Selection of the Reference Gene for Expression Normalization in Papaver somniferum L. under Abiotic Stress and Hormone Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatment
2.2. Selection of Candidate Reference Genes and Primer Design
2.3. Total RNA Extraction and RT-qPCR Analysis
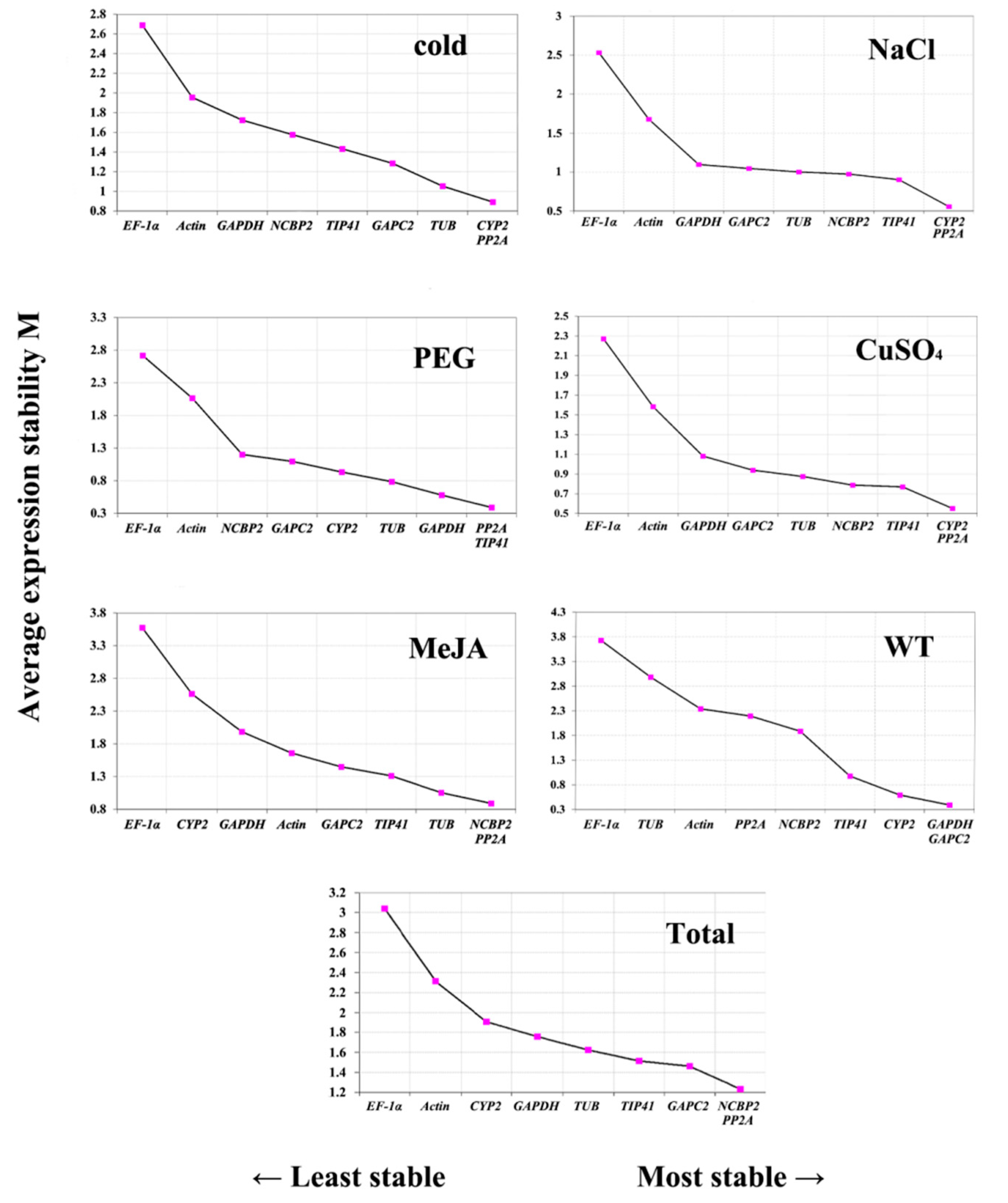
2.4. Gene Expression Stability Analysis
2.5. Statistical Analysis
3. Results
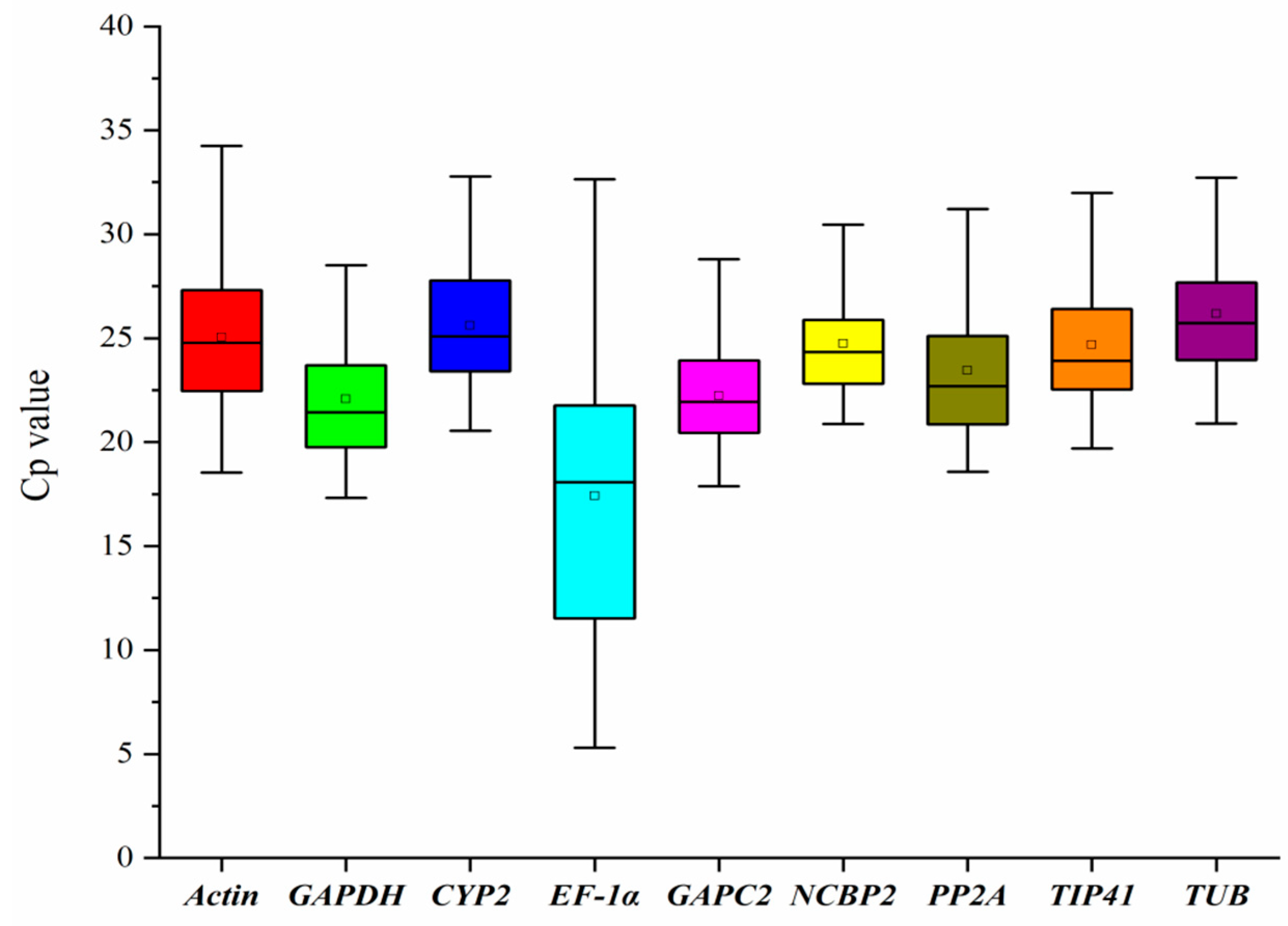
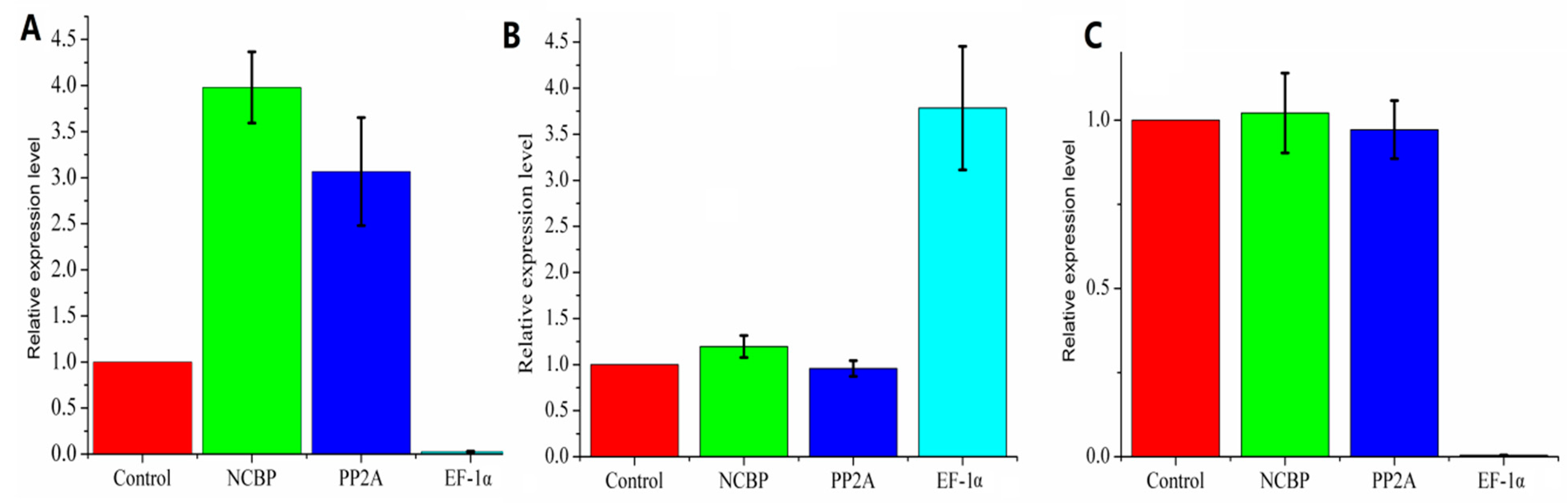
3.1. Expression Profile of the Reference Genes
3.2. Expression Stability of the Candidate Reference Gene
3.2.1. BestKeeper Analysis
3.2.2. NormFinder Analysis
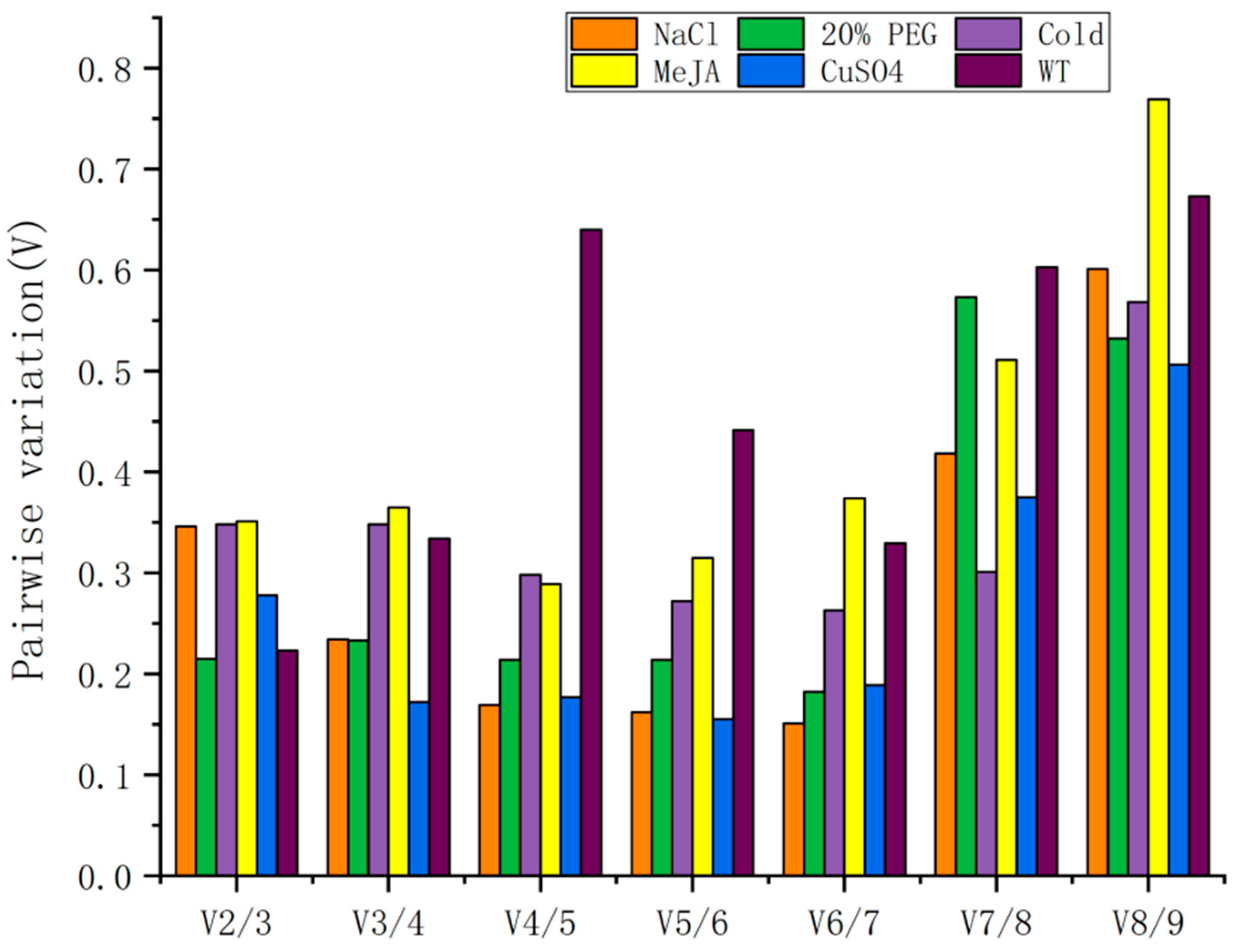
3.2.3. geNorm Analysis
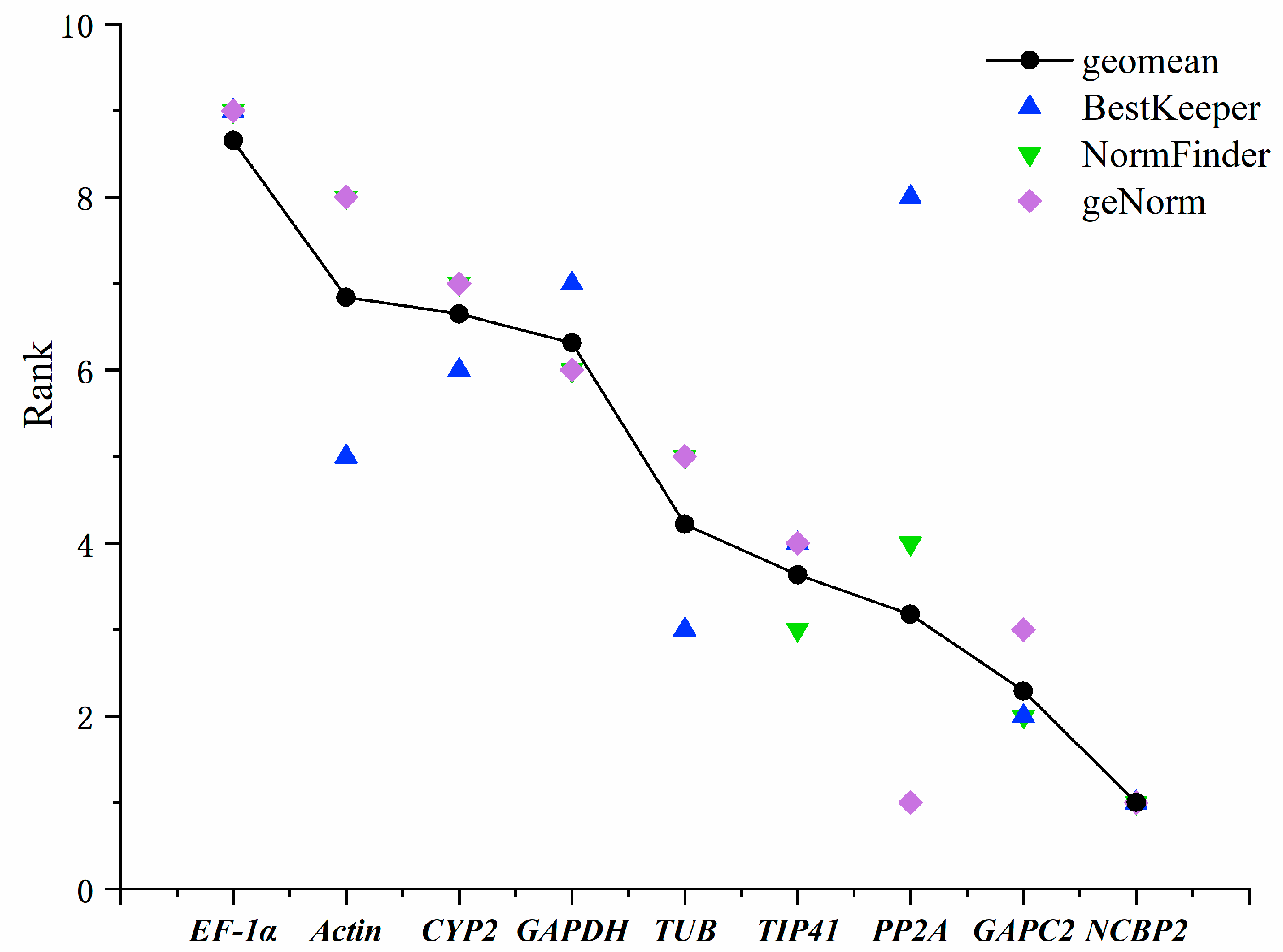
3.3. Comprehensive Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dang, T.T.; Farrow, S.; Onoyovwi, A.; Facchini, P. Biochemical Genomics for Gene Discovery in Benzylisoquinoline Alkaloid Biosynthesis in Opium Poppy and Related Species. Methods Enzymol. 2012, 515, 231–266. [Google Scholar]
- Shukla, S.; Yadav, H.K.; Rastogi, A.; Mishra, B.K.; Singh, S.P. Alkaloid Diversity in Relation to Breeding for Specific Alkaloids in Opium Poppy (Papaver somniferum L.). Crezh J. Genet. Plant Breed. 2010, 46, 164–169. [Google Scholar] [CrossRef]
- Beaudoin, G.; Facchini, P. Benzylisoquinoline alkaloid biosynthesis in opium poppy. Planta 2014, 240, 19–32. [Google Scholar] [CrossRef]
- Marciano, M.; Panicker, S.; Liddil, G.; Lindgren, D.; Sweder, K. Development of a Method to Extract Opium Poppy (Papaver somniferum L.) DNA from Heroin. Sci. Rep. 2018, 8, 2590. [Google Scholar] [CrossRef]
- Krošlák, E.; Maliar, T.; Nemeček, P.; Viskupicova, J.; Maliarová, M.; Havrlentova, M.; Kraic, J. Antioxidant and Proteinase Inhibitory Activities of Selected Poppy (Papaver somniferum L.) Genotypes. Chem. Biodivers. 2017, 14, e1700176. [Google Scholar] [CrossRef]
- Jablonická, V.; Ziegler, J.; Vatehova, Z.; Lišková, D.; Obložinský, M.; Heilmann, M. Inhibition of phospholipases influences the metabolism of wound-induced benzylisoquinoline alkaloids in Papaver somniferum L. J. Plant Physiol. 2018, 223, 1–8. [Google Scholar] [CrossRef]
