PPARGC1A rs8192678 and NRF1 rs6949152 Polymorphisms Are Associated with Muscle Fiber Composition in Women
Abstract
1. Introduction
2. Subjects and Methods
2.1. Subjects
2.2. Genotyping
2.3. Muscle Biopsy
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Brooke, M.H.; Kaiser, K.K. Muscle fiber types: How many and what kind? Arch. Neurol. 1970, 23, 369–379. [Google Scholar] [CrossRef]
- Kumagai, H.; Tobina, T.; Ichinoseki-Sekine, N.; Kakigi, R.; Tsuzuki, T.; Zempo, H.; Shiose, K.; Yoshimura, E.; Kumahara, H.; Ayabe, M.; et al. Role of selected polymorphisms in determining muscle fiber composition in Japanese men and women. J. Appl. Physiol. 2018, 124, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Loenneke, J.P.; Jo, E.; Wilson, G.J.; Zourdos, M.C.; Kim, J.-S. The effects of endurance, strength, and power training on muscle fiber type shifting. J. Strength Cond. Res. 2012, 26, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Kriketos, A.D.; Pan, D.A.; Lillioja, S.; Cooney, G.J.; Baur, L.A.; Milner, M.R.; Sutton, J.R.; Jenkins, A.B.; Bogardus, C.; Storlien, L.H. Interrelationships between muscle morphology, insulin action, and adiposity. Am. J. Physiol. 1996, 270 Pt 2, R1332–R1339. [Google Scholar] [CrossRef]
- Toft, I.; Bønaa, K.H.; Lindal, S.; Jenssen, T. Insulin kinetics, insulin action, and muscle morphology in lean or slightly overweight persons with impaired glucose tolerance. Metabolism 1998, 47, 848–854. [Google Scholar] [CrossRef]
- Essén-Gustavsson, B.; Henriksson, J. Enzyme levels in pools of microdissected human muscle fibres of identified type: Adaptive response to exercise. Acta Physiol. Scand. 1984, 120, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Bergh, U.; Thorstensson, A.; Sjödin, B.; Hulten, B.; Piehl, K.; Karlsson, J. Maximal oxygen uptake and muscle fiber types in trained and untrained humans. Med. Sci. Sports 1978, 10, 151–154. [Google Scholar]
- Foster, C.; Costill, D.L.; Daniels, J.T.; Fink, W.J. Skeletal muscle enzyme activity, fiber composition and VO2 max in relation to distance running performance. Eur. J. Appl. Physiol. Occup. Physiol. 1978, 39, 73–80. [Google Scholar] [CrossRef]
- Fry, A.C.; Schilling, B.K.; Staron, R.S.; Hagerman, F.C.; Hikida, R.S.; Thrush, J.T. Muscle fiber characteristics and performance correlates of male Olympic-style weightlifters. J. Strength Cond. Res. 2003, 17, 746–754. [Google Scholar]
- Widrick, J.J.; Stelzer, J.E.; Shoepe, T.C.; Garner, D.P. Functional properties of human muscle fibers after short-term resistance exercise training. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R408–R416. [Google Scholar] [CrossRef]
