YARS2 Missense Variant in Belgian Shepherd Dogs with Cardiomyopathy and Juvenile Mortality
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Breed Nomenclature
2.3. Animal Selection
2.4. DNA Extraction
2.5. Linkage and Homozygosity Mapping
2.6. Whole Genome Sequencing of an Affected Malinois
2.7. Variant Calling and Filtering
2.8. Gene Analysis
2.9. Sanger Sequencing
3. Results
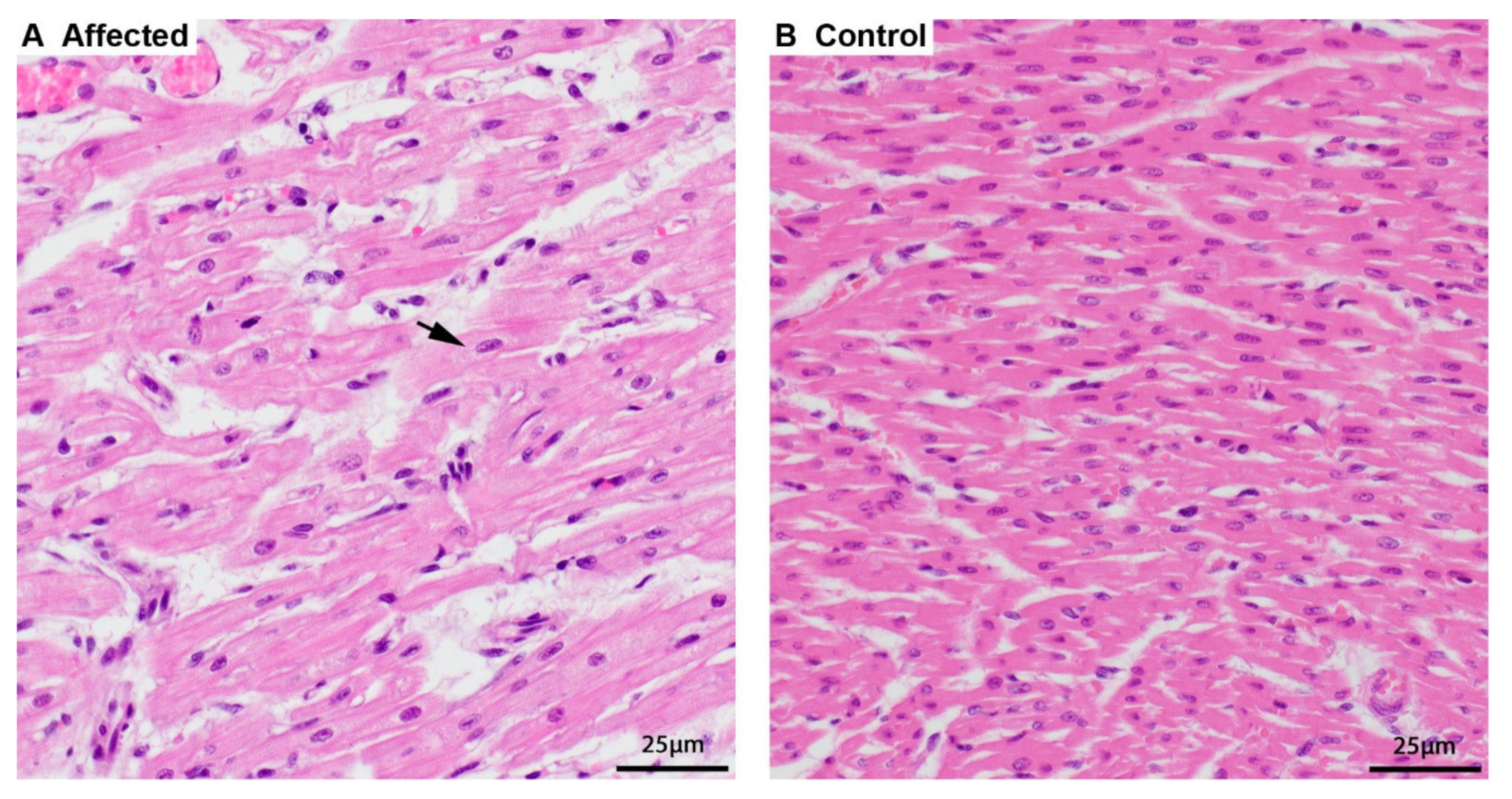
3.1. Phenotype Description
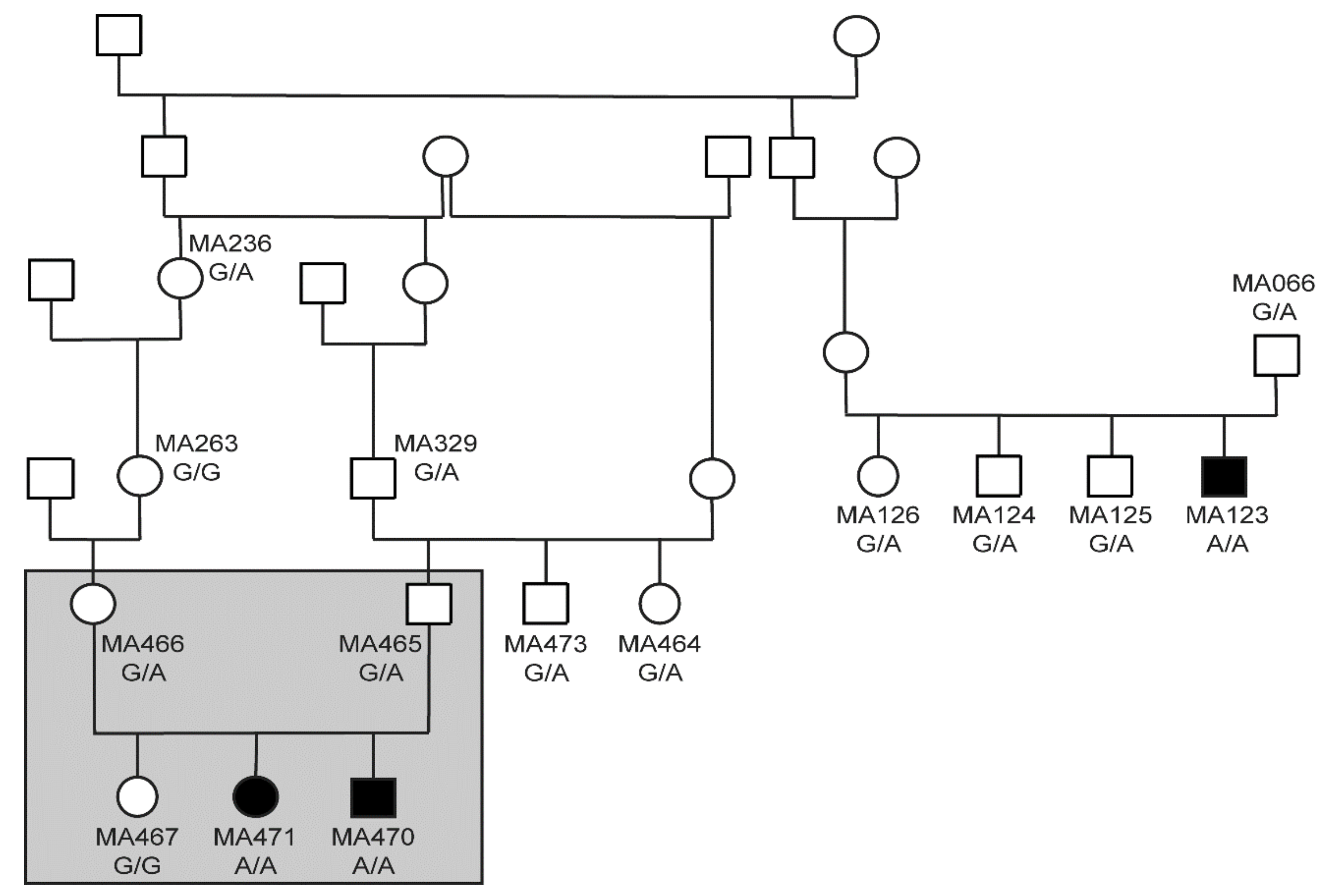
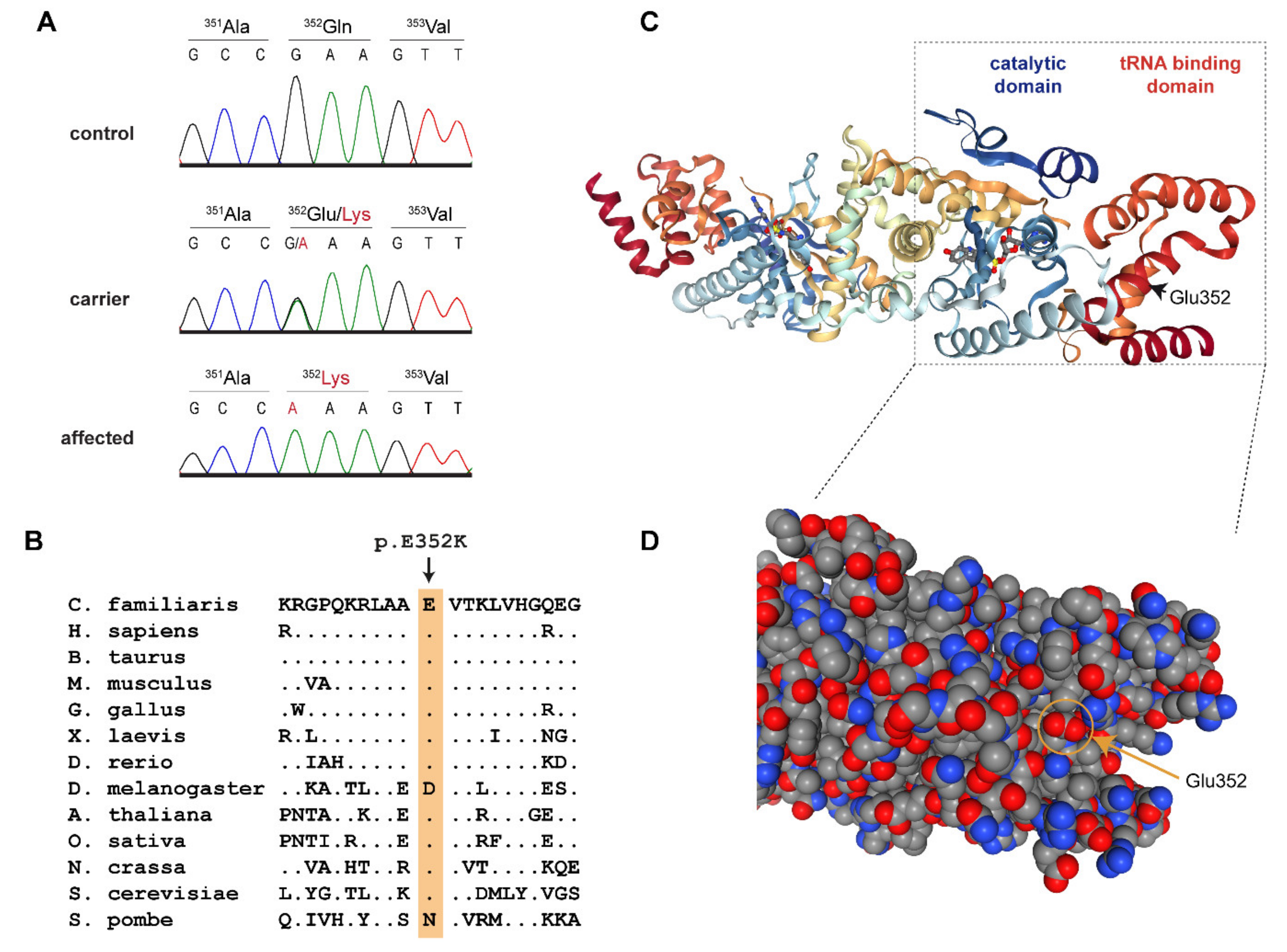
3.2. Genetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Indrebø, A.; Trangerud, C.; Moe, L. Canine neonatal mortality in four large breeds. Acta Vet. Scand. 2007, 49, S2. [Google Scholar] [CrossRef]
- Chastant-Maillard, S.; Guillemot, C.; Feugier, A.; Mariani, C.; Grellet, A.; Mila, H. Reproductive performance and pre-weaning mortality: Preliminary analysis of 27,221 purebred female dogs and 204,537 puppies in France. Reprod. Dom. Anim. 2017, 52, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Münnich, A.; Küchenmeister, U. Causes, diagnosis and therapy of common diseases in neonatal puppies in the first days of life: Cornerstones of practical approach. Reprod. Dom. Anim. 2014, 49, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Nobre Pacifico Pereira, K.H.; Cruz Dos Santos Correia, L.E.; Ritir Oliveira, E.L.; Bernardo, R.B.; Nagib Jorge, M.L.; Mezzena Gobato, M.L.; Ferreira de Souza, F.; Rocha, N.S.; Chiacchio, S.B.; Gomes Lourenço, M.L. Incidence of congenital malformations and impact on the mortality of neonatal canines. Theriogenology 2019, 140, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Buonavoglia, C. Canine parvovirus—A review of epidemiological and diagnostic aspects, with emphasis on type 2c. Vet. Microbiol. 