Ancestry Prediction Comparisons of Different AISNPs for Five Continental Populations and Population Structure Dissection of the Xinjiang Hui Group via a Self-Developed Panel
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Information
2.2. DNA Extraction and Primer Design
2.3. Library Preparation, NGS and Data Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Depth of Coverage and the ACR of the 30 AISNPs in the Hui Group
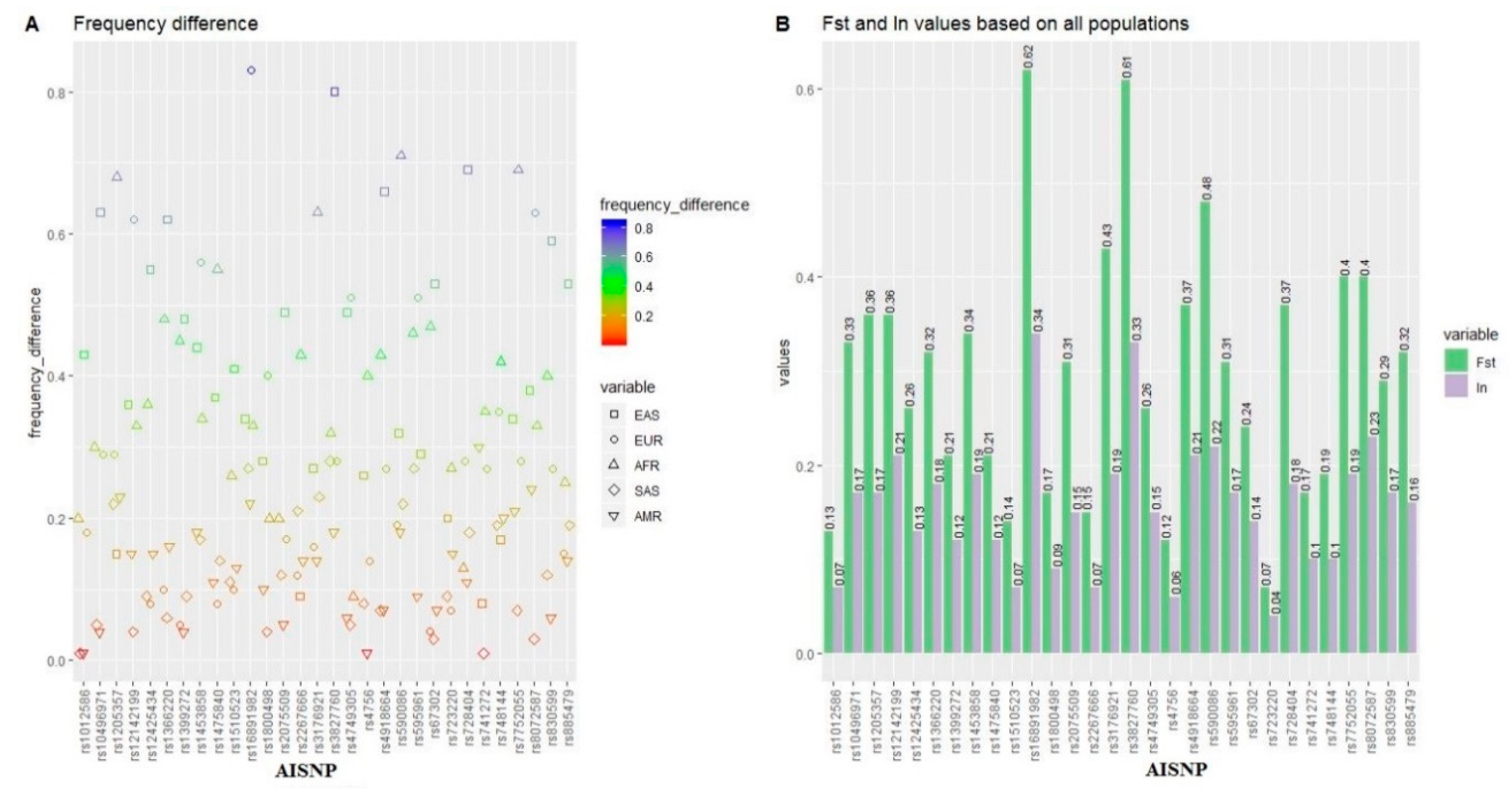
3.2. Genetic Divergences of the 30 AISNPs among the Five Continental Populations
3.3. Ancestry Resolution Comparisons of Different AISNPs among Five Continental Populations
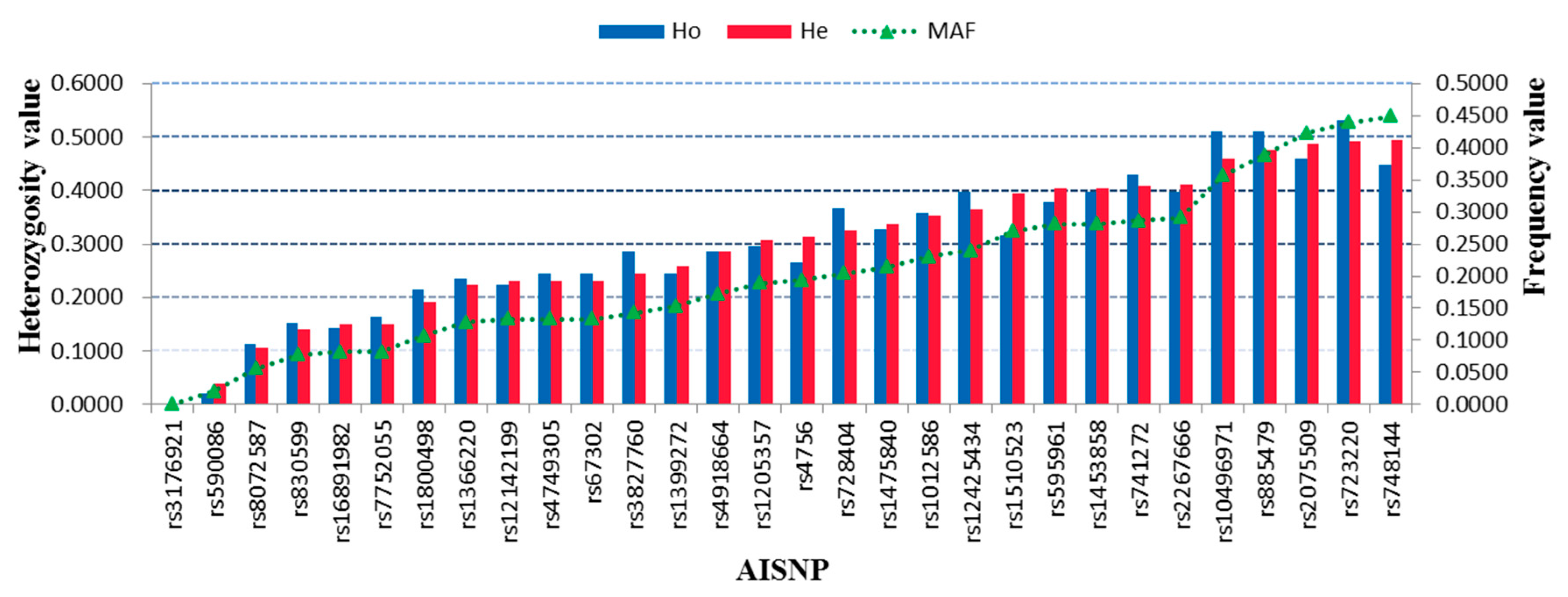
3.4. Genetic Distributions and Forensic Parameters of 30 AISNPs in the Hui Group
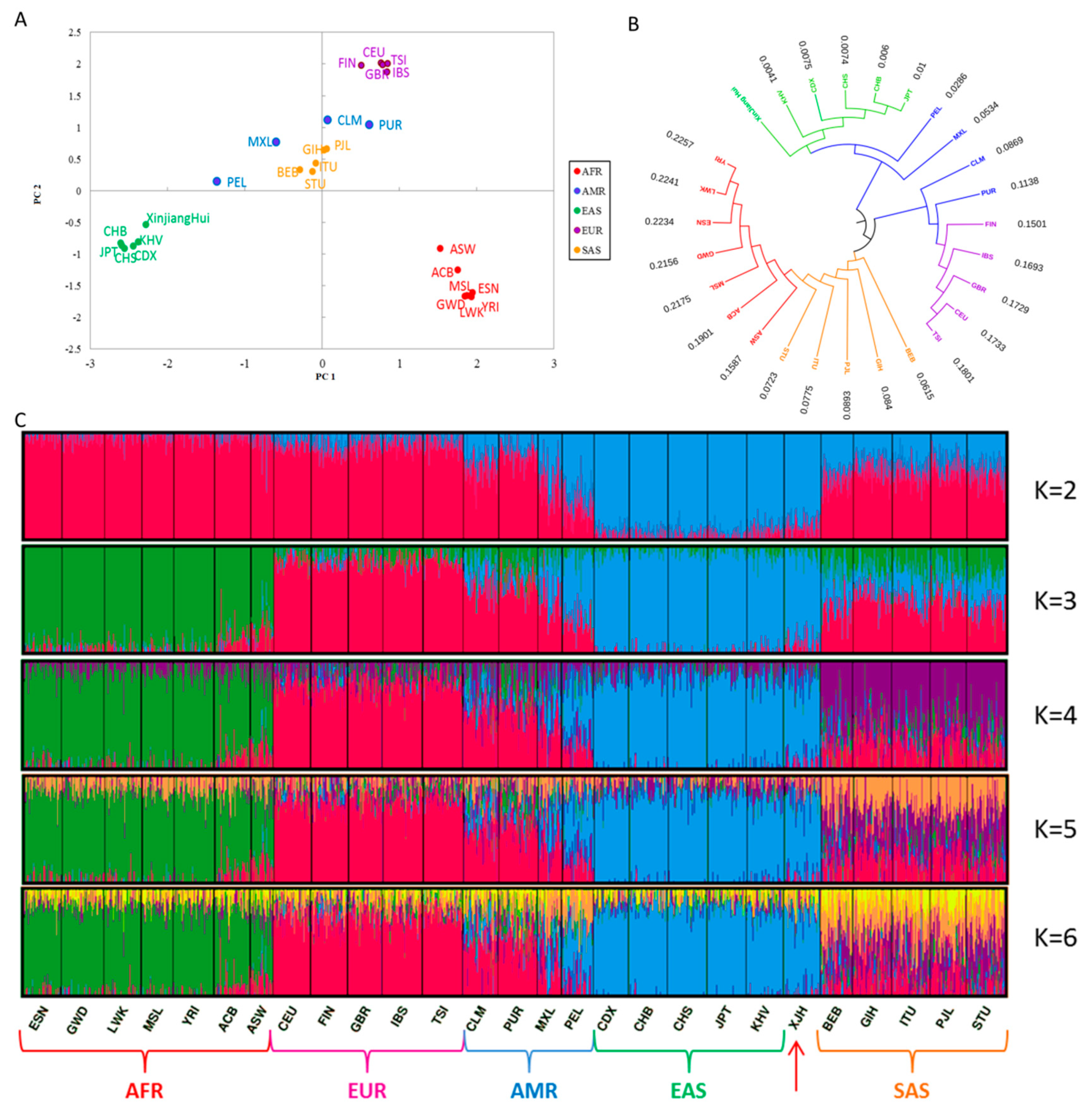
3.5. Phylogenetic Relationships and Population Structure Analyses of the Hui Group and Other Continental Populations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Data Availability Statement
References
- Tishkoff, S.A.; Kidd, K.K. Implications of biogeography of human populations for ‘race’ and medicine. Nat. Genet. 2004, 36, S21–S27. [Google Scholar] [CrossRef]
- Phillips, C. Forensic genetic analysis of bio-geographical ancestry. Forensic Sci. Int. Genet. 2015, 18, 49–65. [Google Scholar] [CrossRef]
- Kidd, K.K.; Speed, W.C.; Pakstis, A.J.; Furtado, M.R.; Fang, R.; Madbouly, A.; Maiers, M.; Middha, M.; Friedlaender, F.R.; Kidd, J.R. Progress toward an efficient panel of SNPs for ancestry inference. Forensic Sci. Int. Genet. 2014, 10, 23–32. [Google Scholar] [CrossRef]
- Phillips, C.; Salas, A.; Sanchez, J.J.; Fondevila, M.; Gomez-Tato, A.; Alvarez-Dios, J.; Calaza, M.; de Cal, M.C.; Ballard, D.; Lareu, M.V.; et al. Inferring ancestral origin using a single multiplex assay of ancestry-informative marker SNPs. Forensic Sci. Int. Genet. 2007, 1, 273–280. [Google Scholar] [CrossRef]