- Andersen, J.L.; Klitgaard, H.; Saltin, B. Myosin heavy chain isoforms in single fibres from m. vastus lateralis of sprinters: Influence of training. Acta Physiol. Scand. 1994, 151, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Wade, A.J.; Marbut, M.M.; Round, J.M. Muscle fibre type and aetiology of obesity. Lancet 1990, 335, 805–808. [Google Scholar] [CrossRef]
- Gerrits, M.F.; Ghosh, S.; Kavaslar, N.; Hill, B.; Tour, A.; Seifert, E.L.; Beauchamp, B.; Gorman, S.; Stuart, J.; Dent, R.; et al. Distinct skeletal muscle fiber characteristics and gene expression in diet-sensitive versus diet-resistant obesity. J. Lipid Res. 2010, 51, 2394–2404. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.J.; Barakat, H.A.; Dohm, G.L.; Pories, W.J.; MacDonald, K.G.; Cunningham, P.R.; Swanson, M.S.; Houmard, J.A. Muscle fiber type is associated with obesity and weight loss. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1191–E1196. [Google Scholar] [CrossRef]
- Frisk-Holmberg, M.; Essen, B.; Fredrikson, M.; Strom, G.; Wibell, L. Muscle fibre composition in relation to blood pressure response to isometric exercise in normotensive and hypertensive subjects. Acta Med. Scand. 1983, 213, 21–26. [Google Scholar] [CrossRef]
- Simoneau, J.A.; Bouchard, C. Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am. J. Physiol. 1989, 257 Pt 1, E567–E572. [Google Scholar] [CrossRef]
- Komi, P.V.; Viitasalo, J.H.; Havu, M.; Thorstensson, A.; Sjodin, B.; Karlsson, J. Skeletal muscle fibres and muscle enzyme activities in monozygous and dizygous twins of both sexes. Acta Physiol. Scand. 1977, 100, 385–392. [Google Scholar]
- Simoneau, J.A.; Bouchard, C. Genetic determinism of fiber type proportion in human skeletal muscle. FASEB J. Publ. Fed. Am. Soc. Exp. Biol. 1995, 9, 1091–1095. [Google Scholar] [CrossRef]
- Ahmetov, I.I.; Vinogradova, O.L.; Williams, A.G. Gene polymorphisms and fiber-type composition of human skeletal muscle. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 292–303. [Google Scholar] [CrossRef]
- Franks, P.W.; Christophi, C.A.; Jablonski, K.A.; Billings, L.K.; Delahanty, L.M.; Horton, E.S.; Knowler, W.C.; Florez, J.C.; Diabetes Prevention Program Research Group. Common variation at PPARGC1A/B and change in body composition and metabolic traits following preventive interventions: The Diabetes Prevention Program. Diabetologia 2014, 57, 485–490. [Google Scholar] [CrossRef]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Virbasius, J.V.; Scarpulla, R.C. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: A potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc. Natl. Acad. Sci. USA 1994, 91, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Virbasius, J.V.; Virbasius, C.A.; Scarpulla, R.C. Identity of GABP with NRF-2, a multisubunit activator of cytochrome oxidase expression, reveals a cellular role for an ETS domain activator of viral promoters. Genes Dev. 1993, 7, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Ahmetov, I.I.; Williams, A.G.; Popov, D.V.; Lyubaeva, E.V.; Hakimullina, A.M.; Fedotovskaya, O.N.; Mozhayskaya, I.A.; Vinogradova, O.L.; Astratenkova, I.V.; Montgomery, H.E.; et al. The combined impact of metabolic gene polymorphisms on elite endurance athlete status and related phenotypes. Hum. Genet. 2009, 126, 751–761. [Google Scholar] [CrossRef]
- Lin, J.; Wu, H.; Tarr, P.T.; Zhang, C.Y.; Wu, Z.; Boss, O.; Michael, L.F.; Puigserver, P.; Isotani, E.; Olson, E.N.; et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 2002, 418, 797–801. [Google Scholar] [CrossRef]
- Ramachandran, B.; Yu, G.; Gulick, T. Nuclear respiratory factor 1 controls myocyte enhancer factor 2A transcription to provide a mechanism for coordinate expression of respiratory chain subunits. J. Biol. Chem. 2008, 283, 11935–11946. [Google Scholar] [CrossRef]
- Joseph, J.S.; Ayeleso, A.O.; Mukwevho, E. Exercise increases hyper-acetylation of histones on the Cis-element of NRF-1 binding to the Mef2a promoter: Implications on type 2 diabetes. Biochem. Biophys. Res. Commun. 2017, 486, 83–87. [Google Scholar] [CrossRef]
- Handschin, C.; Chin, S.; Li, P.; Liu, F.; Maratos-Flier, E.; LeBrasseur, N.K.; Yan, Z.; Spiegelman, B.M. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1 alpha muscle-specific knock-out animals. J. Biol. Chem. 2007, 282, 30014–30021. [Google Scholar] [CrossRef]
- Eynon, N.; Meckel, Y.; Sagiv, M.; Yamin, C.; Amir, R.; Sagiv, M.; Goldhammer, E.; Duarte, J.A.; Oliveira, J. Do PPARGC1A and PPARα polymorphisms influence sprint or endurance phenotypes? Scand. J. Med. Sci. Sport 2010, 20, e145–e150. [Google Scholar] [CrossRef]
- Lucia, A.; Gómez-Gallego, F.; Barroso, I.; Rabadán, M.; Bandrés, F.; San Juan, A.F.; Chicharro, J.L.; Ekelund, U.; Brage, S.; Earnest, C.P.; et al. PPARGC1A genotype (Gly482Ser) predicts exceptional endurance capacity in European men. J. Appl. Physiol. 2005, 99, 344–348. [Google Scholar] [CrossRef]
- Maciejewska, A.; Sawczuk, M.; Cieszczyk, P.; Mozhayskaya, I.A.; Ahmetov, I.I. The PPARGC1A gene Gly482Ser in Polish and Russian athletes. J. Sports Sci. 2012, 30, 101–113. [Google Scholar] [CrossRef]
