrossRef]
- Bartha, D.; Kevey, B.; Tiborcz, V. Current and 20th century distributions of Vitis sylvestris in Hungary. Folia Oecol. 2012, 39, 99–106. [Google Scholar]
- Jahnke, G.; Nagy, Z.A.; Koltai, G.; Hajdu, E.; Májer, J. Preservation and Characterization of Woodland Grape (Vitis vinifera Ssp. Sylvestris GMEL.) Genotypes of the Szigetköz, Hungary. Germplasm: Characteristics, Diversity and Preservation; Nova Science Publishers: Hauppauge, NY, USA, 2016; pp. 27–45. [Google Scholar]
- Rusjan, D.; Bubola, M.; Janjanin, D.; Užila, Z.; Radeka, S.; Poljuha, D.; Pelengić, R.; Javornik, D.; Štajner, N. Ampelographic characterisation of grapevine accessions denominated “Refošk”, “Refosco”, “Teran” and “Terrano” (Vitis vinifera L.) from Slovenia, Croatia and Italy. Vitis 2015, 54, 77–80. [Google Scholar] [CrossRef]
- Maletić, E.; Pejić, I.; Karoglan Kontić, J.; Zdunić, G.; Preiner, D.; Šimon, S.; Andabaka, Ž.; Žulj Mihaljević, M.; Bubola, M.; Marković, Z.; et al. Ampelographic and genetic characterization of Croatian grapevine varieties. Vitis 2015, 54, 93–98. [Google Scholar] [CrossRef]
- Maul, E.; Eibach, R.; Zyprian, E.; Töpfer, R. The prolific grape variety (Vitis vinifera L.) ‘Heunisch Weiss’ (= ‘Gouais blanc’): Bud mutants, “colored” homonyms and further offspring. Vitis 2015, 54, 79–86. [Google Scholar] [CrossRef]
- Lacombe, T.; Boursiquot, J.-M.; Laucou, V.; Di Vecchi-Staraz, M.; Péros, J.-P.; This, P. Large-scale parentage analysis in an extended set of grapevine cultivars (Vitis vinifera L.). Theor. Appl. Genet. 2013, 126, 401–414. [Google Scholar] [CrossRef]
- Regner, F.; Stadlhuber, A.; Eisenheld, C.; Kaserer, H. Considerations about the evolution of grapevine and the role of Traminer. Acta Hortic. 2000, 528, 179–184. [Google Scholar] [CrossRef]
- Ledesma-Krist, G.M.; Schumann, F.; Maul, E. Die Wildrebenpopulation auf der Rheininsel Ketsch—Eine wertvolle genetische Ressource. DWJ 2014, 66, 106–118. [Google Scholar]
- Maul, E.; Sudharma, K.N.; Ganesch, A.; Brühl, U.; Hundemer, M.; Kecke, S.; Mahler-Ries, A.; Marx, G.; Schreiber, T.; Walk, M.; et al. 30 years VIVC—Vitis International Variety Catalogue. In Proceedings of the XI International Conference on Grapevine Breeding and Genetics, Beijing, China, July 28–August 2 2014; Available online: www.vivc.de (accessed on 18 June 2020).
- Zyprian, E.; Topfer, R. Development of Microsatellite-Derived Markers for Grapevine Genotyping and Genetic Mapping; NCBI GeneBank: Bethesda, MD, USA, 2005. [Google Scholar]
- Di Gaspero, G.; Peterlunger, E.; Testolin, R.; Edwards, K.J.; Cipriani, G. Conservation of microsatellite loci within the genus Vitis. Theor. Appl. Genet. 2000, 101, 301–308. [Google Scholar] [CrossRef]
- Sefc, K.M.; Regner, F.; Turetschek, E.; Glössl, J.; Steinkellner, H. Identification of microsatellite sequences in Vitis riparia and their applicability for genotyping of different Vitis species. Genome 1999, 42, 367–373. [Google Scholar] [CrossRef]
- Merdinoglu, D.; Butterlin, G.; Bevilacqua, L.; Chiquet, V.; Adam-Blondon, A.-F.; Decroocq, S. Development and characterization of a large set of microsatellite markers in grapevine (Vitis vinifera L.) suitable for multiplex PCR. Mol. Breed. 2005, 15, 349–366. [Google Scholar] [CrossRef]
- Bowers, J.E.; Dangl, G.S.; Vignani, R.; Meredith, C.P. Isolation and characterization of new polymorphic simple sequence repeat loci in grape (Vitis vinifera L.). Genome 1996, 39, 628–633. [Google Scholar] [CrossRef]
