DDB2 Genetic Risk Factor for Ocular Squamous Cell Carcinoma Identified in Three Additional Horse Breeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Ophthalmic Examination
2.2. Evaluation of Retrospective Data
2.3. Genetic Testing
3. Results
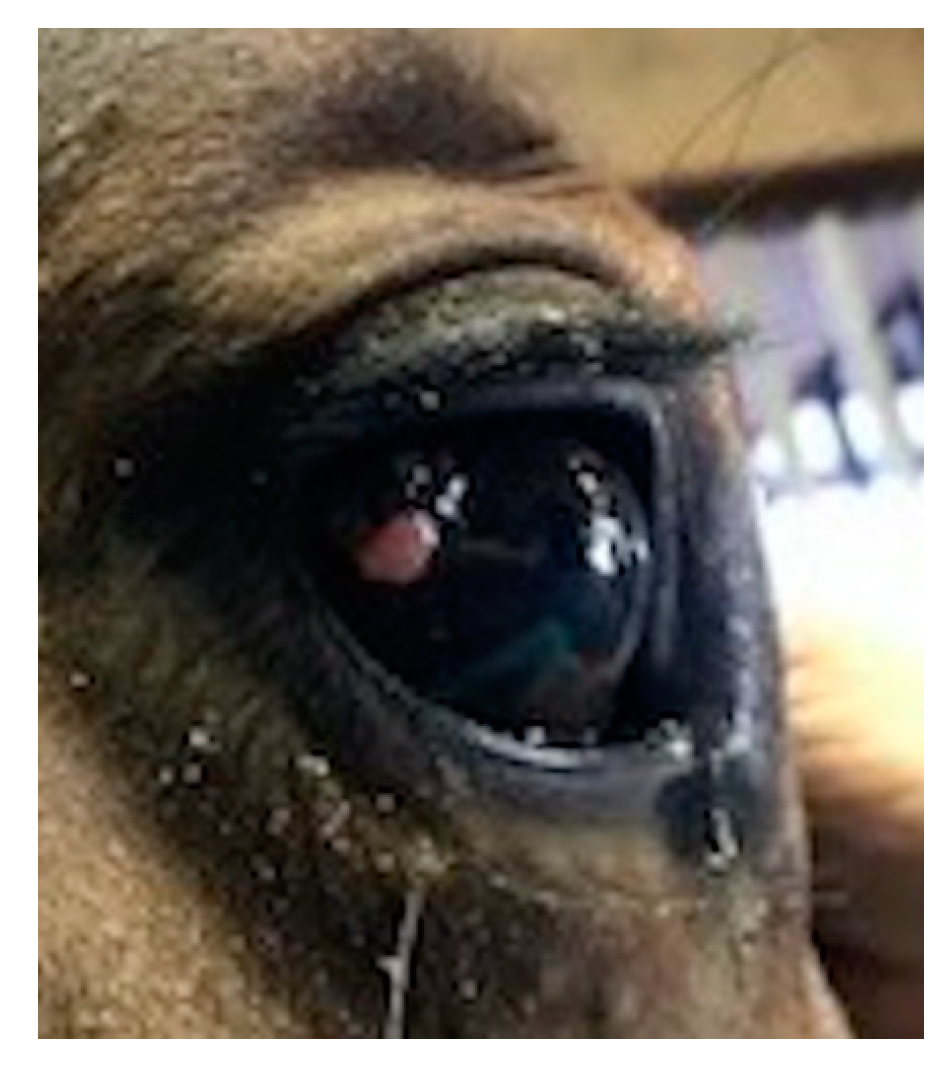
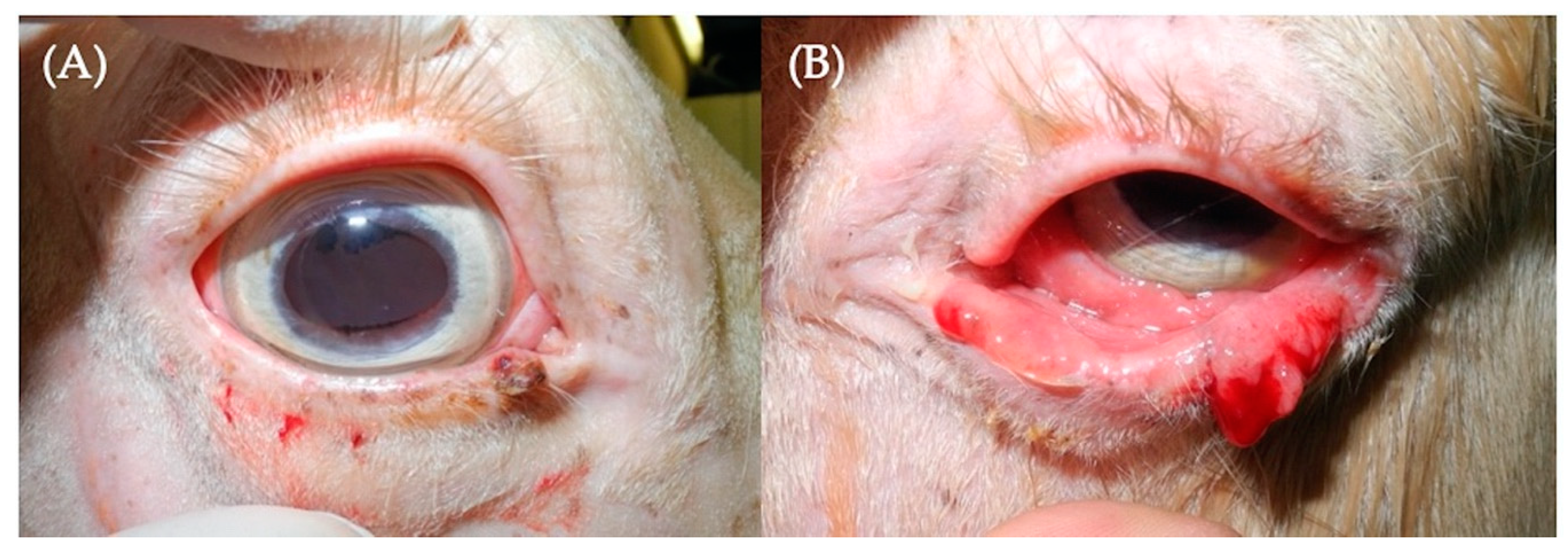
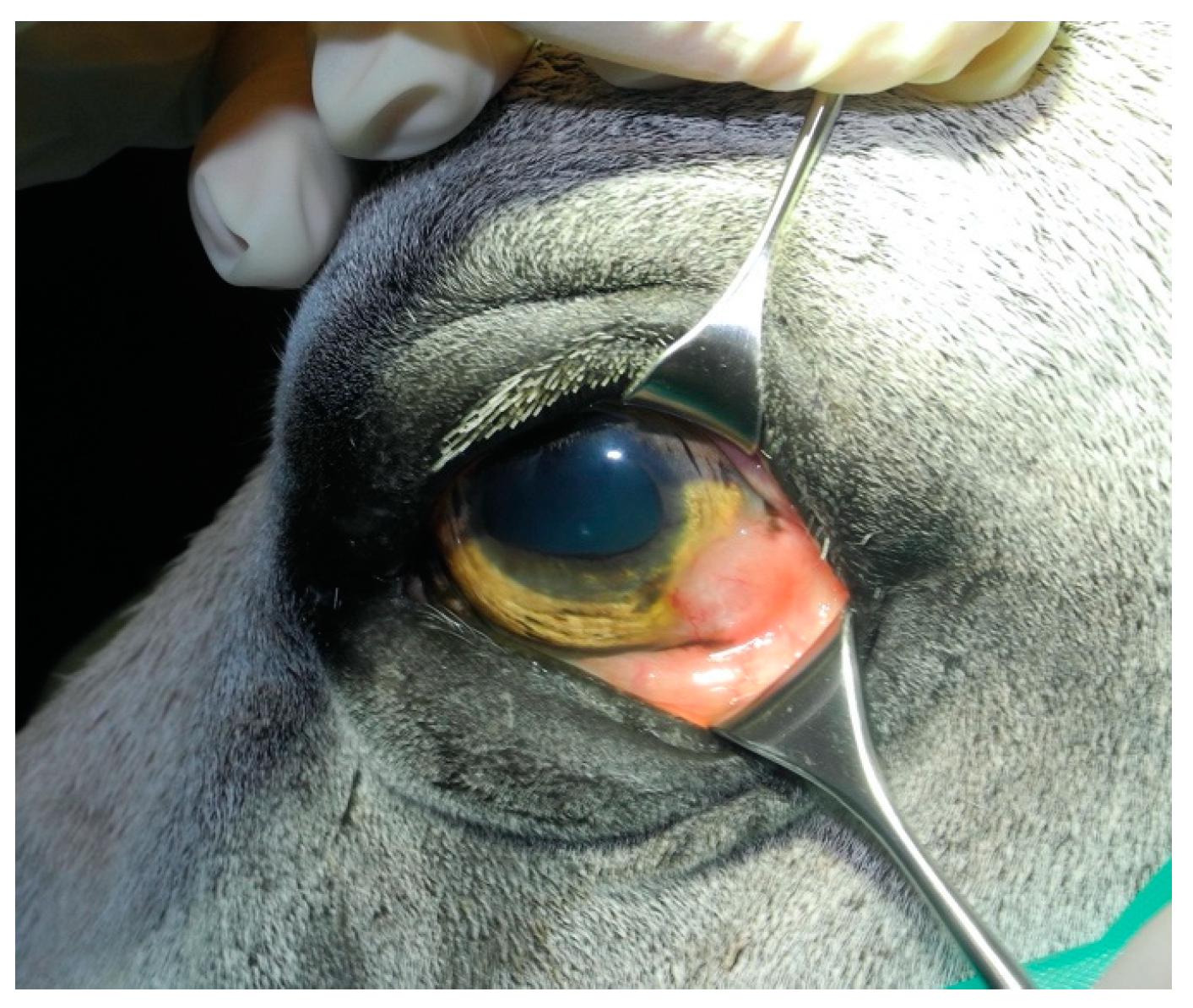
3.1. Case Presentations
3.1.1. Case 1
3.1.2. Case 2
3.1.3. Case 3
3.2. Retrospective Signalment Data
3.3. Genetic Testing
3.3.1. DDB2 and Coat Color Genotypes
3.3.2. Allele frequency of DDB2 c.1013C>T in Connemara Ponies and Holsteiners
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Strafuss, A. Squamous cell carcinoma in horses. J. Am. Vet. Med. Assoc. 1976, 168, 61–62. [Google Scholar] [PubMed]
- Knowles, E.J.; Tremaine, W.H.; Pearson, G.R.; Mair, T.S. A database survey of equine tumours in the United Kingdom. Equine Vet. J. 2015, 48, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, P.A.; Wobeser, B.; Martin, L.E.R.; Dennis, M.M.; Duncan, C.G. Cutaneous neoplastic lesions of equids in the central United States and Canada: 3,351 biopsy specimens from 3,272 equids (2000–2010). J. Am. Vet. Med. Assoc. 2013, 242, 99–104. [Google Scholar] [CrossRef]
- Bellone, R.R.; Liu, J.; Petersen, J.L.; Mack, M.; Singer-Berk, M.; Drögemüller, C.; Malvick, J.; Wallner, B.; Brem, G.; Penedo, M.C.; et al. A missense mutation in damage-specific DNA binding protein 2 is a genetic risk factor for limbal squamous cell carcinoma in