Expression of the Long Noncoding RNA GAS5 Correlates with Liver Fibrosis in Patients with Nonalcoholic Fatty Liver Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Samples
2.2. Diagnosis of NAFLD and Pathologic Evaluations
2.3. RNA Extraction
2.4. Quantitative Real-Time PCR
2.5. Statistical Analysis
3. Results
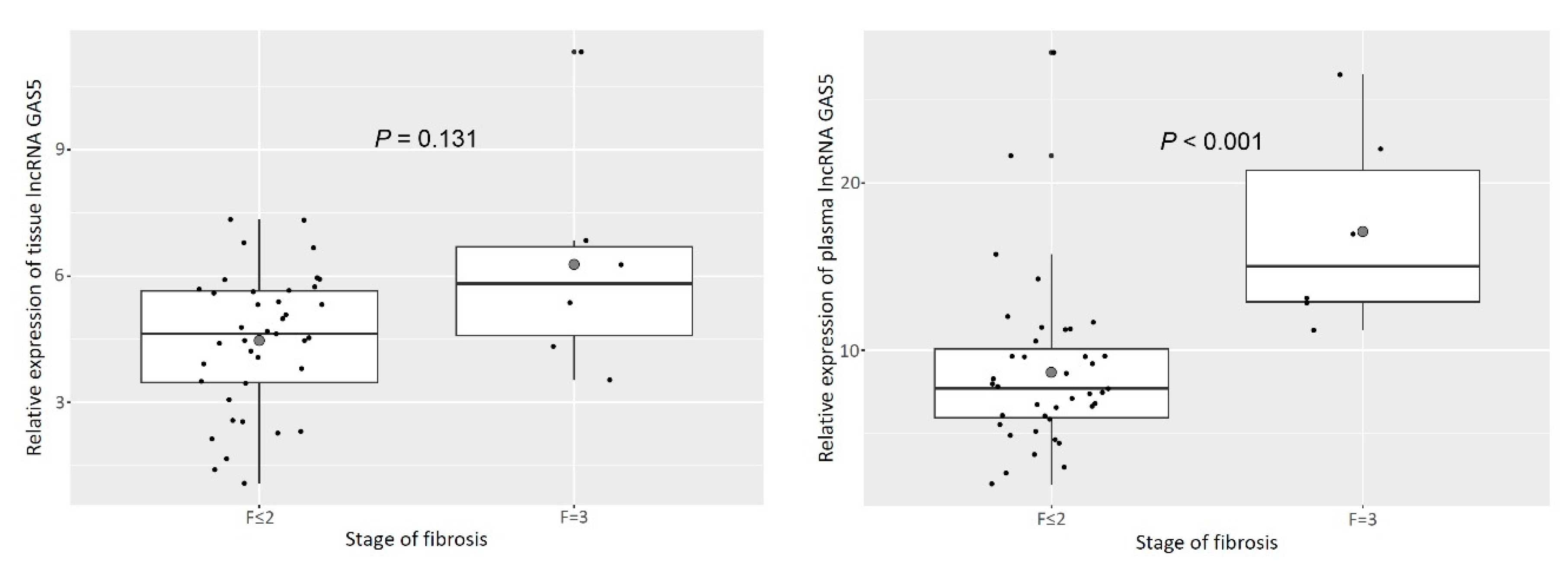
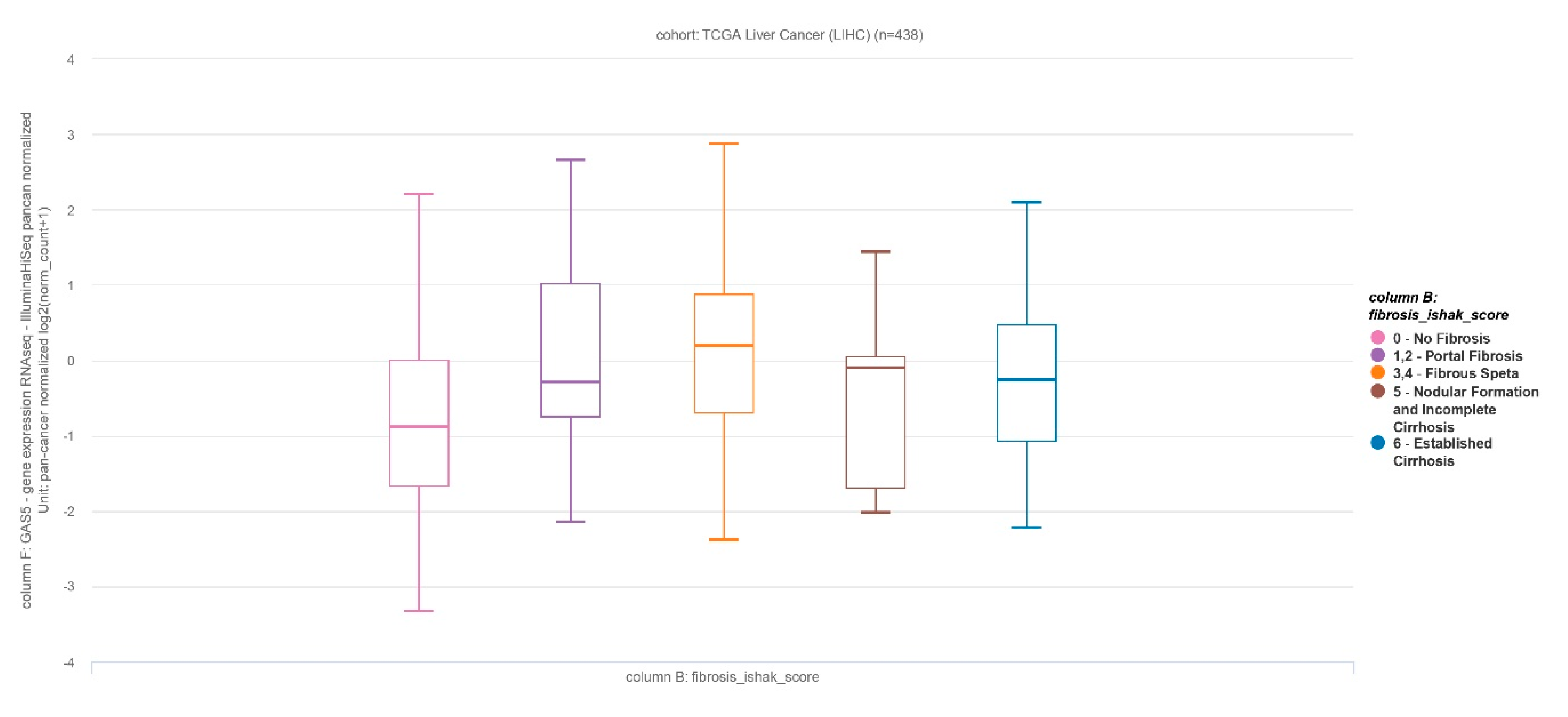
3.1. Tissue and Plasma GAS5 Was UpRegulated in Patients with Advanced Liver Fibrosis
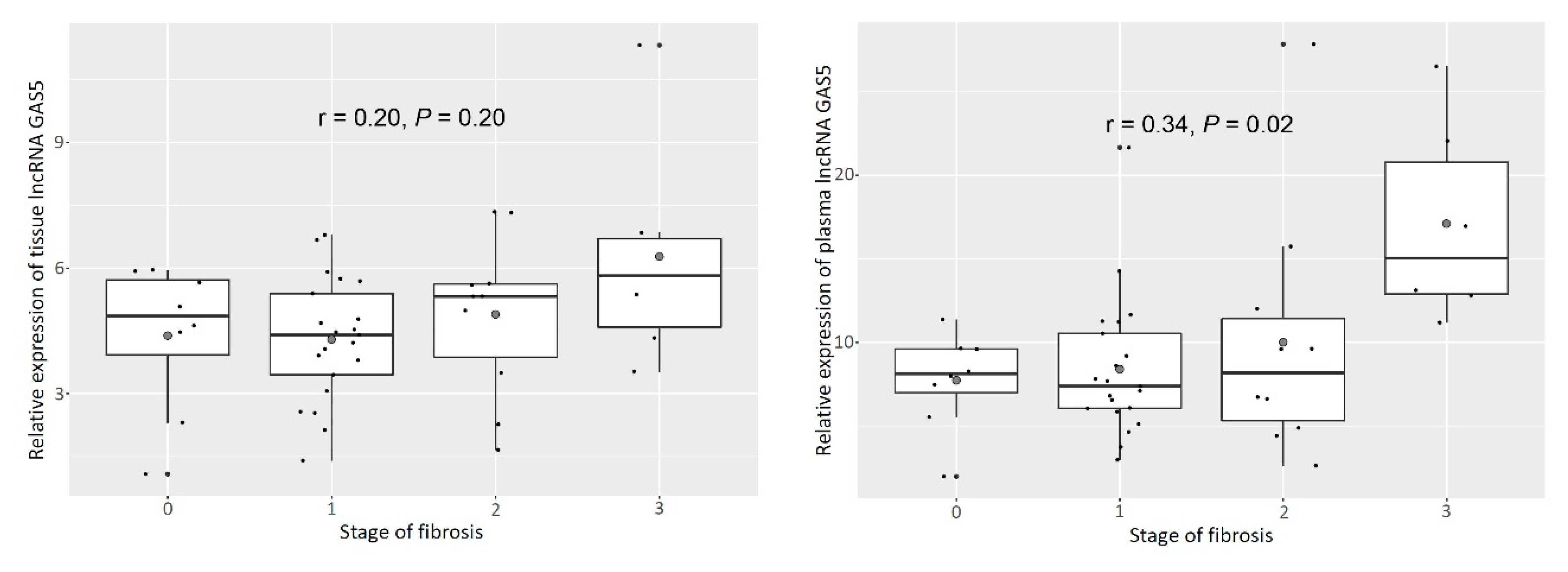
3.2. Tissue and Plasma GAS5 Increased as Fibrosis Progressed in Patients with NAFLD
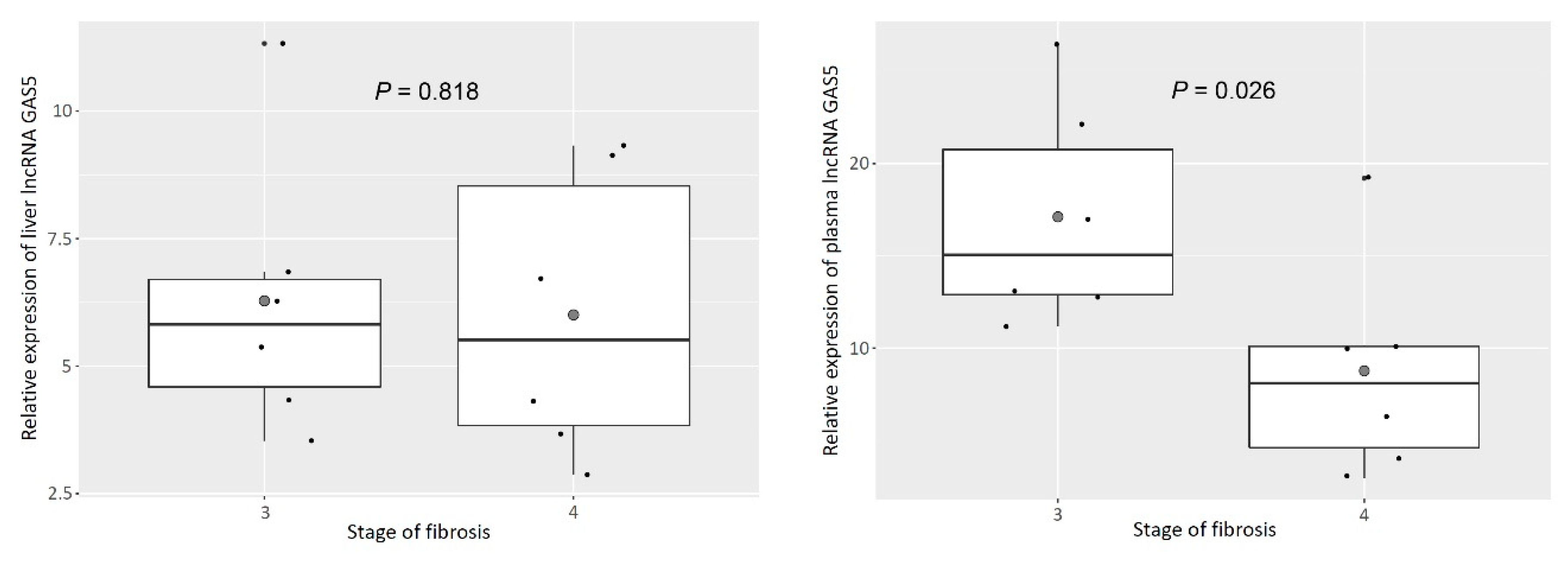
3.3. Plasma GAS5 Was Downregulated in Patients with Cirrhosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Williams, R.; Aspinall, R.J.; Bellis, M.; Camps-Walsh, G.; Cramp, M.; Dhawan, A.; Ferguson, J.; Forton, D.; Foster, G.R.; Gilmore, S.I.; et al. Addressing liver disease in the UK: A blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet 2014, 384, 1953–1997. [Google Scholar] [CrossRef]
- NatCen Social Research, University College London Department of Epidemiology and Public Health. Health Survey for England, 2012; UK Data Archive: Colchester, UK, 2014. [Google Scholar] [CrossRef]
- Ascha, M.S.; Hanouneh, I.A.; Lopez, R.; Tamimi, T.A.-R.; Feldstein, A.F.; Zein, N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010, 51, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Björnsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver Fibrosis, but No Other Histologic Features, is Associated with Long-term Outcomes of Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2015, 149, 389–397.e310. [Google Scholar] [CrossRef] [PubMed]