- Bowers, J.E.; Dangl, G.S.; Meredith, C.P. Development and characterization of additional microsatellite DNA markers for grape. Am. J. Enol. Vitic. 1999, 50, 243–246. [Google Scholar]
- Thomas, M.R.; Scott, N.S. Microsatellite repeats in grapevine reveal DNA polymorphisms when analysed as sequence-tagged sites (STSs). Theor. Appl. Genet. 1993, 86, 985–990. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Earl, D.A.; Vonholdt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Ramasamy, R.K.; Ramasamy, S.; Bindroo, B.B.; Naik, V.G. STRUCTURE PLOT: A program for drawing elegant STRUCTURE bar plots in user friendly interface. SpringerPlus 2014, 3, 431. [Google Scholar] [CrossRef] [PubMed]
- Bitz, L.; Zinelabidine, L.H.; Rühl, E.H.; Bitz, O. Microsatellite analysis of traditional eastern grapevine varieties and wild accessions from Geisenheim collection in Germany. Vitis 2015, 54, 17–21. [Google Scholar]
- Myles, S.; Boyko, A.R.; Owens, C.L.; Brown, P.J.; Grassi, F.; Aradhya, M.K.; Prins, B.; Reynolds, A.; Chia, J.-M.; Ware, D.; et al. Genetic structure and domestication history of the grape. Proc. Natl. Acad. Sci. USA 2011, 108, 3530–3535. [Google Scholar] [CrossRef]
- Aradhya, M.K.; Dangl, G.S.; Prins, B.H.; Boursiquot, J.M.; Walker, M.A.; Meredith, C.P.; Simon, C.J. Genetic structure and differentiation in cultivated grape, Vitis vinifera L. Genet. Res. 2003, 81, 179–192. [Google Scholar] [CrossRef]
- Miller, A.J.; Gross, B.L. From forest to the field: Perennial fruit crop domestication. Am. J. Bot. 2011, 98, 1389–1414. [Google Scholar] [CrossRef]
- Ramos-Madrigal, J.; Wiborg Runge, A.K.; Bouby, L.; Lacombe, T.; Samaniego Castruita, J.A.; Adam-Blondon, A.F.; Figueiral, I.; Hallavant, C.; Martinez-Zapater, J.M.; Schaal, C.; et al. Palaeogenomic insights into the origins of French grapevine diversity. Nat. Plants 2019, 5, 595–603. [Google Scholar] [CrossRef]
- Mercati, F.; De Lorenzis, G.; Brancadoro, L.; Lupini, A.; Abenavoli, M.R.; Barbagallo, M.G.; Di Lorenzo, R.; Scienza, A.; Sunseri, F. High-throughput 18K SNP array to assess genetic variability of the main grapevine cultivars from Sicily. Tree Genet. Genomes 2016, 12, 59. [Google Scholar] [CrossRef]
| Locus | N | Na a | Ne | Ho | He | F |
|---|---|---|---|---|---|---|
| Zag62 | 242 | 7 | 2.818 | 0.488 | 0.645 | 0.244 |
| ZAG79 | 243 | 11 | 2.315 | 0.486 | 0.568 | 0.145 |
| VVIV67 | 234 | 18 | 6.512 | 0.697 | 0.846 | 0.177 |
| VVIN16 | 243 | 5 | 2.460 | 0.506 | 0.593 | 0.147 |
| VVIP60 | 241 | 12 | 4.390 | 0.664 | 0.772 | 0.140 |
| VVMD25 | 239 | 11 | 4.720 | 0.674 | 0.788 | 0.145 |
| VVIN73 | 241 | 6 | 2.062 | 0.465 | 0.515 | 0.098 |
| VVMD5 | 237 | 10 | 4.449 | 0.692 | 0.775 | 0.107 |
| VVIB01 | 235 | 7 | 3.130 | 0.485 | 0.681 | 0.287 |
| VVMD24 | 234 | 8 | 4.257 | 0.654 | 0.765 | 0.145 |
| VVMD27 | 242 | 8 | 2.515 | 0.500 | 0.602 | 0.170 |
| VVIQ52 | 236 | 4 | 1.957 | 0.364 | 0.489 | 0.255 |
| VVS2 | 240 | 13 | 4.009 | 0.608 | 0.751 | 0.190 |
| VVIV37 | 235 | 11 | 4.029 | 0.626 | 0.752 | 0.168 |
| VMC4F3.1 | 236 | 19 | 3.754 | 0.648 | 0.734 | 0.116 |
| VMC1B11 | 240 | 11 | 3.590 | 0.538 | 0.721 | 0.255 |
| VVMD21 | 209 | 11 | 2.709 | 0.416 | 0.631 | 0.340 |
| VVMD28 | 236 | 14 | 3.720 | 0.623 | 0.731 | 0.148 |
| VVIP31 | 243 | 13 | 4.941 | 0.671 | 0.798 | 0.159 |
| VVMD7 | 241 | 13 | 4.815 | 0.755 | 0.792 | 0.047 |
| Mean | 237.4 | 11 | 3.658 | 0.578 | 0.698 | 0.174 |
| Population | N | Na a | Ne | Ho | He | F | |
