Ascorbic Acid 2-Glucoside Pretreatment Protects Cells from Ionizing Radiation, UVC, and Short Wavelength of UVB
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Chemicals
2.2. Gamma-Ray and Ultraviolet Exposure
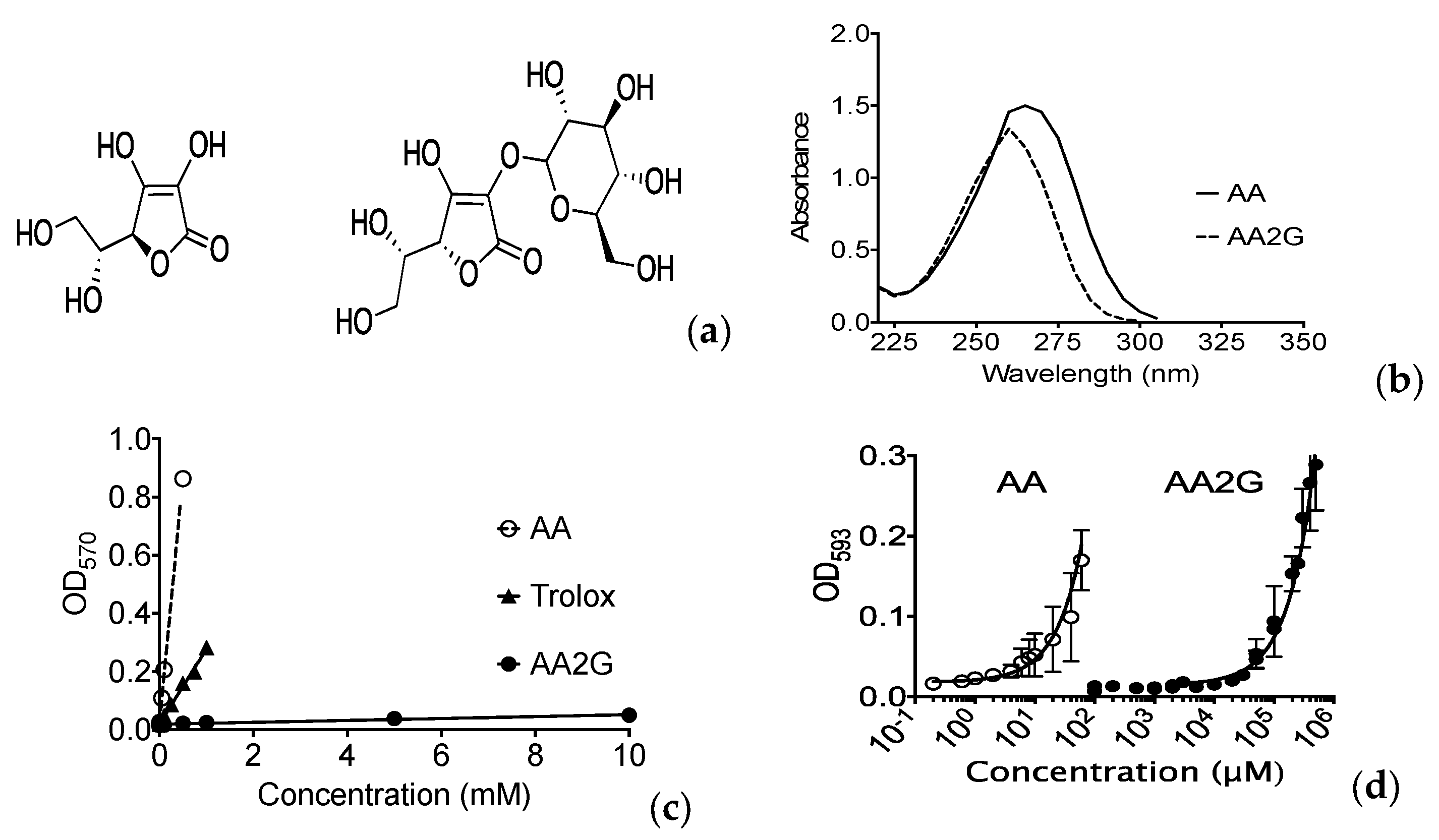
2.3. In Vitro AA2G Properties
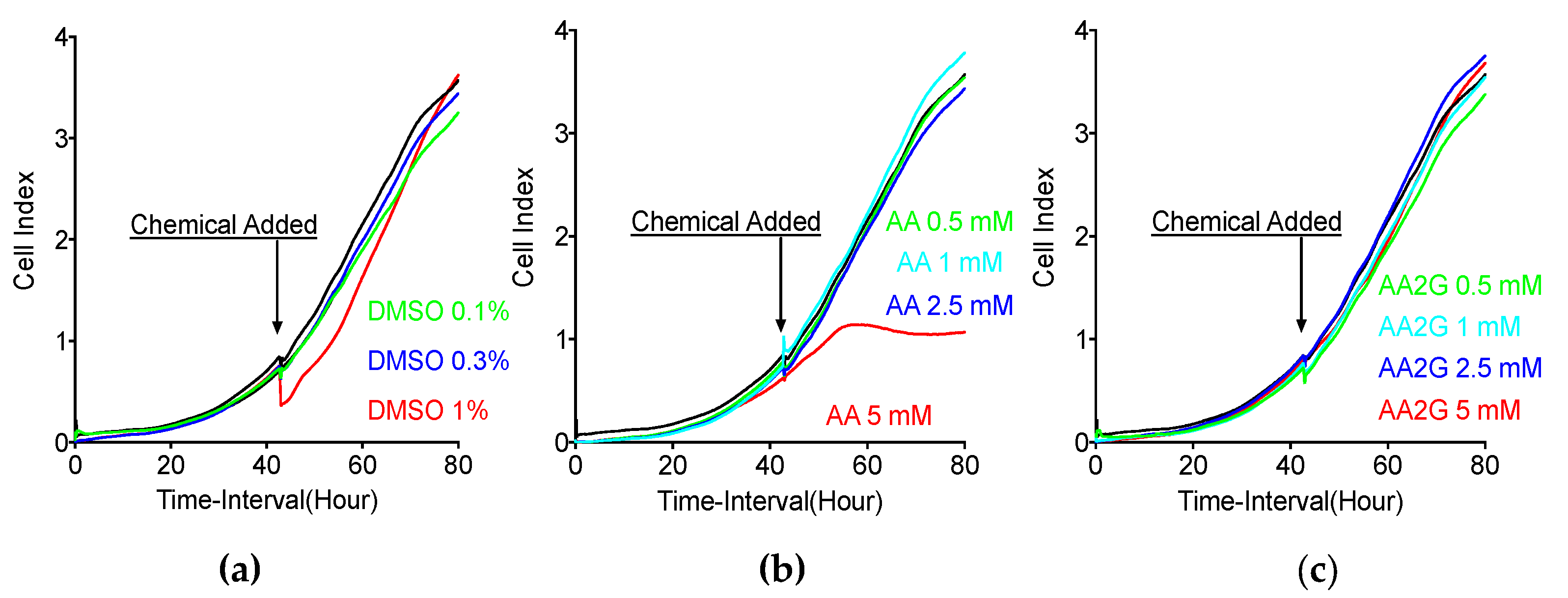
2.4. Real-Time Cell Electronic Sensing (RT-CES)
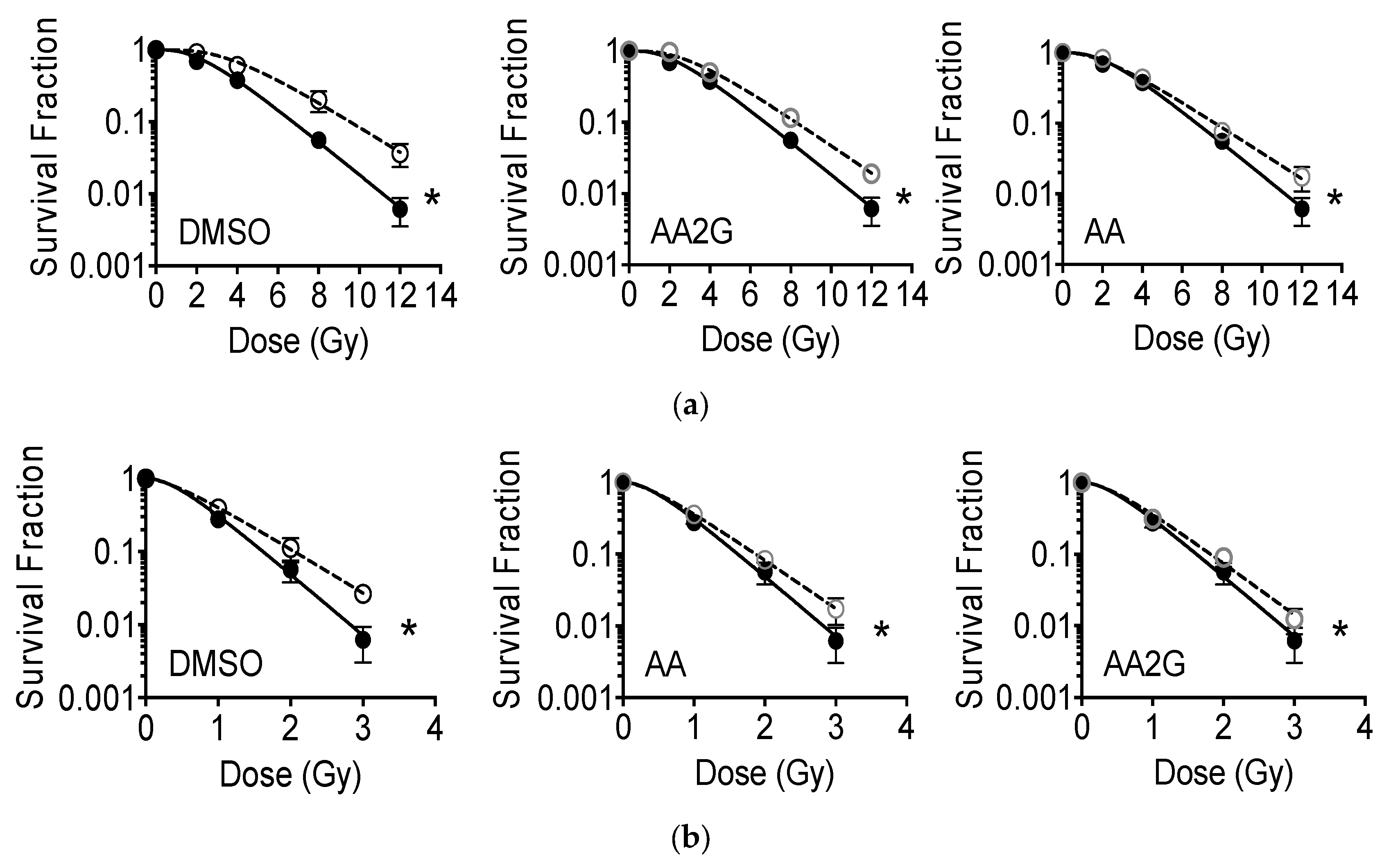
2.5. Cell Survival Analysis
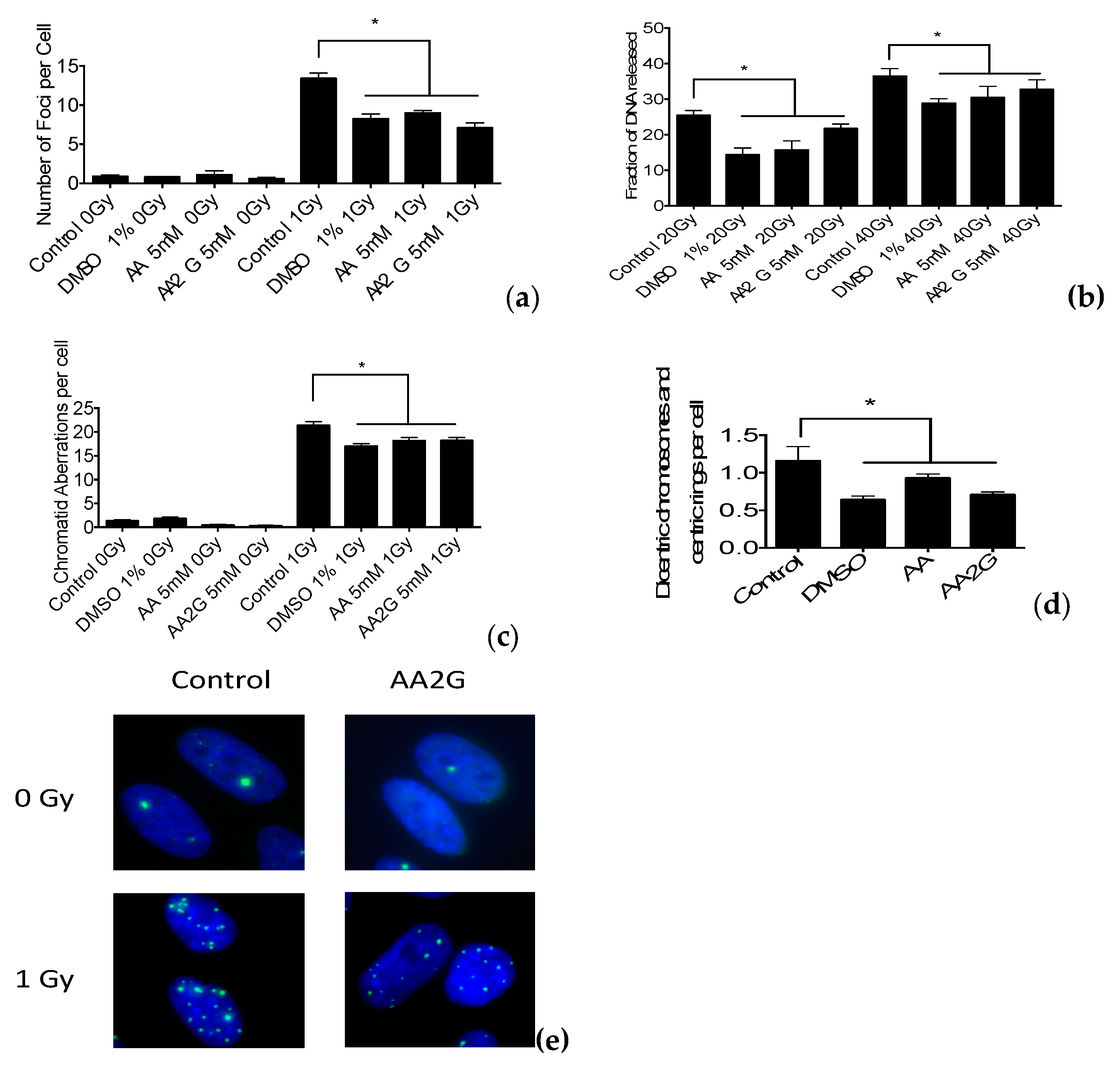
2.6. DNA Double Strand Break Measurements
2.7. G2-premature Chromosome Condensation (PCC) Assay
2.8. Chromosomal Aberration Analysis
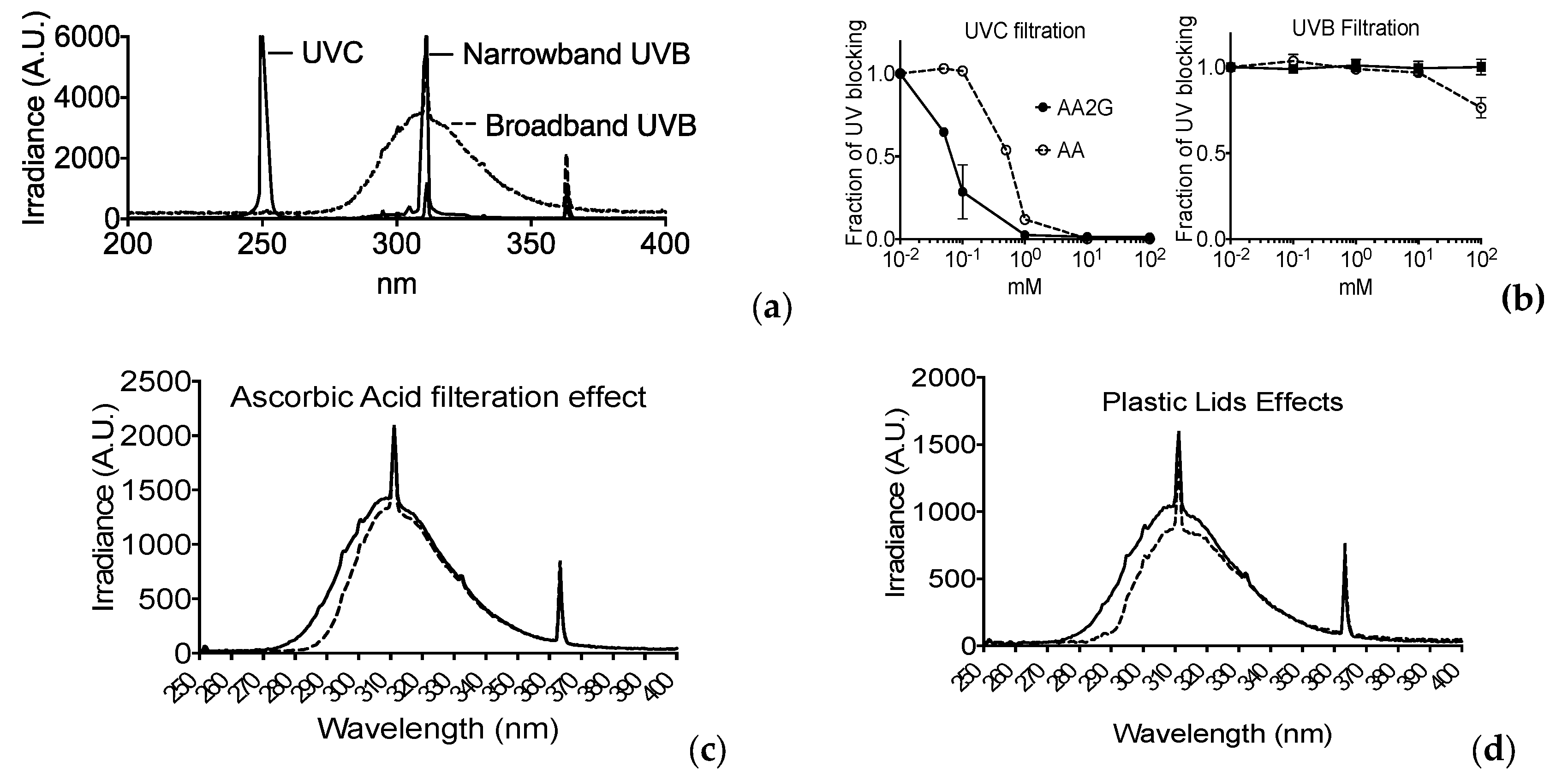
2.9. Filtration Analysis
2.10. Statistical Analysis
3. Results
3.1. Antioxidant Effects
3.2. Cytotoxicity Analysis with RT-CES
3.3. Cell Survival after Gamma-Rays
3.4. DNA Damage Induction after Gamma-Ray Exposure
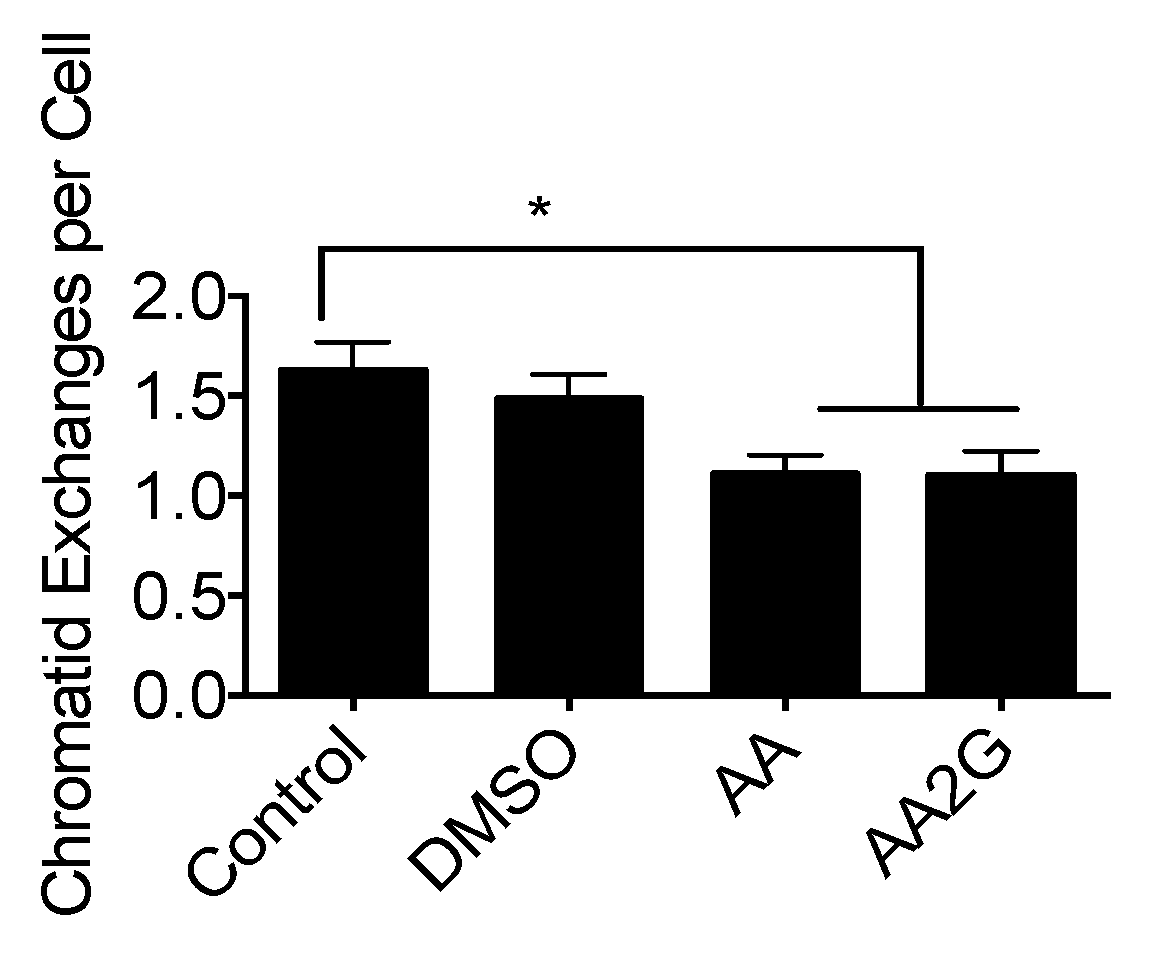
3.5. Chromosomal Aberrations after Gamma-Ray Exposure
3.6. Filtration of UV Light
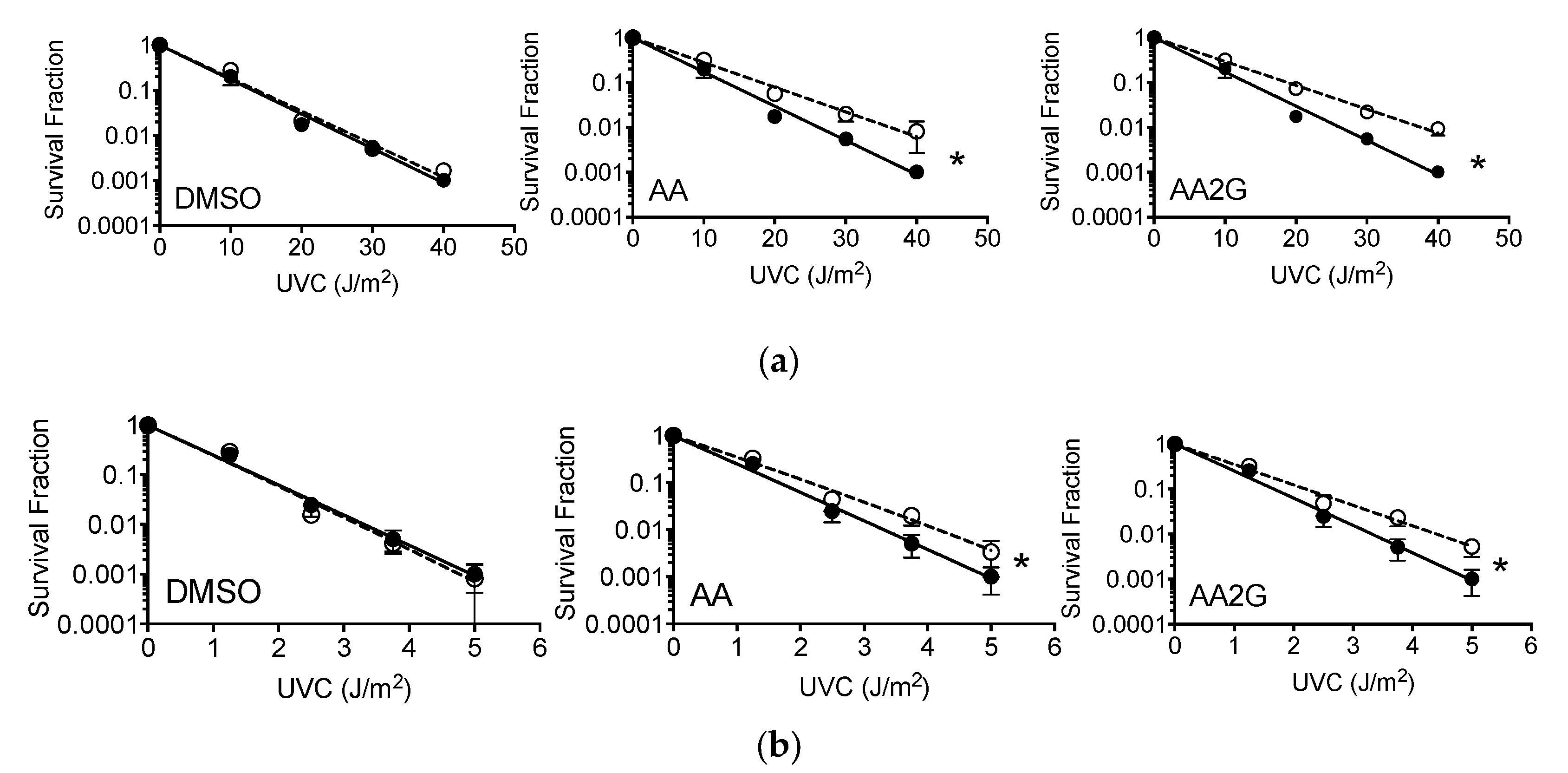
3.7. Cell Survival after UVC
3.8. Chromosomal Aberrations after UVC Exposure
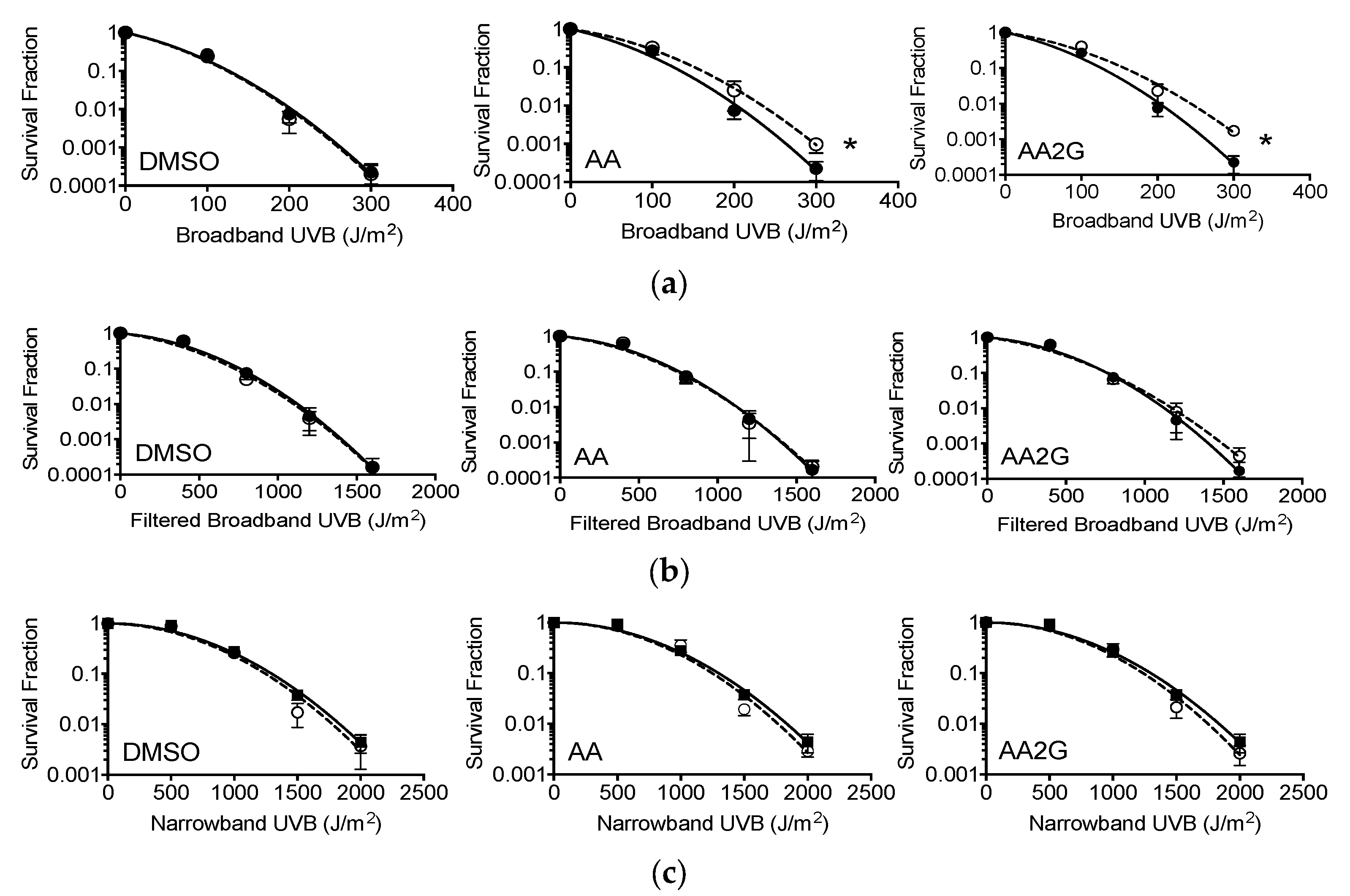
3.9. Cell Survival after Broadband and Narrowband UVB
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chapman, J.D.; Reuvers, A.P.; Borsa, J.; Greenstock, C.L. Chemical radioprotection and radiosensitization of mammalian cells growing in vitro. Radiat. Res. 1973, 56, 291–306. [Google Scholar] [CrossRef]
