Abstract
Malignant peripheral nerve sheath tumors (MPNST) are rare, aggressive soft tissue sarcomas that occur with significantly increased incidence in people with the neuro-genetic syndrome neurofibromatosis type I (NF1). These complex karyotype sarcomas are often difficult to resect completely due to the involvement of neurovascular bundles, and are relatively chemotherapy- and radiation-insensitive. The lifetime risk of developing MPNST in the NF1 population has led to great efforts to characterize the genetic changes that drive the development of these tumors and identify mutations that may be used for diagnostic or therapeutic purposes. Advancements in genetic sequencing and genomic technologies have greatly enhanced researchers’ abilities to broadly and deeply investigate aberrations in human MPNST genomes. Here, we review genetic sequencing efforts in human MPNST samples over the past three decades. Particularly for NF1-associated MPNST, these overall sequencing efforts have converged on a set of four common genetic changes that occur in most MPNST, including mutations in neurofibromin 1 (NF1), CDKN2A, TP53, and members of the polycomb repressor complex 2 (PRC2). However, broader genomic studies have also identified recurrent but less prevalent genetic variants in human MPNST that also contribute to the molecular landscape of MPNST and may inform further research. Future studies to further define the molecular landscape of human MPNST should focus on collaborative efforts across multiple institutions in order to maximize information gathered from large numbers of well-annotated MPNST patient samples, both in the NF1 and the sporadic MPNST populations.
1. Clinical Overview of MPNST
MPNST are aggressive soft tissue sarcomas originating from Schwann cells in the peripheral nervous system [1,2]. Half of MPNST occur in patients with the cancer predisposition syndrome NF1, caused by germline loss of function (LOF) of one copy of the tumor suppressor gene NF1. In patients with NF1, most MPNST arise from within plexiform neurofibromas (pNF), which are pre-malignant tumors of the peripheral nerve [3,4,5]. pNF can, themselves, be a major source of disfigurement or dysfunction. MPNST can also occur sporadically or following radiation treatment in the general population, although the incidence of the latter is substantially lower. MPNST carry a high risk of sarcoma-specific death; in the absence of complete surgical resection with wide negative margins, the five-year event-free survival is ~30% [6,7]. Conventional chemotherapy and radiation often do not improve patient outcomes [8].
2. Germline Loss of NF1: Correlations with NF1 Phenotype
NF1 is one of the most common monogenic inherited syndromes with an incidence of approximately 1:3000 live births [9]. This neurocutaneous syndrome is characterized by several hallmark skin findings (café au lait macules, axillary freckling, cutaneous neurofibromas), may involve additional organ systems (including CNS, musculoskeletal, and vascular manifestations) [10], and predisposes patients to an increased risk of malignancy, with an estimated lifetime cancer risk ~60% [11]. One of the hallmark lesions in NF1 patients is the pNF, a complex lesion that grows along major nerve bundles. While benign, pNF can result in significant anatomic, functional, cosmetic, and psychological effects [12]. In patients with NF1, MPNST may arise within existing pNF and are often accompanied by rapid growth, increased pain, or other nervous system deficits. Studies correlating the pathologic changes and genetic alterations in the peripheral nerves of NF1 patients or model organisms, along the spectrum from healthy to pNF to atypical neurofibroma (ANF) to MPNST, have aided in understanding the roles of specific genetic mutations in MPNST tumorigenesis [13].
NF1 syndrome is characterized by a wide variation in phenotypic expression which partially reflects the large number of mutations in the NF1 gene that have been identified in people with the condition [14,15,16]. The NF1 gene was originally cloned nearly three decades ago [17]. It is a large gene, approximately 350 kb in length, located on human chromosome 17q11.2. There may be multiple splice variants [18] but the primary gene product codes for the NF1 protein of 2,818 amino acids, which acts as a GTPase-activating protein (GAP) for RAS oncogenes [19,20,21,22]. Loss of NF1, therefore, leads to constitutive activation of RAS signaling [23,24] (Figure 1), likely accounting for the pro-tumor phenotype observed in patients with NF1 [25].
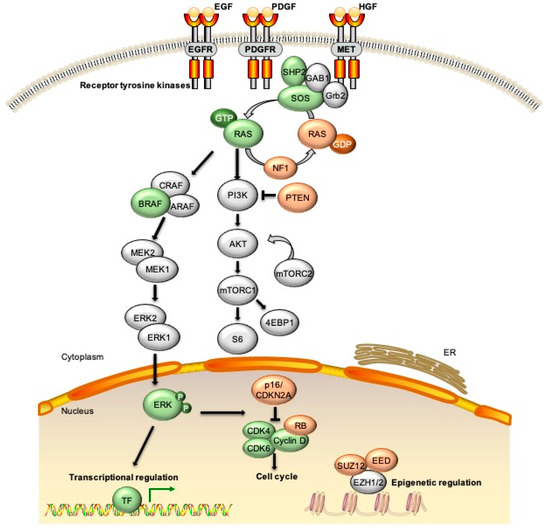
Figure 1.
Signaling pathways altered due to genetic changes observed in malignant peripheral nerve sheath tumors (MPNST). The most common alterations in MPNST are loss of function of multiple tumor suppressors including NF1, p16/CDKN2A, TP53, and SUZ12/EED. Loss of NF1, as well as epigenetic changes due to loss of PRC2 components, leads to increased signaling through the RAS/RAF/MEK and PI3K/AKT pathways. Additional molecular events observed in subsets of MPNST include mutations in BRAF, amplification of EGFR or MET receptor tyrosine kinases (RTKs), and changes to chromatin structure through mutations in alpha thalassemia/mental retardation syndrome X (ATRX) and other epigenetic modifiers. EGF/EGFR = epidermal growth factor/receptor; PDGF/PDGFR = platelet derived growth factor/receptor; HGF = hepatocyte growth factor; ERK = extracellular signal regulated kinase; CDK = cyclin dependent kinase; RB = retinoblastoma; TF = transcription factor; ER = endoplasmic reticulum.
Historically, the diagnosis of neurofibromatosis was based on clinical symptoms and physical findings, without a requirement for clinical genetic testing [26]. With the advances in detailed sequencing efforts, however, disruption of a copy of NF1 in the germline may be identified in the majority of patients with NF1 [27]. Mutational analysis demonstrates a very high rate of mutations occurring in NF1, as evidenced by the fact that approximately 50% of cases of NF1 appear to be de novo. To date, hundreds of mutations associated with the syndrome have been characterized [9,14]. Identification of the specific LOF mutation in patients can be helpful for testing family members, particularly offspring of those affected, and for counseling patients about syndrome-specific risks.
Genotype–phenotype correlations associated with specific germline NF1 alterations have been observed in a limited number of cases. Two examples are associated with limited risk for MPNST. A small in-frame deletion (c.2970_2972del(p.Met992del)) leading to loss of a methionine in the cysteine-serine rich domain (CSRD) of NF1 is associated with suppression of cutaneous neurofibroma (cNF) and clinically apparent pNF formation, though these individuals have an increased risk for learning disabilities (48%) and brain tumors (~5%) [28,29]. Several missense mutations affecting arginine 1809 (e.g. p.Arg1809Cys) have also been characterized in multiple unrelated families. These patients have a high prevalence of developmental delay and learning disabilities as well as short stature and pulmonic stenosis, but few cutaneous or plexiform neurofibromas, and low risk of malignancy [30].
