Identification of Caprine KRTAP28-1 and Its Effect on Cashmere Fiber Diameter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Goats Investigated and Cashmere Data Collection
2.2. Search for the Caprine KAP28-1 Gene
2.3. Polymerase Chain Reaction-Single Strand Conformation Polymorphism (PCR-SSCP) Analysis of Caprine KRTAP28-1
2.4. Sequencing of KRTAP28-1 Variants and Sequence Analyses
2.5. Statistical Analyses
3. Results
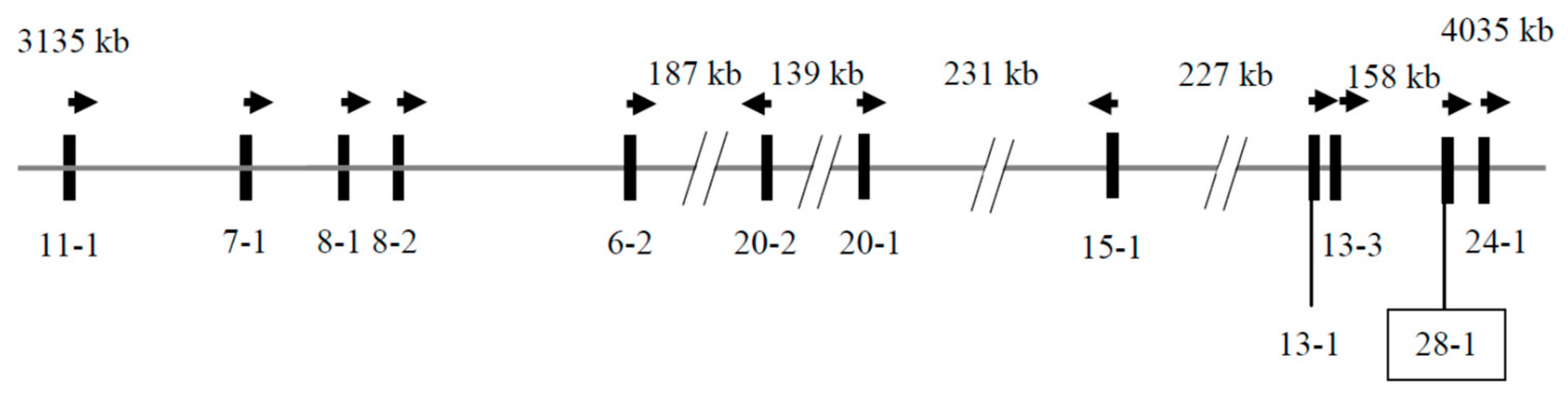
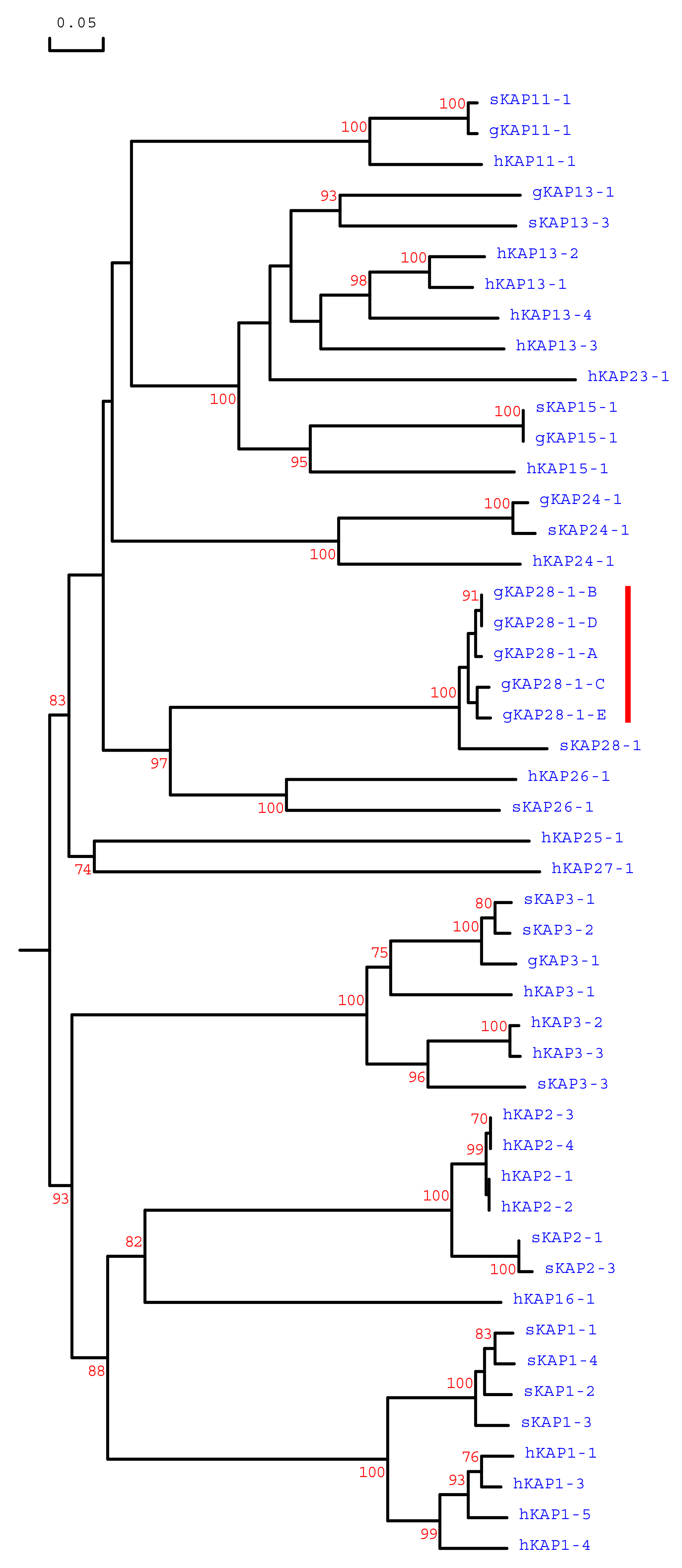
3.1. Identification of KRTAP28-1 in the Goat Genome
3.2. Detection of Sequence Variation in Caprine KRTAP28-1
3.3. Variant and Genotype Frequencies of KRTAP28-1 in the Longdong Cashmere Goats
3.4. Effect of Variation in KRTAP28-1 on Cashmere Traits
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- China National Commission of Animal Genetic Resources. Animal Genetic Resources in China: Sheep and Goats, 1st ed.; China Agricultural Press: Beijing, China, 2011; p. 127. [Google Scholar]
- Gong, H.; Zhou, H.; Forrest, R.H.; Li, S.; Wang, J.; Dyer, J.M.; Luo, Y.; Hickford, J.G. Wool keratin-associated protein genes in Sheep—A Review. Genes 2016, 7, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, M.A.; Schweizer, J. Human KAP genes, only the half of it? Extensive size polymorphisms in hair keratin-associated protein genes. J. Investig. Dermatol. 2005, 124, vii–ix. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, M.A.; Langbein, L.; Praetzel-Wunder, S.; Giehl, K. Characterization and expression analysis of the hair keratin associated protein KAP26.1. Br. J. Dermatol. 2008, 159, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Gong, H.; Zhou, H.; Tao, J.; Hickford, J.G.H. A nucleotide substitution in the ovine KAP20-2 gene leads to a premature stop codon that affects wool fibre curvature. Anim. Genet. 2018, 49, 357–358. [Google Scholar] [CrossRef]
- Bai, L.; Wang, J.; Zhou, H.; Gong, H.; Tao, J.; Hickford, J.G.H. Identification of Ovine KRTAP28-1 and Its Association with Wool Fibre Diameter. Animals 2019, 9, 142. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.; Zhou, H.; Bai, L.; Li, W.; Li, S.; Wang, J.; Luo, Y.; Hickford, J.G.H. Associations between variation in the ovine high glycine-tyrosine keratin-associated protein gene KRTAP20-1 and wool traits. J. Anim. Sci. 2019, 97, 587–595. [Google Scholar] [CrossRef]
