Claudins in Renal Physiology and Pathology
Abstract
:1. Introduction
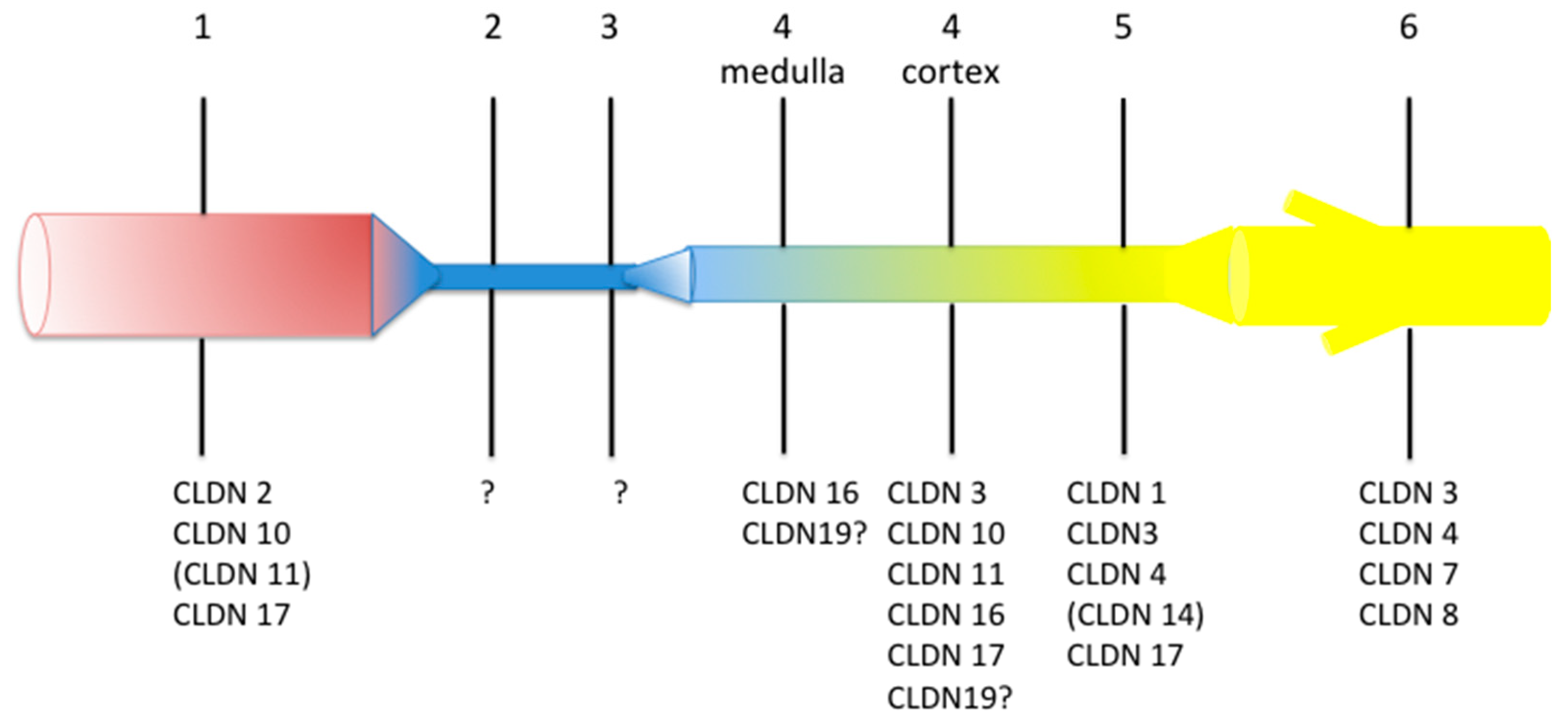
2. Structure and Function of Tight Junction in the Kidney
3. Claudin 10b and the HELIX Syndrome
3.1. Phenotype
3.2. Variants/Pathogenesis
3.3. Prognosis and Treatment
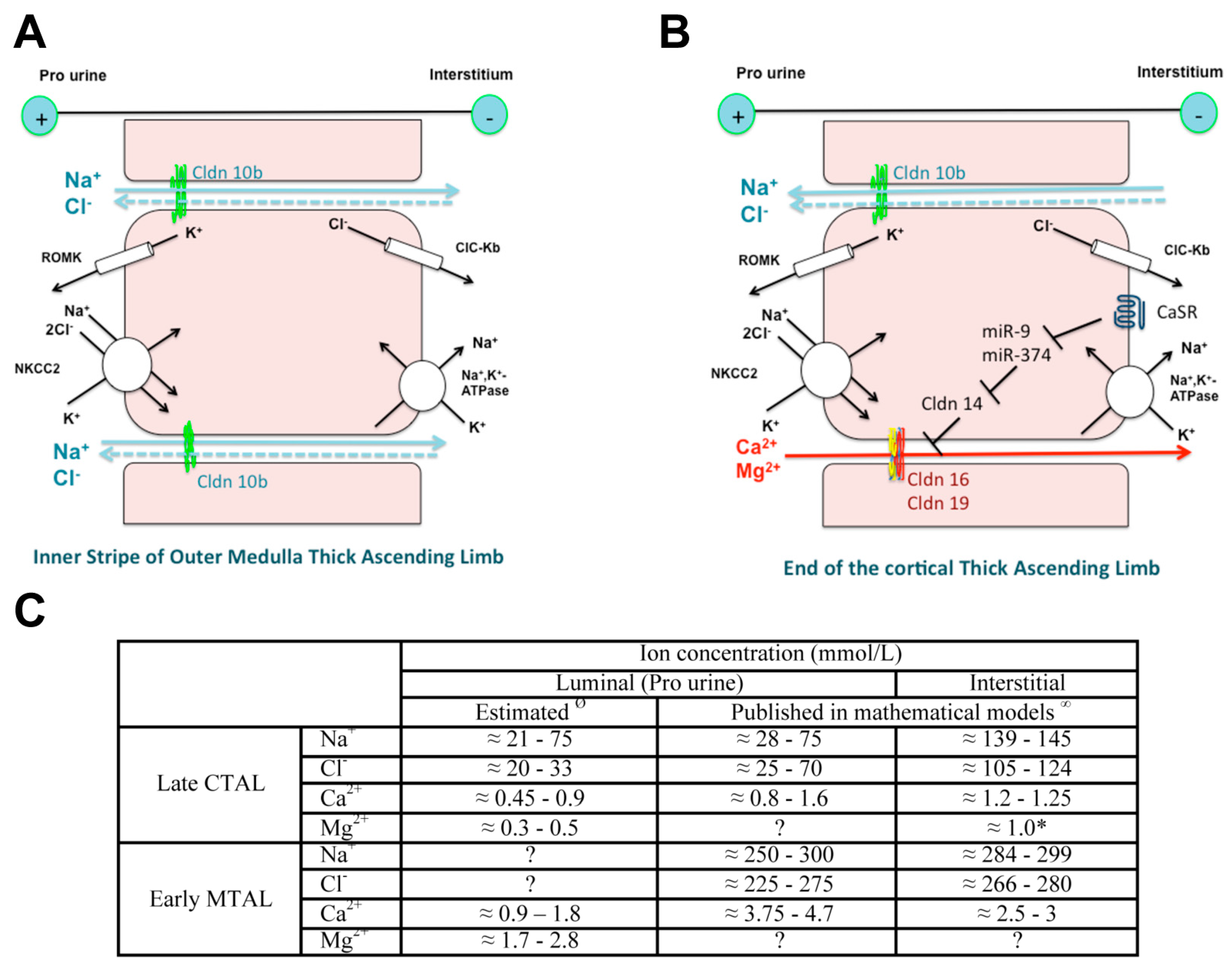
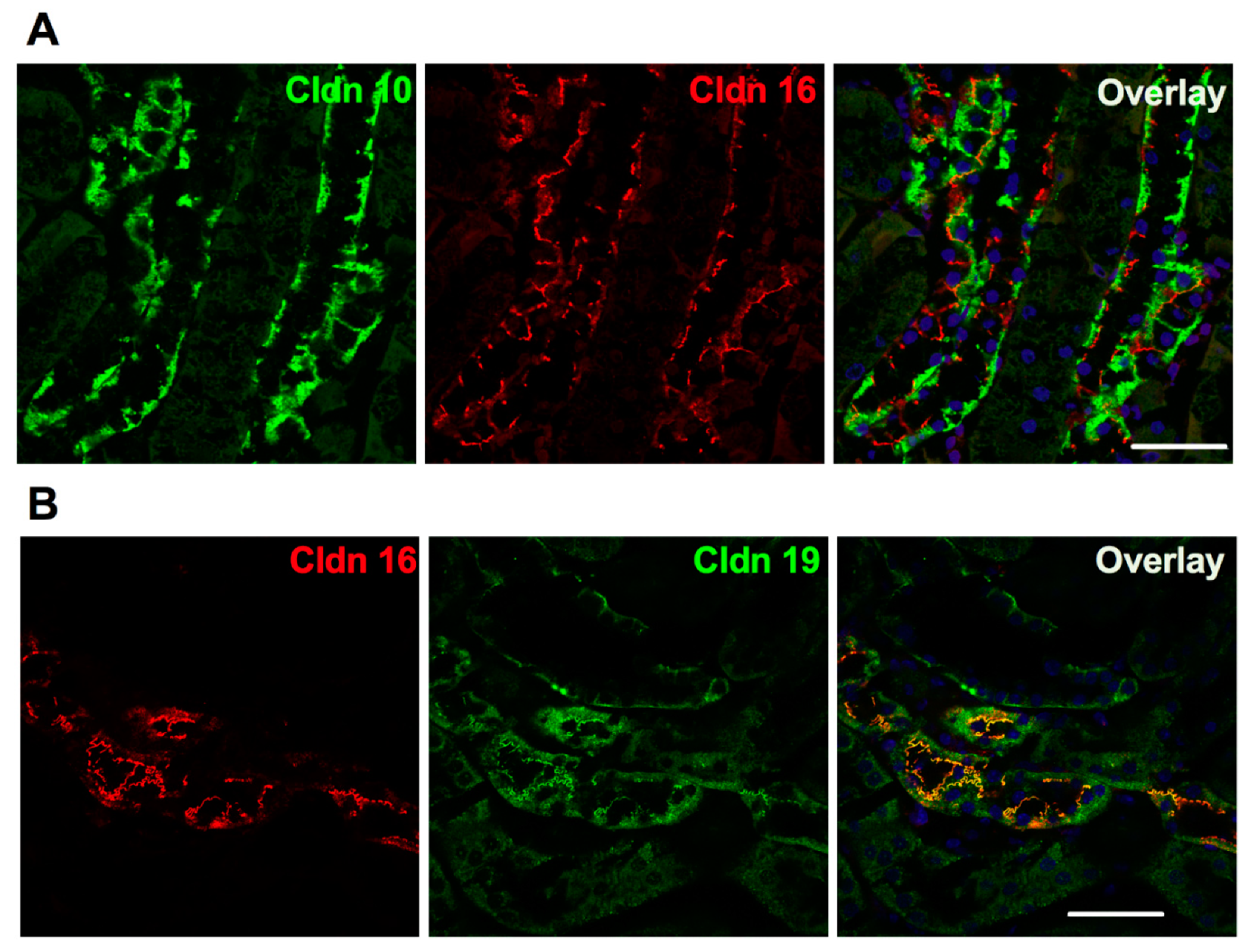
4. Claudin 16, Claudin 19 and Familial Hypomagnesemia with Hypercalciuria and Nephrocalcinosis (FHHNC)
4.1. Phenotype
4.2. Prognosis and Treatment
4.3. Variants/Pathogenesis
5. Claudin 14
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CLDN | human claudin protein |
| CLDN | human claudin gene/mRNA |
| Cldn | rodent claudin protein |
| Cldn | rodent claudin gene/mRNA)) |
References
- Pei, L.; Solis, G.; Nguyen, M.T.; Kamat, N.; Magenheimer, L.; Zhuo, M.; Li, J.; Curry, J.; McDonough, A.A.; Fields, T.A.; et al. Paracellular epithelial sodium transport maximizes energy efficiency in the kidney. J. Clin. Investig. 2016, 126, 2509–2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muto, S. Physiological roles of claudins in kidney tubule paracellular transport. Am. J. Physiol. Ren. Physiol. 2017, 312, F9–F24. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.W. Barrier function of epithelia. Am. J. Physiol. 1981, 241, G275–G288. [Google Scholar] [CrossRef] [PubMed]
- Van Itallie, C.M.; Anderson, J.M. The molecular physiology of tight junction pores. Physiology 2004, 19, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Reuss, L. Tight junction permeability to ions and water. In Tight Junctions, 2nd ed.; Cereijido, M., Anderson, J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 61–88. [Google Scholar]
- Knipp, G.T.; Ho, N.F.; Barsuhn, C.L.; Borchardt, R.T. Paracellular diffusion in Caco-2 cell monolayers: Effect of perturbation on the transport of hydrophilic compounds that vary in charge and size. J. Pharm. Sci. 1997, 86, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.; Rowland, M.; Warhurst, G. Functional modeling of tight junctions in intestinal cell monolayers using polyethylene glycol oligomers. Am. J. Physiol. Cell Physiol. 2001, 281, C388–C397. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, R.; Sugano, K.; Takata, N.; Tachibana, T.; Higashida, A.; Nabuchi, Y.; Aso, Y. Correction of permeability with pore radius of tight junctions in Caco-2 monolayers improves the prediction of the dose fraction of hydrophilic drugs absorbed by humans. Pharm. Res. 2004, 21, 749–755. [Google Scholar] [CrossRef]
- Guo, P.; Weinstein, A.M.; Weinbaum, S. A dual-pathway ultrastructural model for the tight junction of rat proximal tubule epithelium. Am. J. Physiol. Ren. Physiol. 2003, 285, F241–F257. [Google Scholar] [CrossRef] [Green Version]
- Yu, A.S.; Cheng, M.H.; Angelow, S.; Gunzel, D.; Kanzawa, S.A.; Schneeberger, E.E.; Fromm, M.; Coalson, R.D. Molecular basis for cation selectivity in claudin-2-based paracellular pores: Identification of an electrostatic interaction site. J. Gen. Physiol. 2009, 133, 111–127. [Google Scholar] [CrossRef] [Green Version]
- Volkov, A.G.; Paula, S.; Deamer, D.W. Two mechanisms of permeation of small neutral molecules and hydrated ions across phospholipid bilayers. Bioelectrochem. Bioenerg. 1997, 42, 153–160. [Google Scholar] [CrossRef]
- Chiba, H.; Osanai, M.; Murata, M.; Kojima, T.; Sawada, N. Transmembrane proteins of tight junctions. Biochim. Biophys. Acta 2008, 1778, 588–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuse, M. Molecular basis of the core structure of tight junctions. Cold Spring Harb. Perspect. Biol. 2010, 2, a002907. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Furuse, M.; Itoh, M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001, 2, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Fujita, K.; Hiiragi, T.; Fujimoto, K.; Tsukita, S. Claudin-1 and -2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 1998, 141, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Furuse, M.; Fujimoto, K.; Tsukita, S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc. Natl. Acad. Sci. USA 1999, 96, 511–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukita, S.; Tanaka, H.; Tamura, A. The Claudins: From Tight Junctions to Biological Systems. Trends Biochem. Sci. 2019, 44, 141–152. [Google Scholar] [CrossRef]