- Patt, H.M.; Tyree, E.B.; Straube, R.L.; Smith, D.E. Cysteine protection against x irradiation. Science 1949, 110, 213–214. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, A.L.; Nelson, D.F.; Gramkowski, M.; Glover, D.; Glick, J.H.; Yuhas, J.M.; Kligerman, M.M. Clinical trials of wr-2721 with radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1982, 8, 561–563. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin c as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Kodama, S.; Suzuki, K.; Matsumoto, T.; Miyazaki, T.; Watanabe, M. Radiation-induced long-lived radicals which cause mutation and transformation. Mutat. Res. 1998, 421, 45–54. [Google Scholar] [CrossRef]
- Ueno, A.; Vannais, D.; Lenarczyk, M.; Waldren, C.A. Ascorbate, added after irradiation, reduces the mutant yield and alters the spectrum of CD59-Mutations in AL Cells Irradiated with High LET Carbon Ions. J. Radiat. Res. (Tokyo) 2002, 43, S245–S249. [Google Scholar] [CrossRef]
- Fujii, Y.; Kato, T.A.; Ueno, A.; Kubota, N.; Fujimori, A.; Okayasu, R. Ascorbic Acid gives different protective effects in human cells exposed to X-rays and heavy ions. Mutat. Res. 2010, 699, 58–61. [Google Scholar] [CrossRef]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic Acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin c pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef]
- Kumano, Y.; Sakamoto, T.; Egawa, M.; Iwai, I.; Tanaka, M.; Yamamoto, I. In vitro and in vivo prolonged biological activities of novel vitamin c derivative, 2-O-alpha-d-glucopyranosyl-l-Ascorbic Acid (aa-2g), in cosmetic fields. J. Nutr. Sci. Vitaminol. 1998, 44, 345–359. [Google Scholar] [CrossRef]