- Gong, H.; Zhou, H.; Wang, J.; Li, S.; Luo, Y.; Hickford, J.G.H. Characterisation of an ovine keratin associated protein (KAP) gene, which would produce a protein rich in glycine and tyrosine, but lacking in cysteine. Genes 2019, 10, 848. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhou, H.; Gong, H.; Zhao, F.; Hu, J.; Luo, Y.; Hickford, J.G. Identification of the Ovine Keratin-Associated Protein 26-1 Gene and Its Association with Variation in Wool Traits. Genes 2017, 8, 225. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhou, H.; Gong, H.; Zhao, F.; Wang, J.; Liu, X.; Luo, Y.; Hickford, J.G. Identification of the Ovine Keratin-Associated Protein 22-1 (KAP22-1) Gene and Its Effect on Wool Traits. Genes 2017, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhou, H.; Luo, Y.; Zhao, M.; Gong, H.; Hao, Z.; Hu, J.; Hickford, J.G.H. Variation in the Caprine KAP24-1 Gene Affects Cashmere Fibre Diameter. Animals 2019, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, N.; Jia, C.; Zhu, X.; Jia, Z. Effect of the polymorphisms of keratin associated protein 8.2 gene on fibre traits in Inner Mongolia cashmere goats. Asian Australas. J. Anim. Sci. 2007, 20, 821–826. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, W.; Zhang, F.; Shao, Y.; Yu, S. The polymorphism of a novel mutation of KAP13.1 gene and its associations with cashmere traits on Xinjiang local goat breed in China. Asian J. Anim. Vet. Adv. 2010, 5, 34–42. [Google Scholar]
- Wang, J.; Che, L.; Hickford, J.G.; Zhou, H.; Hao, Z.; Luo, Y.; Hu, J.; Liu, X.; Li, S. Identification of the Caprine Keratin-Associated Protein 20-2 (KAP20-2) Gene and Its Effect on Cashmere Traits. Genes 2017, 8, 328. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Hao, Z.; Zhou, H.; Luo, Y.; Hu, J.; Liu, X.; Li, S.; Hickford, J.G.H. A keratin-associated protein (KAP) gene that is associated with variation in cashmere goat fleece weight. Small Rumin. Res. 2018, 167, 104–109. [Google Scholar] [CrossRef]
- Zhou, H.; Hickford, J.G.H.; Fang, Q. A two-step procedure for extracting genomic DNA from dried blood spots on filter paper for polymerase chain reaction amplification. Anal. Biochem. 2006, 354, 159–161. [Google Scholar] [CrossRef]
- Byun, S.O.; Fang, Q.; Zhou, H.; Hickford, J.G.H. An effective method for silver-staining DNA in large numbers of polyacrylamide gels. Anal. Biochem. 2009, 385, 174–175. [Google Scholar] [CrossRef]