- Mineta, K.; Yamamoto, Y.; Yamazaki, Y.; Tanaka, H.; Tada, Y.; Saito, K.; Tamura, A.; Igarashi, M.; Endo, T.; Takeuchi, K.; et al. Predicted expansion of the claudin multigene family. FEBS Lett. 2011, 585, 606–612. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Nishizawa, T.; Tani, K.; Yamazaki, Y.; Tamura, A.; Ishitani, R.; Dohmae, N.; Tsukita, S.; Nureki, O.; Fujiyoshi, Y. Crystal structure of a claudin provides insight into the architecture of tight junctions. Science 2014, 344, 304–307. [Google Scholar] [CrossRef]
- Gong, Y.; Yu, M.; Yang, J.; Gonzales, E.; Perez, R.; Hou, M.; Tripathi, P.; Hering-Smith, K.S.; Hamm, L.L.; Hou, J. The Cap1-claudin-4 regulatory pathway is important for renal chloride reabsorption and blood pressure regulation. Proc. Natl. Acad. Sci. USA 2014, 111, E3766–E3774. [Google Scholar] [CrossRef] [Green Version]
- Piontek, J.; Winkler, L.; Wolburg, H.; Muller, S.L.; Zuleger, N.; Piehl, C.; Wiesner, B.; Krause, G.; Blasig, I.E. Formation of tight junction: Determinants of homophilic interaction between classic claudins. FASEB J. 2008, 22, 146–158. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Tani, K.; Tamura, A.; Tsukita, S.; Fujiyoshi, Y. Model for the architecture of claudin-based paracellular ion channels through tight junctions. J. Mol. Biol. 2015, 427, 291–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberini, G.; Benfenati, F.; Maragliano, L. A refined model of claudin-15 tight junction paracellular architecture by molecular dynamics simulations. PLoS ONE 2017, 12, e0184190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, M.; Sasaki, H.; Furuse, M.; Ozaki, H.; Kita, T.; Tsukita, S. Junctional adhesion molecule (JAM) binds to PAR-3: A possible mechanism for the recruitment of PAR-3 to tight junctions. J. Cell Biol. 2001, 154, 491–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, M.; Furuse, M.; Morita, K.; Kubota, K.; Saitou, M.; Tsukita, S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J. Cell Biol. 1999, 147, 1351–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, D.B.; Lu, Y.; Choate, K.A.; Velazquez, H.; Al-Sabban, E.; Praga, M.; Casari, G.; Bettinelli, A.; Colussi, G.; Rodriguez-Soriano, J.; et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 1999, 285, 103–106. [Google Scholar] [CrossRef]
- Kirk, A.; Campbell, S.; Bass, P.; Mason, J.; Collins, J. Differential expression of claudin tight junction proteins in the human cortical nephron. Nephrol. Dial. Transplant. 2010, 25, 2107–2119. [Google Scholar] [CrossRef] [Green Version]
- Hadj-Rabia, S.; Brideau, G.; Al-Sarraj, Y.; Maroun, R.C.; Figueres, M.L.; Leclerc-Mercier, S.; Olinger, E.; Baron, S.; Chaussain, C.; Nochy, D.; et al. Multiplex epithelium dysfunction due to CLDN10 mutation: The HELIX syndrome. Genet. Med. 2018, 20, 190–201. [Google Scholar] [CrossRef] [Green Version]
- Krug, S.M.; Gunzel, D.; Conrad, M.P.; Rosenthal, R.; Fromm, A.; Amasheh, S.; Schulzke, J.D.; Fromm, M. Claudin-17 forms tight junction channels with distinct anion selectivity. Cell Mol. Life Sci. 2012, 69, 2765–2778. [Google Scholar] [CrossRef]
- Gunzel, D.; Yu, A.S. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef] [Green Version]
- Yu, A.S. Claudins and the kidney. J. Am. Soc. Nephrol. 2015, 26, 11–19. [Google Scholar] [CrossRef] [Green Version]
- HGMD Professional. 2019. Available online: http://www.hgmd.org/ (accessed on 12 December 2019).