|---|---|---|---|---|---|---|---|
| P02 | Mean | 9.0 | 3.700 | 2.583 | 0.586 | 0.576 | 0.016 |
| SE | 0.1 | 0.263 | 0.186 | 0.062 | 0.028 | 0.081 | |
| P03 | Mean | 20.6 | 5.300 | 2.735 | 0.576 | 0.606 | 0.051 |
| SE | 0.2 | 0.252 | 0.170 | 0.034 | 0.025 | 0.035 | |
| P04 | Mean | 18.6 | 4.850 | 2.796 | 0.531 | 0.572 | 0.069 |
| SE | 0.2 | 0.406 | 0.279 | 0.046 | 0.041 | 0.040 | |
| P05 | Mean | 12.6 | 4.050 | 2.513 | 0.456 | 0.538 | 0.146 |
| SE | 0.2 | 0.294 | 0.208 | 0.053 | 0.045 | 0.067 | |
| P06 | Mean | 16.9 | 4.700 | 2.785 | 0.593 | 0.603 | 0.021 |
| SE | 0.2 | 0.282 | 0.201 | 0.046 | 0.029 | 0.054 | |
| P07 | Mean | 7.0 | 3.100 | 2.000 | 0.464 | 0.444 | 0.013 |
| SE | 0.0 | 0.191 | 0.160 | 0.059 | 0.039 | 0.089 | |
| P08 | Mean | 75.7 | 4.550 | 2.034 | 0.437 | 0.447 | 0.020 |
| SE | 1.2 | 0.328 | 0.149 | 0.048 | 0.045 | 0.026 | |
| Cultivars | Mean | 75.2 | 8.600 | 4.471 | 0.759 | 0.733 | -0.038 |
| SE | 0.5 | 0.682 | 0.399 | 0.030 | 0.029 | 0.011 | |
| Total | Mean | 29.4 | 4.856 | 2.739 | 0.550 | 0.565 | 0.036 |
| SE | 2.1 | 0.177 | 0.098 | 0.018 | 0.014 | 0.020 |
| P01 | P02 | P03 | P04 | P05 | P06 | P07 | P08 | Cultivars | |
|---|---|---|---|---|---|---|---|---|---|
| P01 | - | 0.260 | 0.039 | 0.130 | 0.174 | 0.286 | 0.126 | 0.154 | 0.604 |
| P02 | 0.163 | - | 0.181 | 0.293 | 0.327 | 0.299 | 0.422 | 0.348 | 0.482 |
| P03 | 0.092 | 0.069 | - | 0.101 | 0.133 | 0.197 | 0.179 | 0.168 | 0.548 |
| P04 | 0.123 | 0.098 | 0.044 | - | 0.157 | 0.227 | 0.227 | 0.263 | 0.554 |
| P05 | 0.137 | 0.116 | 0.059 | 0.067 | - | 0.179 | 0.130 | 0.218 | 0.600 |
| P06 | 0.159 | 0.091 | 0.062 | 0.074 | 0.071 | - | 0.242 | 0.313 | 0.489 |
| P07 | 0.131 | 0.157 | 0.084 | 0.098 | 0.076 | 0.107 | - | 0.118 | 0.551 |
| P08 | 0.139 | 0.137 | 0.077 | 0.102 | 0.100 | 0.116 | 0.074 | - | 0.625 |
| Cultivars | 0.187 | 0.102 | 0.099 | 0.110 | 0.127 | 0.094 | 0.141 | 0.150 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdunić, G.; Lukšić, K.; Nagy, Z.A.; Mucalo, A.; Hančević, K.; Radić, T.; Butorac, L.; Jahnke, G.G.; Kiss, E.; Ledesma-Krist, G.; et al. Genetic Structure and Relationships among Wild and Cultivated Grapevines from Central Europe and Part of the Western Balkan Peninsula. Genes 2020, 11, 962. https://doi.org/10.3390/genes11090962
Zdunić G, Lukšić K, Nagy ZA, Mucalo A, Hančević K, Radić T, Butorac L, Jahnke GG, Kiss E, Ledesma-Krist G, et al. Genetic Structure and Relationships among Wild and Cultivated Grapevines from Central Europe and Part of the Western Balkan Peninsula. Genes. 2020; 11(9):962. https://doi.org/10.3390/genes11090962
Chicago/Turabian StyleZdunić, Goran, Katarina Lukšić, Zora Annamaria Nagy, Ana Mucalo, Katarina Hančević, Tomislav Radić, Lukrecija Butorac, Gizella Gyorffyne Jahnke, Erzsebet Kiss, Gloria Ledesma-Krist, and et al. 2020. "Genetic Structure and Relationships among Wild and Cultivated Grapevines from Central Europe and Part of the Western Balkan Peninsula" Genes 11, no. 9: 962. https://doi.org/10.3390/genes11090962
APA StyleZdunić, G., Lukšić, K., Nagy, Z. A., Mucalo, A., Hančević, K., Radić, T., Butorac, L., Jahnke, G. G., Kiss, E., Ledesma-Krist, G., Regvar, M., Likar, M., Piltaver, A., Žulj Mihaljević, M., Maletić, E., Pejić, I., Werling, M., & Maul, E. (2020). Genetic Structure and Relationships among Wild and Cultivated Grapevines from Central Europe and Part of the Western Balkan Peninsula. Genes, 11(9), 962. https://doi.org/10.3390/genes11090962