By contrast, two other NF1 genotypes have been strongly associated with a higher risk of MPNST. Microdeletion of a 1.4 Mbp segment of chromosome 17 due to homologous recombination within duplication regions of the chromosome leads to deletion of 14 functional genes [31,32]. Individuals with the microdeletion syndrome (approximately 5% of NF1 cases) tend to present with a more severe NF1 phenotype [33], including dysmorphic features, developmental delay, intellectual disability, increased number of neurofibromas, and a two-fold higher lifetime risk for MPNST (16–26%, compared to approximately 8–13% risk in the general NF1 population) [34,35]. Missense mutations in NF1 protein codons 844–848 (including Leu844, Cys845, Ala846, Leu847, and Gly848; located in the CSRD) occur in ~0.8% of studied NF1 cases and are also reported as a risk factor for severe phenotypic presentation. These patients have higher numbers of clinically apparent major pNF, symptomatic spinal neurofibromas, optic pathway gliomas, and skeletal abnormalities, and up to 10% develop malignancy, including MPNST [36]
3. Sequencing Efforts in Human MPNST Samples: Improvements in Technology with Variability in Study Design
A collated summary of human MPNST sequencing efforts over the past two decades is shown in Table 1. MPNST have complex karyotypes with multiple chromosomal losses and gains and structural anomalies; a single recurrent translocation for diagnostic purposes has not been defined for MPNST as it has for some other mesenchymal tumors [37]. Expanded knowledge of MPNST gene alterations originated in the era of targeted gene evaluation using sequencing specific to the NF1 locus or a small number of related genes. More recent studies have employed whole exome, whole genome, or targeted next-generation sequencing (NGS) on discovery cohorts for MPNST, with follow up studies performed by targeted gene sequencing in validation cohorts. Whole exome sequencing (WES) efforts have also been performed on patient tumors with paired neurofibroma or blood samples in a minority of cases. Individual studies vary with respect to how much additional clinical information is available (e.g., clinical background, treatment effect, comparison to neurofibroma or blood leukocytes). Some studies include sporadic and radiation-associated cases, while others focus purely on NF1-associated MPNST. In addition, in several studies multiple MPNST samples are derived from the same patient or fragments of the same tumor. These differences in study design, sample collection and annotation, and data analysis likely account for some of the differences and depth of discovery in genomic alterations across the literature. Taken together, however, a clear picture emerges of several characteristic alterations (i.e., CDKN2A, genes encoding PRC2 components) involved in evolution of benign nerve sheath tumor to MPNST. Less frequent alterations (i.e., BRAF, MET) identified in smaller subsets also merit additional attention in follow up evaluations, particularly as new diagnostic and treatment strategies for these tumors are being developed.

Table 1.
Genomic sequencing studies for most common genetic alterations in human MPNST. All MPNST or neurofibromas in study reported under n. Reported sequencing results given as in reference (cases or percentages) for human MPNST specimens.
4. Somatic NF1 Mutations in Tumors Including MPNST
Consistent with its role as a classical tumor suppressor gene, loss of heterozygosity (LOH) or “second-hit” somatic mutations in the inherited wild-type NF1 allele have been detected in a variety of tumors in patients with NF1, including pheochromocytomas [50], breast cancer [51], and hematologic malignancies [52]. Somatic LOH analysis using PCR markers performed on the NF1 locus in dermal neurofibromas identified deletions in a subset of tumors in several early studies [53,54]; those cases known to be familial were analyzed further and shown to have deletions in the non-germline allele, demonstrating that somatic inactivation of NF1 occurs in these benign lesions. Several studies have compared germline and somatic NF1 mutations in MPNST. In a single study which investigated 34 MPNST from 27 NF1 patients, germline mutations were identified by lymphocyte DNA in 22 cases—these included one large 1.4 Mbp genomic deletion, one two-exon deletion, and smaller mutations (missense, nonsense, frameshift, and splicing anomalies) in the remainder [55]. In the same cohort, somatic NF1 mutations were identified in 31 out of 34 MPNST samples—of these, 28 (91% tumors) were large genomic deletions that partially or entirely deleted the NF1 gene. The authors speculate that in some cases somatic NF1 mutations arise upon aberrant intrachromosomal recombination of the NF1 gene during mitosis. Similarly, another report screened 47 MPNST from patients with or without NF1 syndrome (n = 25 and 22 cases, respectively). Of the somatic NF1 mutations identified (n = 10/25 NF1-associated and 9/22 sporadic), approximately 55–60% involved large genomic copy number changes (i.e., deletions) in both NF1 and sporadic MPNST [32]. By contrast, in MPNST analyzed from NF1 patients with the 1.4 Mbp germline NF1 microdeletion, the NF1 somatic hit is typically a small (e.g., missense) mutation [31]. Interestingly, in a single patient with clinical NF1 syndrome who developed asynchronous cNF, a primary breast tumor, and later gluteal MPNST, WES revealed three distinct NF1 somatic mutations compared to the germline mutation noted in the blood [51].
5. Acquired Mutations during Transformation from pNF
5.1. Loss of CDKN2A/B: Correlations with the pNF to ANF Transition
NF1 LOH is considered to be an initiating event in pNF formation as confirmed in several animal models [56]. Several additional mutations are necessary for malignant transformation. ANF (now re-classified as atypical neurofibromatous neoplasms of uncertain biological potential, ANNUBP) are precursor lesions to NF1-associated MPNST, representing an intermediate step from the malignant transformation of pNF into MPNST [57,58,59]. Alterations to chromosome 9q have been observed in a high proportion of ANF and MPNST [48,60]; one study noted deletion at 9p21.3, identified in 94% (15/16) of ANF and in 70% (16/23) of high-grade MPNST but not in pNF [57]. This locus encompasses several candidate tumor suppressors, including CDKN2A/B. CDKN2A encodes two gene products each the result of differential splicing: p16ink4a (a negative regulator of CDK4 and CDK6 cyclin dependent kinases) and p19Arf, a negative regulator of the TP53 E3 ligase MDM2. Several early studies on human NF1-associated MPNST specimens identified deletions within the short arm of chromosome 9, in the region of CDKN2A, as well as low expression of p19, while these were not detected in neurofibroma samples [61,62]. A more recent study identified frequent somatic deletions of CDKN2A/B (69%) and SMARCA2 (42%), apart from recurrent NF1 somatic mutations (81%), in 16 ANF [48]. These studies indicate that CDKN2A/B deletion is the first step in the progression of pNF toward ANF and eventually MPNST.
5.2. LOH and Mutation in the Tumor Suppressor TP53: Not Universal in Human MPNST
Copy number variation and mutations in the tumor suppressor gene TP53 have been identified in some cases of NF1-associated MPNST. Early studies on small subsets of NF1-associated neurofibrosarcomas identified deletions on chromosome 17 outside of the NF1 locus [63,64], which included the coding region for TP53. Screening for TP53 inactivation in a panel of 20 MPNST identified LOH in over half of the tumors tested [55]. The first genetically-engineered mouse (GEM) model for MPNST made use of LOH of both NF1 and TP53 from mouse chromosome 11 as the tumor initiating event [65]. Numerous subsequent studies have focused on identifying the true incidence of TP53 mutation in human MPNST; from compiled data on 25 studies including 114 MPNST (both NF1-associated and sporadic), TP53 mutations were observed in 14% of MPNST, with LOH in 39% of cases (Table 1) [39]. WES of NF1 tumor samples from a single patient with pNF, MPNST, and metastatic sites also identified loss of one copy of TP53 in the MPNST and metastatic lesion, but not the primary pNF [66]. Genetic changes in TP53 are thus present in some MPNST but not necessary for all cases of pNF malignant transformation.