- Murakami, K.; Muto, N.; Fukazawa, K.; Yamamoto, I. Comparison of Ascorbic Acid and Ascorbic Acid 2-O-alpha-glucoside on the cytotoxicity and bioavailability to low density cultures of fibroblasts. Biochem. Pharmacol. 1992, 44, 2191–2197. [Google Scholar] [PubMed]
- Yamamoto, I.; Muto, N. Bioavailability and biological activity of l-Ascorbic Acid 2-O-alpha-glucoside. J. Nutr. Sci. Vitaminol. 1992, 38, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Kinashi, Y.; Sakurai, Y.; Masunaga, S.; Suzuki, M.; Nagata, K.; Ono, K. Ascorbic Acid reduced mutagenicity at the hprt locus in cho cells against thermal neutron radiation. Appl. Radiat. Isot. 2004, 61, 929–932. [Google Scholar] [CrossRef]
- Chandrasekharan, D.K.; Kagiya, T.V.; Nair, C.K. Radiation protection by 6-palmitoyl Ascorbic Acid-2-glucoside: Studies on DNA damage in vitro, ex vivo, in vivo and oxidative stress in vivo. J. Radiat. Res. 2009, 50, 203–212. [Google Scholar] [CrossRef][Green Version]
- Mathew, D.; Nair, C.K.; Jacob, J.A.; Biswas, N.; Mukherjee, T.; Kapoor, S.; Kagiya, T.V. Ascorbic Acid monoglucoside as antioxidant and radioprotector. J. Radiat. Res. 2007, 48, 369–376. [Google Scholar] [CrossRef]
- Kinashi, Y.; Tanaka, H.; Masunaga, S.; Suzuki, M.; Kashino, G.; Yong, L.; Takahashi, S.; Ono, K. Ascorbic Acid 2-glucocide reduces micronucleus induction in distant splenic t lymphocytes following head irradiation. Mutat. Res. 2010, 695, 69–74. [Google Scholar] [CrossRef]
- Haskins, A.H.; Buglewicz, D.J.; Hirakawa, H.; Fujimori, A.; Aizawa, Y.; Kato, T.A. Palmitoyl Ascorbic Acid 2-glucoside has the potential to protect mammalian cells from high-let carbon-ion radiation. Sci. Rep. 2018, 8, 13822. [Google Scholar] [CrossRef]