- Winzer, T.; Gazda, V.; He, Z.; Kaminski, F.; Kern, M.; Larson, T.; Li, Y.; Meade, F.; Teodor, R.; Vaistij, F.; et al. A Papaver somniferum 10-Gene Cluster for Synthesis of the Anticancer Alkaloid Noscapine. Science 2012, 336, 1704–1708. [Google Scholar] [CrossRef]
- Dang, T.T.; Chen, X.; Facchini, P. Acetylation serves as a protective group in noscapine biosynthesis in opium poppy. Nat. Chem. Biol. 2014, 11, 104–106. [Google Scholar] [CrossRef]
- Dastmalchi, M.; Chang, L.; Torres, M.; Ng, K.; Facchini, P. Codeinone reductase isoforms with differential stability, efficiency and product selectivity in opium poppy. Plant J. 2018, 95, 631–647. [Google Scholar] [CrossRef]
- Xie, Y.; Sen, B.; Wang, G. Mining terpenoids production and biosynthetic pathway in Thraustochytrids. Bioresour. Technol. 2017, 244, 1269–1280. [Google Scholar] [CrossRef]
- Enciso, M.; Carrascosa, J.; Sarasa, J.; Martínez-Ortiz, P.; Munné, S.; Horcajadas, J.; Aizpurua, J. Development of a new comprehensive and reliable endometrial receptivity map (ER Map/ER Grade) based on RT-qPCR gene expression analysis. Hum. Reprod. 2018, 33, 220–228. [Google Scholar] [CrossRef]
- Wolfinger, R.; Beedanagari, S.; Boitier, E.; Chen, T.; Couttet, P.; Ellinger-Ziegelbauer, H.; Guillemain, G.; Mariet, C.; Mouritzen, P.; O’Lone, R.; et al. Two approaches for estimating the lower limit of quantitation (LLOQ) of microRNA levels assayed as exploratory biomarkers by RT-qPCR. BMC Biotechnol. 2018, 18, 6. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, J.; Xu, S.; Wang, W.; Liu, T.; Han, C.; Chen, Y.; Kong, L. Selection of Reference Genes for Gene Expression Normalization in Peucedanum praeruptorum Dunn under Abiotic Stresses, Hormone Treatments and Different Tissues. PLoS ONE 2016, 11, e0152356. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, J.; Liu, Y.; Chen, J.; Lin, H.; Huang, Y.; Bian, X.; Zhao, Y. Selection and validation of appropriate reference genes for real-time quantitative PCR analysis in Momordica charantia. Phytochemistry 2019, 164, 1–11. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, X.; Sun, M.; Cong, H.; Qiao, F. Evaluation of reference genes for normalizing RT-qPCR in leaves and suspension cells of Cephalotaxus hainanensis under various stimuli. Plant Methods 2019, 15, 31. [Google Scholar] [CrossRef]
- Nguyen, D.; Eamens, A.; Grof, C. Reference gene identification for reliable normalisation of quantitative RT-PCR data in Setaria viridis. Plant Methods 2018, 14, 24. [Google Scholar] [CrossRef]
- Expósito-Rodríguez, M.; Borges, A.A.; Borges-Pérez, A.; Pérez, J.A. Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biol. 2008, 8, 131. [Google Scholar] [CrossRef]
- Nolan, T.; Hands, R.; Bustin, S. Quantification of mRNA using real-time rt-PCR. Nat. Protoc. 2006, 1, 1559–1582. [Google Scholar] [CrossRef]
- Ferdous, J.; Li, Y.; Reid, N.; Langridge, P.; Shi, B.-J.; Tricker, P. Identification of Reference Genes for Quantitative Expression Analysis of MicroRNAs and mRNAs in Barley under Various Stress Conditions. PLoS ONE 2015, 10, e0118503. [Google Scholar] [CrossRef]
- Thellin, O.; Zorzi, W.; Lakaye, B.; Borman, B.; Coumans, B.; Hennen, G.; Grisar, T.; Igout, A.; Heinen, E. Housekeeping genes as internal standards: Use and limits. J. Biotechnol. 1999, 75, 291–295. [Google Scholar] [CrossRef]
- Zhu, X.; Yuan, M.; Shakeel, M.; Zhang, Y.-J.; Wang, S.; Wang, X.; Zhan, S.; Kang, T.; Li, J. Selection and Evaluation of Reference Genes for Expression Analysis Using qRT-PCR in the Beet Armyworm Spodoptera exigua (Hubner) (Lepidoptera: Noctuidae). PLoS ONE 2014, 9, e84730. [Google Scholar] [CrossRef]
- Arya, S.; Jain, G.; Upadhyay, S.; Singh, H.; Yadav, S.; Dixit, S.; Verma, P. Reference genes validation in Phenacoccus solenopsis under various biotic and abiotic stress conditions. Sci. Rep. 2017, 7, 13520. [Google Scholar] [CrossRef]