2012, 155, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.W.; Kiupel, M.; Balzer, H.J.; Agerholm, J.S. Prevalence of canid herpesvirus-1 infection in stillborn and dead neonatal puppies in Denmark. Acta Vet. Scand. 2015, 57, 1. [Google Scholar] [CrossRef]
- Nielen, A.L.; van der Gaag, I.; Knol, B.W.; Schukken, Y.H. Investigation of mortality and pathological changes in a 14-month birth cohort of boxer puppies. Vet. Rec. 1998, 142, 601–606. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D. PLINK: A tool set for whole genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Abecasis, G.R.; Cherny, S.S.; Cookson, W.O.; Cardon, L.R. Merlin—Rapid analysis of dense genetic maps using sparse gene flow trees. Nat. Genet. 2002, 30, 97–101. [Google Scholar] [CrossRef]
- Jagannathan, V.; Drögemüller, C.; Leeb, T.; Dog Biomedical Variant Database Consortium (DBVDC). A comprehensive biomedical variant catalogue based on whole genome sequences of 582 dogs and eight wolves. Anim. Genet. 2019, 50, 695–704. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang le, L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Zhao, W.M.; Tang, B.X.; Wang, Y.Q.; Wang, L.; Zhang, Z.; Yang, H.C.; Liu, Y.H.; Zhu, J.W.; Irwin, D.M.; et al. DoGSD: The dog and wolf genome SNP database. Nucleic Acids Res. 2015, 43, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Plassais, J.; Kim, J.; Davis, B.W.; Karyadi, D.M.; Hogan, A.N.; Harris, A.C.; Decker, B.; Parker, H.G.; Ostrander, E.A. Whole genome sequencing of canids reveals genomic regions under selection and variants influencing morphology. Nat. Commun. 2019, 10, 1489. [Google Scholar] [CrossRef] [PubMed]