- Nishida, Y.; Iyadomi, M.; Higaki, Y.; Tanaka, H.; Kondo, Y.; Otsubo, H.; Horita, M.; Hara, M.; Tanaka, K. Association between the PPARGC1A polymorphism and aerobic capacity in Japanese middle-aged men. Intern. Med. 2015, 54, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Andrulionyte, L.; Peltola, P.; Chiasson, J.-L.; Laakso, M. Single nucleotide polymorphisms of PPARD in combination with the Gly482Ser substitution of PGC-1A and the Pro12Ala substitution of PPARG2 predict the conversion from impaired glucose tolerance to type 2 diabetes: The STOP-NIDDM trial. Diabetes 2006, 55, 2148–2152. [Google Scholar] [CrossRef]
- Hara, K.; Tobe, K.; Okada, T.; Kadowaki, H.; Akanuma, Y.; Ito, C.; Kimura, S.; Kadowaki, T. A genetic variation in the PGC-1 gene could confer insulin resistance and susceptibility to Type II diabetes. Diabetologia 2002, 45, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Vohl, M.-C.; Houde, A.; Lebel, S.; Hould, F.-S.; Marceau, P. Effects of the peroxisome proliferator-activated receptor-gamma co-activator-1 Gly482Ser variant on features of the metabolic syndrome. Mol. Genet. Metab. 2005, 86, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Steinbacher, P.; Feichtinger, R.G.; Kedenko, L.; Kedenko, I.; Reinhardt, S.; Schönauer, A.L.; Leitner, I.; Sänger, A.M.; Stoiber, W.; Kofler, B.; et al. The single nucleotide polymorphism Gly482Ser in the PGC-1alpha gene impairs exercise-induced slow-twitch muscle fibre transformation in humans. PLoS ONE 2015, 10, e0123881. [Google Scholar] [CrossRef]
- He, Z.; Hu, Y.; Feng, L.; Bao, D.; Wang, L.; Li, Y.; Wang, J.; Liu, G.; Xi, Y.; Wen, L.; et al. Is there an association between PPARGC1A genotypes and endurance capacity in Chinese men? Scand. J. Med. Sci. Sports 2008, 18, 195–204. [Google Scholar] [CrossRef]
- Rico-Sanz, J.; Rankinen, T.; Rice, T.; Leon, A.S.; Skinner, J.S.; Wilmore, J.H.; Rao, D.C.; Bouchard, C. Quantitative trait loci for maximal exercise capacity phenotypes and their responses to training in the HERITAGE Family Study. Physiol. Genomics 2004, 16, 256–260. [Google Scholar] [CrossRef]
- Natsume, T.; Ozaki, H.; Kakigi, R.; Kobayashi, H.; Naito, H. Effects of training intensity in electromyostimulation on human skeletal muscle. Eur. J. Appl. Physiol. 2018, 118, 1339–1347. [Google Scholar] [CrossRef]
- He, Z.; Hu, Y.; Feng, L.; Li, Y.; Liu, G.; Xi, Y.; Wen, L.; Lucia, A. NRF-1 genotypes and endurance exercise capacity in young Chinese men. Br. J. Sports Med. 2008, 42, 361–366. [Google Scholar] [CrossRef]
- Yvert, T.; Miyamoto-Mikami, E.; Murakami, H.; Miyachi, M.; Kawahara, T.; Fuku, N. Lack of replication of associations between multiple genetic polymorphisms and endurance athlete status in Japanese population. Physiol. Rep. 2016, 4, e13003. [Google Scholar] [CrossRef] [PubMed]