- Godic, A.; Poljsak, B.; Adamic, M.; Dahmane, R. The role of antioxidants in skin cancer prevention and treatment. Oxidative Med. Cell. Longev. 2014, 2014, 860479. [Google Scholar] [CrossRef]
- Gasperlin, M.; Gosenca, M. Main approaches for delivering antioxidant vitamins through the skin to prevent skin ageing. Expert Opin. Drug Deliv. 2011, 8, 905–919. [Google Scholar] [CrossRef]
- Chen, L.; Hu, J.Y.; Wang, S.Q. The role of antioxidants in photoprotection: A critical review. J. Am. Acad. Dermatol. 2012, 67, 1013–1024. [Google Scholar] [CrossRef]
- Ringvold, A.; Anderssen, E.; Kjonniksen, I. Distribution of ascorbate in the anterior bovine eye. Investig. Ophthalmol. Vis. Sci. 2000, 41, 20–23. [Google Scholar] [PubMed]
- Miyai, E.; Yanagida, M.; Akiyama, J.; Yamamoto, I. Ascorbic Acid 2-O-alpha-glucoside-induced redox modulation in human keratinocyte cell line, scc: Mechanisms of photoprotective effect against ultraviolet light b. Biol. Pharm. Bull. 1997, 20, 632–636. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyai, E.; Yanagida, M.; Akiyama, J.; Yamamoto, I. Ascorbic Acid 2-O-alpha-glucoside, a stable form of Ascorbic Acid, rescues human keratinocyte cell line, scc, from cytotoxicity of ultraviolet light b. Biol. Pharm. Bull. 1996, 19, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.R.; Qin, H.H.; Wu, W.Y.; He, S.J.; Xu, J.H. Vitamin c protects against uv irradiation-induced apoptosis through reactivating silenced tumor suppressor genes p21 and p16 in a tet-dependent DNA demethylation manner in human skin cancer cells. Cancer Biother. Radiopharm. 2014, 29, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Jeggo, P.A.; Kemp, L.M. X-ray-sensitive mutants of chinese hamster ovary cell line. Isolation and cross-sensitivity to other DNA-damaging agents. Mutat. Res. 1983, 112, 313–327. [Google Scholar] [CrossRef]