- Van Itallie, C.M.; Rogan, S.; Yu, A.; Vidal, L.S.; Holmes, J.; Anderson, J.M. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am. J. Physiol. Ren. Physiol. 2006, 291, F1288–F1299. [Google Scholar] [CrossRef] [PubMed]
- Bongers, E.; Shelton, L.M.; Milatz, S.; Verkaart, S.; Bech, A.P.; Schoots, J.; Cornelissen, E.A.M.; Bleich, M.; Hoenderop, J.G.J.; Wetzels, J.F.M.; et al. A Novel Hypokalemic-Alkalotic Salt-Losing Tubulopathy in Patients with CLDN10 Mutations. J. Am. Soc. Nephrol. 2017, 28, 3118–3128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klar, J.; Piontek, J.; Milatz, S.; Tariq, M.; Jameel, M.; Breiderhoff, T.; Schuster, J.; Fatima, A.; Asif, M.; Sher, M.; et al. Altered paracellular cation permeability due to a rare CLDN10B variant causes anhidrosis and kidney damage. PLoS Genet. 2017, 13, e1006897. [Google Scholar] [CrossRef] [PubMed]
- Meyers, N.; Nelson-Williams, C.; Malaga-Dieguez, L.; Kaufmann, H.; Loring, E.; Knight, J.; Lifton, R.P.; Trachtman, H. Hypokalemia Associated With a Claudin 10 Mutation: A Case Report. Am. J. Kidney Dis. 2019, 73, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Stenson, P.D.; Mort, M.; Ball, E.V.; Evans, K.; Hayden, M.; Heywood, S.; Hussain, M.; Phillips, A.D.; Cooper, D.N. The Human Gene Mutation Database: Towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies. Hum. Genet. 2017, 136, 665–677. [Google Scholar] [CrossRef] [Green Version]
- Gunzel, D.; Stuiver, M.; Kausalya, P.J.; Haisch, L.; Krug, S.M.; Rosenthal, R.; Meij, I.C.; Hunziker, W.; Fromm, M.; Muller, D. Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. J. Cell Sci. 2009, 122, 1507–1517. [Google Scholar] [CrossRef] [Green Version]
- Breiderhoff, T.; Himmerkus, N.; Stuiver, M.; Mutig, K.; Will, C.; Meij, I.C.; Bachmann, S.; Bleich, M.; Willnow, T.E.; Muller, D. Deletion of claudin-10 (Cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis. Proc. Natl. Acad. Sci. USA 2012, 109, 14241–14246. [Google Scholar] [CrossRef] [Green Version]
- Milatz, S.; Piontek, J.; Hempel, C.; Meoli, L.; Grohe, C.; Fromm, A.; Lee, I.M.; El-Athman, R.; Gunzel, D. Tight junction strand formation by claudin-10 isoforms and claudin-10a/-10b chimeras. Ann. N Y Acad. Sci. 2017, 1405, 102–115. [Google Scholar] [CrossRef]
- Gunzel, D.; Yu, A.S. Function and regulation of claudins in the thick ascending limb of Henle. Pflug. Arch. 2009, 458, 77–88. [Google Scholar] [CrossRef] [Green Version]
- Angelow, S.; Schneeberger, E.E.; Yu, A.S. Claudin-8 expression in renal epithelial cells augments the paracellular barrier by replacing endogenous claudin-2. J. Membr. Biol. 2007, 215, 147–159. [Google Scholar] [CrossRef]
- Breiderhoff, T.; Himmerkus, N.; Drewell, H.; Plain, A.; Gunzel, D.; Mutig, K.; Willnow, T.E.; Muller, D.; Bleich, M. Deletion of claudin-10 rescues claudin-16-deficient mice from hypomagnesemia and hypercalciuria. Kidney Int. 2018, 93, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, S.; Rossignol, P.; Grelac, F.; Chalumeau, C.; Klein, C.; Laghmani, K.; Chambrey, R.; Bruneval, P.; Duong, J.P.; Poggioli, J.; et al. Differentiated thick ascending limb (TAL) cultured cells derived from SV40 transgenic mice express functional apical NHE2 isoform: Effect of nitric oxide. Pflug. Arch. 2003, 446, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.; Schneider, L.; Peters, M.; Misselwitz, J.; Ronnefarth, G.; Boswald, M.; Bonzel, K.E.; Seeman, T.; Sulakova, T.; Kuwertz-Broking, E.; et al. Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J. Am. Soc. Nephrol. 2001, 12, 1872–1881. [Google Scholar] [PubMed]
- Hou, J.; Paul, D.L.; Goodenough, D.A. Paracellin-1 and the modulation of ion selectivity of tight junctions. J. Cell Sci. 2005, 118, 5109–5118. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Renigunta, A.; Konrad, M.; Gomes, A.S.; Schneeberger, E.E.; Paul, D.L.; Waldegger, S.; Goodenough, D.A. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J. Clin. Investig. 2008, 118, 619–628. [Google Scholar] [CrossRef] [Green Version]
- Gunzel, D.; Amasheh, S.; Pfaffenbach, S.; Richter, J.F.; Kausalya, P.J.; Hunziker, W.; Fromm, M. Claudin-16 affects transcellular Cl-secretion in MDCK cells. J. Physiol. 2009, 587, 3777–3793. [Google Scholar] [CrossRef]
- Konrad, M.; Hou, J.; Weber, S.; Dotsch, J.; Kari, J.A.; Seeman, T.; Kuwertz-Broking, E.; Peco-Antic, A.; Tasic, V.; Dittrich, K.; et al. CLDN16 genotype predicts renal decline in familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J. Am. Soc. Nephrol. 2008, 19, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Godron, A.; Harambat, J.; Boccio, V.; Mensire, A.; May, A.; Rigothier, C.; Couzi, L.; Barrou, B.; Godin, M.; Chauveau, D.; et al. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis: Phenotype-genotype correlation and outcome in 32 patients with CLDN16 or CLDN19 mutations. Clin. J. Am. Soc. Nephrol. 2012, 7, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Claverie-Martin, F.; Garcia-Nieto, V.; Loris, C.; Ariceta, G.; Nadal, I.; Espinosa, L.; Fernandez-Maseda, A.; Anton-Gamero, M.; Avila, A.; Madrid, A.; et al. Claudin-19 mutations and clinical phenotype in Spanish patients with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. PLoS ONE 2013, 8, e53151. [Google Scholar] [CrossRef]
- Sikora, P.; Zaniew, M.; Haisch, L.; Pulcer, B.; Szczepanska, M.; Moczulska, A.; Rogowska-Kalisz, A.; Bienias, B.; Tkaczyk, M.; Ostalska-Nowicka, D.; et al. Retrospective cohort study of familial hypomagnesaemia with hypercalciuria and nephrocalcinosis due to CLDN16 mutations. Nephrol. Dial. Transplant. 2015, 30, 636–644. [Google Scholar] [CrossRef] [Green Version]
- Alparslan, C.; Oncel, E.P.; Akbay, S.; Alaygut, D.; Mutlubas, F.; Tatli, M.; Konrad, M.; Yavascan, O.; Kasap-Demir, B. A novel homozygous W99G mutation in CLDN-16 gene causing familial hypomagnesemic hypercalciuric nephrocalcinosis in Turkish siblings. Turk. J. Pediatrics 2018, 60, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, A.; Jeunemaitre, X.; Coudol, P.; Dechaux, M.; Froissart, M.; May, A.; Demontis, R.; Fournier, A.; Paillard, M.; Houillier, P. Paracellin-1 is critical for magnesium and calcium reabsorption in the human thick ascending limb of Henle. Kidney Int. 2001, 59, 2206–2215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeb, A.; Abood, S.A.; Simon, J.; Dastoor, H.; Pearce, S.H.; Sayer, J.A. A novel CLDN16 mutation in a large family with familial hypomagnesaemia with hypercalciuria and nephrocalcinosis. BMC Res. Notes 2013, 6, 527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Zhao, X.; Paiardini, A.; Lang, Y.; Bottillo, I.; Shao, L. Familial hypomagnesaemia, Hypercalciuria and Nephrocalcinosis associated with a novel mutation of the highly conserved leucine residue 116 of Claudin 16 in a Chinese patient with a delayed diagnosis: A case report. BMC Nephrol. 2018, 19, 181. [Google Scholar] [CrossRef]
- Konrad, M.; Schaller, A.; Seelow, D.; Pandey, A.V.; Waldegger, S.; Lesslauer, A.; Vitzthum, H.; Suzuki, Y.; Luk, J.M.; Becker, C.; et al. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am. J. Hum. Genet. 2006, 79, 949–957. [Google Scholar] [CrossRef] [Green Version]