- Bamman, M.M.; Hill, V.J.; Adams, G.R.; Haddad, F.; Wetzstein, C.J.; Gower, B.A.; Ahmed, A.; Hunter, G.R. Gender differences in resistance-training-induced myofiber hypertrophy among older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Glenmark, B.; Hedberg, G.; Jansson, E. Changes in muscle fibre type from adolescence to adulthood in women and men. Acta Physiol. Scand. 1992, 146, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Velarde, M.C. Mitochondrial and sex steroid hormone crosstalk during aging. Longev. Health 2014, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Q.; Cammarata, P.R.; Baines, C.P.; Yager, J.D. Regulation of mitochondrial respiratory chain biogenesis by estrogens/estrogen receptors and physiological, pathological and pharmacological implications. Biochim. Biophys. Acta. 2009, 1793, 1540–1570. [Google Scholar] [CrossRef]
- Klinge, C.M. Estrogenic control of mitochondrial function and biogenesis. J. Cell. Biochem. 2008, 105, 1342–1351. [Google Scholar] [CrossRef]
- Colom, B.; Alcolea, M.P.; Valle, A.; Oliver, J.; Roca, P.; Garcia-Palmer, F.J. Skeletal muscle of female rats exhibit higher mitochondrial mass and oxidative-phosphorylative capacities compared to males. Cell. Physiol. Biochem. 2007, 19, 205–212. [Google Scholar] [CrossRef]
- Witt, H.; Schubert, C.; Jaekel, J.; Fliegner, D.; Penkalla, A.; Tiemann, K.; Stypmann, J.; Roepcke, S.; Brokat, S.; Mahmoodzadeh, S.; et al. Sex-specific pathways in early cardiac response to pressure overload in mice. J. Mol. Med. 2008, 86, 1013–1024. [Google Scholar] [CrossRef]
- Tcherepanova, I.; Puigserver, P.; Norris, J.D.; Spiegelman, B.M.; McDonnell, D.P. Modulation of estrogen receptor-alpha transcriptional activity by the coactivator PGC-1. J. Biol. Chem. 2000, 275, 16302–16308. [Google Scholar] [CrossRef]
- Mattingly, K.A.; Ivanova, M.M.; Riggs, K.A.; Wickramasinghe, N.S.; Barch, M.J.; Klinge, C.M. Estradiol stimulates transcription of nuclear respiratory factor-1 and increases mitochondrial biogenesis. Mol. Endocrinol. 2008, 22, 609–622. [Google Scholar] [CrossRef]
- Wright, D.C.; Han, D.-H.; Garcia-Roves, P.M.; Geiger, P.C.; Jones, T.E.; Holloszy, J.O. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J. Biol. Chem. 2007, 282, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Gleyzer, N.; Vercauteren, K.; Scarpulla, R.C. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol. Cell. Biol. 2005, 25, 1354–1366. [Google Scholar] [CrossRef] [PubMed]