- Gong, H.; Zhou, H.; Hickford, J.G.H. Diversity of the glycine/tyrosine-rich keratin-associated protein 6 gene (KAP6) family in sheep. Mol. Biol. Rep. 2011, 38, 31–35. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, H.; Hickford, J.G.H.; Gong, H.; Wang, J.; Hu, J.; Liu, X.; Li, S.; Hao, Z.; Luo, Y. Variation in the caprine keratin-associated protein 15-1 (KAP15-1) gene affects cashmere fibre diameter. Arch. Anim. Breed. 2019, 62, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Powell, B.C.; Rogers, G.E. The role of keratin proteins and their genes in the growth, structure and properties of hair. In Formation and Structure of Human Hair; Jollès, P., Zahn, H., Höcker, H., Eds.; Birkhäuser Verlag: Basel, Switzerland, 1997; pp. 59–148. [Google Scholar]
- Matsunaga, R.; Abe, R.; Ishii, D.; Watanabe, S.; Kiyoshi, M.; Nöcker, B.; Tsuchiya, M.; Tsumoto, K. Bidirectional binding property of high glycine-tyrosine keratin-associated protein contributes to the mechanical strength and shape of hair. J. Struct. Biol. 2013, 183, 484–494. [Google Scholar] [CrossRef]
- Ku, N.O.; Liao, J.; Chou, C.F.; Omary, M.B. Implications of intermediate filament protein phosphorylation. Cancer Metastasis Rev. 1996, 15, 429–444. [Google Scholar] [CrossRef]
- McGaughey, G.B.; Gagné, M.; Rappé, A.K. π-Stacking interactions. Alive and well in proteins. J. Biol. Chem. 1998, 273, 15458–15463. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhou, H.; Forrest, R.H.J.; Hu, J.; Liu, X.; Li, S.; Luo, Y.; Hickford, J.G.H. Variation in the ovine MYF5 gene and its effect on carcass lean meat yield in New Zealand Romney sheep. Meat Sci. 2017, 131, 146–151. [Google Scholar] [CrossRef]



| Cashmere Trait (unit) | Variant | Absent | Present | p Value 1 | ||
|---|---|---|---|---|---|---|
| Mean ± SE | n | Mean ± SE | n | |||
| Cashmere weight (g) | A | 411 ± 3.9 | 152 | 412 ± 3.4 | 233 | 0.730 |
| B | 413 ± 3.4 | 226 | 409 ± 3.8 | 159 | 0.322 | |
| C | 412 ± 3.3 | 252 | 410 ± 4.1 | 133 | 0.729 | |
| D | 411 ± 3.2 | 291 | 412 ± 4.8 | 94 | 0.843 | |
| Mean fiber diameter (μm) | A | 13.7 ± 0.04 | 152 | 13.5 ± 0.03 | 233 | 0.001 |
| B | 13.5 ± 0.03 | 226 | 13.6 ± 0.04 | 159 | 0.050 | |
| C | 13.5 ± 0.03 | 252 | 13.6 ± 0.04 | 133 | 0.073 | |
| D | 13.6 ± 0.03 | 291 | 13.6 ± 0.05 | 94 | 0.467 | |
| Crimped fiber length (cm) | A | 4.3 ± 0.04 | 152 | 4.2 ± 0.04 | 233 | 0.084 |