- Yuan, M.; Lu, Y.; Zhu, X.; Wan, H.; Shakeel, M.; Zhan, S.; Jin, B.-R.; Li, J. Selection and Evaluation of Potential Reference Genes for Gene Expression Analysis in the Brown Planthopper, Nilaparvata lugens (Hemiptera: Delphacidae) Using Reverse-Transcription Quantitative PCR. PLoS ONE 2014, 9, e86503. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, X.; Chen, S.; Zheng, L.; He, X.; Liu, M.; Qiao, G.; Wang, Y.; Zhuo, R. Selection of suitable reference genes for quantitative real-time PCR gene expression analysis in Salix matsudana under different abiotic stresses. Sci. Rep. 2017, 7, 40290. [Google Scholar] [CrossRef]
- Zhu, W.; Lin, Y.; Liao, H.; Wang, Y. Selection of Reference Genes for Gene Expression Studies Related to Intramuscular Fat Deposition in Capra hircus Skeletal Muscle. PLoS ONE 2015, 10, e0121280. [Google Scholar] [CrossRef]
- Huang, L.; Yan, H.; Jiang, X.; Yin, G.; Zhang, X.; Qi, X.; Zhang, Y.; Yan, Y.; Ma, X.; Peng, Y. Identification of Candidate Reference Genes in Perennial Ryegrass for Quantitative RT-PCR under Various Abiotic Stress Conditions. PLoS ONE 2014, 9, e93724. [Google Scholar] [CrossRef]
- de Andrade, L.; Brito, M.; Junior, R.; Marchiori, P.; Nobile, P.; Martins, A.; Ribeiro, R.; Creste, S. Reference genes for normalization of qPCR assays in sugarcane plants under water deficit. Plant Methods 2017, 13, 28. [Google Scholar] [CrossRef]
- Tichopad, A.; Prgomet, C.; Neuvians, T. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper-Excel-Based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Roy, N.; De Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, 00341–003411. [Google Scholar] [CrossRef]
- Andersen, C.; Jensen, J.; Ørntoft, T. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Li, M.-Z.; Luo, J.; Chen, F.; Gong, Y.; Li, Y.; Wei, Y.; Su, Y.; Kong, L. Transcriptomic profiles of 33 opium poppy samples in different tissues, growth phases, and cultivars. Sci. Data 2019, 6, 66. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Alagöz, Y.; Gurkok Tan, T.; Zhang, B.; Unver, T. Manipulating the Biosynthesis of Bioactive Compound Alkaloids for Next-Generation Metabolic Engineering in Opium Poppy Using CRISPR-Cas 9 Genome Editing Technology. Sci. Rep. 2016, 6, 30910. [Google Scholar] [CrossRef]