horses. Int. J. Cancer 2017, 141, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Dugan, S.J.; Roberts, S.M.; Curtis, C.R.; Severin, G. Prognostic factors and survival of horses with ocular/adnexal squamous cell carcinoma: 147 cases (1978–1988). J. Am. Vet. Med. Assoc. 1991, 198, 298–303. [Google Scholar]
- Grahn, B.H.; Bauer, B.S.; Sandmeyer, L.S. Diagnostic Ophthalmology. Can. Vet. J. 2014, 55, 697–698. [Google Scholar]
- Kaps, S.; Richter, M.; Philipp, M.; Bart, M.; Eule, C.; Spiess, B.M. Primary invasive ocular squamous cell carcinoma in a horse. Vet. Ophthalmol. 2005, 8, 193–197. [Google Scholar] [CrossRef]
- Dugan, S.J.; Curtis, C.R.; Roberts, S.M.; Severin, G. Epidemiologic study of ocular/adnexal squamous cell carcinoma in horses. J. Am. Vet. Med. Assoc. 1991, 198, 251–256. [Google Scholar]
- Pazzi, K.A.; Kraegel, S.A.; Griffey, S.M.; Theon, A.P.; Madewell, B.R. Analysis of the equine tumor suppressor gene p53 in the normal horse and in eight cutaneous squamous cell carcinomas. Cancer Lett. 1996, 107, 125–130. [Google Scholar] [CrossRef]
- Lassaline, M.; Cranford, T.L.; Latimer, C.A.; Bellone, R.R. Limbal squamous cell carcinoma in Haflinger horses. Vet. Ophthalmol. 2014, 18, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bellone, R.R.; Singer-Berk, M.; Artandi, S.E. A novel DDB2 mutation causes defective DNA binding of UV-DDB and prevalent equine squamous cell carcinoma. DNA Repair. 2020, 97. [Google Scholar] [CrossRef]