- Ekstedt, M.; Hagström, H.; Nasr, P.; Fredrikson, M.; Stal, P.; Kechagias, S.; Hultcrantz, R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Jun, D.W.; Saeed, W.K.; Nguyen, M.H. Non-alcoholic fatty liver diseases: Update on the challenge of diagnosis and treatment. Clin. Mol. Hepatol. 2016, 22, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Spizzo, R.; Almeida, M.I.; Colombatti, A.; Calin, G.A. Long non-coding RNAs and cancer: A new frontier of translational research? Oncogene 2012, 31, 4577–4587. [Google Scholar] [CrossRef]
- Mercer, T.R.; Dinger, M.E.; Mattick, J. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef]
- Smith, C.M.; Steitz, J.A. Classification of gas5 as a Multi-Small-Nucleolar-RNA (snoRNA) Host Gene and a Member of the 5′-Terminal Oligopyrimidine Gene Family Reveals Common Features of snoRNA Host Genes. Mol. Cell. Boil. 1998, 18, 6897–6909. [Google Scholar] [CrossRef]
- Mourtada-Maarabouni, M.; Pickard, M.R.; Hedge, V.L.; Farzaneh, F.; Williams, G. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene 2008, 28, 195–208. [Google Scholar] [CrossRef]
- Sun, M.; Jin, F.-Y.; Chen, J.; Kong, R.; Li, J.-H.; Xu, T.-P.; Liu, Y.-W.; Zhang, E.-B.; Liu, X.-H.; De, W. Decreased expression of long noncoding RNA GAS5 indicates a poor prognosis and promotes cell proliferation in gastric cancer. BMC Cancer 2014, 14, 319. [Google Scholar] [CrossRef]
- Yacqub-Usman, K.; Pickard, M.R.; Williams, G. Reciprocal regulation of GAS5 lncRNA levels and mTOR inhibitor action in prostate cancer cells. Prostate 2015, 75, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Lv, T.; Shi, X.; Liu, H.; Zhu, Q.; Zeng, J.; Yang, W.; Yin, J.; Song, Y. Circulating long noncoding RNA GAS5 is a novel biomarker for the diagnosis of nonsmall cell lung cancer. Medicine 2016, 95, e4608. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.; Miladinovic, B.; Patel, A.A.; Deland, L.; Mastorides, S.; Patel, N.A. Circulating long noncoding RNA GAS5 levels are correlated to prevalence of type 2 diabetes mellitus. BBA Clin. 2015, 4, 102–107. [Google Scholar] [CrossRef]