- Li, J.; Lee, E.-J.; Chang, L.; Facchini, P. Genes encoding norcoclaurine synthase occur as tandem fusions in the Papaveraceae. Sci. Rep. 2016, 6, 39256. [Google Scholar] [CrossRef]
- Wan, H.; Zhao, Z.; Qian, C.; Sui, Y.; Malik, A.A.; Chen, J. Selection of appropriate reference genes for gene expression studies by quantitative real-time polymerase chain reaction in cucumber. Anal. Biochem. 2010, 399, 257–261. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, N.; Si, H.; Calderón-Urrea, A. Selection and validation of reference genes for RT-qPCR analysis in potato under abiotic stress. Plant Methods 2017, 13, 35. [Google Scholar] [CrossRef]
- Leal, M.F.; Belangero, P.S.; Figueiredo, E.A.; Cohen, C.; Loyola, L.C.; Andreoli, C.V.; Smith, M.C.; de Castro Pochini, A.; Ejnisman, B.; Cohen, M. Identification of Suitable Reference Genes for Gene Expression Studies in Tendons from Patients with Rotator Cuff Tear. PLoS ONE 2015, 10, e0118821. [Google Scholar] [CrossRef]
- Borowski, J.; Galli, V.; Messias, R.; Perin, E.; Buss, J.; Silva, S.; Rombaldi, C. Selection of candidate reference genes for real-time PCR studies in lettuce under abiotic stresses. Planta 2014, 239, 1187–1200. [Google Scholar] [CrossRef]
- Long, X.-Y.; He, B.; Gao, X.; Qin, Y.; Jianghua, Y.; Fang, Y.; Qi, J.; Tang, C. Validation of reference genes for quantitative real-time PCR during latex regeneration in rubber tree. Gene 2015, 563, 190–195. [Google Scholar] [CrossRef]
- Amorim, L.; Ferreira-Neto, J.; Neto, J.; Pandolfi, V.; Araújo, F.; Matos, M.; Santos, M.; Kido, E.; Iseppon, A. Cowpea and abiotic stresses: Identification of reference genes for transcriptional profiling by qPCR. Plant Methods 2018, 14, 88. [Google Scholar] [CrossRef]
- Basu, S.; Pereira, A.; Pinheiro, D.; Wang, H.; Valencia-Jiménez, A.; Siegfried, B.; Louis, J.; Zhou, X.; Velez, A. Evaluation of reference genes for real-time quantitative PCR analysis in southern corn rootworm, Diabrotica undecimpunctata howardi (Barber). Sci. Rep. 2019, 9, 10703. [Google Scholar] [CrossRef]