| B | 4.2 ± 0.04 | 226 | 4.2 ± 0.04 | 159 | 0.438 | |
| C | 4.2 ± 0.03 | 252 | 4.2 ± 0.04 | 133 | 0.652 | |
| D | 4.2 ± 0.03 | 291 | 4.2 ± 0.05 | 94 | 0.951 |
| Cashmere Trait (unit) | Variant | Absent | One Copy | Two Copies | p Value | |||
|---|---|---|---|---|---|---|---|---|
| Mean ± SE | n | Mean ± SE | n | Mean ± SE | n | |||
| Cashmere weight (g) | A | 411 ± 3.9 | 152 | 410 ± 3.9 | 154 | 417 ± 5.2 | 79 | 0.431 |
| B | 413 ± 3.4 | 226 | 408 ± 4.1 | 134 | 411 ± 4.1 | 25 | 0.585 | |
| C | 412 ± 3.3 | 252 | 410 ± 4.3 | 117 | 411 ± 3.6 | 16 | 0.632 | |
| D | 411 ± 3.2 | 291 | 412 ± 5.1 | 80 | 410 ± 6.2 | 14 | 0.967 | |
| Mean fiber diameter (μm) | A | 13.7 ± 0.04Aa | 152 | 13.5 ± 0.04ABb | 154 | 13.4 ± 0.05Bc | 79 | <0.001 |
| B | 13.5 ± 0.03 | 226 | 13.6 ± 0.04 | 134 | 13.6 ± 0.06 | 25 | 0.141 | |
| C | 13.6 ± 0.03 | 252 | 13.6 ± 0.04 | 117 | 13.7 ± 0.06 | 16 | 0.094 | |
| D | 13.6 ± 0.03 | 291 | 13.6 ± 0.05 | 80 | 13.7 ± 0.05 | 14 | 0.523 | |
| Crimped fiber length (cm) | A | 4.2 ± 0.04 | 152 | 4.2 ± 0.04 | 154 | 4.2 ± 0.06 | 79 | 0.220 |
| B | 4.2 ± 0.04 | 226 | 4.2 ± 0.04 | 134 | 4.3 ± 0.05 | 25 | 0.454 | |
| C | 4.2 ± 0.03 | 252 | 4.2 ± 0.04 | 117 | 4.3 ± 0.04 | 16 | 0.656 | |
| D | 4.2 ± 0.03 | 291 | 4.2 ± 0.05 | 80 | 4.2 ± 0.06 | 14 | 0.976 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhou, H.; Hickford, J.G.H.; Zhao, M.; Gong, H.; Hao, Z.; Shen, J.; Hu, J.; Liu, X.; Li, S.; et al. Identification of Caprine KRTAP28-1 and Its Effect on Cashmere Fiber Diameter. Genes 2020, 11, 121. https://doi.org/10.3390/genes11020121
Wang J, Zhou H, Hickford JGH, Zhao M, Gong H, Hao Z, Shen J, Hu J, Liu X, Li S, et al. Identification of Caprine KRTAP28-1 and Its Effect on Cashmere Fiber Diameter. Genes. 2020; 11(2):121. https://doi.org/10.3390/genes11020121
Chicago/Turabian StyleWang, Jiqing, Huitong Zhou, Jon G. H. Hickford, Mengli Zhao, Hua Gong, Zhiyun Hao, Jiyuan Shen, Jiang Hu, Xiu Liu, Shaobin Li, and et al. 2020. "Identification of Caprine KRTAP28-1 and Its Effect on Cashmere Fiber Diameter" Genes 11, no. 2: 121. https://doi.org/10.3390/genes11020121
APA StyleWang, J., Zhou, H., Hickford, J. G. H., Zhao, M., Gong, H., Hao, Z., Shen, J., Hu, J., Liu, X., Li, S., & Luo, Y. (2020). Identification of Caprine KRTAP28-1 and Its Effect on Cashmere Fiber Diameter. Genes, 11(2), 121. https://doi.org/10.3390/genes11020121