- Yin, Q.; Wu, A.; Liu, M. Plasma Long Non-Coding RNA (lncRNA) GAS5 is a New Biomarker for Coronary Artery Disease. Med. Sci. Monit. 2017, 23, 6042–6048. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zheng, J.; Mao, Y.; Dong, P.; Lu, Z.-Q.; Li, G.; Guo, C.; Liu, Z.; Fan, X. Long Non-coding RNA Growth Arrest-specific Transcript 5 (GAS5) Inhibits Liver Fibrogenesis through a Mechanism of Competing Endogenous RNA*. J. Boil. Chem. 2015, 290, 28286–28298. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Hur, K.; Toiyama, Y.; Okugawa, Y.; Ide, S.; Imaoka, H.; Boland, C.R.; Goel, A. Circulating microRNA-203 predicts prognosis and metastasis in human colorectal cancer. Gut 2015, 66, 654–665. [Google Scholar] [CrossRef]
- Hu, L.; Ye, H.; Huang, G.; Luo, F.; Liu, Y.; Liu, Y.; Yang, X.; Shen, J.; Liu, Q.; Zhang, J. Long noncoding RNA GAS5 suppresses the migration and invasion of hepatocellular carcinoma cells via miR-21. Tumor Boil. 2015, 37, 2691–2702. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, J. GAS5 Regulates RECK Expression and Inhibits Invasion Potential of HCC Cells by Sponging miR-135b. BioMed Res. Int. 2019, 2019, 2973289. [Google Scholar] [CrossRef]
- Wang, C.; Ke, S.; Li, M.; Lin, C.; Liu, X.; Pan, Q. Downregulation of LncRNA GAS5 promotes liver cancer proliferation and drug resistance by decreasing PTEN expression. Mol. Genet. Genom. 2019, 295, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Xu, C.; Zhao, P.; Qi, Z. Long non-coding RNA GAS5 inhibited hepatitis C virus replication by binding viral NS3 protein. Virology 2016, 492, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Li, S.; Wang, X.; Si, L.; Ma, R.; Bao, L.; Bo, A. lncRNA GAS5 restrains CCl4-induced hepatic fibrosis by targeting miR-23a through the PTEN/PI3K/Akt signaling pathway. Am. J. Physiol. Liver Physiol. 2019, 316, G539–G550. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yang, D.; Fan, X.; Zhang, M.; Li, Y.; Gu, X.; Yang, M. The Roles and Mechanisms of lncRNAs in Liver Fibrosis. Int. J. Mol. Sci. 2020, 21, 1482. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wan, L.-Y.; Liang, J.-J.; Zhang, Y.-Q.; Ai, W.-B.; Wu, J. The roles of lncRNA in hepatic fibrosis. Cell Biosci. 2018, 8, 63. [Google Scholar] [CrossRef]