- Thompson, L.H.; Brookman, K.W.; Salazar, E.P.; Fuscoe, J.C.; Weber, C.A. DNA repair genes of mammalian cells. Basic Life Sci. 1986, 39, 349–358. [Google Scholar]
- Thompson, L.H.; Carrano, A.V.; Sato, K.; Salazar, E.P.; White, B.F.; Stewart, S.A.; Minkler, J.L.; Siciliano, M.J. Identification of nucleotide-excision-repair genes on human chromosomes 2 and 13 by functional complementation in hamster-human hybrids. Somat. Cell Mol. Genet. 1987, 13, 539–551. [Google Scholar] [CrossRef]
- Genet, S.C.; Fujii, Y.; Maeda, J.; Kaneko, M.; Genet, M.D.; Miyagawa, K.; Kato, T.A. Hyperthermia inhibits homologous recombination repair and sensitizes cells to ionizing radiation in a time and temperature dependent manner. J. Cell. Physiol. 2012, 228, 1473–1481. [Google Scholar] [CrossRef]
- Suzuki, F.; Han, A.; Lankas, G.R.; Utsumi, H.; Elkind, M.M. Spectral dependencies of killing, mutation, and transformation in mammalian cells and their relevance to hazards caused by solar ultraviolet radiation. Cancer Res. 1981, 41, 4916–4924. [Google Scholar]
- Ceriotti, L.; Ponti, J.; Colpo, P.; Sabbioni, E.; Rossi, F. Assessment of cytotoxicity by impedance spectroscopy. Biosens. Bioelectron. 2007, 22, 3057–3063. [Google Scholar] [CrossRef]
- Maeda, J.; Bell, J.J.; Genet, S.C.; Fujii, Y.; Genet, M.D.; Brents, C.A.; Genik, P.C.; Kato, T.A. Potentially lethal damage repair in drug arrested g2-phase cells after radiation exposure. Radiat. Res. 2014, 182, 448–457. [Google Scholar] [CrossRef]