- Arteaga, M.E.; Hunziker, W.; Teo, A.S.; Hillmer, A.M.; Mutchinick, O.M. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis: Variable phenotypic expression in three affected sisters from Mexican ancestry. Ren. Fail. 2015, 37, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Hampson, G.; Konrad, M.A.; Scoble, J. Familial hypomagnesaemia with hypercalciuria and nephrocalcinosis (FHHNC): Compound heterozygous mutation in the claudin 16 (CLDN16) gene. BMC Nephrol. 2008, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Hanssen, O.; Castermans, E.; Bovy, C.; Weekers, L.; Erpicum, P.; Dubois, B.; Bours, V.; Krzesinski, J.M.; Jouret, F. Two novel mutations of the CLDN16 gene cause familial hypomagnesaemia with hypercalciuria and nephrocalcinosis. Clin. Kidney J. 2014, 7, 282–285. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.H.; Choi, H.J.; Cho, H.Y.; Lee, J.H.; Ha, I.S.; Cheong, H.I.; Choi, Y. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis associated with CLDN16 mutations. Pediatrics Nephrol. 2005, 20, 1490–1493. [Google Scholar] [CrossRef]
- Lv, F.; Xu, X.J.; Wang, J.Y.; Liu, Y.; Jiang, Y.; Wang, O.; Xia, W.B.; Xing, X.P.; Li, M. A novel mutation in CLDN16 results in rare familial hypomagnesaemia with hypercalciuria and nephrocalcinosis in a Chinese family. Clin. Chim. Acta 2016, 457, 69–74. [Google Scholar] [CrossRef]
- Perdomo-Ramirez, A.; Aguirre, M.; Davitaia, T.; Ariceta, G.; Ramos-Trujillo, E.; RenalTube, G.; Claverie-Martin, F. Characterization of two novel mutations in the claudin-16 and claudin-19 genes that cause familial hypomagnesemia with hypercalciuria and nephrocalcinosis. Gene 2019, 689, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Poussou, R.; Cochat, P.; Le Pottier, N.; Roncelin, I.; Liutkus, A.; Blanchard, A.; Jeunemaitre, X. Report of a family with two different hereditary diseases leading to early nephrocalcinosis. Pediatrics Nephrol. 2008, 23, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Vianna, J.G.P.; Simor, T.G.; Senna, P.; De Bortoli, M.R.; Costalonga, E.F.; Seguro, A.C.; Luchi, W.M. Atypical presentation of familial hypomagnesemia with hypercalciuria and nephrocalcinosis in a patient with a new claudin-16 gene mutation. Clin. Nephrol. Case Stud. 2019, 7, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.; Hoffmann, K.; Jeck, N.; Saar, K.; Boeswald, M.; Kuwertz-Broeking, E.; Meij, I.I.; Knoers, N.V.; Cochat, P.; Sulakova, T.; et al. Familial hypomagnesaemia with hypercalciuria and nephrocalcinosis maps to chromosome 3q27 and is associated with mutations in the PCLN-1 gene. Eur. J. Hum. Genet. 2000, 8, 414–422. [Google Scholar] [CrossRef] [Green Version]
- Faguer, S.; Chauveau, D.; Cintas, P.; Tack, I.; Cointault, O.; Rostaing, L.; Vargas-Poussou, R.; Ribes, D. Renal, ocular, and neuromuscular involvements in patients with CLDN19 mutations. Clin. J. Am. Soc. Nephrol. 2011, 6, 355–360. [Google Scholar] [CrossRef] [Green Version]
- Al-Shibli, A.; Konrad, M.; Altay, W.; Al Masri, O.; Al-Gazali, L.; Al Attrach, I. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC): Report of three cases with a novel mutation in CLDN19 gene. Saudi J. Kidney Dis. Transplant. 2013, 24, 338–344. [Google Scholar] [CrossRef]
- Peng, S.; Rao, V.S.; Adelman, R.A.; Rizzolo, L.J. Claudin-19 and the barrier properties of the human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1392–1403. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.B.; Xu, T.; Peng, S.; Singh, D.; Ghiassi-Nejad, M.; Adelman, R.A.; Rizzolo, L.J. Disease-associated mutations of claudin-19 disrupt retinal neurogenesis and visual function. Commun. Biol. 2019, 2, 113. [Google Scholar] [CrossRef]
- Bardet, C.; Courson, F.; Wu, Y.; Khaddam, M.; Salmon, B.; Ribes, S.; Thumfart, J.; Yamaguti, P.M.; Rochefort, G.Y.; Figueres, M.L.; et al. Claudin-16 Deficiency Impairs Tight Junction Function in Ameloblasts, Leading to Abnormal Enamel Formation. J. Bone Miner. Res. 2016, 31, 498–513. [Google Scholar] [CrossRef] [Green Version]
- Yamaguti, P.M.; Neves, F.A.; Hotton, D.; Bardet, C.; de La Dure-Molla, M.; Castro, L.C.; Scher, M.D.; Barbosa, M.E.; Ditsch, C.; Fricain, J.C.; et al. Amelogenesis imperfecta in familial hypomagnesaemia and hypercalciuria with nephrocalcinosis caused by CLDN19 gene mutations. J. Med. Genet. 2017, 54, 26–37. [Google Scholar] [CrossRef]
- Miyamoto, T.; Morita, K.; Takemoto, D.; Takeuchi, K.; Kitano, Y.; Miyakawa, T.; Nakayama, K.; Okamura, Y.; Sasaki, H.; Miyachi, Y.; et al. Tight junctions in Schwann cells of peripheral myelinated axons: A lesson from claudin-19-deficient mice. J. Cell Biol. 2005, 169, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, L.; Khosravi, M.; Dumitriu, S.; Klootwijk, E.; Kleta, R.; Yaqoob, M.M.; Walsh, S.B. A novel claudin-16 mutation, severe bone disease, and nephrocalcinosis. Lancet 2014, 383, 98. [Google Scholar] [CrossRef]
- Naeem, M.; Hussain, S.; Akhtar, N. Mutation in the tight-junction gene claudin 19 (CLDN19) and familial hypomagnesemia, hypercalciuria, nephrocalcinosis (FHHNC) and severe ocular disease. Am. J. Nephrol. 2011, 34, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Pang, Q.; Xing, X.; Wang, X.; Li, Y.; Li, J.; Wu, X.; Li, M.; Wang, O.; Jiang, Y.; et al. First report of a novel missense CLDN19 mutations causing familial hypomagnesemia with hypercalciuria and nephrocalcinosis in a Chinese family. Calcif. Tissue Int. 2015, 96, 265–273. [Google Scholar] [CrossRef]
- Sanjad, S.A.; Hariri, A.; Habbal, Z.M.; Lifton, R.P. A novel PCLN-1 gene mutation in familial hypomagnesemia with hypercalciuria and atypical phenotype. Pediatrics Nephrol. 2007, 22, 503–508. [Google Scholar] [CrossRef]
- Seeley, H.H.; Loomba-Albrecht, L.A.; Nagel, M.; Butani, L.; Bremer, A.A. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis in three siblings having the same genetic lesion but different clinical presentations. World J. Pediatrics 2012, 8, 177–180. [Google Scholar] [CrossRef]
- Zimmermann, B.; Plank, C.; Konrad, M.; Stohr, W.; Gravou-Apostolatou, C.; Rascher, W.; Dotsch, J. Hydrochlorothiazide in CLDN16 mutation. Nephrol. Dial. Transplant. 2006, 21, 2127–2132. [Google Scholar] [CrossRef] [Green Version]
- Alexander, R.T.; Dimke, H. Effect of diuretics on renal tubular transport of calcium and magnesium. Am. J. Physiol. Ren. Physiol. 2017, 312, F998–F1015. [Google Scholar] [CrossRef] [Green Version]
- Muller, D.; Kausalya, P.J.; Bockenhauer, D.; Thumfart, J.; Meij, I.C.; Dillon, M.J.; van’t Hoff, W.; Hunziker, W. Unusual clinical presentation and possible rescue of a novel claudin-16 mutation. J. Clin. Endocrinol. Metab. 2006, 91, 3076–3079. [Google Scholar] [CrossRef] [Green Version]
- Muller, D.; Kausalya, P.J.; Meij, I.C.; Hunziker, W. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis: Blocking endocytosis restores surface expression of a novel Claudin-16 mutant that lacks the entire C-terminal cytosolic tail. Hum. Mol. Genet. 2006, 15, 1049–1058. [Google Scholar] [CrossRef] [Green Version]