5.3. Loss of PRC2 or H3K27me3: Recurrently and Specifically Occurs in MPNST
Components of the epigenetic regulatory PRC2are recurrently and specifically inactivated in MPNST (Table 1). Chi and colleagues identified genomic alterations in EED (37%, or 19/52) and SUZ12 (48% or 25/52) in MPNST, alongside frequent somatic alterations in CDKN2A (81%, 42/52) and NF1 (87%, 45/52) [43]. Bettegowda and colleagues simultaneously reported PRC2 loss via EED (2%, 1/50) and SUZ12 (32%, 16/50) mutations in 50 MPNST [42]. De Raedt et al. similarly reported alterations in EED in 29% (15/51) and SUZ12 in 63% (32/51) of NF1-associated MPNST [41]. PRC2-component loss in MPNST is associated with complete loss of histone H3 trimethylation at lysine 27 (H3K27me3) and increased level of H3K27 acetylation (H3K27Ac), which can serve as biomarkers to improve upon the accuracy of the diagnosis of MPNST [41,43]. SUZ12 loss potentiates the effects of NF1 loss by amplifying RAS-driven transcription through effects on chromatin that triggers an epigenetic switch [41]. Further detail on the role and function of PRC2 elements in MPNST is found in the review article by Zhang et al. dedicated to this topic, also included in this Special Issue on Genomics and Models of Nerve Sheath Tumors [67]. Collectively, the highly recurrent and specific inactivation of PRC2 components, NF1, and CDKN2A/B posits their critical and potentially cooperative roles in MPNST pathogenesis.
6. Less Common Recurrent Variants Identified with Modern Sequencing Investigations of MPNST
MPNST demonstrate complex genomic imbalances and chromosomal aberrations [58,59]. In addition to the common deletions of tumor suppressor genes NF1, CDKN2A, TP53 and LOF in the PRC2 genes EED and SUZ12, several other recurrent genomic events have been identified in NF1-associated and sporadic MPNST. Significant findings from these studies are highlighted in Table 2 and described below.

Table 2.
Less frequent genomic alterations identified in MPNST.
6.1. BRAF Mutation: An Alternate Mechanism for Activation of RAS Signaling
In addition to loss of NF1 and PRC2function, BRAF mutations are reported as an alternate mechanism for aberrant activation of RAS signaling in MPNST, albeit at a lower frequency (ranging from 0–9.7%) [32,47,69,78,79], and occurring more commonly in sporadic than NF1-associated cases [78]. Strongly activating kinase mutations (BRAF V600E) occurred in five out of ten BRAF-mutant NF1-wild type MPNST (n = 84; Table 2) [47]. BRAF amplification has also been described, with a frequency of 31% in another study cohort consisting of 51 MPNST [40]. Brohl et al. suggest that the relative strength of RAS-activating mutations may determine whether BRAF and NF1 mutations (or NRAS/KRAS and NF1) co-occur and thereby serve together to result in ERK signaling hyperactivation [45].
6.2. EGFR, MET and Other Receptor Tyrosine Kinases: Frequent Copy Number Gains in MPNST
A variety of oncogenic receptor tyrosine kinases (RTK) are frequently altered in MPNST. In MPNST, alterations in RTK usually take the form of amplification, rather than single nucleotide variations that result in constitutively activated kinases (Table 2). Several early aCGH studies revealed amplifications of HGF, MET, EGFR, PDGFRA, and IGF1R in approximately 25% to 40% of analyzed MPNST [38,40]. These studies and others [71,72,80] suggest a putative role of these genes and their respective biological pathways in the initiation and/or progression of MPNST.
Notably, HGF and its receptor MET, co-located at chromosome 7q, are highly expressed in a relatively large panel of human MPNST samples, and increased phospho-MET expression level directly correlates with shorter MPNST patient survival [81]. A single patient study revealed progressive amplifications of HGF, MET and EGFR in a patient with MPNST harboring early NF1 and TP53 loss, using longitudinal genomic analysis from pNF, to MPNST, to metastatic recurrence. These studies further justify investigation of the role of RTK signaling, in particular HGF/MET, on the progression of MPNST.
6.3. AURKA Amplification
Dramatic upregulation (7.9-fold) of AURKA (the gene encoding aurora kinase A) was observed through RAS-driven transcriptome analysis on a GEM model and 14 human MPNST samples compared with normal nerves. Further analysis using SNP-array and qPCR confirmed copy number gains in the AURKA locus in eight out of 13 primary MPNST and five out of five MPNST cell lines but not neurofibromas [74]. Reducing the expression and activity of Aurora kinase using shRNA knockdown and a kinase inhibitor MLN8237, respectively, inhibits MPNST cell survival in vitro and in vivo, and supports the role of aurora kinase as a rational therapeutic target for MPNST [82].
6.4. Tyrosine Kinase 2 Overexpression in MPNST
NGS on a set of seven NF1-associated MPNST identified a predicted pathogenic mutation in tyrosine kinase 2 (TYK2) in two out of seven tumors [75]. TYK2 P1104A mutated tumors demonstrated strong immunoreactivity, whereas TYK2 wild type tumors were not immunoreactive. Strong TYK2 expression as assayed by immunohistochemical staining was observed in 63% of MPNST in an independent tissue set, while only 11% of pNF samples stained for TYK2. Ablation of TYK2 expression in human and murine MPNST cells resulted in increased cell death in vitro and decreased tumor growth in a murine model [83]. The example of TYK2 suggests the role that sequencing efforts can play in development of novel markers of MPNST biology.
6.5. ATRX Mutation and Evidence for Alternative Lengthening of Telomeres
In addition to the role of PRC2 in MPNST chromatin regulation, the chromatin regulator ATRX (Alpha Thalassemia/Mental Retardation Syndrome X) has been identified as mutated in a subset of MPNST [75]. Loss of ATRX function is involved in alternative lengthening of telomeres (ALT), a telomerase-independent means of telomere maintenance which prevents tumor cell senescence and promotes tumorigenesis. Subsequent studies on a larger subset of MPNST identified decreased nuclear expression of ATRX and demonstrated a correlation between aberrant ATRX expression and decreased overall survival in NF1-associated MPNST [84]. In a separate study a small subset (n = 3) of NF1-associated MPNST that were ALT-positive were analyzed by NGS and found to have ATRX mutations in two out of three cases [76]. While this study did not identify inferior overall survival (OS) for ALT-positive MPNST compared to those with normal telomere length, short telomeres were significantly correlated with improved OS.
6.6. Beyond SUZ12: Less Common Variant Mutations in Other Chromatin Modifying Genes
In addition to loss of SUZ12 and EED, several studies have demonstrated additional alterations in PRC2 components or associated chromatin modifying genes. Sohier and colleagues detected a novel sequence change in the histone lysine demethylase KDM2B by WES (c3376C > T) in one out of eight human MPNST. This change is thought to potentially impact protein function; in an additional set of 14 tumors assayed by qPCR, KDM2B expression was reduced [44]. Whole genome and whole exome sequencing on an additional subset of NF1-associated MPNST identified mutations in additional chromatin associated genes including CHD4, AEBP2, EPC1, and EZH2, particularly in tumors with intact SUZ12 [42].
6.7. Evidence for Alterations in the HIPPO Pathway in a Subset of MPNST and Schwann Cell Derived Tumors
Several studies have found evidence for alterations in the HIPPO–YAP pathway in MPNST. Analysis of aCGH from 51 MPNST samples [40] revealed an increase in the copy number of HIPPO effector gene loci, including TAZ, CTGF and BIRC5 and a loss of HIPPO inhibitory gene loci, such as LATS2 and AMOTL2 [77]. In agreement with these findings, transcriptome sequencing of human MPNST samples from two additional patient cohorts revealed elevated YAP-activated gene expression in MPNST relative to normal nerves and NF1-associated neurofibromas [85,86]. Genomic alterations in the HIPPO pathway appear to occur in additional NF1 patient tissues including somatic mutations in seven of 33 cNF described in a recent study and as germline mutations (e.g., missense, frameshift and occasionally insertion) in seven of nine NF1 patients from the same dataset [87]. Together these studies validate the role of HIPPO pathway in neurofibroma biology and as a driver of MPNST tumorigenesis [77,87].