- López-Ferrando, V.; Gazzo, A.; de la Cruz, X.; Orozco, M.; Gelpí, J.L. PMut: A web-based tool for the annotation of pathological variants on proteins, 2017 update. Nucleic Acids Res. 2017, 45, W222–W228. [Google Scholar] [CrossRef]
- Sim, N.L.; Kumar, P.; Hu, J.; Henikoff, S.; Schneider, G.; Ng, P.C. SIFT web server: Predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 2012, 40, W452–W457. [Google Scholar] [CrossRef] [PubMed]
- Bonnefond, L.; Frugier, M.; Touzé, E.; Lorber, B.; Florentz, C.; Giegé, R.; Sauter, C.; Rudinger-Thirion, J. Crystal structure of human mitochondrial tyrosyl-tRNA synthetase reveals common and idiosyncratic features. Structure 2007, 15, 1505–1516. [Google Scholar] [CrossRef]
- PDB Entry 2PID—Crystal Structure of Human Mitochondrial Tyrosyl-tRNA Synthetase in Complex with an Adenylate Analog. Available online: http://www.rcsb.org/pdb/results/results.do?tabtoshow=Current&qrid=D9008E42 (accessed on 17 December 2019).
- Yaremchuk, A.; Kriklivyi, I.; Tukalo, M.; Cusack, S. Class I tyrosyl-tRNA synthetase has a class II mode of cognate tRNA recognition. EMBO J. 2002, 21, 3829–3840. [Google Scholar] [CrossRef]
- Boczonadi, V.; Jennings, M.J.; Horvath, R. The role of tRNA synthetases in neurological and neuromuscular disorders. FEBS Lett. 2018, 592, 703–717. [Google Scholar] [CrossRef]
- Riley, L.G.; Cooper, S.; Hickey, P.; Rudinger-Thirion, J.; McKenzie, M.; Compton, A.; Lim, S.C.; Thorburn, D.; Ryan, M.T.; Giege, R. Mutation of the mitochondrial tyrosyl-tRNA synthetase gene, YARS2, causes myopathy, lactic acidosis, and sideroblastic anemia—MLASA syndrome. Am. J. Hum. Genet. 2010, 87, 52–59. [Google Scholar] [CrossRef]
- Riley, L.G.; Menezes, M.J.; Rudinger-Thirion, J.; Duff, R.; de Lonlay, P.; Rotig, A.; Tchan, M.C.; Davis, M.; Cooper, S.T.; Christodoulou, J. Phenotypic variability and identification of novel YARS2 mutations in YARS2 mitochondrial myopathy, lactic acidosis and sideroblastic anaemia. Orphanet J. Rare Dis. 2013, 8, 193. [Google Scholar] [CrossRef]