- Marunaka, K.; Fujii, N.; Kimura, T.; Furuta, T.; Hasegawa, H.; Matsunaga, T.; Endo, S.; Ikari, A. Rescue of tight junctional localization of a claudin-16 mutant D97S by antimalarial medicine primaquine in Madin-Darby canine kidney cells. Sci. Rep. 2019, 9, 9647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trujillano, D.; Bertoli-Avella, A.M.; Kumar Kandaswamy, K.; Weiss, M.E.; Koster, J.; Marais, A.; Paknia, O.; Schroder, R.; Garcia-Aznar, J.M.; Werber, M.; et al. Clinical exome sequencing: Results from 2819 samples reflecting 1000 families. Eur. J. Hum. Genet. 2017, 25, 176–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guran, T.; Akcay, T.; Bereket, A.; Atay, Z.; Turan, S.; Haisch, L.; Konrad, M.; Schlingmann, K.P. Clinical and molecular characterization of Turkish patients with familial hypomagnesaemia: Novel mutations in TRPM6 and CLDN16 genes. Nephrol. Dial. Transplant. 2012, 27, 667–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdomo-Ramirez, A.; de Armas-Ortiz, M.; Ramos-Trujillo, E.; Suarez-Artiles, L.; Claverie-Martin, F. Exonic CLDN16 mutations associated with familial hypomagnesemia with hypercalciuria and nephrocalcinosis can induce deleterious mRNA alterations. BMC Med. Genet. 2019, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.A.; Schueler, M.; Halbritter, J.; Gee, H.Y.; Porath, J.D.; Lawson, J.A.; Airik, R.; Shril, S.; Allen, S.J.; Stein, D.; et al. Whole exome sequencing identifies causative mutations in the majority of consanguineous or familial cases with childhood-onset increased renal echogenicity. Kidney Int. 2016, 89, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Staiger, K.; Staiger, H.; Haas, C.; Thamer, C.; Risler, T.; Machicao, F.; Haring, H.U. Hypomagnesemia and nephrocalcinosis in a patient with two heterozygous mutations in the CLDN16 gene. J. Nephrol. 2007, 20, 107–110. [Google Scholar] [PubMed]
- Tajima, T.; Nakae, J.; Fujieda, K. Two heterozygous mutations of CLDN16 in a Japanese patient with FHHNC. Pediatrics Nephrol. 2003, 18, 1280–1282. [Google Scholar] [CrossRef]
- Yavarna, T.; Al-Dewik, N.; Al-Mureikhi, M.; Ali, R.; Al-Mesaifri, F.; Mahmoud, L.; Shahbeck, N.; Lakhani, S.; AlMulla, M.; Nawaz, Z.; et al. High diagnostic yield of clinical exome sequencing in Middle Eastern patients with Mendelian disorders. Hum. Genet. 2015, 134, 967–980. [Google Scholar] [CrossRef]
- Kasapkara, C.S.; Tumer, L.; Okur, I.; Hasanoglu, A. A novel mutation of the claudin 16 gene in familial hypomagnesemia with hypercalciuria and nephrocalcinosis mimicking rickets. Genet. Couns. 2011, 22, 187–192. [Google Scholar]
- Margabandhu, S.; Doshi, M. Familial Hypomagnesemia, Hypercalciuria and Nephrocalcinosis with Novel Mutation. Indian J. Nephrol. 2019, 29, 57–61. [Google Scholar] [CrossRef]
- Kutluturk, F.; Temel, B.; Uslu, B.; Aral, F.; Azezli, A.; Orhan, Y.; Konrad, M.; Ozbey, N. An unusual patient with hypercalciuria, recurrent nephrolithiasis, hypomagnesemia and G227R mutation of Paracellin-1. An unusual patient with hypercalciuria and hypomagnesemia unresponsive to thiazide diuretics. Horm. Res. 2006, 66, 175–181. [Google Scholar] [PubMed]
- Daga, A.; Majmundar, A.J.; Braun, D.A.; Gee, H.Y.; Lawson, J.A.; Shril, S.; Jobst-Schwan, T.; Vivante, A.; Schapiro, D.; Tan, W.; et al. Whole exome sequencing frequently detects a monogenic cause in early onset nephrolithiasis and nephrocalcinosis. Kidney Int. 2018, 93, 204–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turkmen, M.; Kasap, B.; Soylu, A.; Bober, E.; Konrad, M.; Kavukcu, S. Paracellin-1 gene mutation with multiple congenital abnormalities. Pediatrics Nephrol. 2006, 21, 1776–1778. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.; Kausalya, P.J.; Claverie-Martin, F.; Meij, I.C.; Eggert, P.; Garcia-Nieto, V.; Hunziker, W. A novel claudin 16 mutation associated with childhood hypercalciuria abolishes binding to ZO-1 and results in lysosomal mistargeting. Am. J. Hum. Genet. 2003, 73, 1293–1301. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Mao, J.; Ye, Q.; Zhu, X.; Zhang, Y.; Ye, Y.; Fu, H.; Shen, H.; Lu, Z.; Xia, Y.; et al. Clinical features and genetic findings in Chinese children with distal renal tubular acidosis. Int. J. Clin. Exp. Pathol. 2018, 11, 3523–3532. [Google Scholar]
- Yamaguti, P.M.; dos Santos, P.A.; Leal, B.S.; Santana, V.B.; Mazzeu, J.F.; Acevedo, A.C.; Neves Fde, A. Identification of the first large deletion in the CLDN16 gene in a patient with FHHNC and late-onset of chronic kidney disease: Case report. BMC Nephrol. 2015, 16, 92. [Google Scholar] [CrossRef] [Green Version]
- Uniprot. Available online: https://www.uniprot.org (accessed on 20 December 2019).
- Claverie-Martin, F.; Vargas-Poussou, R.; Muller, D.; Garcia-Nieto, V. Clinical utility gene card for: Familial hypomagnesemia with hypercalciuria and nephrocalcinosis with/without severe ocular involvement. Eur. J. Hum. Genet. 2015, 23. [Google Scholar] [CrossRef] [Green Version]
- Almeida, J.R.; Machado Gde, A.; dos Santos, M.M.; Lopes Pde, F.; de Matos, J.P.; Neves, A.C.; Lugon, J.R. Five years results after intrafamilial kidney post-transplant in a case of familial hypomagnesemia due to a claudin-19 mutation. J. Bras. Nefrol. 2014, 36, 401–405. [Google Scholar] [CrossRef]
- Haisch, L.; Almeida, J.R.; Abreu da Silva, P.R.; Schlingmann, K.P.; Konrad, M. The role of tight junctions in paracellular ion transport in the renal tubule: Lessons learned from a rare inherited tubular disorder. Am. J. Kidney Dis. 2011, 57, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Martin-Nunez, E.; Cordoba-Lanus, E.; Gonzalez-Acosta, H.; Oliet, A.; Izquierdo, E.; Claverie-Martin, F. Haplotype analysis of CLDN19 single nucleotide polymorphisms in Spanish patients with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. World J. Pediatrics 2015, 11, 272–275. [Google Scholar] [CrossRef]
- Khan, A.O.; Patel, N.; Ghazi, N.G.; Alzahrani, S.S.; Arold, S.T.; Alkuraya, F.S. Familial non-syndromic macular pseudocoloboma secondary to homozygous CLDN19 mutation. Ophthalmic Genet. 2018, 39, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, Z.; Karabas, L.; Konrad, M. Hypomagnesemia-hypercalciuria-nephrocalcinosis and ocular findings: A new claudin-19 mutation. Turk. J. Pediatrics 2012, 54, 168–170. [Google Scholar]
- Sharma, S.; Place, E.; Lord, K.; Leroy, B.P.; Falk, M.J.; Pradhan, M. Claudin 19-based familial hypomagnesemia with hypercalciuria and nephrocalcinosis in a sibling pair. Clin. Nephrol. 2016, 85, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Dimke, H.; Schnermann, J. Axial and cellular heterogeneity in electrolyte transport pathways along the thick ascending limb. Acta Physiol. 2018, 223, e13057. [Google Scholar] [CrossRef] [PubMed]
- Greger, R. Chloride reabsorption in the rabbit cortical thick ascending limb of the loop of Henle. A sodium dependent process. Pflug. Arch. 1981, 390, 38–43. [Google Scholar] [CrossRef]
- Greger, R.; Schlatter, E. Properties of the basolateral membrane of the cortical thick ascending limb of Henle’s loop of rabbit kidney. A model for secondary active chloride transport. Pflug. Arch. 1983, 396, 325–334. [Google Scholar] [CrossRef]
- Greger, R.; Schlatter, E. Properties of the lumen membrane of the cortical thick ascending limb of Henle’s loop of rabbit kidney. Pflug. Arch. 1983, 396, 315–324. [Google Scholar] [CrossRef]
- Carney, S.L.; Wong, N.L.; Quamme, G.A.; Dirks, J.H. Effect of magnesium deficiency on renal magnesium and calcium transport in the rat. J. Clin. Investig. 1980, 65, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Le Grimellec, C.; Roinel, N.; Morel, F. Simultaneous Mg, Ca, P,K,Na and Cl analysis in rat tubular fluid. I. During perfusion of either inulin or ferrocyanide. Pflug. Arch. 1973, 340, 181–196. [Google Scholar] [CrossRef]
- Costanzo, L.S.; Windhager, E.E. Calcium and sodium transport by the distal convoluted tubule of the rat. Am. J. Physiol. 1978, 235, F492–F506. [Google Scholar] [CrossRef]