- Yu, F.; Lu, Z.-Q.; Chen, B.; Dong, P.; Zheng, J. Identification of a Novel lincRNA-p21-miR-181b-PTEN Signaling Cascade in Liver Fibrosis. Mediat. Inflamm. 2016, 2016, 9856538. [Google Scholar] [CrossRef]
- Yu, F.; Guo, Y.; Chen, B.; Shi, L.; Dong, P.; Zhou, M.; Zheng, J. LincRNA-p21 Inhibits the Wnt/β-Catenin Pathway in Activated Hepatic Stellate Cells via Sponging MicroRNA-17-5p. Cell. Physiol. Biochem. 2017, 41, 1970–1980. [Google Scholar] [CrossRef]
- Barsotti, A.M.; Beckerman, R.; Laptenko, O.; Huppi, K.; Caplen, N.J.; Prives, C. p53-dependent Induction of PVT1 and miR-1204*. J. Boil. Chem. 2011, 287, 2509–2519. [Google Scholar] [CrossRef]
- Yu, F.; Chen, B.; Dong, P.; Zheng, J. HOTAIR Epigenetically Modulates PTEN Expression via MicroRNA-29b: A Novel Mechanism in Regulation of Liver Fibrosis. Mol. Ther. 2017, 25, 205–217. [Google Scholar] [CrossRef]
- Yu, F.; Lu, Z.-Q.; Cai, J.; Huang, K.; Chen, B.; Li, G.; Dong, P.; Zheng, J. MALAT1 functions as a competing endogenous RNA to mediate Rac1 expression by sequestering miR-101b in liver fibrosis. Cell Cycle 2015, 14, 3885–3896. [Google Scholar] [CrossRef]
- Shen, X.; Guo, H.; Xu, J.; Wang, J. Inhibition of lncRNA HULC improves hepatic fibrosis and hepatocyte apoptosis by inhibiting the MAPK signaling pathway in rats with nonalcoholic fatty liver disease. J. Cell. Physiol. 2019, 234, 18169–18179. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Xiao, Z.; Wang, Y.; Zheng, M.; Song, T.; Cai, X.; Sun, B.; Ye, L.; Zhang, X. Long Noncoding RNA HULC Modulates Abnormal Lipid Metabolism in Hepatoma Cells through an miR-9-Mediated RXRA Signaling Pathway. Cancer Res. 2015, 75, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Mayama, T.; Marr, A.; Kino, T. Differential Expression of Glucocorticoid