- Liang, W.; Zou, X.; Carballar-Lejarazú, R.; Wu, L.; Sun, W.; Yuan, X.; Wu, S.; Li, P.; Ding, H.; Ni, L.; et al. Selection and evaluation of reference genes for qRT-PCR analysis in Euscaphis konishii Hayata based on transcriptome data. Plant Methods 2018, 14, 42. [Google Scholar] [CrossRef]
- Huang, T.; Long, J.; Liu, S.-W.; Yang, Z.-W.; Zhu, Q.-J.; Zhao, X.-L.; Peng, C. Selection and Validation of Reference Genes for mRNA Expression by Quantitative Real-Time PCR Analysis in Neolamarckia cadamba. Sci. Rep. 2018, 8, 9311. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, Z.; Hu, B.; Yang, Z.; Xu, B.; Zhuang, L.; Huang, B. Selection and validation of reference genes for target gene analysis with quantitative RT-PCR in leaves and roots of bermudagrass under four different abiotic stresses. Physiol. Plant 2014, 155, 138–148. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, B.; Tan, Z.; Liu, J.; Yang, Z.; Li, Z.; Huang, B. Selection of reference genes for quantitative real-time PCR normalization in creeping bentgrass involved in four abiotic stresses. Plant Cell Rep. 2015, 34, 1825–1834. [Google Scholar] [CrossRef]
| Gene | Gene Name | PCR | Primer Sequences (5′-3′) (Forward/Reverse) | Length (bp)/Tm (°C) | PCR Efficiency |
|---|---|---|---|---|---|
| Actin | Actin-11 | AT3G12110 | GCTGTCCTTTCCCTCTACGC/AGGGCATCAGTAAGGTCACG | 155/88.1 | 1.867 |
| CYP2 | Cyclophilin 2 | AT4G33060 | TGTGCTAATGCTGGAACGCC/TCTCGACCTCCGCCAAATTC | 148/83.6 | 1.851 |
| EF-1α | Elongation factor 1-α | AT5G60390 | CCTGGACAGATAGGTAATGG/TAATACCAGCATCACCGTTC | 135/86.8 | 1.885 |
| GAPC2 | Glyceraldehyde-3-phosphate dehydrogenase C2 | AT3G04120 | CCACAAATTGCCTTGCTCCC/TGGGTGGCAGTGATGGAGTG | 97/82.7 | 1.871 |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase | AT1G42970 | GCCAAGGCTGTGTCATTAGT/AAAGTCCCTTCTTCTCGACG | 115/85.4 | 1.866 |
| NCBP2 | Nuclear cap binding protein 2 | AT5G44200 | AAACAGCAAAACCCCCTGTG/CCCCAATCAAAATCCACACG | 129/84.2 | 1.885 |
| PP2A | Protein phosphatase 2A gene | AT3G25800 | TCTTCGTGCGGGTTGTTTCC/CGGATCTCCTGACCATTGGC | 118/84.9 | 1.880 |