- Wei, Y.L.; Wei, L.; Zhao, L.; Sun, Q.F.; Jiang, L.; Zhang, T.; Liu, H.B.; Chen, J.G.; Ye, J.; Hu, L.; et al. A single-tube 27-plex SNP assay for estimating individual ancestry and admixture from three continents. Int. J. Legal Med. 2016, 130, 27–37. [Google Scholar] [CrossRef]
- Lan, Q.; Shen, C.; Jin, X.; Guo, Y.; Xie, T.; Chen, C.; Cui, W.; Fang, Y.; Yang, G.; Zhu, B. Distinguishing three distinct biogeographic regions with an in-house developed 39-AIM-InDel panel and further admixture proportion estimation for Uyghurs. Electrophoresis 2019. [Google Scholar] [CrossRef]
- Fondevila, M.; Phillips, C.; Santos, C.; Freire Aradas, A.; Vallone, P.M.; Butler, J.M.; Lareu, M.V.; Carracedo, A. Revision of the SNPforID 34-plex forensic ancestry test: Assay enhancements, standard reference sample genotypes and extended population studies. Forensic Sci. Int. Genet. 2013, 7, 63–74. [Google Scholar] [CrossRef]
- Bruijns, B.; Tiggelaar, R.; Gardeniers, H. Massively parallel sequencing techniques for forensics: A review. Electrophoresis 2018, 39, 2642–2654. [Google Scholar] [CrossRef]
- de Knijff, P. From next generation sequencing to now generation sequencing in forensics. Forensic Sci. Int. Genet. 2019, 38, 175–180. [Google Scholar] [CrossRef]
- Lan, Q.; Chen, J.; Guo, Y.; Xie, T.; Fang, Y.; Jin, X.; Cui, W.; Zhou, Y.; Zhu, B. Genetic structure and polymorphism analysis of Xinjiang Hui ethnic minority based on 21 STRs. Mol. Biol. Rep. 2018, 45, 99–108. [Google Scholar] [CrossRef]
- Gladney, D.C. Ethnic Identity in China: The Making of a Muslim Minority Nationality; Wadsworth Publishing Company: Cambridge, MA, USA, 1998. [Google Scholar]
- Akiner, S. Familiar Strangers: A History of Muslims in Northwest China by Jonathan N. Lipman. Am. Hist. Rev. 1998, 38, 1–4. [Google Scholar]