- Kato, T.A.; Okayasu, R.; Jeggo, P.A.; Fujimori, A. Aspm influences DNA double-strand break repair and represents a potential target for radiotherapy. Int. J. Radiat. Biol. 2011, 87, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Yurkon, C.R.; Maeda, J.; Genet, S.C.; Kubota, N.; Fujimori, A.; Mori, T.; Maruo, K.; Kato, T.A. Comparative study of radioresistance between feline cells and human cells. Radiat. Res. 2013, 180, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, E.; Asakawa, Y.; Kosaka, H. Inhibition of protein-serine threonine phosphatases directly induces premature chromosome condensation in mammalian somatic-cells. Biomed. Res. Tokyo 1995, 16, 63–68. [Google Scholar] [CrossRef]
- Cartwright, I.M.; Genet, M.D.; Kato, T.A. A simple and rapid fluorescence in situ hybridization microwave protocol for reliable dicentric chromosome analysis. J. Radiat. Res. 2012, 54, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.A. Human lymphocyte metaphase chromosome preparation for radiation-induced chromosome aberration analysis. Methods Mol. Biol. 2019, 1984, 1–6. [Google Scholar] [PubMed]
- Tobey, R.A.; Ley, K.D. Regulation of initiation of DNA synthesis in chinese hamster cells. I. Production of stable, reversible g1-arrested populations in suspension culture. J. Cell Biol. 1970, 46, 151–157. [Google Scholar] [CrossRef]
- Nagasawa, H.; Little, J.B.; Inkret, W.C.; Carpenter, S.; Thompson, K.; Raju, M.R.; Chen, D.J.; Strniste, G.F. Cytogenetic effects of extremely low doses of plutonium-238 alpha-particle irradiation in cho k-1 cells. Mutat. Res. 1990, 244, 233–238. [Google Scholar] [CrossRef]