- Wittschieben, B.Ø.; Iwai, S.; Wood, R.D. DDB1-DDB2 (Xeroderma Pigmentosum Group E) Protein Complex Recognizes a Cyclobutane Pyrimidine Dimer, Mismatches, Apurinic/Apyrimidinic Sites, and Compound Lesions in DNA. J. Biol. Chem. 2005, 280, 39982–39989. [Google Scholar] [CrossRef] [PubMed]
- Kapetanaki, M.G.; Guerrero-Santoro, J.; Bisi, D.C.; Hsieh, C.L.; Rapi’c-Otrin, V.; Levine, A.S. The DDB1-CUL4ADDB2 ubiquitin ligase is deficient in xeroderma pigmentosum group E and targets histone H2A at UV-damaged DNA sites. Proc. Natl. Acad. Sci. USA 2006, 103, 2588–2593. [Google Scholar] [CrossRef] [PubMed]
- Luijsterburg, M.S.; Goedhart, J.; Moser, J.; Kool, H.; Geverts, B.; Houtsmuller, A.B.; Mullenders, L.; Vermeulen, W.; Van Driel, R. Dynamic in vivo interaction of DDB2 E3 ubiquitin ligase with UV-damaged DNA is independent of damage-recognition protein XPC. J. Cell Sci. 2007, 120, 2706–2716. [Google Scholar] [CrossRef] [PubMed]
- Singer-Berk, M.; Knickelbein, K.E.; Vig, S.; Liu, J.; Bentley, E.; Nunnery, C.; Reilly, C.; Dwyer, A.; Drögemüller, C.; Unger, L.; et al. Genetic risk for squamous cell carcinoma of the nictitating membrane parallels that of the limbus in Haflinger horses. Anim. Genet. 2018, 49, 457–460. [Google Scholar] [CrossRef]
- Knickelbein, K.E.; Lassaline, M.; Singer-Berk, M.; Reilly, C.M.; Clode, A.B.; Famula, T.R.; Michau, T.M.; Bellone, R.R. A missense mutation in damage-specific DNA binding protein 2 is a genetic risk factor for ocular squamous cell carcinoma in Belgian horses. Equine Vet. J. 2020, 52, 34–40. [Google Scholar] [CrossRef]