- Xie, M.; Song, F.; Li, J.; Lang, M.; Luo, H.; Wang, Z.; Wu, J.; Li, C.; Tian, C.; Wang, W.; et al. Genetic substructure and forensic characteristics of Chinese Hui populations using 157 Y-SNPs and 27 Y-STRs. Forensic Sci. Int. Genet. 2019, 41, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Guo, Y.; Chen, L.; Fang, Y.; Tai, Y.; Zhou, Y.; Qiu, P.; Zhu, B. A set of autosomal multiple InDel markers for forensic application and population genetic analysis in the Chinese Xinjiang Hui group. Forensic Sci. Int. Genet. 2018, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Guo, Y.; Xie, T.; Jin, X.; Lan, Q.; Zhou, Y.; Zhu, B. Forensic molecular genetic diversity analysis of Chinese Hui ethnic group based on a novel STR panel. Int. J. Legal Med. 2018, 132, 1297–1299. [Google Scholar] [CrossRef]
- Zhu, B.F.; Zhang, Y.D.; Liu, W.J.; Meng, H.T.; Yuan, G.L.; Lv, Z.; Dong, N.; Li, Q.; Yang, C.H.; Zhang, Y.H.; et al. Genetic diversity and haplotype structure of 24 Y-chromosomal STR in Chinese Hui ethnic group and its genetic relationships with other populations. Electrophoresis 2014, 35, 1993–2000. [Google Scholar] [CrossRef]
- Meng, H.T.; Han, J.T.; Zhang, Y.D.; Liu, W.J.; Wang, T.J.; Yan, J.W.; Huang, J.F.; Du, W.A.; Guo, J.X.; Wang, H.D.; et al. Diversity study of 12 X-chromosomal STR loci in Hui ethnic from China. Electrophoresis 2014, 35, 2001–2007. [Google Scholar] [CrossRef]
- He, G.; Wang, Z.; Wang, M.; Luo, T.; Liu, J.; Zhou, Y.; Gao, B.; Hou, Y. Forensic ancestry analysis in two Chinese minority populations using massively parallel sequencing of 165 ancestry-informative SNPs. Electrophoresis 2018, 39, 2732–2742. [Google Scholar] [CrossRef]
- Guo, Y.X.; Jin, X.Y.; Xia, Z.Y.; Chen, C.; Cui, W.; Zhu, B.F. A small NGS-SNP panel of ancestry inference designed to distinguish African, European, East and South Asian populations. Electrophoresis 2020. [Google Scholar] [CrossRef]