Receptor Noncoding RNA Repressor Gas5 in Autoimmune and Inflammatory Diseases. Horm. Metab. Res. 2016, 48, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Lucafò, M.; Di Silvestre, A.; Romano, M.; Avian, A.; Antonelli, R.; Martelossi, S.; Naviglio, S.; Tommasini, A.; Stocco, G.; Ventura, A.; et al. Role of the Long Non-Coding RNA Growth Arrest-Specific 5 in Glucocorticoid Response in Children with Inflammatory Bowel Disease. Basic Clin. Pharmacol. Toxicol. 2017, 122, 87–93. [Google Scholar] [CrossRef]
- Keenan, C.; Schuliga, M.; Stewart, A.G. Pro-inflammatory mediators increase levels of the noncoding RNA GAS5 in airway smooth muscle and epithelial cells. Can. J. Physiol. Pharmacol. 2015, 93, 203–206. [Google Scholar] [CrossRef]
- Shen, J.; Hodges, T.R.; Song, R.; Gong, Y.; Calin, G.A.; Heimberger, A.B.; Zhao, H. Serum HOTAIR and GAS5 levels as predictors of survival in patients with glioblastoma. Mol. Carcinog. 2017, 57, 137–141. [Google Scholar] [CrossRef]
- Han, L.; Ma, P.; Liu, S.-M.; Zhou, X. Circulating long noncoding RNA GAS5 as a potential biomarker in breast cancer for assessing the surgical effects. Tumor Boil. 2015, 37, 6847–6854. [Google Scholar] [CrossRef]
- Fayda, M.; Işin, M.; Tambas, M.; Guveli, M.; Meral, R.; Altun, M.; Sahin, D.; Ozkan, G.; Sanli, Y.; Isin, H.; et al. Do circulating long non-coding RNAs (lncRNAs) (LincRNA-p21, GAS 5, HOTAIR) predict the treatment response in patients with head and neck cancer treated with chemoradiotherapy? Tumor Boil. 2015, 37, 3969–3978. [Google Scholar] [CrossRef]
- Wu, G.-C.; Li, J.; Leng, R.-X.; Li, X.-P.; Li, X.-M.; Wang, D.-G.; Pan, H.-F.; Ye, D.-Q. Identification of long non-coding RNAs GAS5, linc0597 and lnc-DC in plasma as novel biomarkers for systemic lupus erythematosus. Oncotarget 2017, 8, 23650–23663. [Google Scholar] [CrossRef]

| Characteristic | F ≤ 2 (N = 39) | F = 3 (N = 6) | P-Value |
|---|---|---|---|
| Male (%) | 23 (59.0) | 2 (33.3) | 0.462 |