- Wende, A.R.; Schaeffer, P.J.; Parker, G.J.; Zechner, C.; Han, D.H.; Chen, M.M.; Hancock, C.R.; Lehman, J.J.; Huss, J.M.; McClain, D.A.; et al. A role for the transcriptional coactivator PGC-1alpha in muscle refueling. J. Biol. Chem. 2007, 282, 36642–36651. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.A.; Daniels, T.G.; Wang, X.; Paul ALin, J.; Spiegelman, B.M.; Stevenson, S.C.; Rangwala, S.M. Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J. Appl. Physiol. 2008, 104, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Michael, L.F.; Wu, Z.; Cheatham, R.B.; Puigserver, P.; Adelmant, G.; Lehman, J.J.; Kelly, D.P.; Spiegelman, B.M. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc. Natl. Acad. Sci. USA 2001, 98, 3820–3825. [Google Scholar] [CrossRef] [PubMed]
- Pandorf, C.E.; Caiozzo, V.J.; Haddad, F.; Baldwin, K.M. A rationale for SDS-PAGE of MHC isoforms as a gold standard for determining contractile phenotype. J. Appl. Physiol. 2010, 108, 222. [Google Scholar]
- Serrano, N.; Colenso-Semple, L.M.; Lazauskus, K.K.; Siu, J.W.; Bagley, J.R.; Lockie, R.G.; Costa, P.B.; Galpin, A.J. Extraordinary fast-twitch fiber abundance in elite weightlifters. PLoS ONE 2019, 14, e0207975. [Google Scholar] [CrossRef]
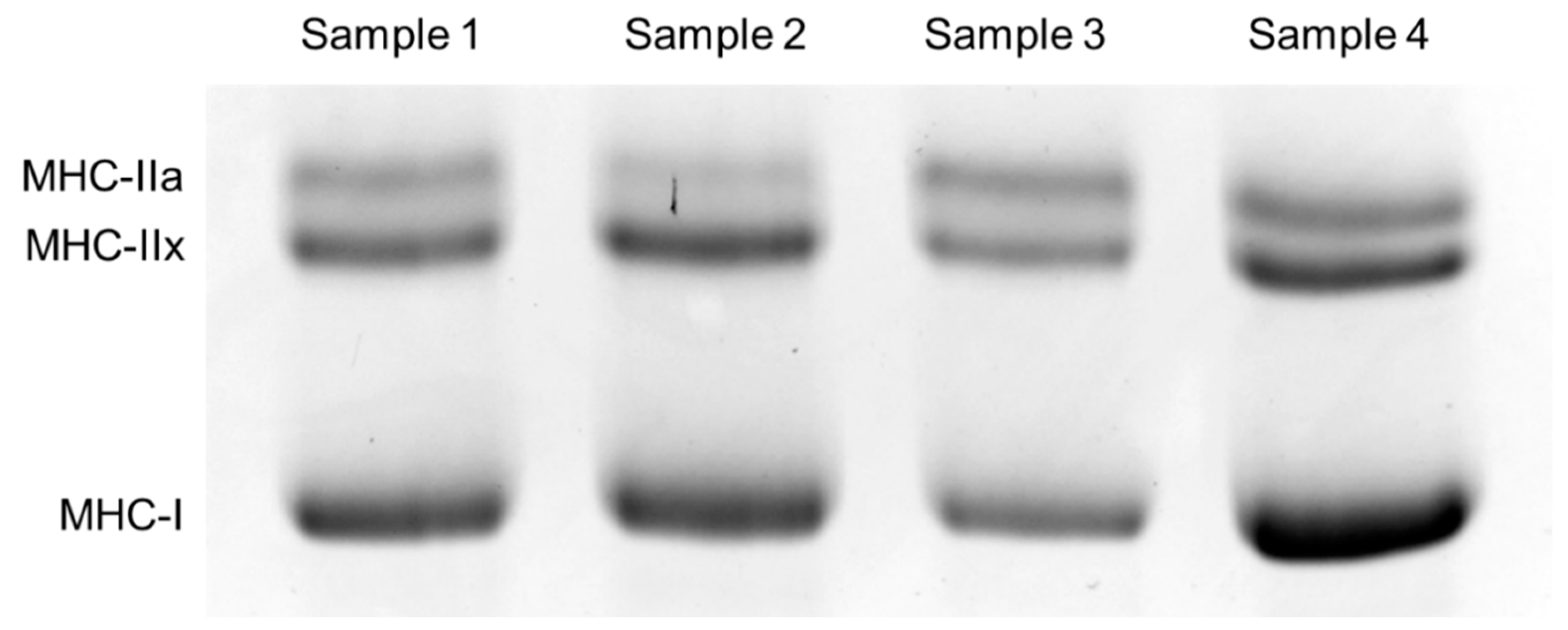

| Men (n = 107) | Women (n = 107) | |
|---|---|---|
| Age (years) | 47.5 ± 17.9 | 48.3 ± 16.3 |
| Height (cm) | 169.7 ± 5.9 | 156.7 ± 5.9 *** |
| Weight (kg) | 74.7 ± 11.2 | 62.5 ± 9.9 *** |
| BMI (kg/m2) | 25.9 ± 3.8 | 25.5 ± 4.3 |
| MHC-I (%) | 40.7 ± 11.5 | 50.3 ± 11.2 *** |
| MHC-IIa (%) | 35.9 ± 8.2 | 30.9 ± 8.2 *** |
| MHC-IIx (%) | 23.4 ± 9.1 | 18.8 ± 8.3 *** |
| Gene Name (rs Number) | Genotype | p (AIC) | ||||
|---|---|---|---|---|---|---|
| PPARGC1A (rs8192678) | Additive | Dominant | Recessive | |||
| Men | GG (n = 31) | GA (n = 51) | AA (n = 22) | GG vs. GA vs. AA | GG + GA vs. AA | GG vs. GA + AA |
| MHC-I (%) | 40.3 ± 10.7 | 41.6 ± 10.8 | 39.3 ± 14.6 | 0.854 (795.8) | 0.985 (795.9) | 0.764 (795.8) |
| MHC-IIa (%) | 37.5 ± 7.3 | 34.4 ± 7.2 | 36.9 ± 11.0 | 0.366 (724.2) | 0.965 (725.1) | 0.150 (722.9) |
| MHC-IIx (%) | 22.2 ± 8.9 | 24.0 ± 9.0 | 23.8 ± 10.0 | 0.589 (759.0) | 0.981 (759.3) | 0.392 (758.6) |
| Women | (n = 36) | (n = 52) | (n = 18) | |||
| MHC-I (%) | 47.7 ± 12.1 | 50.3 ± 11.0 | 54.4 ± 7.7 | 0.042 (809.1) * | 0.084 (810.3) | 0.112 (810.7) |
| MHC-IIa (%) | 32.3 ± 8.3 | 30.1 ± 8.0 | 30.4 ± 9.0 | 0.368 (750.3) | 0.854 (751.1) | 0.241 (749.7) |
| MHC-IIx (%) | 20.1 ± 8.9 | 19.6 ± 7.4 | 15.2 ± 8.0 | 0.072 (749.2) | 0.033 (747.8) * | 0.348 (751.7) |
| NRF1 (rs6949152) | Additive | Dominant | Recessive | |||