- Genomes Project, C.; Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; et al. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 3. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Koboldt, D.C.; Chen, K.; Wylie, T.; Larson, D.E.; McLellan, M.D.; Mardis, E.R.; Weinstock, G.M.; Wilson, R.K.; Ding, L. VarScan: Variant detection in massively parallel sequencing of individual and pooled samples. Bioinformatics 2009, 25, 2283–2285. [Google Scholar] [CrossRef] [PubMed]
- Rousset, F. genepop’007: A complete re-implementation of the genepop software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [Google Scholar] [CrossRef]
- Guo, S.W.; Thompson, E.A. Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics 1992, 48, 361–372. [Google Scholar] [CrossRef]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef]
- Ota, T. Dispan: Genetic Distance and Phylogenetic Analysis; The Pennsylvania State University: Philadelphia, PA, USA, 1993. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef]
- Kopelman, N.M.; Mayzel, J.; Jakobsson, M.; Rosenberg, N.A.; Mayrose, I. Clumpak: A program for identifying clustering modes and packaging population structure inferences across K. Mol. Ecol. Resour. 2015, 15, 1179–1191. [Google Scholar] [CrossRef]
- Wendt, F.R.; Churchill, J.D.; Novroski, N.M.M.; King, J.L.; Ng, J.; Oldt, R.F.; McCulloh, K.L.; Weise, J.A.; Smith, D.G.; Kanthaswamy, S.; et al. Genetic analysis of the Yavapai Native Americans from West-Central Arizona using the Illumina MiSeq FGx forensic genomics system. Forensic Sci. Int. Genet. 2016, 24, 18–23. [Google Scholar] [CrossRef]
- Guo, F.; Yu, J.; Zhang, L.; Li, J. Massively parallel sequencing of forensic STRs and SNPs using the Illumina((R)) ForenSeq DNA Signature Prep Kit on the MiSeq FGx Forensic Genomics System. Forensic Sci. Int. Genet. 2017, 31, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Zhu, J.; Chen, P.; Chen, D.; Wang, H.; Liang, W.; Zhang, L. Estimate the heterozygote balance of microhaplotype marker with massively parallel sequencing. Forensic Sci. Int. Genet. Suppl. Ser. 2017, 6, e375–e376. [Google Scholar] [CrossRef]
