Investigation of Mating Pheromone–Pheromone Receptor Specificity in Lentinula edodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Modification of Yeast Host Strain
2.3. Total RNA Extraction and PCR Conditions
2.4. Construction of RCB-Expressing Yeast Strains
2.5. Pheromone Response Assay with Synthetic PHBs
2.6. Effect of Synthetic PHBs on Lentinula Edodes S1–10
3. Results
3.1. Construction of Yeast Model System for the Investigation of Lentinula edodes Mating Pheromone Receptor–Pheromone Interactions
3.2. Interaction between Lentinula edodes RCBs and PHBs in the Yeast Model System
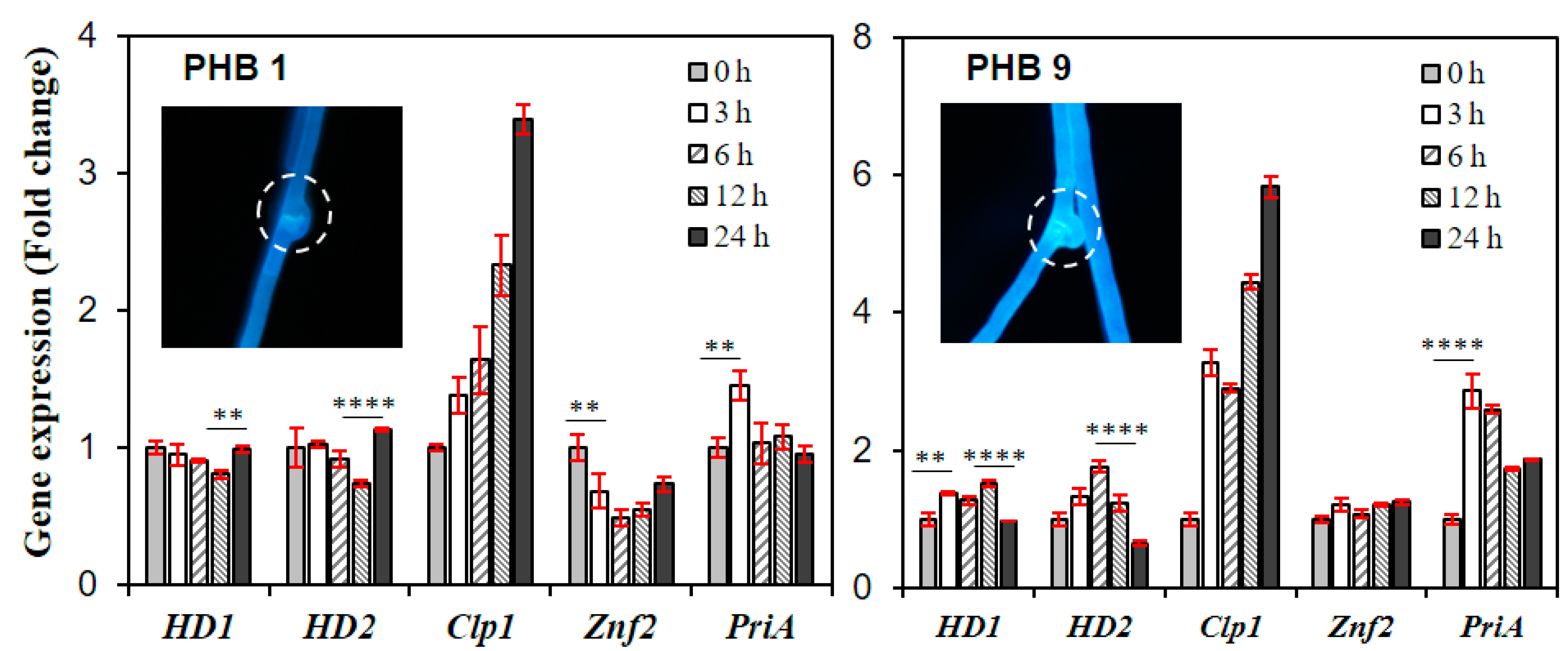
3.3. Effects of PHBs on the Activation of the Lentinula edodes Mating Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Casselton, L.A.; Olesnicky, N.S. Molecular genetics of mating recognition in basidiomycete fungi. Microbiol. Mol. Biol. Rev. 1998, 62, 55–70. [Google Scholar] [CrossRef]
- Bakkeren, G.; Kämper, J.; Schirawski, J. Sex in smut fungi: Structure, function and evolution of mating-type complexes. Fungal Genet. Biol. 2008, 45, S15–S21. [Google Scholar] [CrossRef]
- Kües, U. From two to many: Multiple mating types in Basidiomycetes. Fungal Biol. Rev. 2015, 29, 126–166. [Google Scholar] [CrossRef]
- Riquelme, M.; Challen, M.P.; Casselton, L.A.; Brown, A.J. The origin of multiple B mating specificities in Coprinus cinereus. Genetics 2005, 170, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, G.A.; Naider, F.; Becker, J.M. Fungal lipopeptide mating pheromones: A model system for the study of protein prenylation. Microbiol. Rev. 1995, 59, 406–422. [Google Scholar] [CrossRef] [PubMed]
- Wendland, J.; Vaillancourt, L.J.; Hegner, J.; Lengeler, K.B.; Laddison, K.J.; Specht, C.A.; Raper, C.A.; Kothe, E. The mating-type locus B alpha 1 of Schizophyllum commune contains a pheromone receptor gene and putative pheromone genes. EMBO J. 1995, 14, 5271–5278. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Sakagami, Y.; Isogai, A.; Suzuki, A. Structures of tremerogens A-9291-I and A-9291-VIII: Peptidal sex hormones of Tremella brasiliensis. Biochemistry 1984, 23, 1399–1404. [Google Scholar] [CrossRef]
- Sakagami, Y.; Isogai, A.; Suzuki, A.; Tamura, S.; Kitada, C.; Fujino, M. Structure of tremerogen A-10, a peptidal hormone inducing conjugation tube formation in Tremella mesenterica. Agric. Biol. Chem. 1979, 43, 2643–2645. [Google Scholar] [CrossRef]
- Sakagami, Y.; Yoshida, M.; Suzuki, A. Peptidal sex hormones inducing conjugation tube formation in compatible mating-type cells of Tremella mesenterica. Science 1981, 212, 1525–1527. [Google Scholar] [CrossRef]
- Brown, A.J.; Casselton, L.A. Mating in mushrooms: Increasing the chances but prolonging the affair. Trend. Genet. 2001, 17, 393–400. [Google Scholar] [CrossRef]
- Nakayama, N.; Miyajima, A.; Arai, K. Nucleotide sequences of ste2 and ste3, cell type-specific sterile genes from Saccharomyces cerevisiae. EMBO J. 1985, 4, 2643–2648. [Google Scholar] [CrossRef] [PubMed]