| Men | AA (n = 77) | AG (n = 21) | GG (n = 6) | AA vs. AG vs. GG | AA + AG vs. GG | AA vs. AG + GG |
| MHC-I (%) | 39.8 ± 11.4 | 44.3 ± 11.6 | 40.2 ± 13.3 | 0.334 (794.9) | 0.713 (795.7) | 0.142 (793.6) |
| MHC-IIa (%) | 36.0 ± 8.5 | 34.8 ± 7.1 | 38.0 ± 9.2 | 0.853 (725.0) | 0.341 (724.1) | 0.794 (725.0) |
| MHC-IIx (%) | 24.2 ± 9.1 | 20.9 ± 9.3 | 21.7 ± 8.3 | 0.188 (757.6) | 0.713 (759.2) | 0.125 (756.9) |
| Women | (n = 59) | (n = 41) | (n = 6) | |||
| MHC-I (%) | 52.7 ± 10.5 | 46.3 ± 11.5 | 51.0 ± 6.6 | 0.033 (808.7) * | 0.891 (813.3) | 0.008 (806.0) ** |
| MHC-IIa (%) | 29.8 ± 8.7 | 32.4 ± 7.4 | 31.2 ± 8.7 | 0.221 (749.6) | 0.886 (751.1) | 0.155 (749.0) |
| MHC-IIx (%) | 17.5 ± 7.7 | 21.3 ± 8.7 | 17.8 ± 5.1 | 0.108 (749.9) | 0.745 (752.5) | 0.035 (748.0) * |
| − | Model | R2 (Adjusted R2) | β for GS (95% CI), p Value |
|---|---|---|---|
| MHC-I | 1 | 0.104 (0.096) | 2.94 (1.26–4.61), p = 0.0007 |
| 2 | 0.140 (0.123) | 2.82 * (1.17–4.47), p = 0.0010 | |
| MHC-IIa | 1 | 0.018 (0.009) | −0.92 (−2.22−0.38), p = 0.1657 |
| 2 | 0.053 (0.035) | −0.83 * (−0.19−0.00), p = 0.2014 | |
| MHC-IIx | 1 | 0.091 (0.083) | −2.02 (−3.26–(−0.78)), p = 0.0016 |
| 2 | 0.096 (0.078) | −1.99 * (−3.24–(−0.75)), p = 0.0020 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yvert, T.; Miyamoto-Mikami, E.; Tobina, T.; Shiose, K.; Kakigi, R.; Tsuzuki, T.; Takaragawa, M.; Ichinoseki-Sekine, N.; Pérez, M.; Kobayashi, H.; et al. PPARGC1A rs8192678 and NRF1 rs6949152 Polymorphisms Are Associated with Muscle Fiber Composition in Women. Genes 2020, 11, 1012. https://doi.org/10.3390/genes11091012
Yvert T, Miyamoto-Mikami E, Tobina T, Shiose K, Kakigi R, Tsuzuki T, Takaragawa M, Ichinoseki-Sekine N, Pérez M, Kobayashi H, et al. PPARGC1A rs8192678 and NRF1 rs6949152 Polymorphisms Are Associated with Muscle Fiber Composition in Women. Genes. 2020; 11(9):1012. https://doi.org/10.3390/genes11091012
Chicago/Turabian StyleYvert, Thomas, Eri Miyamoto-Mikami, Takuro Tobina, Keisuke Shiose, Ryo Kakigi, Takamasa Tsuzuki, Mizuki Takaragawa, Noriko Ichinoseki-Sekine, Margarita Pérez, Hiroyuki Kobayashi, and et al. 2020. "PPARGC1A rs8192678 and NRF1 rs6949152 Polymorphisms Are Associated with Muscle Fiber Composition in Women" Genes 11, no. 9: 1012. https://doi.org/10.3390/genes11091012
APA StyleYvert, T., Miyamoto-Mikami, E., Tobina, T., Shiose, K., Kakigi, R., Tsuzuki, T., Takaragawa, M., Ichinoseki-Sekine, N., Pérez, M., Kobayashi, H., Tanaka, H., Naito, H., & Fuku, N. (2020). PPARGC1A rs8192678 and NRF1 rs6949152 Polymorphisms Are Associated with Muscle Fiber Composition in Women. Genes, 11(9), 1012. https://doi.org/10.3390/genes11091012