- Knickelbein, K.E.; Lassaline, M.; Bellone, R.R. Limbal squamous cell carcinoma in a Rocky Mountain Horse: Case report and investigation of genetic contribution. Vet. Ophthalmol. 2018, 22, 201–205. [Google Scholar] [CrossRef]
- Singer-Berk, M.H.; Knickelbein, K.E.; Lounsberry, Z.T.; Crausaz, M.; Vig, S.; Joshi, N.; Britton, M.; Settles, M.L.; Reilly, C.M.; Bentley, E.; et al. Additional Evidence for DDB2 T338M as a Genetic Risk Factor for Ocular Squamous Cell Carcinoma in Horses. Int. J. Genom. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Kainzbauer, C.; Rushton, J.O.; Tober, R.; Scase, T.; Nell, B.; Sýkora, S.; Brandt, S. Bovine papillomavirus type 1 and Equus caballus papillomavirus 2 in equine squamous cell carcinoma of the head and neck in a Connemara mare. Equine Vet. J. 2011, 44, 112–115. [Google Scholar] [CrossRef]
- Bogedale, K.; Jagannathan, V.; Gerber, V.; Unger, L. Differentially expressed microRNAs, including a large microRNA cluster on chromosome 24, are associated with equine sarcoid and squamous cell carcinoma. Vet. Comp. Oncol. 2019, 17, 155–164. [Google Scholar] [CrossRef]
- Smith, K.M.; Scase, T.J.; Miller, J.L.; Donaldson, D.; Sansom, J. Expression of cyclooxygenase-2 by equine ocular and adnexal squamous cell carcinomas. Vet. Ophthalmol. 