- Ha, B.; Kim, S.; Kim, M.; Ro, H.S. Activation of the mating pheromone response pathway of Lentinula edodes by synthetic pheromones. Mycobiology 2018, 46, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Bardwell, L.; Cook, J.G.; Inouye, C.J.; Thorner, J. Signal propagation and regulation in the mating pheromone response pathway of the yeast Saccharomyces cerevisiae. Dev. Biol. 1994, 166, 363–379. [Google Scholar] [CrossRef] [PubMed]
- Christopher, G.A.; Thorner, J. Heterotrimeric G protein-coupled receptor signaling in yeast mating pheromone response. J. Biol. Chem. 2016, 291, 7788–7795. [Google Scholar]
- Roberts, C.J.; Nelson, B.; Marton, M.J.; Stoughton, R.; Meyer, M.R.; Bennett, H.A.; He, Y.D.; Dai, H.; Walker, W.L.; Hughes, T.R.; et al. Signaling and circuitry of multiple MAPK pathways revealed by a matrix of global gene expression profiles. Science 2000, 287, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Madden, K.; Snyder, M. Cell polarity and morphogenesis in budding yeast. Ann. Rev. Microbiol. 1998, 52, 687–744. [Google Scholar] [CrossRef]
- Raudaskoski, M.; Kothe, E. Basidiomycete mating type genes and pheromone signaling. Euk. Cell 2010, 9, 847–859. [Google Scholar] [CrossRef]
- Olesnicky, N.S.; Brown, A.J.; Dowell, S.J.; Casselton, L.A. A constitutively active G-protein-coupled receptor causes mating self-compatibility in the mushroom Coprinus. EMBO J. 1999, 18, 2756–2763. [Google Scholar] [CrossRef]
- Feretzaki, M.; Heitman, J. Genetic circuits that govern bisexual and unisexual reproduction in Cryptococcus neoformans. PLoS Genet. 2013, 9, e1003688. [Google Scholar] [CrossRef]
- Lin, X.; Jackson, J.C.; Feretzaki, M.; Xue, C.; Heitman, J. Transcription factor Mat2 and Znf2 operate cellular circuits orchestrating opposite- and same sex mating in Cryptococcus neoformans. PLoS Genet. 2010, 6, e1000953. [Google Scholar] [CrossRef]
- Heimel, K.; Scherer, M.; Vranes, M.; Wahl, R.; Pothiratana, C.; Schuler, D.; Vincon, V.; Finkernagel, F.; Flor-Parra, I.; Kämper, J. The transcription factor Rbf1 is the master regulator for b-mating type controlled pathogenic development in Ustilago maydis. PLoS Pathog. 2010, 6, e1001035. [Google Scholar] [CrossRef] [PubMed]
- Mead, E.; Hull, C.M. Transcriptional control of sexual development in Cryptococcus neoformans. J. Microbiol. 2016, 54, 339–346. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, S.F.; Chaure, P.T.; Halsall, J.R.; Olesnicky, N.S.; Leibbrandt, A.; Connerton, I.F.; Casselton, L.A. A large pheromone and receptor gene complex determines multiple B mating type specificities in Coprinus cinereus. Genetics 1998, 148, 1081–1090. [Google Scholar] [PubMed]
- Ruirong, Y.; Hiroyuki, M.; Takashi, T.; Norihiro, S.; Tadanori, A. A-mating type gene expression can drive clamp formation in the bipolar mushroom Pholiota microspore (Pholiota nameko). Euk. Cell 2010, 9, 1109–1119. [Google Scholar]
- Inada, K.; Morimoto, Y.; Arima, T.; Murata, Y.; Kamada, T. The clp1 gene of the mushroom Coprinus cinereus is essential for A-regulated sexual development. Genetics 2001, 157, 133–140. [Google Scholar]