- Edwards, B.R.; Baer, P.G.; Sutton, R.A.; Dirks, J.H. Micropuncture study of diuretic effects on sodium and calcium reabsorption in the dog nephron. J. Clin. Investig. 1973, 52, 2418–2427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stumpe, K.O.; Lowitz, H.D.; Ochwadt, B. Fluid reabsorption in Henle’sloop and urinary excretion of sodium and water in normal rats and rats with chronic hypertension. J. Clin. Investig. 1970, 49, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Richter, K.; Blantz, R.C.; Thomson, S.; Osswald, H. Glomerular hyperfiltration in experimental diabetes mellitus: Potential role of tubular reabsorption. J. Am. Soc. Nephrol. 1999, 10, 2569–2576. [Google Scholar] [PubMed]
- Vallon, V.; Osswald, H.; Blantz, R.C.; Thomson, S. Potential role of luminal potassium in tubuloglomerular feedback. J. Am. Soc. Nephrol. 1997, 8, 1831–1837. [Google Scholar]
- Schnermann, J.; Briggs, J.; Schubert, G. In situ studies of the distal convoluted tubule in the rat. I. Evidence for NaCl secretion. Am. J. Physiol. 1982, 243, F160–F166. [Google Scholar] [CrossRef]
- Luke, R.G.; Wright, F.S.; Fowler, N.; Kashgarian, M.; Giebisch, G.H. Effects of potassium depletion on renal tubular chloride transport in the rat. Kidney Int. 1978, 14, 414–427. [Google Scholar] [CrossRef] [Green Version]
- Edwards, A.; Castrop, H.; Laghmani, K.; Vallon, V.; Layton, A.T. Effects of NKCC2 isoform regulation on NaCl transport in thick ascending limb and macula densa: A modeling study. Am. J. Physiol. Ren. Physiol. 2014, 307, F137–F146. [Google Scholar] [CrossRef] [Green Version]
- Edwards, A. Regulation of calcium reabsorption along the rat nephron: A modeling study. Am. J. Physiol. Ren. Physiol. 2015, 308, F553–F566. [Google Scholar] [CrossRef]
- Layton, A.T.; Vallon, V.; Edwards, A. A computational model for simulating solute transport and oxygen consumption along the nephrons. Am. J. Physiol. Ren. Physiol. 2016, 311, F1378–F1390. [Google Scholar] [CrossRef]
- Tournus, M.; Seguin, N.; Perthame, B.; Thomas, S.R.; Edwards, A. A model of calcium transport along the rat nephron. Am. J. Physiol. Ren. Physiol. 2013, 305, F979–F994. [Google Scholar] [CrossRef] [Green Version]
- Nieves-Gonzalez, A.; Clausen, C.; Layton, A.T.; Layton, H.E.; Moore, L.C. Transport efficiency and workload distribution in a mathematical model of the thick ascending limb. Am. J. Physiol. Ren. Physiol. 2013, 304, F653–F664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinstein, A.M. A mathematical model of rat ascending Henle limb. III. Tubular function. Am. J. Physiol Ren. Physiol 2010, 298, F543–F556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinstein, A.M. A mathematical model of rat proximal tubule and loop of Henle. Am. J. Physiol. Ren. Physiol. 2015, 308, F1076–F1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milatz, S.; Himmerkus, N.; Wulfmeyer, V.C.; Drewell, H.; Mutig, K.; Hou, J.; Breiderhoff, T.; Muller, D.; Fromm, M.; Bleich, M.; et al. Mosaic expression of claudins in thick ascending limbs of Henle results in spatial separation of paracellular Na+ and Mg2+ transport. Proc. Natl. Acad. Sci. USA 2017, 114, E219–E227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plain, A.; Wulfmeyer, V.C.; Milatz, S.; Klietz, A.; Hou, J.; Bleich, M.; Himmerkus, N. Corticomedullary difference in the effects of dietary Ca2+ on tight junction properties in thick ascending limbs of Henle’s loop. Pflug. Arch. 2016, 468, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Ikari, A.; Hirai, N.; Shiroma, M.; Harada, H.; Sakai, H.; Hayashi, H.; Suzuki, Y.; Degawa, M.; Takagi, K. Association of paracellin-1 with ZO-1 augments the reabsorption of divalent cations in renal epithelial cells. J. Biol. Chem. 2004, 279, 54826–54832. [Google Scholar] [CrossRef] [Green Version]
- Ikari, A.; Matsumoto, S.; Harada, H.; Takagi, K.; Hayashi, H.; Suzuki, Y.; Degawa, M.; Miwa, M. Phosphorylation of paracellin-1 at Ser217 by protein kinase A is essential for localization in tight junctions. J. Cell Sci. 2006, 119, 1781–1789. [Google Scholar] [CrossRef] [Green Version]
- Kausalya, P.J.; Amasheh, S.; Gunzel, D.; Wurps, H.; Muller, D.; Fromm, M.; Hunziker, W. Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of Claudin-16. J. Clin. Investig. 2006, 116, 878–891. [Google Scholar] [CrossRef]
- Ikari, A.; Kinjo, K.; Atomi, K.; Sasaki, Y.; Yamazaki, Y.; Sugatani, J. Extracellular Mg(2+) regulates the tight junctional localization of claudin-16 mediated by ERK-dependent phosphorylation. Biochim. Biophys. Acta 2010, 1798, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Tang, V.W.; Goodenough, D.A. Paracellular ion channel at the tight junction. Biophys. J. 2003, 84, 1660–1673. [Google Scholar] [CrossRef] [Green Version]
- Angelow, S.; El-Husseini, R.; Kanzawa, S.A.; Yu, A.S. Renal localization and function of the tight junction protein, claudin-19. Am. J. Physiol. Ren. Physiol. 2007, 293, F166–F177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Y.; Renigunta, V.; Zhou, Y.; Sunq, A.; Wang, J.; Yang, J.; Renigunta, A.; Baker, L.A.; Hou, J. Biochemical and biophysical analyses of tight junction permeability made of claudin-16 and claudin-19 dimerization. Mol. Biol. Cell 2015, 26, 4333–4346. [Google Scholar] [CrossRef] [PubMed]
- Will, C.; Breiderhoff, T.; Thumfart, J.; Stuiver, M.; Kopplin, K.; Sommer, K.; Gunzel, D.; Querfeld, U.; Meij, I.C.; Shan, Q.; et al. Targeted deletion of murine Cldn16 identifies extra- and intrarenal compensatory mechanisms of Ca2+ and Mg2+ wasting. Am. J. Physiol. Ren. Physiol. 2010, 298, F1152–F1161. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Renigunta, A.; Gomes, A.S.; Hou, M.; Paul, D.L.; Waldegger, S.; Goodenough, D.A. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc. Natl. Acad. Sci. USA 2009, 106, 15350–15355. [Google Scholar] [CrossRef] [Green Version]
- Himmerkus, N.; Shan, Q.; Goerke, B.; Hou, J.; Goodenough, D.A.; Bleich, M. Salt and acid-base metabolism in claudin-16 knockdown mice: Impact for the pathophysiology of FHHNC patients. Am. J. Physiol. Ren. Physiol. 2008, 295, F1641–F1647. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Shan, Q.; Wang, T.; Gomes, A.S.; Yan, Q.; Paul, D.L.; Bleich, M.; Goodenough, D.A. Transgenic RNAi depletion of claudin-16 and the renal handling of magnesium. J. Biol. Chem. 2007, 282, 17114–17122. [Google Scholar] [CrossRef] [Green Version]
- Wilcox, E.R.; Burton, Q.L.; Naz, S.; Riazuddin, S.; Smith, T.N.; Ploplis, B.; Belyantseva, I.; Ben-Yosef, T.; Liburd, N.A.; Morell, R.J.; et al. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell 2001, 104, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Bashir, Z.E.; Latief, N.; Belyantseva, I.A.; Iqbal, F.; Riazuddin, S.A.; Khan, S.N.; Friedman, T.B.; Riazuddin, S.; Riazuddin, S. Phenotypic variability of CLDN14 mutations causing DFNB29 hearing loss in the Pakistani population. J. Hum. Genet. 2013, 58, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Ben-Yosef, T.; Belyantseva, I.A.; Saunders, T.L.; Hughes, E.D.; Kawamoto, K.; Van Itallie, C.M.; Beyer, L.A.; Halsey, K.; Gardner, D.J.; Wilcox, E.R.; et al. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum. Mol. Genet. 2003, 12, 2049–2061. [Google Scholar] [CrossRef] [Green Version]