- Santos, C.; Phillips, C.; Gomez-Tato, A.; Alvarez-Dios, J.; Carracedo, A.; Lareu, M.V. Inference of Ancestry in Forensic Analysis II: Analysis of Genetic Data. Methods Mol. Biol. 2016, 1420, 255–285. [Google Scholar] [CrossRef] [PubMed]
- Kohnemann, S.; Sibbing, U.; Pfeiffer, H.; Hohoff, C. A rapid mtDNA assay of 22 SNPs in one multiplex reaction increases the power of forensic testing in European Caucasians. Int. J. Legal Med. 2008, 122, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Takezaki, N.; Nei, M. Genetic distances and reconstruction of phylogenetic trees from microsatellite DNA. Genetics 1996, 144, 389–399. [Google Scholar] [PubMed]
- Yao, H.B.; Wang, C.C.; Tao, X.; Shang, L.; Wen, S.Q.; Zhu, B.; Kang, L.; Jin, L.; Li, H. Genetic evidence for an East Asian origin of Chinese Muslim populations Dongxiang and Hui. Sci. Rep. 2016, 6, 38656. [Google Scholar] [CrossRef]
- Zhou, B.; Wen, S.; Sun, H.; Zhang, H.; Shi, R. Genetic affinity between Ningxia Hui and eastern Asian populations revealed by a set of InDel loci. R. Soc. Open Sci. 2020, 7, 190358. [Google Scholar] [CrossRef]

| Panels | Intercontinental Populations | AFR | AMR | EAS | EUR | SAS |
|---|---|---|---|---|---|---|
| this study | AFR | 0.9864 | 0.0076 | 0.0000 | 0.0015 | 0.0045 |
| AMR | 0.0058 | 0.6858 | 0.0605 | 0.1816 | 0.0663 | |
| EAS | 0.0000 | 0.0000 | 1.0000 | 0.0000 | 0.0000 | |
| EUR | 0.0000 | 0.0139 | 0.0000 | 0.9801 | 0.0060 | |
| SAS | 0.0000 | 0.0736 | 0.0000 | 0.0143 | 0.9121 | |
| 55 AISNPs | AFR | 0.9924 | 0.0076 | 0.0000 | 0.0000 | 0.0000 |
| AMR | 0.0058 | 0.8069 | 0.0029 | 0.1527 | 0.0317 | |
| EAS | 0.0000 | 0.0000 | 1.0000 | 0.0000 | 0.0000 | |
| EUR | 0.0000 | 0.0000 | 0.0000 | 1.0000 | 0.0000 | |
| SAS | 0.0000 | 0.0102 | 0.0000 | 0.0041 | 0.9857 | |