- Chapman, J.D.; Doern, S.D.; Reuvers, A.P.; Gillespie, C.J.; Chatterjee, A.; Blakely, E.A.; Smith, K.C.; Tobias, C.A. Radioprotection by dmso of mammalian cells exposed to X-rays and to heavy charged-particle beams. Radiat. Environ. Biophys. 1979, 16, 29–41. [Google Scholar] [CrossRef]
- Douki, T. The variety of uv-induced pyrimidine dimeric photoproducts in DNA as shown by chromatographic quantification methods. Photochem. Photobiol. Sci. 2013, 12, 1286–1302. [Google Scholar] [CrossRef]
- Steiner, M.G.; Babbs, C.F. Quantitation of the hydroxyl radical by reaction with dimethyl sulfoxide. Arch. Biochem. Biophys. 1990, 278, 478–481. [Google Scholar] [CrossRef]
- Zhang, X.; Rosenstein, B.S.; Wang, Y.; Lebwohl, M.; Mitchell, D.M.; Wei, H. Induction of 8-oxo-7,8-dihydro-2′-deoxyguanosine by ultraviolet radiation in calf thymus DNA and hela cells. Photochem. Photobiol. 1997, 65, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Zelle, B.; Reynolds, R.J.; Kottenhagen, M.J.; Schuite, A.; Lohman, P.H. The influence of the wavelength of ultraviolet radiation on survival, mutation induction and DNA repair in irradiated chinese hamster cells. Mutat. Res. 1980, 72, 491–509. [Google Scholar] [CrossRef]
| Cells | D10 of Gamma-Ray (Gy)/Protection Ratio | |||
|---|---|---|---|---|
| Control | DMSO | AA | AA2G | |
| CHO | 6.7 | 9.5/1.4 | 7.6/1.1 | 8.2/1.2 |
| xrs5 | 1.6 | 2.1/1.3 | 1.9/1.2 | 1.8/1.1 |
| Cells | D10 of UVC (J/m2)/Protection Ratio | |||
|---|---|---|---|---|
| Control | DMSO | AA | AA2G | |