- Duran, D.; Zeng, X.; Jin, S.C.; Choi, J.; Nelson-Williams, C.; Yatsula, B.; Gaillard, J.; Furey, C.G.; Lu, Q.; Timberlake, A.T.; et al. Mutations in Chromatin Modifier and Ephrin Signaling Genes in Vein of Galen Malformation. Neuron 2019, 101, 429–443 e424. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Renigunta, V.; Himmerkus, N.; Zhang, J.; Renigunta, A.; Bleich, M.; Hou, J. Claudin-14 regulates renal Ca(+)(+) transport in response to CaSR signalling via a novel microRNA pathway. EMBO J. 2012, 31, 1999–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimke, H.; Desai, P.; Borovac, J.; Lau, A.; Pan, W.; Alexander, R.T. Activation of the Ca2+-sensing receptor increases renal claudin-14 expression and urinary Ca2+ excretion. Am. J. Physiol. Ren. Physiol. 2013, 304, F761–F769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corre, T.; Olinger, E.; Harris, S.E.; Traglia, M.; Ulivi, S.; Lenarduzzi, S.; Belge, H.; Youhanna, S.; Tokonami, N.; Bonny, O.; et al. Common variants in CLDN14 are associated with differential excretion of magnesium over calcium in urine. Pflugers Arch. 2017, 469, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Hou, J. Claudin-14 underlies Ca(+)(+)-sensing receptor-mediated Ca(+)(+) metabolism via NFAT-microRNA-based mechanisms. J. Am. Soc. Nephrol. 2014, 25, 745–760. [Google Scholar] [CrossRef] [Green Version]
- Thorleifsson, G.; Holm, H.; Edvardsson, V.; Walters, G.B.; Styrkarsdottir, U.; Gudbjartsson, D.F.; Sulem, P.; Halldorsson, B.V.; de Vegt, F.; d’Ancona, F.C.; et al. Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat. Genet. 2009, 41, 926–930. [Google Scholar] [CrossRef]
- Guha, M.; Bankura, B.; Ghosh, S.; Pattanayak, A.K.; Ghosh, S.; Pal, D.K.; Puri, A.; Kundu, A.K.; Das, M. Polymorphisms in CaSR and CLDN14 Genes Associated with Increased Risk of Kidney Stone Disease in Patients from the Eastern Part of India. PLoS ONE 2015, 10, e0130790. [Google Scholar] [CrossRef] [Green Version]
- Oddsson, A.; Sulem, P.; Helgason, H.; Edvardsson, V.O.; Thorleifsson, G.; Sveinbjornsson, G.; Haraldsdottir, E.; Eyjolfsson, G.I.; Sigurdardottir, O.; Olafsson, I.; et al. Common and rare variants associated with kidney stones and biochemical traits. Nat. Commun. 2015, 6, 7975. [Google Scholar] [CrossRef] [Green Version]
- Ure, M.E.; Heydari, E.; Pan, W.; Ramesh, A.; Rehman, S.; Morgan, C.; Pinsk, M.; Erickson, R.; Herrmann, J.M.; Dimke, H.; et al. A variant in a cis-regulatory element enhances claudin-14 expression and is associated with pediatric-onset hypercalciuria and kidney stones. Hum. Mutat. 2017, 38, 649–657. [Google Scholar] [CrossRef]
- Toka, H.R.; Genovese, G.; Mount, D.B.; Pollak, M.R.; Curhan, G.C. Frequency of rare allelic variation in candidate genes among individuals with low and high urinary calcium excretion. PLoS ONE 2013, 8, e71885. [Google Scholar] [CrossRef] [Green Version]
- Arcidiacono, T.; Simonini, M.; Lanzani, C.; Citterio, L.; Salvi, E.; Barlassina, C.; Spotti, D.; Cusi, D.; Manunta, P.; Vezzoli, G. Claudin-14 Gene Polymorphisms and Urine Calcium Excretion. Clin. J. Am. Soc. Nephrol. 2018, 13, 1542–1549. [Google Scholar] [CrossRef] [Green Version]
- Loupy, A.; Ramakrishnan, S.K.; Wootla, B.; Chambrey, R.; de la Faille, R.; Bourgeois, S.; Bruneval, P.; Mandet, C.; Christensen, E.I.; Faure, H.; et al. PTH-independent regulation of blood calcium concentration by the calcium-sensing receptor. J. Clin. Investig. 2012, 122, 3355–3367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Reference | Bongers [34] | Klar [35] | Hadj-Rabia [28] | Meyers [36] | Overall (%) |
|---|---|---|---|---|---|
| Area of Origin | Europe | Pakistan | North Africa, Pakistan | South America | |
| Consanguinity | No | Yes | Yes | Yes | |
| Hypohidrosis | N.D. | 13/13 | 6/6 | 1/1 | 20/20 (100%) |
| Electrolyte imbalance | 2/2 | 6/7 | 6/6 | 1/1 | 15/16 (94%) |
| Hypolacrimia | N.D. | 13/13 | 6/6 | 1/1 | 20/20 (100%) |
| Ichthyosis | N.D. | N.D. | 6/6 | 0/1 | 6/7 (86%) |
| Xerostomia | N.D. | 13/13 | 6/6 | 1/1 | 20/20 (100%) |
| Plasma abnormalities | |||||
| Hypokalemia | 2/2 | 0/7 | 3/6 | 1/1 | 6/16 (38%) |
| Hypermagnesemia | 1/2 | 6/7 | 6/6 | 1/1 | 14/16 (88%) |
| eGFR < 60 mL/min/1.73 m2 | 1/2 | 0/3 | 1/6 | 1/1 | 3/12 (25%) |
| Secondary hyperaldosteronism | N.D. | N.D. | 6/6 | Hyperaldosteronism without hyperreninism | |
| Nephrolithiasis | 0/2 | 4/13 | 0/6 | 0/1 | 4/22 (18%) |
| Missense/Nonsense Mutations | ||||||
|---|---|---|---|---|---|---|
| Ref. | Nucleotide Change | Amino Acid Change | Protein Change | Variant Class | Exon | Domain |
| [28] | c.2T>C | Met1Thr | p.M1? | DM | 1b | Helical |
| [35] | c.144C>G | Asn48Lys | p.N48K | DM | 1b | ECS1 |
| [34] | c.217G>A | Asp73Asn | p.D73N | DM? | 1b | ECS1 |
| [36] | c.238A>G | Arg80Gly | p.R80G | DM | 2 | ECS1 |
| [28] | c.386C>T | Ser131Leu | p.S131L | DM | 3 | Helical |
| [34] | c.446C>G | Pro149Arg | p.P149R | DM? | 3 | ECS2 |
| Splicing Mutations | ||||||
| [34] | c.465–1G>A | p.E157_T192del | DM? | 4 | Helical | |
| Claudin | Cell Line | Transfection | TER | PNa/PCl | PNa | PCl | PMg/PCl | PCa/PCl | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Mouse Cldn10b | MDCK II | Stable | NS | NS | NS | NS | [33] | ||
| Mouse Cldn10b | LLC-PK1 | Stable | ↘ | NS | ↗ | NS | [33] | ||
| Mouse Cldn10b | MDCK-C7 | Stable | ↘ | ↗ | ↗ | ↗ | [38] | ||
| Human CLDN10b | MDCK-C7 | Stable | ↘ | ↗ | ↗ | ↗ | [38] | ||
| Human CLDN10b | MDCK-C7 | Stable | ↘ | [40] | |||||
| Mouse Cldn10a | MCDK II | Stable | NS | ↘ | ↘ | ↗ | [33] | ||
| Mouse Cldn10a | LLC-PK1 | Stable | ↘ | NS | NS | ↗ | [33] | ||
| Mouse Cldn10a | MDCK II | Stable | NS | ↘ | [38] | ||||
| Mouse Cldn10a | MDCK-C7 | Stable | NS | NS | NS | NS | [38] | ||
| Human CLDN10a | MDCK-C7 | Stable | ↘ | NS | NS | NS | [38] | ||
| Human CLDN10a | MDCK-C7 | Stable | NS | [40] |
| Missense/Nonsense Mutations | ||||||
|---|---|---|---|---|---|---|
| Ref. | Nucleotide Change | Amino Acid Change | Protein Change | Variant Class | Exon | Domain |
| [84] | c.114C>A | Cys38Term | p.C38 * | DM a | 1 | N term |
| [78] | c.211A>G | Met71Val | p.M71V | DM | 1 | N term |
| [26] | c.212T>G | Met71Arg | p.M71R | DM | 1 | N term |
| [49] | c.212T>C | Met71Thr | p.M71T | DM | 1 | N term |
| [50] | c.239G>A | Cys80Tyr | p.C80Y | DM | 1 | TM1 |
| [49] | c.263G>A | Gly88Glu | p.G88E | DM | 1 | TM1 |
| [85] | c.290A>G | Asp97Gly | p.D97G | DM | 1 | ECS1 |
| [53] | c.295T>G | Trp99Gly | p.W99G | DM | 1 | ECS1 |
| [49,52] | c.330C>G | Ser110Arg b | p.S110R | DM | 2 | ECS1 |
| [49,86] | c.341G>A | Arg114Gln | p.R114Q | DM | 2 | ECS1 |
| [60] | c.340C>T | Arg114Term | p.R114 * | DM | 2 | ECS1 |
| [56] | c.346C>G | Leu116Val | p.L116V | DM | 2 | ECS1 |
| [45,66] | c.350G>A | Trp117Term | p.W117 * | DM | 2 | ECS1 |
| [87] | c.354G>A | Trp118Term | p.W118 * | DM | 2 | ECS1 |
| [52,88] | c.358T>C | Cys120Arg | p.C120R | DM | 2 | ECS1 |
| [49] | c.385C>T | Arg129Cys | p.R129C | DM | 2 | ECS1 |
| [50,54,64,71,86] | c.416C>T | Ala139Val | p.A139V | DM | 2 | ECS1 |
| [45,49,66,86] | c.421C>G | His141Asp | p.H141D | DM | 2 | ECS1 |
| [45,46,47,52,66,86] | c.434T>C | Leu145Pro | p.L145P | DM | 3 | ECS1 |
| [86,89] | c.446G>A | Arg149Gln | p.R149Q | DM | 3 | ECS1 |
| [45,46,86] | c.446G>T | Arg149Leu | p.R149L | DM | 3 | ECS1 |
| [26,50] | c.445C>T | Arg149Term | p.R149 * | DM | 3 | ECS1 |
| [45,46,47,49,52,66,86,88] | c.453G>T | Leu151Phe | p.L151F | DM | 3 | ECS1or TM2? |
| [45,49,66,86] | c.452T>G | Leu151Trp | p.L151W | DM | 3 | ECS1or TM2? |