| TIP41 | TIP41-like protein | AT4G34270 | GTTCCTGCTGCTGCCAAATG/GATGTTCCCTTCCCCCTCTG | 135/82.2 | 1.884 |
| TUB | Beta-6-tubulin | AT5G12250 | TTGCTTCAGGACCCTCAAGC/GTTGCCCAGGGAACCTAAGG | 107/85.8 | 1.887 |
| Rank | Cold | PEG | NaCl | MeJA | CuSO4 | WT | Total |
|---|---|---|---|---|---|---|---|
| 1 | GAPC2 | NCBP2 | NCBP2 | TUB | Actin | NCBP2 | NCBP2 |
| 5.93 ± 1.35 | 7.66±1.88 | 6.13 ± 1.45 | 6.96 ± 1.89 | 6.75 ± 1.76 | 9.24 ± 2.36 | 8.26 ± 2.04 | |
| 2 | TIP41 | CYP2 | TUB | NCBP2 | GAPDH | GAPC2 | GAPC2 |
| 7.59 ± 1.88 | 10.58 ± 2.67 | 6.64 ± 1.65 | 7.51 ± 1.94 | 8.26 ± 1.90 | 9.92 ± 2.21 | 8.99 ± 2.00 | |
| 3 | Actin | TUB | TIP41 | GAPC2 | TUB | CYP2 | TUB |
| 8.48 ± 2.04 | 11.08 ± 2.91 | 7.44 ± 1.75 | 7.95 ± 1.84 | 8.27 ± 2.14 | 10.15 ± 2.75 | 9.05 ± 2.37 | |
| 4 | NCBP2 | TIP41 | GAPC2 | TIP41 | GAPC2 | TUB | TIP41 |
| 8.70 ± 2.17 | 11.46 ± 2.85 | 7.57 ± 1.56 | 8.10 ± 2.07 | 8.77 ± 1.94 | 11.16 ± 2.88 | 9.49 ± 2.34 | |
| 5 | TUB | PP2A | CYP2 | PP2A | NCBP2 | GAPDH | ACTIN |
| 9.14 ± 2.45 | 11.65 ± 2.67 | 8.92 ± 2.21 | 9.39 ± 2.37 | 10.07 ± 2.46 | 11.59 ± 2.49 | 10.75 ± 2.69 | |
| 6 | PP2A | Actin | GAPDH | GAPDH | TIP41 | PP2A | CYP2 |
| 9.31 ± 2.18 | 12.35 ± 3.20 | 9.35 ± 1.91 | 11.48 ± 2.60 | 10.34 ± 2.49 | 11.92 ± 2.85 | 10.78 ± 2.76 | |
| 7 | CYP2 | GAPC2 | PP2A | Actin | CYP2 | Actin | GAPDH |
| 9.72 ± 2.54 | 12.73 ± 2.85 | 9.72 ± 2.18 | 11.51 ± 2.83 | 11.20 ± 2.85 | 12.93 ± 3.22 | 10.97 ± 2.42 | |
| 8 | GAPDH | GAPDH | Actin | CYP2 | PP2A | TIP41 | PP2A |
| 11.27 ± 2.45 | 12.93 ± 2.95 | 12.84 ± 3.18 | 12.36 ± 3.21 | 11.48 ± 2.65 | 12.97 ± 3.51 | 11.13 ± 2.61 | |
| 9 | EF-1 | EF-1 | EF-1 | EF-1 | EF-1 | EF-1 | EF-1 |
| 26.25 ± 4.55 | 29.27 ± 4.98 | 25.23 ± 4.04 | 30.66 ± 5.83 | 23.21 ± 4.23 | 29.51 ± 4.71 | 27.64 ± 4.81 |
| Rank | NaCl | PEG | Cold | MeJA | CuSO4 | WT | Total |
|---|---|---|---|---|---|---|---|
| 1 | TIP41 | PP2A | PP2A | TUB | GAPC2 | CYP2 | NCBP2 |
| 0.228 | 0.136 | 0.627 | 0.339 | 0.189 | 0.569 | 0.608 | |
| 2 | NCBP2 | CYP2 | TIP41 | TIP41 | NCBP2 | GAPC2 | GAPC2 |