- Fowler, T.J.; Mitton, M.F.; Vaillancourt, L.J.; Raper, C.A. Changes in mate recognition through alterations of pheromones and receptors in the multisexual mushroom fungus Schizophyllum commune. Genetics 2001, 158, 1491–1503. [Google Scholar]
- Ha, B.; Moon, Y.J.; Song, Y.; Kim, S.; Kim, M.; Yoon, C.W.; Ro, H.S. Molecular analysis of B mating type diversity in Lentinula edodes. Sci. Hortic. 2019, 243, 55–63. [Google Scholar] [CrossRef]
- McCaffrey, G.; Clay, F.J.; Kelsay, K.; Sprague, G.F., Jr. Identification and regulation of a gene required for cell fusion during mating of the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 1987, 7, 2680–2690. [Google Scholar] [CrossRef]
- Dohlman, H.G.; Song, J.; Ma, D.; Courchesne, W.E.; Thorner, J. Sst2, a negative regulator of pheromone signaling in the yeast Saccharomyces cerevisiae: Expression, localization, and genetic interaction and physical association with Gpa1 (the G-protein alpha subunit). Mol. Cell. Biol. 1996, 16, 5194–5209. [Google Scholar] [CrossRef]
- Chasse, S.A.; Flanary, P.; Parnell, S.C.; Hao, N.; Cha, J.Y.; Siderovski, D.P.; Dohlman, H.G. Genome-scale analysis reveals Sst2 as the principal regulator of mating pheromone signaling in the yeast Saccharomyces cerevisiae. Euk. Cell 2006, 5, 330–346. [Google Scholar] [CrossRef]
- Stajich, J.E.; Wilke, S.K.; Ahrén, D.; Au, C.H.; Birren, B.W.; Borodovsky, M.; Burns, C.; Canbäck, B.; Casselton, L.A.; Cheng, C.K.; et al. Insights into evolution of multicellular fungi from the assembled chromosomes of the mushroom Coprinopsis cinerea (Coprinus cinereus). Proc. Natl. Acad. Sci. USA 2010, 107, 11889–11894. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lian, L.; Xu, P.; Chou, T.; Mukhtar, I.; Osakina, A.; Waqas, M.; Chen, B.; Liu, X.; Liu, F.; et al. Advances in understanding mating type gene organization in the mushroom-forming fungus Flammulina velutipes. G3-Genes Genomes Genet. 2016, 6, 3635–3645. [Google Scholar]
- Olesnicky, N.S.; Brown, A.J.; Honda, Y.; Dyos, S.L.; Dowell, S.J.; Casselton, L.A. Self-compatible B mutants in Coprinus with altered pheromone-receptor specificities. Genetics 2000, 156, 1025–1033. [Google Scholar] [PubMed]
- Fowler, T.J.; DeSimone, S.M.; Mitton, M.F.; Kurjan, J.; Raper, C.A. Multiple sex pheromones and receptors of a mushroom-producing fungus elicit mating in yeast. Mol. Biol. Cell 1999, 10, 2559–2572. [Google Scholar] [CrossRef]
- Wu, L.; van Peer, A.; Song, W.; Wang, H.; Chen, M.; Tan, Q.; Song, C.; Zhang, M.; Bao, D. Cloning of the Lentinula edodes B mating-type locus and identification of the genetic structure controlling B mating. Gene 2013, 531, 270–278. [Google Scholar] [CrossRef]
- Dawe, A.L.; Becker, J.M.; Jiang, Y.; Naider, F.; Eummer, J.T.; Mu, Y.Q.; Gibbs, R.A. Novel modifications to the farnesyl moiety of the a-factor lipopeptide pheromone from Saccharomyces cerevisiae: A role for isoprene modifications in ligand presentation. Biochemistry 1997, 36, 12036–12044. [Google Scholar] [CrossRef]