- Nakajima, J.; Eminoglu, T.F.; Vatansever, G.; Nakashima, M.; Tsurusaki, Y.; Saitsu, H.; Kawashima, H.; Matsumoto, N.; Miyake, N. A novel homozygous YARS2 mutation causes severe myopathy, lactic acidosis, and sideroblastic anemia 2. J. Hum. Genet. 2014, 59, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Sasarman, F.; Nishimura, T.; Thiffault, I.; Shoubridge, E.A. A novel mutation in YARS2 causes myopathy with lactic acidosis and sideroblastic anemia. Hum. Mutat. 2012, 8, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Shahni, R.; Wedatilake, Y.; Cleary, M.A.; Lindley, K.J.; Sibson, K.R.; Rahman, S. A distinct mitochondrial myopathy, lactic acidosis and sideroblastic anemia (MLASA) phenotype associates with YARS2 mutations. Am. J. Med. Genet. A 2013, 9, 2334–2338. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.G.; Heeney, M.M.; Rudinger-Thirion, J.; Frugier, M.; Campagna, D.R.; Zhou, R.; Hale, G.A.; Hilliard, L.M.; Kaplan, J.A.; Kwiatkowski, J.L.; et al. The phenotypic spectrum of germline YARS2 variants: From isolated sideroblastic anemia to mitochondrial myopathy, lactic acidosis and sideroblastic anemia 2. Haematologica 2018, 103, 2008–2015. [Google Scholar] [CrossRef] [PubMed]
- Hadji Rasouliha, S.; Barrientos, L.; Anderegg, L.; Klesty, C.; Lorenz, J.; Chevallier, L.; Jagannathan, V.; Rösch, S.; Leeb, T. A RAPGEF6 variant constitutes a major risk factor for laryngeal paralysis in dogs. PLoS Genet. 2019, 15, e1008416. [Google Scholar] [CrossRef] [PubMed]
| Filtering Step | Variants |
|---|---|
| Homozygous variants in whole genome | 2,757,566 |