| Age, year | 41.0 (33.5–54.5) | 60.5 (57.0–64.0) | 0.015 * |
| Steatosis | 0.284 | ||
| 5–33% (%) | 13 (33.3) | 4 (66.7) | |
| >33–66% (%) | 16 (41.0) | 1 (16.7) | |
| >66% (%) | 10 (25.6) | 1 (16.7) | |
| Lobular inflammation | 0.496 | ||
| <2 foci per 200 × field (%) | 25 (64.1) | 4 (66.7) | |
| 2–4 foci per 200 × field (%) | 12 (30.8) | 1 (16.7) | |
| >4 foci per 200 × field (%) | 2 (5.1) | 1 (16.7) | |
| Ballooning | 0.144 | ||
| None (%) | 8 (20.5) | 0 (0.0) | |
| Few ballooned cells (%) | 20 (51.3) | 2 (33.3) | |
| Many cells/prominent ballooning (%) | 11 (28.2) | 4 (66.7) | |
| Nonalcoholic steatohepatitis | 31 (79.5) | 6 (100.0) | 0.516 |
| NAS ≥ 5 (%) | 17 (43.6) | 4 (66.7) | 0.538 |
| Weight, kg | 79.0 (70.0–97.0) | 72.0 (59.3–84.0) | 0.226 |
| BMI, kg/m2 | 27.8 (26.1–32.4) | 27.9 (26.5–30.4) | 0.694 |
| Systolic blood pressure, mmHg | 138 (127–148) | 132.0 (132–133) | 0.573 |
| Hypertension | 19 (48.7) | 4 (66.7) | 0.704 |
| Diabetes | 10 (25.6) | 5 (83.3) | 0.020 * |
| Platelets, ×103/mm | 240.0 (200.0–286.0) | 167.0 (142.0–192.0) | 0.023 |
| AST, IU/L | 63.0 (51.5–82.0) | 119.0 (74.0–165.0) | 0.019 * |
| ALT, IU/L | 96.0 (70.5–128.5) | 76.0 (58.0–173.0) | 0.593 |
| Bilirubin, mg/dL | 0.6 (0.5–0.7) | 0.7 (0.7–0.9) | 0.681 |
| Albumin, g/dL | 4.6 (4.4–4.8) | 4.5 (4.1–4.7) | 0.350 |
| Gamma GTP, mg/dL | 60.0 (46.0–123.0) | 61.0 (52.0-92.0) | 0.820 |
| Creatinine, mg/dL | 0.8 (0.7–0.9) | 0.7 (0.7–0.9) | 0.494 |
| Fasting blood glucose, mg/dL | 108.0 (98.0–123.0) | 118.5 (113.0–127.0) | 0.142 |
| Total cholesterol, mg/dL | 183.0 (164.0–204.0) | 167.5 (160.0–211.0) | 0.687 |
| HDL, mg/dL | 38.5 (31.0–49.0) | 48.5 (43.0–56.0) | 0.098 |
| LDL, mg/dL | 118.5 (93.0–145.0) | 108.5 (100.0–151.0) | 0.857 |
| Triglyceride, mg/dL | 183.0 (123.0–295.0) | 102.0 (70.0–118.0) | 0.005 * |
| Pathologic Feature | Pathologic Grade (Number) | P-Value | |
|---|---|---|---|
| Steatosis ≥ 2 | <2 (21) | ≥2 (30) | |