- Sherrill, C.; Khouri, O.; Zeman, S.; Roise, D. Synthesis and biological activity of fluorescent yeast pheromones. Biochemistry 1995, 34, 3553–3560. [Google Scholar] [CrossRef]
- Marcus, S.; Caldwell, G.A.; Miller, D.; Xue, C.B.; Naider, F.; Becker, J.M. Significance of C-terminal cysteine modifications to the biological activity of the Saccharomyces cerevisiae a-factor mating pheromone. Mol. Cell. Biol. 1991, 11, 3603–3612. [Google Scholar] [CrossRef][Green Version]
- Xue, C.B.; Becker, J.M.; Naider, F. Synthesis of S-alkyl and C-terminal analogs of the Saccharomyces cerevisiae a-factor. Influence of temperature on the stability of Fmoc and OFm groups toward HF. Int. J. Pept. Protein Res. 1991, 37, 476–486. [Google Scholar] [CrossRef]
- Diaz-Rodriguez, V.; Hsu, E.T.; Ganusova, E.; Werst, E.R.; Becker, J.M.; Hrycyna, C.A.; Distefano, M.D. a-Factor analogues containing alkyne- and azide-functionalized isoprenoids are efficiently enzymatically processed and retain wild-type bioactivity. Bioconjug. Chem. 2018, 29, 316–323. [Google Scholar] [CrossRef]




| Strain Name | Genotype |
|---|---|
| W303a | MAT-a ade2-1 ura3-1 his3-11, 15 trp1-1 leu2-3, 112 can1-100 |
| RCY1419 | W303a ste2Δ::KanMX6RCB1-4 |
| RCY1420 | W303a sst2Δ::HIS3 |
| RCY1421 | W303a sst2Δ::HIS3, ste2Δ::KanMX6RCB1-4 |
| RCY1423 | W303a sst2Δ::HIS3, ste2Δ::KanMX6RCB2-1 |
| RCY1429 | W303a sst2Δ::HIS3, ste2Δ::KanMX6RCB1-2 |
| RCY1432 | W303a sst2Δ::HIS3, gpaLe::TRP1 |
| RCY1433 | W303a sst2Δ::HIS3, gpaLe::TRP1, ste2Δ::KanMX6RCB1-2 |
| RCY1434 | W303a sst2Δ::HIS3, gpaLe::TRP1, ste2Δ::KanMX6RCB1-4 |
| RCY1440 | W303a sst2Δ::HIS3, gpaLe::TRP1, ste2Δ::KanMX6RCB2-1 |
| Sublocus | PHBs | Sequence | Sublocus | PHBs | Sequence |
|---|---|---|---|---|---|
| Bα1 | PHB1 | EHDTADSTNIGYAC-OME * | Bβ1 | PHB7 | EAIGAGDATAFC-OME |
| PHB2 | EHDTSDSGYTGYC-OME | PHB4 | EAGGGDAIAFC-OME | ||
| Bα2 | PHB5 | EHPSDSGAVADFGYC-OME | Bβ2 | PHB3 | EAVGSGDIIGFC-OME |
| PHB6 | EHADESGSVALLGGYC-OME | PHB4 | EAGGGDAIAFC-OME | ||
| Bα3 | PHB8 | EHDTNDSAFLGFC-OME | Bβ3 | PHB9 | EAVGSGDIIGFC-OME |
| Bα4 | PHB11 | ERPSNSGAVADFGYC-OME | PHB10 | EAIGAADGSAFC-OME | |
| PHB12 | EHDSSDSTDIGYC-OME | ||||
| Bα5 | PHB13 | EHPSETSSDANFGSYC-OME | |||
| PHB14 | EHDTSDSTDIGYC-OME | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Ha, B.; Kim, M.; Ro, H.-S. Investigation of Mating Pheromone–Pheromone Receptor Specificity in Lentinula edodes. Genes 2020, 11, 506. https://doi.org/10.3390/genes11050506
Kim S, Ha B, Kim M, Ro H-S. Investigation of Mating Pheromone–Pheromone Receptor Specificity in Lentinula edodes. Genes. 2020; 11(5):506. https://doi.org/10.3390/genes11050506
Chicago/Turabian StyleKim, Sinil, Byeongsuk Ha, Minseek Kim, and Hyeon-Su Ro. 2020. "Investigation of Mating Pheromone–Pheromone Receptor Specificity in Lentinula edodes" Genes 11, no. 5: 506. https://doi.org/10.3390/genes11050506
APA StyleKim, S., Ha, B., Kim, M., & Ro, H.-S. (2020). Investigation of Mating Pheromone–Pheromone Receptor Specificity in Lentinula edodes. Genes, 11(5), 506. https://doi.org/10.3390/genes11050506