| 33 AISNPs | AFR | 0.9849 | 0.0151 | 0.0000 | 0.0000 | 0.0000 |
| AMR | 0.0086 | 0.7983 | 0.0375 | 0.1124 | 0.0432 | |
| EAS | 0.0000 | 0.0040 | 0.9940 | 0.0000 | 0.0020 | |
| EUR | 0.0000 | 0.0159 | 0.0000 | 0.9801 | 0.0040 | |
| SAS | 0.0000 | 0.0409 | 0.0000 | 0.0061 | 0.9530 | |
| 27 AISNPs | AFR | 0.9879 | 0.0091 | 0.0000 | 0.0000 | 0.0030 |
| AMR | 0.0058 | 0.6426 | 0.0778 | 0.1499 | 0.1239 | |
| EAS | 0.0000 | 0.0020 | 0.9980 | 0.0000 | 0.0000 | |
| EUR | 0.0000 | 0.0119 | 0.0000 | 0.9801 | 0.0080 | |
| SAS | 0.0000 | 0.1493 | 0.0000 | 0.0429 | 0.8078 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.-Y.; Guo, Y.-X.; Chen, C.; Cui, W.; Liu, Y.-F.; Tai, Y.-C.; Zhu, B.-F. Ancestry Prediction Comparisons of Different AISNPs for Five Continental Populations and Population Structure Dissection of the Xinjiang Hui Group via a Self-Developed Panel. Genes 2020, 11, 505. https://doi.org/10.3390/genes11050505
Jin X-Y, Guo Y-X, Chen C, Cui W, Liu Y-F, Tai Y-C, Zhu B-F. Ancestry Prediction Comparisons of Different AISNPs for Five Continental Populations and Population Structure Dissection of the Xinjiang Hui Group via a Self-Developed Panel. Genes. 2020; 11(5):505. https://doi.org/10.3390/genes11050505
Chicago/Turabian StyleJin, Xiao-Ye, Yu-Xin Guo, Chong Chen, Wei Cui, Yan-Fang Liu, Yun-Chun Tai, and Bo-Feng Zhu. 2020. "Ancestry Prediction Comparisons of Different AISNPs for Five Continental Populations and Population Structure Dissection of the Xinjiang Hui Group via a Self-Developed Panel" Genes 11, no. 5: 505. https://doi.org/10.3390/genes11050505
APA StyleJin, X.-Y., Guo, Y.-X., Chen, C., Cui, W., Liu, Y.-F., Tai, Y.-C., & Zhu, B.-F. (2020). Ancestry Prediction Comparisons of Different AISNPs for Five Continental Populations and Population Structure Dissection of the Xinjiang Hui Group via a Self-Developed Panel. Genes, 11(5), 505. https://doi.org/10.3390/genes11050505