| Tissue GAS5 | 4.5 (3.8–6.3) | 4.9 (2.6–5.7) | 0.602 |
| Plasma GAS5 | 9.2 (6.6–12.0) | 7.9 (5.1–11.2) | 0.274 |
| Inflamation ≥ 2 | <2 (32) | ≥2 (29) | |
| Tissue GAS5 | 4.5 (3.3–5.6) | 5.3 (3.8–6.8) | 0.148 |
| Plasma GAS5 | 8.1 (6.0–11.3) | 9.2 (6.2–11.1) | 0.885 |
| Presence of NASH | Not NASH (8) | NASH (43) | |
| Tissue GAS5 | 4.2 (2.4–4.6) | 5.1 (3.7–5.9) | 0.090 |
| Plasma GAS5 | 8.4 (6.8–10.4) | 8.0 (6.0–11.7) | 0.990 |
| Severe NASH | Not Severe NASH (27) | Severe NASH (24) | |
| Tissue GAS5 | 4.6 (3.7–5.7) | 5.2 (3.3–6.3) | 0.674 |
| Plasma GAS5 | 9.2 (6.6–11.3) | 7.4 ( 5.0–12.0) | 0.448 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.-H.; Lee, J.H.; Kim, G.; Lee, E.; Lee, Y.R.; Jang, S.Y.; Lee, H.W.; Chun, J.M.; Han, Y.S.; Yoon, J.S.; et al. Expression of the Long Noncoding RNA GAS5 Correlates with Liver Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Genes 2020, 11, 545. https://doi.org/10.3390/genes11050545
Han M-H, Lee JH, Kim G, Lee E, Lee YR, Jang SY, Lee HW, Chun JM, Han YS, Yoon JS, et al. Expression of the Long Noncoding RNA GAS5 Correlates with Liver Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Genes. 2020; 11(5):545. https://doi.org/10.3390/genes11050545
Chicago/Turabian StyleHan, Man-Hoon, Jee Hyun Lee, Gyeonghwa Kim, Eunhye Lee, Yu Rim Lee, Se Young Jang, Hye Won Lee, Jae Min Chun, Young Seok Han, Jun Sik Yoon, and et al. 2020. "Expression of the Long Noncoding RNA GAS5 Correlates with Liver Fibrosis in Patients with Nonalcoholic Fatty Liver Disease" Genes 11, no. 5: 545. https://doi.org/10.3390/genes11050545
APA StyleHan, M.-H., Lee, J. H., Kim, G., Lee, E., Lee, Y. R., Jang, S. Y., Lee, H. W., Chun, J. M., Han, Y. S., Yoon, J. S., Kang, M. K., Lee, W. K., Kweon, Y. O., Tak, W. Y., Park, S. Y., Park, J. G., & Hur, K. (2020). Expression of the Long Noncoding RNA GAS5 Correlates with Liver Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Genes, 11(5), 545. https://doi.org/10.3390/genes11050545