7. Beyond Genomics: The State of Understanding MPNST Transcriptomes, Proteomes, Epigenomes, and Metabolomes
In addition to the genomic alterations described above, these and other studies on human MPNST have revealed downstream effects on MPNST gene product expression and signaling. These investigations have confirmed or supplemented the genomic data by assaying downstream pathway effects in human MPNST. Several studies have broadly analyzed gene expression in human MPNST samples using microarray or RNAseq approaches [48,88]; these data can be examined in relation to known genetic changes to generate additional hypotheses for effects on downstream signaling pathways. Recent work compared gene expression in multiple functional pathways across pNF, ANF/ANNUBP, and MPNST and found that some ANNUBP share signaling pathway characteristics that more closely resemble pNF (e.g., ERK/MAPK) and others (e.g., AKT/mTOR) are more similar to MPNST [88]. Phospho-proteome arrays may be used to investigate kinase signaling in relation to various genomic alterations or therapeutic interventions in MPNST; to date this has primarily been used in MPNST cell lines or animal models (see article by Grit et al. in this Special Issue on Genomics and Models of Nerve Sheath Tumors) [89]. Methylation analysis on MPNST has revealed overall decreased histone and DNA methylation [90], and has also revealed how methylation changes in MPNST can affect expression of other tumor suppressor genes (e.g., PTEN) in MPNST [91]. Parallel methylation analysis and proteomic analysis on a set of nine MPNST samples characterized the relationship between PRC2 LOF on histone and DNA modification and consequent gene product expression. This work found that PRC2 loss was associated with increased pro-growth and immune evasion protein expression [92]. To date global metabolomics profiling has not been reported on human MPNST specimens; several recent efforts have examined metabolic shifts in animal models of MPNST in response to preclinical therapeutic interventions [93,94,95].
8. Translating Molecular Landscape of MPNST into Improved Therapies for Patients
One overarching goal of improved molecular characterization of MPNST is to translate genomic discoveries into improved treatments for this classically chemo-refractory tumor. As a result of improved understanding of MPNST genomic variants, several targeted therapies have been trialed in preclinical MPNST models. For example, the MET-specific tyrosine kinase inhibitor capmatinib has shown promise, particularly in combination with the MEK inhibitor trametinib, in an NF1-MET driven MPNST GEM model [70]. BRAF mutant MPNST may also respond to targeted therapy; one case report described a dramatic response to the RAF inhibitor vemurafenib in a patient with sporadic metastatic MPNST harboring the BRAF V600E mutation [96]. Efforts to target histone acetylation in a preclinical MPNST model with loss of SUZ12 shrank tumors when combined with MEK inhibition [41], while other DNA methyltransferase inhibitors appear to affect immune surveillance of MPNST [92]. It is likely that in the near future MPNST clinical trials will incorporate therapies inhibiting components of the epigenetic machinery.
9. Conclusions
Significant research efforts over the past three decades have significantly advanced the state of knowledge of the genetic landscape of human MPNST. Particularly in NF1-associated MPNST, it is generally accepted that alterations in NF1, CDKN2A, TP53, and SUZ12 are involved in tumor progression from benign to malignant tumors. However, less frequent alterations in genes with complementary function have been described in subsets of tumors, and additional tumor-driving mutations may be present in sporadic or recurrent/metastatic tumor samples. Future genomic studies should aim to incorporate as many well-annotated samples as feasible and clearly report on differences between NF1-associated and sporadic MPNST subtypes. Exciting future work will also incorporate additional technologies to improve our understanding of the downstream consequences of genomic alterations for MPNST biology and aid in development of improved treatments.
Author Contributions
All authors researched the published literature, conceived, wrote, and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
K.M.L. is supported by a Young Investigator Award from CureSearch for Children′s Cancer. C.A.P. receives research funding from the Neurofibromatosis Therapeutic Acceleration Program (NTAP).
Conflicts of Interest
The authors have no conflicts of interest to declare, with the exception of Christine A. Pratilas, who served as a co-editor for this special issue of Genes. Christine Pratilas has abstained from editorial duties relating to this manuscript. Christine A. Pratilas is a paid consultant for Genentech/ Roche.
References
- Carroll, S.L. Molecular mechanisms promoting the pathogenesis of Schwann cell neoplasms. Acta Neuropathol. 2012, 123, 321–348. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ghosh, P.; Charnay, P.; Burns, D.K.; Parada, L.F. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science 2002, 296, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Ferner, R.E.; Gutmann, D.H. International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res. 2002, 62, 1573–1577. [Google Scholar]
- Evans, D.G.; Baser, M.E.; McGaughran, J.; Sharif, S.; Howard, E.; Moran, A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J. Med. Genet. 2002, 39, 311–314. [Google Scholar] [CrossRef] [PubMed]
- McCaughan, J.A.; Holloway, S.M.; Davidson, R.; Lam, W.W. Further evidence of the increased risk for malignant peripheral nerve sheath tumour from a Scottish cohort of patients with neurofibromatosis type 1. J. Med. Genet. 2007, 44, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Kattan, M.W.; Leung, D.H.; Brennan, M.F. Postoperative nomogram for 12-year sarcoma-specific death. J. Clin. Oncol. 2002, 20, 791–796. [Google Scholar] [CrossRef]
- Fletcher, C.D.; McKee, P.H. Sarcomas—A clinicopathological guide with particular reference to cutaneous manifestation. II. Malignant nerve sheath tumour, leiomyosarcoma and rhabdomyosarcoma. Clin. Exp. Dermatol 1985, 10, 201–216. [Google Scholar] [CrossRef]