| 0.228 | 0.222 | 0.651 | 0.364 | 0.200 | 0.690 | 0.615 | |
| 3 | GAPC2 | TIP41 | CYP2 | NCBP2 | TUB | GAPDH | TIP41 |
| 0.394 | 0.389 | 0.677 | 0.504 | 0.339 | 0.873 | 0.643 | |
| 4 | TUB | GAPDH | GAPC2 | GAPC2 | TIP41 | TIP41 | PP2A |
| 0.450 | 0.589 | 0.698 | 0.664 | 0.437 | 1.214 | 0.750 | |
| 5 | CYP2 | NCBP2 | TUB | PP2A | PP2A | NCBP2 | TUB |
| 0.588 | 0.590 | 0.892 | 0.769 | 0.488 | 1.282 | 1.028 | |
| 6 | GAPDH | GAPC2 | NCBP2 | GAPDH | CYP2 | PP2A | GAPDH |
| 0.654 | 0.819 | 1.032 | 1.449 | 0.628 | 1.482 | 1.136 | |
| 7 | PP2A | TUB | Actin | Actin | GAPDH | Actin | CYP2 |
| 0.685 | 0.919 | 1.422 | 1.695 | 0.890 | 1.657 | 1.410 | |
| 8 | Actin | Actin | GAPDH | CYP2 | Actin | TUB | Actin |
| 2.465 | 3.211 | 1.517 | 3.008 | 2.177 | 3.565 | 2.317 | |
| 9 | EF-1 | EF-1 | EF-1 | EF-1 | EF-1 | EF-1 | EF-1 |
| 3.731 | 3.286 | 3.523 | 4.774 | 3.142 | 4.155 | 3.694 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Li, C.; Zhang, J.; Chen, F.; Gong, Y.; Li, Y.; Su, Y.; Wei, Y.; Zhao, Y. Selection of the Reference Gene for Expression Normalization in Papaver somniferum L. under Abiotic Stress and Hormone Treatment. Genes 2020, 11, 124. https://doi.org/10.3390/genes11020124
Zhang Z, Li C, Zhang J, Chen F, Gong Y, Li Y, Su Y, Wei Y, Zhao Y. Selection of the Reference Gene for Expression Normalization in Papaver somniferum L. under Abiotic Stress and Hormone Treatment. Genes. 2020; 11(2):124. https://doi.org/10.3390/genes11020124
Chicago/Turabian StyleZhang, Zhaoping, Changjian Li, Junqing Zhang, Fang Chen, Yongfu Gong, Yanrong Li, Yujie Su, Yujie Wei, and Yucheng Zhao. 2020. "Selection of the Reference Gene for Expression Normalization in Papaver somniferum L. under Abiotic Stress and Hormone Treatment" Genes 11, no. 2: 124. https://doi.org/10.3390/genes11020124
APA StyleZhang, Z., Li, C., Zhang, J., Chen, F., Gong, Y., Li, Y., Su, Y., Wei, Y., & Zhao, Y. (2020). Selection of the Reference Gene for Expression Normalization in Papaver somniferum L. under Abiotic Stress and Hormone Treatment. Genes, 11(2), 124. https://doi.org/10.3390/genes11020124