2008, 11, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Schwink, K. Factors influencing morbidity and outcome of equine ocular squamous cell carcinoma. Equine Vet. J. 1987, 19, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Michau, T.M.; Davidson, M.G.; Gilger, B.C. Carbon dioxide laser photoablation adjunctive therapy following superficial lamellar keratectomy and bulbar conjunctivectomy for the treatment of corneolimbal squamous cell carcinoma in horses: A review of 24 cases. Vet. Ophthalmol. 2011, 15, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Mosunic, C.B.; Moore, P.A.; Carmicheal, K.P.; Chandler, M.J.; Vidyashankar, A.; Zhao, Y.; Roberts, R.E.; Dietrich, U.M. Effects of treatment with and without adjuvant radiation therapy on recurrence of ocular and adnexal squamous cell carcinoma in horses: 157 cases (1985–2002). J. Am. Vet. Med. Assoc. 2004, 225, 1733–1738. [Google Scholar] [CrossRef]
- Plummer, C.E.; Smith, S.; Andrew, S.E.; Lassaline, M.E.; Gelatt, K.N.; Brooks, D.E.; Kallberg, M.E.; Ollivier, F.J. Combined keratectomy, strontium-90 irradiation and permanent bulbar conjunctival grafts for corneolimbal squamous cell carcinomas in horses (1990–2002): 38 horses. Vet. Ophthalmol. 2007, 10, 37–42. [Google Scholar] [CrossRef]
- Veterinary Genetic Laboratory: Ocular Squamous Cell Carcinoma (SCC) in Haflinger and Belgian Horses. Available online: https://vgl.ucdavis.edu/test/haflinger-belgian-scc (accessed on 14 November 2020).
- Ensembl Genome Browser: Horse (EquCab3.0), Gene: DDB2. Available online: https://uswest.ensembl.org/Equus_caballus/Gene/Variation_Gene/Table?db=core;g=ENSECAG00000008711;r=12:11631920-11806687;t=ENSECAT00000071106 (accessed on 14 November 2020).