- Higham, C.S.; Steinberg, S.M.; Dombi, E.; Perry, A.; Helman, L.J.; Schuetze, S.M.; Ludwig, J.A.; Staddon, A.; Milhem, M.M.; Rushing, D.; et al. SARC006: Phase II Trial of Chemotherapy in Sporadic and Neurofibromatosis Type 1 Associated Chemotherapy-Naive Malignant Peripheral Nerve Sheath Tumors. Sarcoma 2017, 2017, 8685638. [Google Scholar] [CrossRef]
- Gutmann, D.H.; Ferner, R.E.; Listernick, R.H.; Korf, B.R.; Wolters, P.L.; Johnson, K.J. Neurofibromatosis type 1. Nat. Rev. Dis. Primers 2017, 3, 17004. [Google Scholar] [CrossRef]
- Ly, K.I.; Blakeley, J.O. The Diagnosis and Management of Neurofibromatosis Type 1. Med. Clin. N. Am. 2019, 103, 1035–1054. [Google Scholar] [CrossRef]
- Uusitalo, E.; Rantanen, M.; Kallionpää, R.A.; Poyhonen, M.; Leppavirta, J.; Yla-Outinen, H.; Riccardi, V.M.; Pukkala, E.; Pitkaniemi, J.; Peltonen, S.; et al. Distinctive Cancer Associations in Patients with Neurofibromatosis Type 1. J. Clin. Oncol. 2016, 34, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.M.; Singh, G.; Akshintala, S.; Baldwin, A.; Dombi, E.; Ukwuani, S.; Goodwin, A.; Liewehr, D.J.; Steinberg, S.M.; Widemann, B.C. Association of Plexiform Neurofibroma Volume Changes and Development of Clinical Morbidities in Neurofibromatosis 1. Neuro. Oncol. 2018, 12, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Stewart, D.R.; Reilly, K.M.; Viskochil, D.; Miettinen, M.M.; Widemann, B.C. Malignant Peripheral Nerve Sheath Tumors State of the Science: Leveraging Clinical and Biological Insights into Effective Therapies. Sarcoma 2017, 2017, 7429697. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.H.; Harper, P.S.; Upadhyaya, M. Molecular genetics of neurofibromatosis type 1 (NF1). J. Med. Genet. 1996, 33, 2–17. [Google Scholar] [CrossRef]
- Jett, K.; Friedman, J.M. Clinical and genetic aspects of neurofibromatosis 1. Genet. Med. 2010, 12, 1–11. [Google Scholar] [CrossRef]
- Anderson, J.L.; Gutmann, D.H. Neurofibromatosis type 1. Handb. Clin. Neurol. 2015, 132, 75–86. [Google Scholar]
- Wallace, M.R.; Marchuk, D.A.; Andersen, L.B.; Letcher, R.; Odeh, H.M.; Saulino, A.M.; Fountain, J.W.; Brereton, A.; Nicholson, J.; Mitchell, A.L.; et al. Type 1 neurofibromatosis gene: Identification of a large transcript disrupted in three NF1 patients. Science 1990, 249, 181–186. [Google Scholar] [CrossRef]
- Andersen, L.B.; Ballester, R.; Marchuk, D.A.; Chang, E.; Gutmann, D.H.; Saulino, A.M.; Camonis, J.; Wigler, M.; Collins, F.S. A conserved alternative splice in the von Recklinghausen neurofibromatosis (NF1) gene produces two neurofibromin isoforms, both of which have GTPase-activating protein activity. Mol. Cell Biol. 1993, 13, 487–495. [Google Scholar] [CrossRef][Green Version]
- Gutmann, D.H.; Wood, D.L.; Collins, F.S. Identification of the neurofibromatosis type 1 gene product. Proc. Natl. Acad. Sci. USA 1991, 88, 9658–9662. [Google Scholar] [CrossRef]
- DeClue, J.E.; Cohen, B.D.; Lowy, D.R. Identification and characterization of the neurofibromatosis type 1 protein product. Proc. Natl. Acad. Sci. USA 1991, 88, 9914–9918. [Google Scholar] [CrossRef]
- Martin, G.A.; Viskochil, D.; Bollag, G.; McCabe, P.C.; Crosier, W.J.; Haubruck, H.; Conroy, L.; Clark, R.; O′Connell, P.; Cawthon, R.M.; et al. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell 1990, 63, 843–849. [Google Scholar] [CrossRef]
- Ballester, R.; Marchuk, D.; Boguski, M.; Letcher, R.; Wigler, M.; Collins, F. The NF1 locus encodes a protein functionally related to mammalian GAP and yeast IRA proteins. Cell 1990, 63, 851–859. [Google Scholar] [CrossRef]
- Basu, T.N.; Gutmann, D.H.; Fletcher, J.A.; Glover, T.W.; Collins, F.S.; Downward, J. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature 1992, 356, 713–715. [Google Scholar] [CrossRef] [PubMed]
- Bollag, G.; Clapp, D.W.; Shih, S.; Adler, F.; Zhang, Y.Y.; Thompson, P.; Lange, B.J.; Freedman, M.H.; McCormick, F.; Jacks, T.; et al. Loss of NF1 results in activation of the Ras signaling pathway and leads to aberrant growth in haematopoietic cells. Nat. Genet. 1996, 12, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Cichowski, K.; Jacks, T. NF1 tumor suppressor gene function: Narrowing the GAP. Cell 2001, 104, 593–604. [Google Scholar] [CrossRef]
- Rosser, T.; Packer, R.J. Neurofibromas in children with neurofibromatosis 1. J. Child Neurol. 2002, 17, 585–651. [Google Scholar] [CrossRef]
- Messiaen, L.M.; Callens, T.; Mortier, G.; Beysen, D.; Vandenbroucke, I.; Van Roy, N.; Speleman, F.; Paepe, A.D. Exhaustive mutation analysis of the NF1 gene allows identification of 95% of mutations and reveals a high frequency of unusual splicing defects. Hum. Mutat. 2000, 15, 541–555. [Google Scholar] [CrossRef]
- Koczkowska, M.; Callens, T.; Gomes, A.; Sharp, A.; Chen, Y.; Hicks, A.D.; Aylsworth, A.S.; Azizi, A.A.; Basel, D.G.; Bellus, G.; et al. Expanding the clinical phenotype of individuals with a 3-bp in-frame deletion of the NF1 gene (c.2970_2972del): An update of genotype-phenotype correlation. Genet. Med. 2019, 21, 867–876. [Google Scholar] [CrossRef]
- Upadhyaya, M.; Huson, S.M.; Davies, M.; Thomas, N.; Chuzhanova, N.; Giovannini, S.; Evans, D.G.; Howard, E.; Kerr, B.; Griffiths, S.; et al. An absence of cutaneous neurofibromas associated with a 3-bp inframe deletion in exon 17 of the NF1 gene (c.2970-2972 delAAT): Evidence of a clinically significant NF1 genotype-phenotype correlation. Am. J. Hum. Genet. 2007, 80, 140–151. [Google Scholar] [CrossRef]
- Rojnueangnit, K.; Xie, J.; Gomes, A.; Sharp, A.; Callens, T.; Chen, Y.; Liu, Y.; Cochran, M.; Abbott, M.A.; Atkin, J.; et al. High Incidence of Noonan Syndrome Features Including Short Stature and Pulmonic Stenosis in Patients carrying NF1 Missense Mutations Affecting p.Arg1809: Genotype-Phenotype Correlation. Hum. Mutat. 2015, 36, 1052–1063. [Google Scholar] [CrossRef]