| Private homozygous variants (absent from 645 control genomes) in whole genome | 1321 |
| Private homozygous variants in 82 Mb critical intervals | 150 |
| Protein-changing private variants in critical intervals | 3 |
| Chr. | Position | Ref. | Alt. | Gene | HGVS-c | HGVS-p |
|---|---|---|---|---|---|---|
| 5 | 41,681,870 | G | A | SREBF1 | c.1678G>A | p.Gly560Ser |
| 27 | 3,744,738 | C | T | LOC106557897 | c.443G>A | p.Arg148His |
| 27 | 16,157,324 | G | A | YARS2 | c.1054G>A | p.Glu352Lys |
| Dogs | A/A | G/A | G/G |
|---|---|---|---|
| Belgian Shepherd cases (n = 3) | 3 | 0 | 0 |
| Belgian Shepherd controls (n = 471) | 0 | 128 | 343 |
| Control dogs other breeds (n = 534) | 0 | 0 | 534 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurtner, C.; Hug, P.; Kleiter, M.; Köhler, K.; Dietschi, E.; Jagannathan, V.; Leeb, T. YARS2 Missense Variant in Belgian Shepherd Dogs with Cardiomyopathy and Juvenile Mortality. Genes 2020, 11, 313. https://doi.org/10.3390/genes11030313
Gurtner C, Hug P, Kleiter M, Köhler K, Dietschi E, Jagannathan V, Leeb T. YARS2 Missense Variant in Belgian Shepherd Dogs with Cardiomyopathy and Juvenile Mortality. Genes. 2020; 11(3):313. https://doi.org/10.3390/genes11030313
Chicago/Turabian StyleGurtner, Corinne, Petra Hug, Miriam Kleiter, Kernt Köhler, Elisabeth Dietschi, Vidhya Jagannathan, and Tosso Leeb. 2020. "YARS2 Missense Variant in Belgian Shepherd Dogs with Cardiomyopathy and Juvenile Mortality" Genes 11, no. 3: 313. https://doi.org/10.3390/genes11030313
APA StyleGurtner, C., Hug, P., Kleiter, M., Köhler, K., Dietschi, E., Jagannathan, V., & Leeb, T. (2020). YARS2 Missense Variant in Belgian Shepherd Dogs with Cardiomyopathy and Juvenile Mortality. Genes, 11(3), 313. https://doi.org/10.3390/genes11030313