| CHO | 13.1 | 13.7/1.1 | 18.1/1.4 | 18.8/1.4 |
| UV135 | 1.66 | 1.63/1.0 | 2.16/1.3 | 2.20/1.3 |
| Conditions | D10 of UVB (J/m2)/Protection Ratio | |||
|---|---|---|---|---|
| Control | DMSO | AA | AA2G | |
| Broadband UVB | 126 | 124/0.98 | 151/1.20 | 156/1.23 |
| Broadband UVB + filter | 758 | 722/0.95 | 741/0.98 | 763/1.01 |
| Narrowband UVB | 1297 | 1248/0.96 | 1248/0.96 | 1236/0.95 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, J.; Allum, A.J.; Mussallem, J.T.; Froning, C.E.; Haskins, A.H.; Buckner, M.A.; Miller, C.D.; Kato, T.A. Ascorbic Acid 2-Glucoside Pretreatment Protects Cells from Ionizing Radiation, UVC, and Short Wavelength of UVB. Genes 2020, 11, 238. https://doi.org/10.3390/genes11030238
Maeda J, Allum AJ, Mussallem JT, Froning CE, Haskins AH, Buckner MA, Miller CD, Kato TA. Ascorbic Acid 2-Glucoside Pretreatment Protects Cells from Ionizing Radiation, UVC, and Short Wavelength of UVB. Genes. 2020; 11(3):238. https://doi.org/10.3390/genes11030238
Chicago/Turabian StyleMaeda, Junko, Allison J. Allum, Jacob T. Mussallem, Coral E. Froning, Alexis H. Haskins, Mark A. Buckner, Chris D. Miller, and Takamitsu A. Kato. 2020. "Ascorbic Acid 2-Glucoside Pretreatment Protects Cells from Ionizing Radiation, UVC, and Short Wavelength of UVB" Genes 11, no. 3: 238. https://doi.org/10.3390/genes11030238
APA StyleMaeda, J., Allum, A. J., Mussallem, J. T., Froning, C. E., Haskins, A. H., Buckner, M. A., Miller, C. D., & Kato, T. A. (2020). Ascorbic Acid 2-Glucoside Pretreatment Protects Cells from Ionizing Radiation, UVC, and Short Wavelength of UVB. Genes, 11(3), 238. https://doi.org/10.3390/genes11030238