- De Raedt, T.; Maertens, O.; Chmaram, M.; Brems, H.; Heyns, I.; Sciot, R.; Majounie, E.; Upadhyaya, M.; De Schepper, S.; Speleman, F.; et al. Somatic loss of wild type NF1 allele in neurofibromas: Comparison of NF1 microdeletion and non-microdeletion patients. Genes Chromosomes Cancer 2006, 45, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Bottillo, I.; Ahlquist, T.; Brekke, H.; Danielsen, S.A.; van den Berg, E.; Mertens, F.; Lothe, R.A.; Dallapiccola, B. Germline and somatic NF1 mutations in sporadic and NF1-associated malignant peripheral nerve sheath tumours. J. Pathol. 2009, 217, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Pasmant, E.; Sabbagh, A.; Spurlock, G.; Laurendeau, I.; Grillo, E.; Hamel, M.J.; Martin, L.; Barbarot, S.; Leheup, B.; Rodriguez, D.; et al. NF1 microdeletions in neurofibromatosis type 1: From genotype to phenotype. Hum. Mutat. 2010, 31, E1506–E1518. [Google Scholar] [CrossRef] [PubMed]
- Kehrer-Sawatzki, H.; Mautner, V.F.; Cooper, D.N. Emerging genotype-phenotype relationships in patients with large NF1 deletions. Hum. Genet. 2017, 136, 349–376. [Google Scholar] [CrossRef]
- De Raedt, T.; Brems, H.; Wolkenstein, P.; Vidaud, D.; Pilotti, S.; Perrone, F.; Mautner, V.; Frahm, S.; Sciot, R.; Legius, E. Elevated risk for MPNST in NF1 microdeletion patients. Am. J. Hum. Genet. 2003, 72, 1288–1292. [Google Scholar] [CrossRef]
- Koczkowska, M.; Chen, Y.; Callens, T.; Gomes, A.; Sharp, A.; Johnson, S.; Hsiao, M.C.; Chen, Z.; Balasubramanian, M.; Barnett, C.P.; et al. Genotype-Phenotype Correlation in NF1: Evidence for a More Severe Phenotype Associated with Missense Mutations Affecting NF1 Codons 844–848. Am. J. Hum. Genet. 2018, 102, 69–87. [Google Scholar] [CrossRef]
- Bridge, R.S., Jr.; Bridge, J.A.; Neff, J.R.; Naumann, S.; Althof, P.; Bruch, L.A. Recurrent chromosomal imbalances and structurally abnormal breakpoints within complex karyotypes of malignant peripheral nerve sheath tumour and malignant triton tumour: A cytogenetic and molecular cytogenetic study. J. Clin. Pathol. 2004, 57, 1172–1178. [Google Scholar] [CrossRef]
- Mantripragada, K.K.; Spurlock, G.; Kluwe, L.; Chuzhanova, N.; Ferner, R.E.; Frayling, I.M.; Dumanski, J.P.; Guha, A.; Mautner, V.; Upadhyaya, M. High-resolution DNA copy number profiling of malignant peripheral nerve sheath tumors using targeted microarray-based comparative genomic hybridization. Clin. Cancer Res. 2008, 14, 1015–1024. [Google Scholar] [CrossRef]
- Verdijk, R.M.; den Bakker, M.A.; Dubbink, H.J.; Hop, W.C.; Dinjens, W.N.; Kros, J.M. TP53 mutation analysis of malignant peripheral nerve sheath tumors. J. Neuropathol. Exp. Neurol. 2010, 69, 16–26. [Google Scholar] [CrossRef]
- Yang, J.; Ylipää, A.; Sun, Y.; Zheng, H.; Chen, K.; Nykter, M.; Trent, J.; Ratner, N.; Lev, D.C.; Zhang, W. Genomic and molecular characterization of malignant peripheral nerve sheath tumor identifies the IGF1R pathway as a primary target for treatment. Clin. Cancer Res. 2011, 17, 7563–7573. [Google Scholar] [CrossRef]
- De Raedt, T.; Beert, E.; Pasmant, E.; Luscan, A.; Brems, H.; Ortonne, N.; Helin, K.; Hornick, J.L.; Mautner, V.; Kehrer-Sawatzki, H.; et al. PRC2 loss amplifies Ras-driven transcription and confers sensitivity to BRD4-based therapies. Nature 2014, 514, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Y.; Jones, S.; Sausen, M.; McMahon, K.; Sharma, R.; Wang, Q.; Belzberg, A.J.; Chaichana, K.; Gallia, G.L.; et al. Somatic mutations of SUZ12 in malignant peripheral nerve sheath tumors. Nat. Genet. 2014, 46, 1170–1172. [Google Scholar] [CrossRef]
- Lee, W.; Teckie, S.; Wiesner, T.; Prieto Granada, C.N.; Lin, M.; Zhu, S.; Cao, Z.; Liang, Y.; Sboner, A.; Tap, W.D.; et al. PRC2 is recurrently inactivated through EED or SUZ12 loss in malignant peripheral nerve sheath tumors. Nat. Genet. 2014, 46, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Sohier, P.; Luscan, A.; Lloyd, A.; Ashelford, K.; Laurendeau, I.; Briand-Suleau, A.; Vidaud, D.; Ortonne, N.; Pasmant, E.; Upadhyaya, M. Confirmation of mutation landscape of NF1-associated malignant peripheral nerve sheath tumors. Genes Chromosomes Cancer 2017, 56, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Brohl, A.S.; Kahen, E.; Yoder, S.J.; Teer, J.K.; Reed, D.R. The genomic landscape of malignant peripheral nerve sheath tumors: Diverse drivers of Ras pathway activation. Sci. Rep. 2017, 7, 14992. [Google Scholar] [CrossRef] [PubMed]
- Zehir, A.; Benayed, R.; Shah, R.H.; Syed, A.; Middha, S.; Kim, H.R.; Srinivasan, P.; Gao, J.; Chakravarty, D.; Devlin, S.M.; et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017, 23, 703–713. [Google Scholar] [CrossRef]
- Kaplan, H.G.; Rostad, S.; Ross, J.S.; Ali, S.M.; Millis, S.Z. Genomic Profiling in Patients with Malignant Peripheral Nerve Sheath Tumors Reveals Multiple Pathways with Targetable Mutations. J. Natl. Compr. Cancer Netw. 2018, 16, 967–974. [Google Scholar] [CrossRef]
- Pemov, A.; Hansen, N.F.; Sindiri, S.; Patidar, R.; Higham, C.S.; Dombi, E.; Miettinen, M.M.; Fetsch, P.; Brems, H.; Chandrasekharappa, S.; et al. Low mutation burden and frequent loss of CDKN2A/B and SMARCA2, but not PRC2, define pre-malignant neurofibromatosis type 1-associated atypical neurofibromas. Neuro. Oncol. 2019, 21, 981–992. [Google Scholar] [CrossRef]
- Pollard, K.; Banerjee, J.; Doan, X.; Wang, J.; Guo, X.; Allaway, R.; Langmead, S.; Slobogean, B.; Meyer, C.F.; Loeb, D.M.; et al. A clinically and genomically annotated nerve sheath tumor biospecimen repository. Sci. Data 2020, 7, 184. [Google Scholar] [CrossRef]
- Xu, W.; Mulligan, L.M.; Ponder, M.A.; Liu, L.; Smith, B.A.; Mathew, C.G.; Ponder, B.A. Loss of NF1 alleles in phaeochromocytomas from patients with type I neurofibromatosis. Genes Chromosomes Cancer 1992, 4, 337–342. [Google Scholar] [CrossRef]
- McPherson, J.R.; Ong, C.K.; Ng, C.C.; Rajasegaran, V.; Heng, H.L.; Yu, W.S.; Tan, B.K.; Madhukumar, P.; Teo, M.C.; Ngeow, J.; et al. Whole-exome sequencing of breast cancer, malignant peripheral nerve sheath tumor and neurofibroma from a patient with neurofibromatosis type 1. Cancer Med. 2015, 4, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Shannon, K.M.; O′Connell, P.; Martin, G.A.; Paderanga, D.; Olson, K.; Dinndorf, P.; McCormick, F. Loss of the normal NF1 allele from the bone marrow of children with type 1 neurofibromatosis and malignant myeloid disorders. N. Engl. J. Med. 1994, 330, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Colman, S.D.; Williams, C.A.; Wallace, M.R. Benign neurofibromas in type 1 neurofibromatosis (NF1) show somatic deletions of the NF1 gene. Nat. Genet. 1995, 11, 90–92. [Google Scholar] [CrossRef]