| [50,54,71,86] | c.485G>T | Gly162Val | p.G162V | DM | 3 | TM2 |
| [26,46] | c.500T>C | Leu167Pro | p.L167P | DM | 3 | TM2 |
| [90] | c.539C>T | Pro180Leu | p.P180L | DM | 3 | ICL |
| [50,87,91] | c.547A>G | Lys183Glu | p.K183E | DM | 3 | ICL |
| [26,46,47,86] | c.571G>A | Gly191Arg | p.G191R | DM | 3 | TM3 |
| [65] | c.592G>C | Gly198Arg | p.G198R | DM | 3 | TM3 |
| [45,86] | c.593G>C | Gly198Ala | p.G198A | DM | 4 | TM3 |
| [26,46,66,86] | c.593G>A | Gly198Asp | p.G198D | DM | 4 | TM3 |
| [63] | c.602G>A | Gly201Glu | p.G201E | DM | 4 | TM3 |
| [92] | c.620G>A | Trp207Term | p.W207 * | DM | 4 | ECS2 |
| [45,46,47,49] | c.625G>A | Ala209Thr | p.A209T | DM | 4 | ECS2 |
| [49,59,89] | c.646C>T | Arg216Cys | p.R216C | DM | 4 | ECS2 |
| [55] | c.647G>A | Arg216His | p.R216H | DM | 4 | ECS2 |
| [49,93] | c.679G>C | Gly227Arg | p.G227R | DM | 4 | ECS2 |
| [26,46,47,94] | c.695T>G | Phe232Cys | p.F232C | DM | 4 | ECS2 |
| [50] | c.697G>C | Gly233Arg | p.G233R | DM | 4 | ECS2 |
| [26,46] | c.698G>A | Gly233Asp | p.G233D | DM | 4 | ECS2 |
| [61] | c.697G>T | Gly233Cys | p.G233C | DM | 4 | ECS2 |
| [52] | c.702G>T | Trp234Cys | p.W234C | DM | 4 | ECS2 |
| [26] | c.704C>T | Ser235Phe | p.S235F | DM | 4 | ECS2 |
| [45,46] | c.703T>C | Ser235Pro | p.S235P | DM | 4 | ECS2 |
| [77] | c.704C>A | Ser235Tyr | p.S235Y | DM | 4 | ECS2 |
| [49,95] | c.710G>A | Trp237Term | p.W237 * | DM | 4 | ECS2 |
| [26,45,46,52,66,71,87] | c.715G>A | Gly239Arg | p.G239R | DM | 4 | ECL2 or TM4? |
| [52] | c.734G>A | Gly245Asp | p.G245D | DM | 4 | TM4 |
| [82] | c.823A>T c | Lys275Term | p.K275 * | DM | 5 | C term |
| [81] | c.831T>G d | Tyr277Term | p.Y277 * | DM | 5 | C term |
| [52] | c.864C>G | Tyr288Term | p.Y288 * | DM | 5 | C term |
| [96] | c.908C>G e | Thr303Arg | p.T303R | DM | 5 | C term |
| Splicing Mutations | ||||||
| Ref. | Nucleotide Change | Splicing Mutation | Variant Class | |||
| [45] | c.325-5T>G | IVS1 as T-G -5 | DM | |||
| [60] | c.427+5G>A | IVS2 ds G-A +5 | DM | |||
| [26] | c.593-2A>G | IVS3 as A-G -2 | DM | |||
| [49,59] | c.784+1G>T | IVS4 ds G-T +1 | DM | |||
| [45] | c.785-14T>G | IVS4 as T-G -14 | DM | |||
| Small Deletions | ||||||
| Nucleotide Change | Protein Change | Variant Class | Exon | Domain | ||
| [97] | c.166delG f | p.(Ala56Leufs*16) | DM? | 1 | N term | |
| [49] | c.235delG g | p.(Ala79fsX90) | 1 | TM1 | ||
| [45] | c.368delA | p.(Asn123Metfs*21) | DM | 2 | ECS1 | |
| [49] | c.408_410delCAT | p.(Ile137del) | DM | 2 | ECS1 | |
| [61] | c.800delG | p.(Arg267Lysfs*7) | DM | 5 | C term | |
| Small Insertions | ||||||
| Nucleotide Change | Protein Change | Variant Class | Exon | Domain | ||
| [74] | c.324+3_324+4insT | Not available | DM | intron 1 | ||
| [71] | c.545_548dupTTAA | p.(Lys183Asnfs*2) | DM | 3 | ICL | |
| Small Indels | ||||||
| Nucleotide Change | Protein Change | Variant Class | Exon | Domain | ||
| [45,66] | c.165_166delGGinsC | p.(Arg55Serfs*17) | R | 1 | N term | |
| [45,46] | c.646_647delCGinsAC | p.(Arg216Thr) | DM | 4 | ECS2 | |
| Gross Deletions | ||||||
| DNA Level | Description | Variant Class | Exon | Domain | ||
| [71,98] | g.DNA | Ex. 2-5 | DM | 2-5 | ||
| Complex Mutations | ||||||
| Description | Variant Class | Exon | Domain | |||
| [62] | c.574_589delins23bp | p.(A192Yfs∗ 25) | DM | 3 | TM3 | |
| Missense/Nonsense Mutations | ||||||
|---|---|---|---|---|---|---|
| Ref. | Nucleotide Change | Amino Acid Change | Protein Change | Variant Class | Exon | Domain |
| [50] | c.54G>A | Trp18Term | p.W18 * | DM | 1 | TM1 |
| [47,50,51,57,58,67,70,72,101,102,103] | c.59G>A a,b | Gly20Asp | p.G20D | DM | 1 | TM1 |
| [50,101,102] | c.83C>T | Pro28Leu | p.P28L | DM | 1 | TM1 |
| [51] | c.122T>C | Ile41Thr | p.I41T | DM | 1 | ECS1 |
| [50,67] | c.130G>A | Val44Met | p.V44M | DM | 1 | ECS1 |
| [47,51,57] | c.169C>G | Gln57Glu | p.Q57E | DM | 1 | ECS1 |
| [50,72] | c.169C>T | Gln57Term | p.Q57 * | DM | 1 | ECS1 |
| [51] | c.223G>T | Gly75Cys | p.G75C | DM? | 1 | ECS1 |
| [51] | c.223G>A | Gly75Ser | p.G75S | DM? | 1 | ECS1 |
| [68,76] c | c.241C>T | Arg81Trp | p.R81W | DM | 2 | ECS1 or TM2? |
| [104] | c.263T>A | Val88Glu | p.V88E | DM | 2 | TM2 |
| [72] | c.269T>G | Leu90Arg | p.L90R | DM | 2 | TM2 |
| [47,57] | c.269T>C | Leu90Pro | p.L90P | DM | 2 | TM2 |
| [51] | c.364G>A | Gly122Arg | p.G122R | DM | 2 | TM3 |
| [63] | c.388G>T | Gly130Asp | p.G130C | DM | 2 | TM3 |
| [63,75] | c.389G>A | Gly130Asp | p.G130D | DM | 3 | TM3 |
| [105] | c.506G>A d | Trp169Term | p.W169 * | DM | 4 | TM4 |
| [94] | c.535G>A | Gly179Ser | p.G179S | DM | 4 | TM4 |
| [72] | c.599G>A | Arg200Gln | p.R200Q | DM? | 4 | C term |
| Small Deletions | ||||||
| Nucleotide Change | Protein Change | Variant Class | Exon | Domain | ||
| [106] | c.140_141delAT | p.(Tyr47 *) | DM | 1 | ECS1 | |
| [50] | c.403_406delACTG | p.(Thr135Leufs*9) | DM | 3 | TM3 | |
| Gross Deletions | ||||||
| DNA Level | Description | Variant Class | Exon | Domain | ||
| [50] | g.DNA | Ex. 1-4 | DM | 1-4 | ||
| Claudin | Cell Line | Transfection | TER | PNa/PCl | PNa | PCl | PMg | PCa | Mg2+ Flux | Ca2+ Flux | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rat Cldn16 | MDCK | stable | ↗ | ↗ *∫◊ | [129] | ||||||
| Rat? Cldn16 | MDCK | stable | ↗ | ↘ | ↘ | NS | ↗ §ø | [130] | |||
| Rat Cldn16 | MDCK Tet-OFF | Inducible expression | ↗ | ↗ §* | [132] | ||||||
| Human ∆70 CLDN16 | LLC-PK1 | Stable? | ↘ | ↗ | ↗ | NS | ↗ † | [46] | |||
| Human ∆70 CLDN16 | MDCK II | Stable? | NS | NS | NS | [46] | |||||
| Full length Human CLDN16 | LLC-PK1 | Stable? | ↘ | ↗ | ↗ | NS | [47] | ||||
| Full length Human CLDN16 | MDCK II | Stable? | NS | NS | [47] | ||||||
| Short and long version of human CLDN16 | MDCK-C7 | Stable | *** | ↗ £ | NS ∫ | [48] | |||||
| Long version of human CLDN16 | MDCK-C7 | Stable | NS | ↗ ∞ | NS ∞ | [48] | |||||
| Full-length human CLDN16 | MDCK-C7 | stable | NS | ↗ ¥ | [131] |
| Claudin | Cell Line | Transfection | TER | PNa/PCl | PNa | PCl | PMg | PCa | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Human CLDN19 | LLC-PK1 | Stable? | ↗ | ↗ | NS | ↘ | † | [47] | |
| Human CLDN19 | MDCK II | Stable? | NS | NS | [47] | ||||
| Mouse Cldn19 | MDCK II Tet-Off cells | Stable inducible expression | ↗ | ↘ | NS | ↘ £∂ | ↘∫∂ | [134] | |
| Human CLDN19 + full length human CLDN16 | LLC-PK1 | Stable? | ↘ | ↗ | ↗ | ↘ | ↘ † | [47] |
| Claudin | Cell Line | Transfection | TER | PNa/PCl | PNa | PCl | PCa | Ca2+ Flux | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Human CLDN14 | MDCK II Tet-Off cells | Stable inducible expression | ↗ | ↘ | ↘ | [142] | |||
| Mouse Cldn14 | OK | Stable | ↗ | ↘ | ↘ | NS | ↘∫ ø | [145] | |
| Mouse Cldn14 | MDCK II Tet-Off cells | Stable inducible expression | ↗ | ↘ | ↘ | NS | ↘∫ | [145] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prot-Bertoye, C.; Houillier, P. Claudins in Renal Physiology and Pathology. Genes 2020, 11, 290. https://doi.org/10.3390/genes11030290
Prot-Bertoye C, Houillier P. Claudins in Renal Physiology and Pathology. Genes. 2020; 11(3):290. https://doi.org/10.3390/genes11030290
Chicago/Turabian StyleProt-Bertoye, Caroline, and Pascal Houillier. 2020. "Claudins in Renal Physiology and Pathology" Genes 11, no. 3: 290. https://doi.org/10.3390/genes11030290
APA StyleProt-Bertoye, C., & Houillier, P. (2020). Claudins in Renal Physiology and Pathology. Genes, 11(3), 290. https://doi.org/10.3390/genes11030290