- Mariat, D.; Taourit, S.; Guérin, G. A mutation in the MATP gene causes the cream coat colour in the horse. Genet. Sel. Evol. 2003, 35, 1–15. [Google Scholar] [CrossRef]
- Pielberg, G.R.; Golovko, A.; Sundström, E.; Curik, I.; Lennartsson, J.; Seltenhammer, M.H.; Druml, T.; Binns, M.; Fitzsimmons, C.; Lindgren, G.; et al. A cis-acting regulatory mutation causes premature hair graying and susceptibility to melanoma in the horse. Nat. Genet 2008, 40, 1004–1009. [Google Scholar] [CrossRef]
- Kafarnik, C.; Rawlings, M.; Dubielzig, R.R. Corneal stromal invasive squamous cell carcinoma: A retrospective morphological description in 10 horses. Vet. Ophthalmol. 2009, 12, 6–12. [Google Scholar] [CrossRef]
| Veterinary Clinic | Breed | Number of Cases (Percent of Cases) | Percent of Total Ophthalmology Caseload | Number of Geldings | Number of Mares | Average Age of Diagnosis |
|---|---|---|---|---|---|---|
| Bailly Vétérinaires Clinique du Lys | Haflinger | 4 (16.7%) | 1.49% | 4 | 0 | 10 ± 4.97 |
| Connemara | 4 (16.7%) | 0.74% | 4 | 0 | 15.75 ± 6.08 | |
| Selle Français | 4 (16.7%) | 56% | 2 | 2 | 7.50 ± 2.89 | |
| Trotteur Français | 2 (8.3%) | 2.23% | 2 | 0 | 12 ± 2.83 | |
| Draft horse | 1 (4.2%) | 0.2% | 0 | 1 | 15 | |
| Paint Horse | 1 (4.2%) | 0.2% | 0 | 1 | 11 | |
| Noriker Draft Horse | 1 (4.2%) | 0.2% | 0 | 1 | 14 | |
| Anglo Arabian | 1 (4.2%) | 0.2% | 1 | 0 | 9 | |
| Appaloosa | 1 (4.2%) | 2.98% | 0 | 1 | 17 | |
| Arabian–Boulonnais cross | 1 (4.2%) | 0.2% | 1 | 0 | 12 | |
| Unknown Breed | 1 (4.2%) | 0.2% | 0 | 1 | 16 | |
| Trait Breton | 1 (4.2%) | 0.2% | 0 | 1 | N/A | |
| Irish Cob | 1 (4.2%) | 0.2% | 1 | 0 | 14 | |
| Welsh | 1 (4.2%) | 0.2% | 1 | 0 | 13 | |
| Totals | 24 | 16 (66.7%) | 8 (33.3%) | 12.09 1± 4.47 | ||
| University of Illinois Veterinary Teaching Hospital | Paint Horse | 12 (36.36%) | 15.74% | 11 | 1 | 13.67 ± 4.01 |
| Quarter Horse | 6 (18.18%) | 26.81% | 3 | 3 | 17.83 ± 7.22 | |
| Appaloosa | 4 (12.12%) | 5.96% | 3 | 1 | 21 ± 5.35 | |
| Arabian | 3 (9.09%) | 6.38% | 2 | 1 | 16 ± 4.58 | |
| Haflinger | 2 (6.06%) | 1.28% | 0 | 2 | 13 ± 1.41 | |
| Mixed Breed Horse | 2 (6.06%) | 2.98% | 1 | 1 | 16 ± 7.07 | |
| Holsteiner–Belgian Warmblood cross | 1 (3.03%) | 0.43% | 0 | 1 | 10 | |
| Clydesdale | 1 (3.03%) | 0.85% | 1 | 0 | 23 | |
| Tennessee Walker | 1 (3.03%) | 4.68% | 1 | 0 | 9 | |
| Thoroughbred | 1 (3.03%) | 7.23% | 0 | 1 | 9 | |
| Totals | 33 | 22 (66.7%) | 11 (33.3%) | 15.52± 5.58 | ||
| Cumulative Totals | 57 | 38 (66.7%) | 19 (33.3%) | 14.11 1± 5.39 |
| Case Number | Breed | Age (Years) | Ocular SCC | DDB2 c.1013C>T Genotype |
|---|---|---|---|---|
| Case 1 | Holsteiner–Belgian Warmblood cross | 10 | Conjunctival in situ OD | T/T |
| Case 2 | Connemara Pony | 7 | Eyelid OD, Limbal and Eyelid OS | T/T |