- Serra, E.; Puig, S.; Otero, D.; Gaona, A.; Kruyer, H.; Ars, E.; Estivill, X.; Lázaro, C. Confirmation of a double-hit model for the NF1 gene in benign neurofibromas. Am. J. Hum. Genet. 1997, 61, 512–519. [Google Scholar] [CrossRef]
- Upadhyaya, M.; Kluwe, L.; Spurlock, G.; Monem, B.; Majounie, E.; Mantripragada, K.; Ruggieri, M.; Chuzhanova, N.; Evans, D.G.; Ferner, R.; et al. Germline and somatic NF1 gene mutation spectrum in NF1-associated malignant peripheral nerve sheath tumors (MPNSTs). Hum. Mutat. 2008, 29, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.C.; Ingram, D.A.; Chen, S.; Zhu, Y.; Yuan, J.; Li, X.; Yang, X.; Knowles, S.; Horn, W.; Li, Y.; et al. Nf1-dependent tumors require a microenvironment containing Nf1+/− and c-kit-dependent bone marrow. Cell 2008, 135, 437–448. [Google Scholar] [CrossRef]
- Beert, E.; Brems, H.; Daniëls, B.; De Wever, I.; Van Calenbergh, F.; Schoenaers, J.; Debiec-Rychter, M.; Gevaert, O.; De Raedt, T.; Van Den Bruel, A.; et al. Atypical neurofibromas in neurofibromatosis type 1 are premalignant tumors. Genes Chromosomes Cancer 2011, 50, 1021–1032. [Google Scholar] [CrossRef]
- Higham, C.S.; Dombi, E.; Rogiers, A.; Bhaumik, S.; Pans, S.; Connor, S.; Miettinen, M.; Sciot, R.; Tirabosco, R.; Brems, H.; et al. The characteristics of 76 atypical neurofibromas as precursors to neurofibromatosis 1 associated malignant peripheral nerve sheath tumors. Neuro. Oncol. 2018, 20, 818–825. [Google Scholar] [CrossRef]
- Miettinen, M.M.; Antonescu, C.R.; Fletcher, C.D.M.; Bhaumik, S.; Pans, S.; Connor, S.; Miettinen, M.; Sciot, R.; Tirabosco, R.; Brems, H.; et al. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum. Pathol. 2017, 67, 1–10. [Google Scholar] [CrossRef]
- Nielsen, G.P.; Stemmer-Rachamimov, A.O.; Ino, Y.; Moller, M.B.; Rosenberg, A.E.; Louis, D.N. Malignant transformation of neurofibromas in neurofibromatosis 1 is associated with CDKN2A/p16 inactivation. Am. J. Pathol. 1999, 155, 1879–1884. [Google Scholar] [CrossRef]
- Kourea, H.P.; Orlow, I.; Scheithauer, B.W.; Cordon-Cardo, C.; Woodruff, J.M. Deletions of the INK4A gene occur in malignant peripheral nerve sheath tumors but not in neurofibromas. Am. J. Pathol. 1999, 155, 1855–1860. [Google Scholar] [CrossRef]
- Berner, J.M.; Sørlie, T.; Mertens, F.; Henriksen, J.; Saeter, G.; Mandahl, N.; Brøgger, A.; Myklebost, O.; Lothe, R.A. Chromosome band 9p21 is frequently altered in malignant peripheral nerve sheath tumors: Studies of CDKN2A and other genes of the pRB pathway. Genes Chromosomes Cancer 1999, 26, 151–160. [Google Scholar] [CrossRef]
- Menon, A.G.; Anderson, K.M.; Riccardi, V.M.; Chung, R.Y.; Whaley, J.M.; Yandell, D.W.; Farmer, G.E.; Freiman, R.N.; Lee, J.K.; Li, F.P.; et al. Chromosome 17p deletions and p53 gene mutations associated with the formation of malignant neurofibrosarcomas in von Recklinghausen neurofibromatosis. Proc. Natl. Acad. Sci. USA 1990, 87, 5435–5439. [Google Scholar] [CrossRef] [PubMed]
- Legius, E.; Dierick, H.; Wu, R.; Hall, B.K.; Marynen, P.; Cassiman, J.J.; Glover, T.W. TP53 mutations are frequent in malignant NF1 tumors. Genes Chromosomes Cancer 1994, 10, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Cichowski, K.; Shih, T.S.; Schmitt, E.; Santiago, S.; Reilly, K.; McLaughlin, M.E.; Bronson, R.T.; Jacks, T. Mouse models of tumor development in neurofibromatosis type 1. Science 1999, 286, 2172–2176. [Google Scholar] [CrossRef]
- Hirbe, A.C.; Dahiya, S.; Miller, C.A.; Li, T.; Fulton, R.S.; Zhang, X.; McDonald, S.; DeSchryver, K.; Duncavage, E.J.; Walrath, J.; et al. Whole Exome Sequencing Reveals the Order of Genetic Changes during Malignant Transformation and Metastasis in a Single Patient with NF1-plexiform Neurofibroma. Clin. Cancer Res. 2015, 21, 4201–4211. [Google Scholar] [CrossRef]
- Zhang, X.; Murray, B.; Mo, G.; Shern, J.F. The Role of Polycomb Repressive Complex in Malignant Peripheral Nerve Sheath Tumor. Genes 2020, 11, 287. [Google Scholar] [CrossRef]
- Schindler, G.; Capper, D.; Meyer, J.; Janzarik, W.; Omran, H.; Herold-Mende, C.; Schmieder, K.; Wesseling, P.; Mawrin, C.; Hasselblatt, M.; et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011, 121, 397–405. [Google Scholar] [CrossRef]
- Je, E.M.; An, C.H.; Yoo, N.J.; Lee, S.H. Mutational analysis of PIK3CA, JAK2, BRAF, FOXL2, IDH1, AKT1 and EZH2 oncogenes in sarcomas. APMIS 2012, 120, 635–639. [Google Scholar] [CrossRef]
- Peacock, J.D.; Pridgeon, M.G.; Tovar, E.A.; Essenburg, C.J.; Bowman, M.; Madaj, Z.; Koeman, J.; Boguslawski, E.A.; Grit, J.; Dodd, R.D.; et al. Genomic Status of MET Potentiates Sensitivity to MET and MEK Inhibition in NF1-Related Malignant Peripheral Nerve Sheath Tumors. Cancer Res. 2018, 78, 3672–3687. [Google Scholar] [CrossRef]
- Du, X.; Yang, J.; Ylipää, A.; Zhu, Z. Genomic amplification and high expression of EGFR are key targetable oncogenic events in malignant peripheral nerve sheath tumor. J. Hematol. Oncol. 2013, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, N.; Malzer, E.; Zietsch, J.; Okuducu, A.F.; Mucha, J.; Mawrin, C.; Mautner, V.F.; Schildhaus, H.U.; von Deimling, A. EGFR and erbB2 in malignant peripheral nerve sheath tumors and implications for targeted therapy. Neuro. Oncol. 2008, 10, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Perrone, F.; Da Riva, L.; Orsenigo, M.; Losa, M.; Jocollè, G.; Millefanti, C.; Pastore, E.; Gronchi, A.; Pierotti, M.A.; Pilotti, S. PDGFRA, PDGFRB, EGFR, and downstream signaling activation in malignant peripheral nerve sheath tumor. Neuro. Oncol. 2009, 11, 725–736. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patel, A.V.; Da Riva, L.; Orsenigo, M.; Rizvi, T.A.; Ecsedy, J.A.; Qian, M.G.; Aronow, B.J.; Perentesis, J.P.; Serra, E.; Cripe, T.P.; et al. Ras-driven transcriptome analysis identifies aurora kinase A as a potential malignant peripheral nerve sheath tumor therapeutic target. Clin. Cancer Res. 2012, 18, 5020–5030. [Google Scholar] [CrossRef] [PubMed]
- Hirbe, A.C.; Kaushal, M.; Sharma, M.K.; Dahiya, S.; Pekmezci, M.; Perry, A.; Gutmann, D.H. Clinical genomic profiling identifies TYK2 mutation and overexpression in patients with neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Cancer 2017, 123, 1194–1201. [Google Scholar] [CrossRef]
- Rodriguez, F.J.; Graham, M.K.; Brosnan-Cashman, J.A.; Cashman, J.A.; Barber, J.R.; Davis, C.; Vizcaino, M.A.; Palsgrove, D.N.; Giannini, C.; Pekmezci, M.; et al. Telomere alterations in neurofibromatosis type 1-associated solid tumors. Acta Neuropathol. Commun. 2019, 7, 139. [Google Scholar] [CrossRef]
- Wu, L.M.N.; Deng, Y.; Wang, J.; Zhao, C.; Wang, J.; Rao, R.; Xu, L.; Zhou, W.; Choi, K.; Rizvi, T.A.; et al. Programming of Schwann Cells by Lats1/2-TAZ/YAP Signaling Drives Malignant Peripheral Nerve Sheath Tumorigenesis. Cancer Cell 2018, 33, 292–308.e7. [Google Scholar] [CrossRef]
- Hirbe, A.C.; Pekmezci, M.; Dahiya, S.; Apicelli, A.J.; Van Tine, B.A.; Perry, A.; Gutmann, D.H. BRAFV600E mutation in sporadic and neurofibromatosis type 1-related malignant peripheral nerve sheath tumors. Neuro. Oncol. 2014, 16, 466–467. [Google Scholar] [CrossRef]
- Serrano, C.; Simonetti, S.; Hernández-Losa, J.; Valverde, C.; Carrato, C.; Bagué, S.; Orellana, R.; Somoza, R.; Moliné, T.; Carles, J.; et al. BRAF V600E and KRAS G12S mutations in peripheral nerve sheath tumours. Histopathology 2013, 62, 499–504. [Google Scholar] [CrossRef]
- DeClue, J.E.; Heffelfinger, S.; Benvenuto, G.; Ling, B.; Li, S.; Rui, W.; Vass, W.C.; Viskochil, D.; Ratner, N. Epidermal growth factor receptor expression in neurofibromatosis type 1-related tumors and NF1 animal models. J. Clin. Investig. 2000, 105, 1233–1241. [Google Scholar] [CrossRef]
- Torres, K.E.; Zhu, Q.S.; Bill, K.; Lopez, G.; Ghadimi, M.P.; Xie, X.; Young, E.D.; Liu, J.; Nguyen, T.; Bolshakov, S.; et al. Activated MET is a molecular prognosticator and potential therapeutic target for malignant peripheral nerve sheath tumors. Clin. Cancer Res. 2011, 17, 3943–3955. [Google Scholar] [CrossRef] [PubMed]
- Mohan, P.; Castellsague, J.; Jiang, J.; Allen, K.; Chen, H.; Nemirovsky, O.; Spyra, M.; Hu, K.; Kluwe, L.; Pujana, M.A.; et al. Genomic imbalance of HMMR/RHAMM regulates the sensitivity and response of malignant peripheral nerve sheath tumour cells to aurora kinase inhibition. Oncotarget 2013, 4, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Godec, A.; Zhang, X.; Zhu, C.; Shao, J.; Tao, Y.; Bu, X.; Hirbe, A.C. TYK2 promotes malignant peripheral nerve sheath tumor progression through inhibition of cell death. Cancer Med. 2019, 8, 5232–5241. [Google Scholar] [CrossRef]
- Lu, H.C.; Eulo, V.; Apicelli, A.J.; Pekmezci, M.; Tao, Y.; Luo, J.; Hirbe, A.C.; Dahiya, S. Aberrant ATRX protein expression is associated with poor overall survival in NF1-MPNST. Oncotarget 2018, 9, 23018–23028. [Google Scholar] [CrossRef][Green Version]
- Jessen, W.J.; Miller, S.J.; Jousma, E.; Wu, J.; Rizvi, T.A.; Brundage, M.E.; Eaves, D.; Widemann, B.; Kim, M.O.; Dombi, E.; et al. MEK inhibition exhibits efficacy in human and mouse neurofibromatosis tumors. J. Clin. Investig. 2013, 123, 340–347. [Google Scholar] [CrossRef]
- Kolberg, M.; Høland, M.; Lind, G.E.; Agesen, T.H.; Skotheim, R.I.; Hall, K.S.; Mandahl, N.; Smeland, S.; Mertens, F.; Davidson, B.; et al. Protein expression of BIRC5, TK1, and TOP2A in malignant peripheral nerve sheath tumours—A prognostic test after surgical resection. Mol. Oncol. 2015, 9, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Mo, J.; Brosseau, J.P.; Shipman, T.; Wang, Y.; Liao, C.P.; Cooper, J.M.; Allaway, R.J.; Gosline, S.; Guinney, J.; et al. Spatiotemporal Loss of NF1 in Schwann Cell Lineage Leads to Different Types of Cutaneous Neurofibroma Susceptible to Modification by the Hippo Pathway. Cancer Discov. 2019, 9, 114–129. [Google Scholar] [CrossRef]
- Kohlmeyer, J.L.; Kaemmer, C.A.; Pulliam, C.; Maharjan, C.K.; Samayoa, A.M.; Major, H.J.; Cornick, K.E.; Knepper-Adrian, V.; Khanna, R.; Sieren, J.C.; et al. RABL6A is an essential driver of MPNSTs that negatively regulates the RB1 pathway and sensitizes tumor cells to CDK4/6 inhibitors. Clin. Cancer Res. 2020, 26, 2997–3011. [Google Scholar] [CrossRef]
- Brossier, N.M.; Prechtl, A.M.; Longo, J.F.; Barnes, S.; Wilson, L.S.; Byer, S.J.; Brosius, S.N.; Carroll, S.L. Classic Ras Proteins Promote Proliferation and Survival via Distinct Phosphoproteome Alterations in Neurofibromin-Null Malignant Peripheral Nerve Sheath Tumor Cells. J. Neuropathol. Exp. Neurol. 2015, 74, 568–586. [Google Scholar] [CrossRef]
- Nix, J.S.; Haffner, M.C.; Ahsan, S.; Hicks, J.; De Marzo, A.M.; Blakeley, J.; Raabe, E.H.; Rodriguez, F.J. Malignant Peripheral Nerve Sheath Tumors Show Decreased Global DNA Methylation. J. Neuropathol. Exp. Neurol. 2018, 77, 958–963. [Google Scholar] [CrossRef]
- Bradtmoller, M.; Hartmann, C.; Zietsch, J.; Jäschke, S.; Mautner, V.F.; Kurtz, A.; Park, S.J.; Baier, M.; Harder, A.; Reuss, D.; et al. Impaired Pten expression in human malignant peripheral nerve sheath tumours. PLoS ONE 2012, 7, e47595. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, J.B.; Marchione, D.M.; Sidoli, S.; Djedid, A.; Lisby, A.; Majewski, J.; Garcia, B.A. Epigenomic Reordering Induced by Polycomb Loss Drives Oncogenesis but Leads to Therapeutic Vulnerabilities in Malignant Peripheral Nerve Sheath Tumors. Cancer Res. 2019, 79, 3205–3219. [Google Scholar] [CrossRef] [PubMed]
- Malone, C.F.; Fromm, J.A.; Maertens, O.; DeRaedt, T.; Ingraham, R.; Cichowski, K. Defining key signaling nodes and therapeutic biomarkers in NF1-mutant cancers. Cancer Discov. 2014, 4, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, T.N.; Patwardhan, P.P.; Cremers, S.; Schwartz, G.K. Targeted inhibition of glutaminase as a potential new approach for the treatment of NF1 associated soft tissue malignancies. Oncotarget 2017, 8, 94054–94068. [Google Scholar] [CrossRef]
- Lemberg, K.M.; Zhao, L.; Wu, Y.; Veeravalli, V.; Alt, J.; Aguilar, J.; Dash, R.P.; Lam, J.; Tenora, L.; Rodriguez, C.; et al. The novel glutamine antagonist prodrug JHU395 has antitumor activity in malignant peripheral nerve sheath tumor. Mol. Cancer Ther. 2019, 19, 397–408. [Google Scholar] [CrossRef]
- Kaplan, H.G. Vemurafenib treatment of BRAF V600E-mutated malignant peripheral nerve sheath tumor. J. Natl. Compr. Cancer Netw. 2013, 11, 1466–1470. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).