| Case 3 | Connemara Pony | 17 | Limbal OS | T/T |
| Case Number | Red Factor | Agouti | Cream | Pearl | Silver | Dun | Champagne | White Spotting Pattern Tests 1 | Gray | Coat Color |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | E/E | A/A | N/N | N/N | N/N | nd2/nd2 | N/N | N/N | N/N | Bay |
| Case 2 | E/E | A/a | Cr/Cr | N/N | N/N | nd1/nd2 | N/N | N/N | N/N | Perlino |
| Case 3 | E/E | A/A | N/Cr | N/N | N/N | nd2/nd2 | N/N | N/PATN1 2 | G/G | Gray |
| Breed | Sample Size | Genotypic Frequency T/T | Genotypic Frequency C/T | Genotypic Frequency C/C | DDB2 c.1013C>T Allele Frequency | T/T | C/T | C/C |
|---|---|---|---|---|---|---|---|---|
| Connemara Pony | 86 | 0.070 1 | 0.29 1 | 0.64 1 | 0.22 1 | 6 | 25 | 55 |
| Holsteiner | 115 | 0 | 0.0087 1 | 0.99 1 | 0.0043 1 | 0 | 1 | 114 |
| Belgian Warmblood | 71 | 0 | 0.028 1 | 0.97 1 | 0.014 1 | 0 | 2 | 69 |
| Variant Id | Position | Ensembl Transcript ID | SNP ID | SIFT Score (SIFT Class) | Reference |
|---|---|---|---|---|---|
| rs68921635 | 12:11728317 | ENSECAT00000058569.1 | DDB2 c.1230G>C p.Leu410Phe | 0.53 (tolerated—low confidence) | [4,16] |
| rs1136080479 | 12:11707134 | ENSECAT00000009017.2 | DDB2 c.379C>G p.Leu127Val | 0.61 (tolerated) | [27] |
| rs1147672039 | 12:11726162 | ENSECAT00000009017.2 | DDB2 c.835T>G p.Ser279Ala | 0.52 (tolerated) | [27] |
| rs1151711657 | 12:11707119 | ENSECAT00000009017.2 | DDB2 c.364G>A p.Ala122Thr | 1 (tolerated) | [27] |
| rs782834602 | 12:11726610 | ENSECAT00000009017.2 | DDB2 c.956A>G p.Gln319Arg | 0.31 (tolerated) | [4,16] |
| rs1139682898 | 12:11726667 | ENSECAT00000009017.2 | DDB2 c.1013C>T p.Thr338Met | 0 (deleterious) | [4,16] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crausaz, M.; Launois, T.; Smith-Fleming, K.; McCoy, A.M.; Knickelbein, K.E.; Bellone, R.R. DDB2 Genetic Risk Factor for Ocular Squamous Cell Carcinoma Identified in Three Additional Horse Breeds. Genes 2020, 11, 1460. https://doi.org/10.3390/genes11121460
Crausaz M, Launois T, Smith-Fleming K, McCoy AM, Knickelbein KE, Bellone RR. DDB2 Genetic Risk Factor for Ocular Squamous Cell Carcinoma Identified in Three Additional Horse Breeds. Genes. 2020; 11(12):1460. https://doi.org/10.3390/genes11121460
Chicago/Turabian StyleCrausaz, Margo, Thomas Launois, Kathryn Smith-Fleming, Annette M. McCoy, Kelly E. Knickelbein, and Rebecca R. Bellone. 2020. "DDB2 Genetic Risk Factor for Ocular Squamous Cell Carcinoma Identified in Three Additional Horse Breeds" Genes 11, no. 12: 1460. https://doi.org/10.3390/genes11121460
APA StyleCrausaz, M., Launois, T., Smith-Fleming, K., McCoy, A. M., Knickelbein, K. E., & Bellone, R. R. (2020). DDB2 Genetic Risk Factor for Ocular Squamous Cell Carcinoma Identified in Three Additional Horse Breeds. Genes, 11(12), 1460. https://doi.org/10.3390/genes11121460





